Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Fe3O4-TiO2 Thin Film Photocatalyst
2.2.1. Synthesis of Magnetite Nanoparticles
2.2.2. Preparation of Fe3O4-TiO2 Nanoparticles
2.2.3. Preparation of Photocatalyst Films
2.3. Photocatalyst Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Fe3O4 and Fe3O4-TiO2 NPs Morphology, Size, Crystallinity and Composition
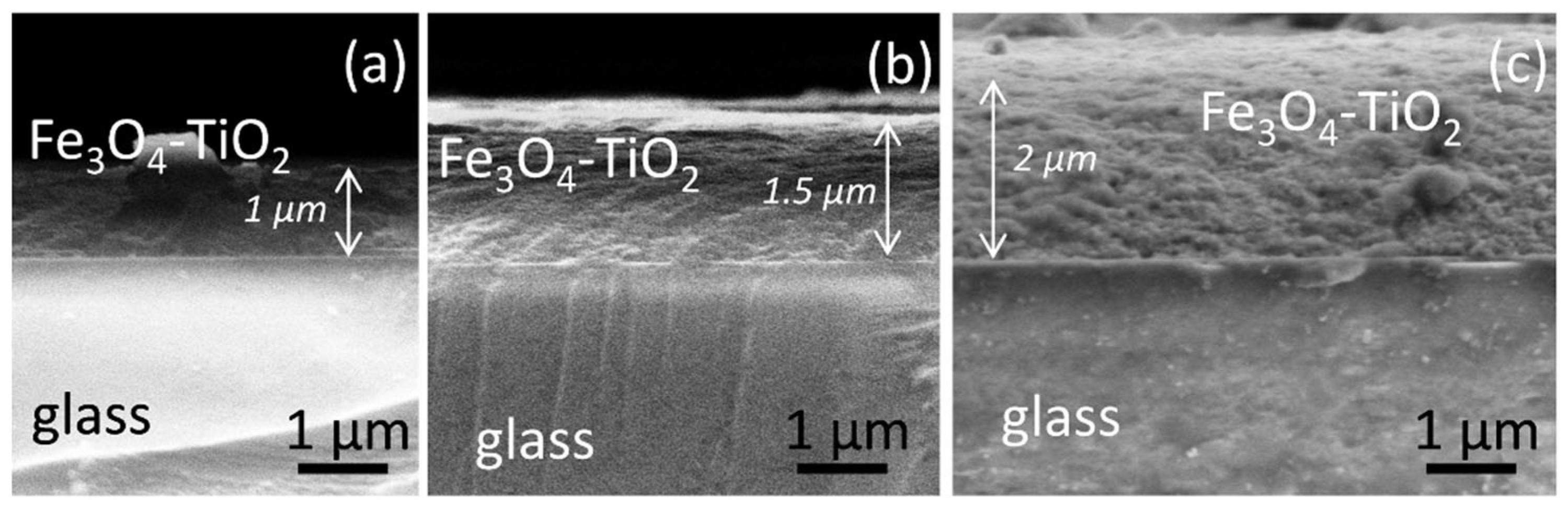
3.2. Thin Film Characterization
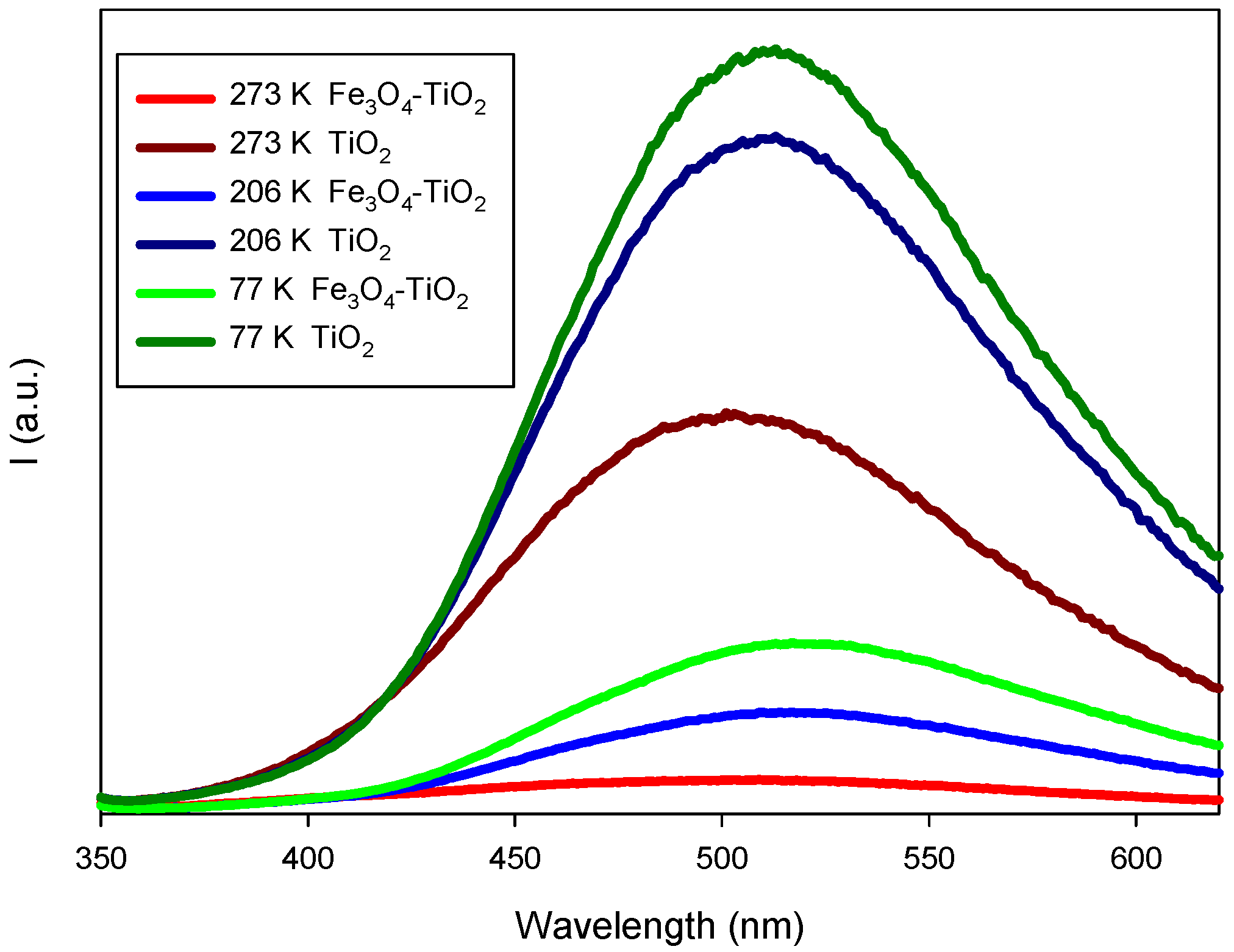
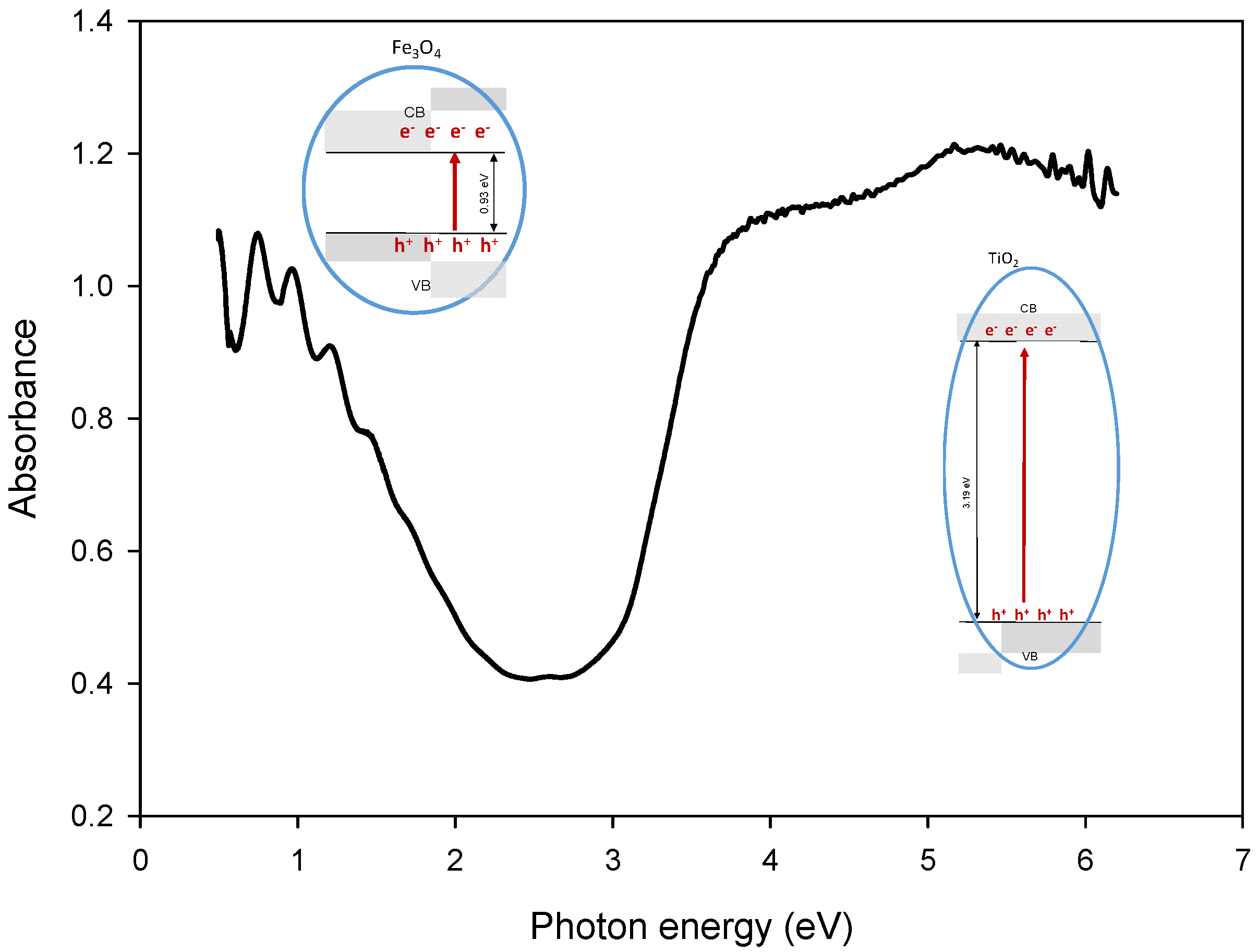
3.2.1. Optical Properties: UV-Vis Spectra, Bandgap Energy and Photoluminescence Studies
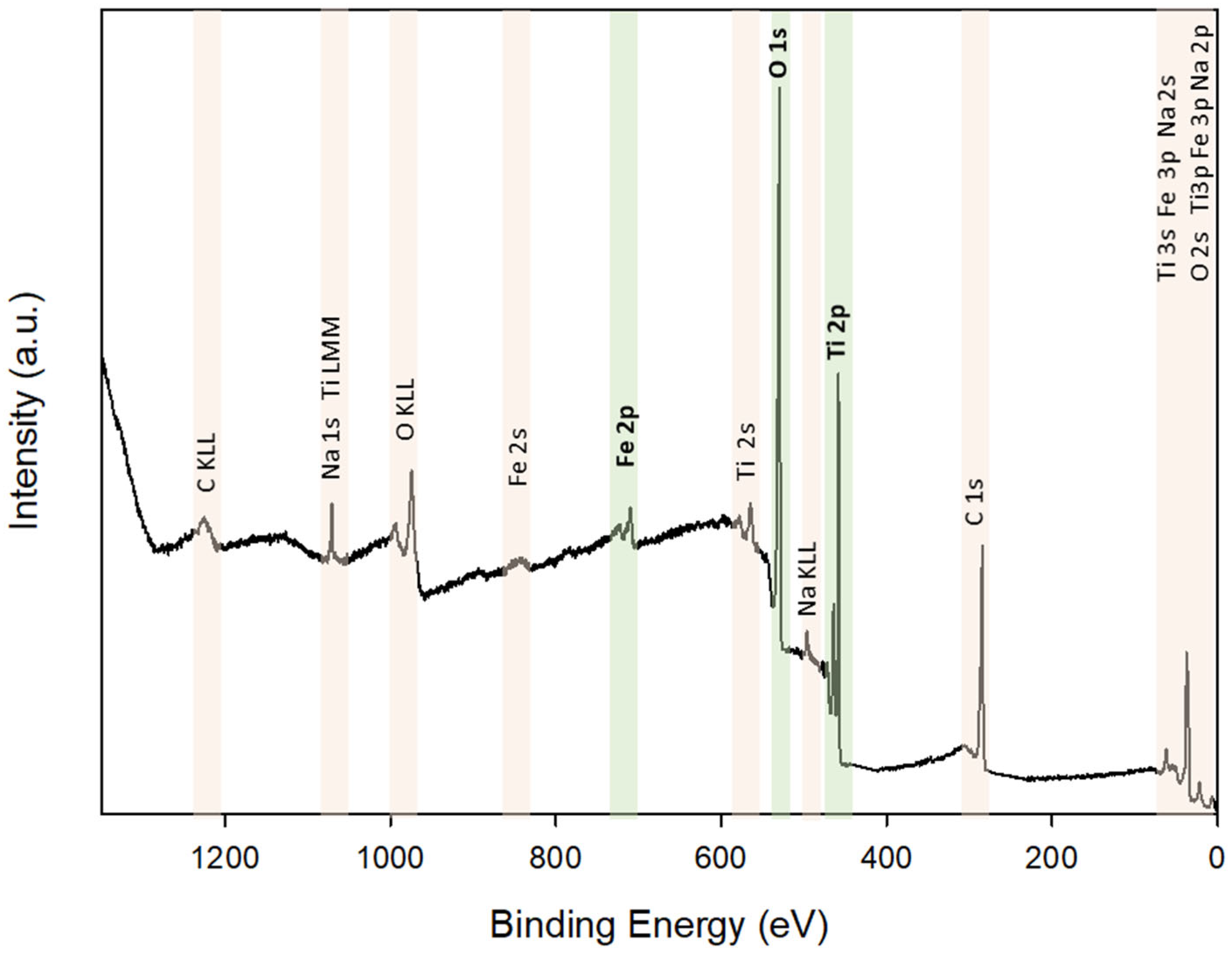
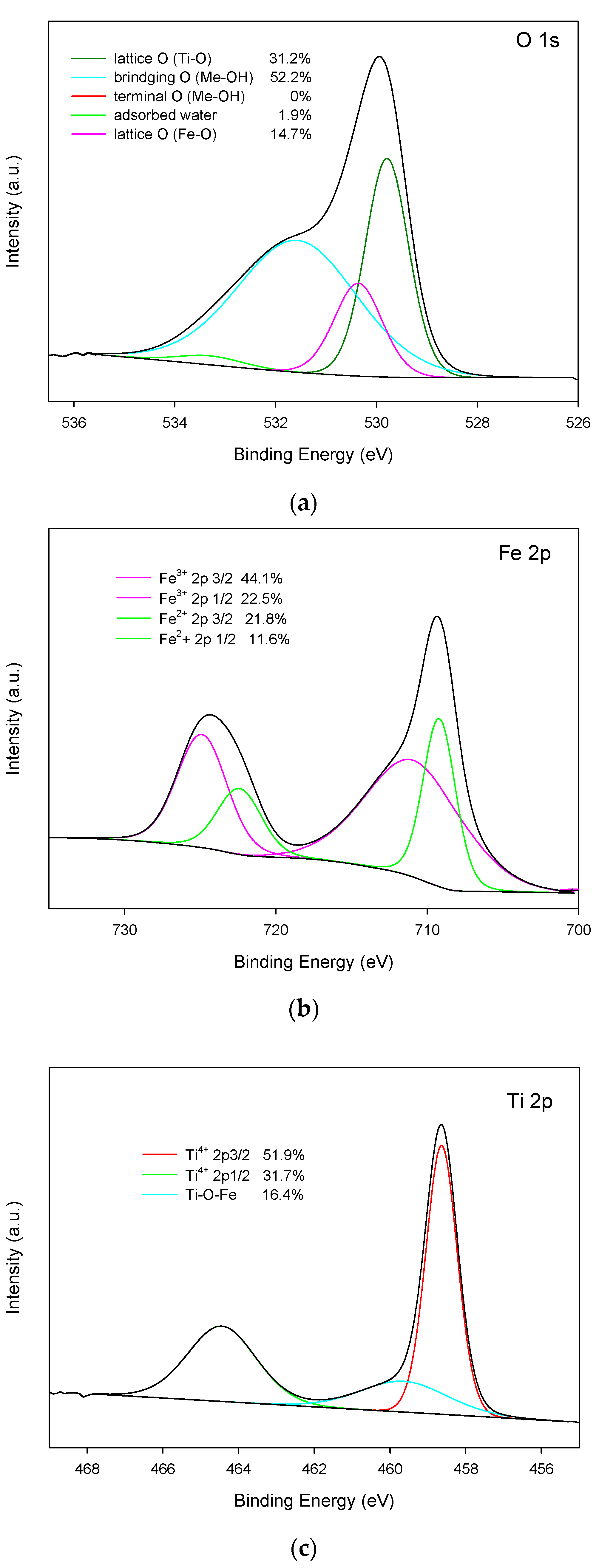
3.2.2. Composition, Structure and Morphology
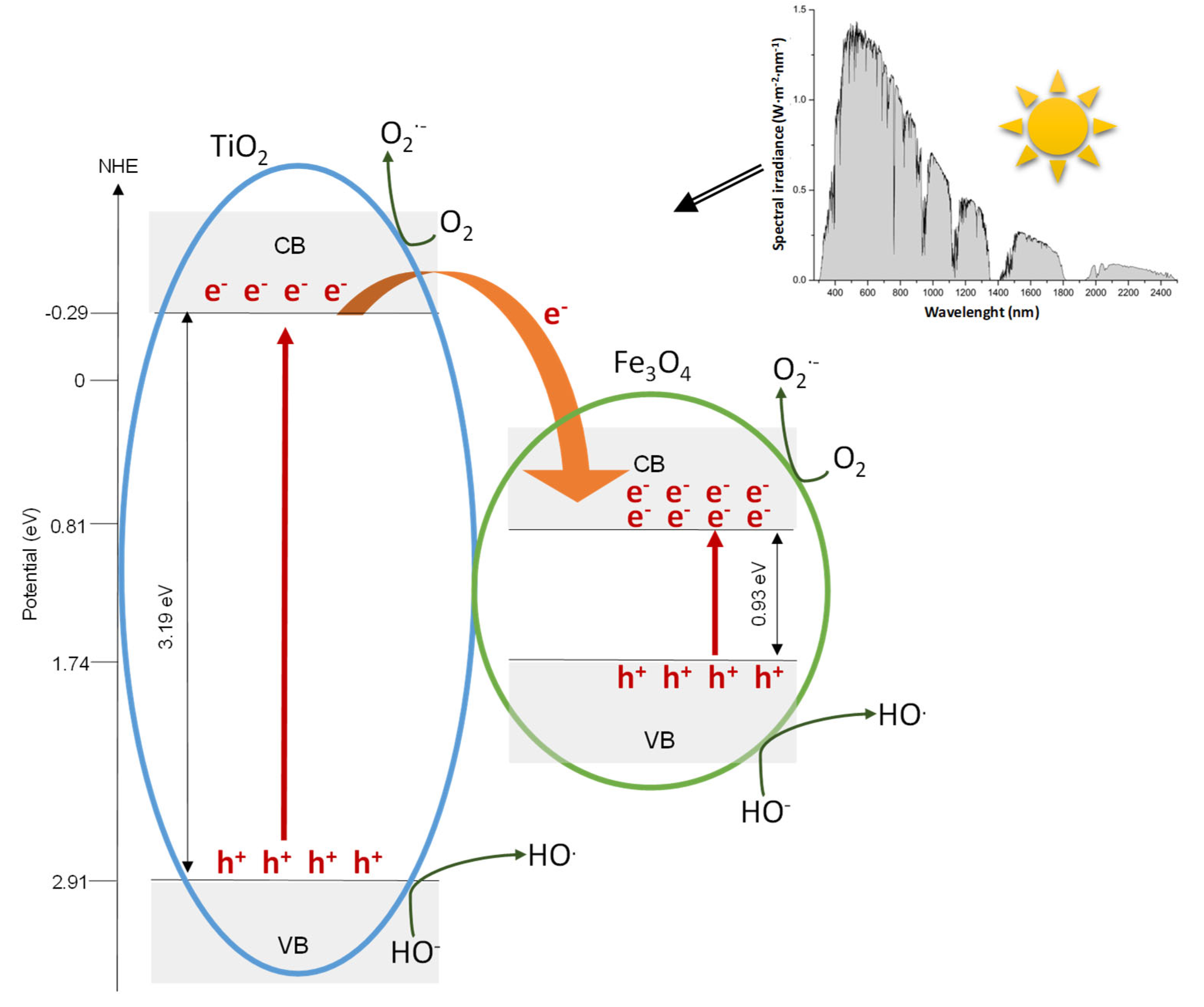
3.3. Proposed Mechanism
- Electron-hole pairs are produced in TiO2 and Fe3O4 nanoparticles.
- Electrons can be easily transferred from the conduction band (CB) of TiO2 to the CB of Fe3O4. It is a consequence of the positive energy difference between conduction bands of Fe3O4 and TiO2 (3.19 and 0.93 eV, respectively).
- The transferred electrons would then migrate to the surface of Fe3O4 to further assist to oxygen reduction to form superoxide radical anion during photocatalysis [60].
- Additionally, electrons in the conduction band of TiO2 will further react with molecular oxygen O2 dissolved to also form the superoxide radical anion O2·− [61].
- Superoxide radical anion could recombine to yield hydrogen peroxide that can react with Fe(II) generated by the electrons transferred from the TiO2 to Fe3O4, via a Fenton reaction, to generate ·OH and Fe(III) [62].
- On the other hand, holes located in the valence band of TiO2 are transferred to the surface of TiO2 generating hydroxyl radicals because of the oxidation of water or surface hydroxyl species [60].
- Radiation corresponding to wavelength range above 388 nm (E < 3.2 eV) is not energetic enough to excite TiO2 and generate electron-hole pairs, whereas Fe3O4 could be easily activated in this spectral range and produces charge carriers. Subsequently, the photogenerated electrons should be involved in the processes previously described in the step 3 of this proposed mechanism.
- The holes that are accumulated in the valence band of Fe3O4 will react with OH− species existing on the surface of the catalyst, producing reactive hydroxyl radicals (·OH).
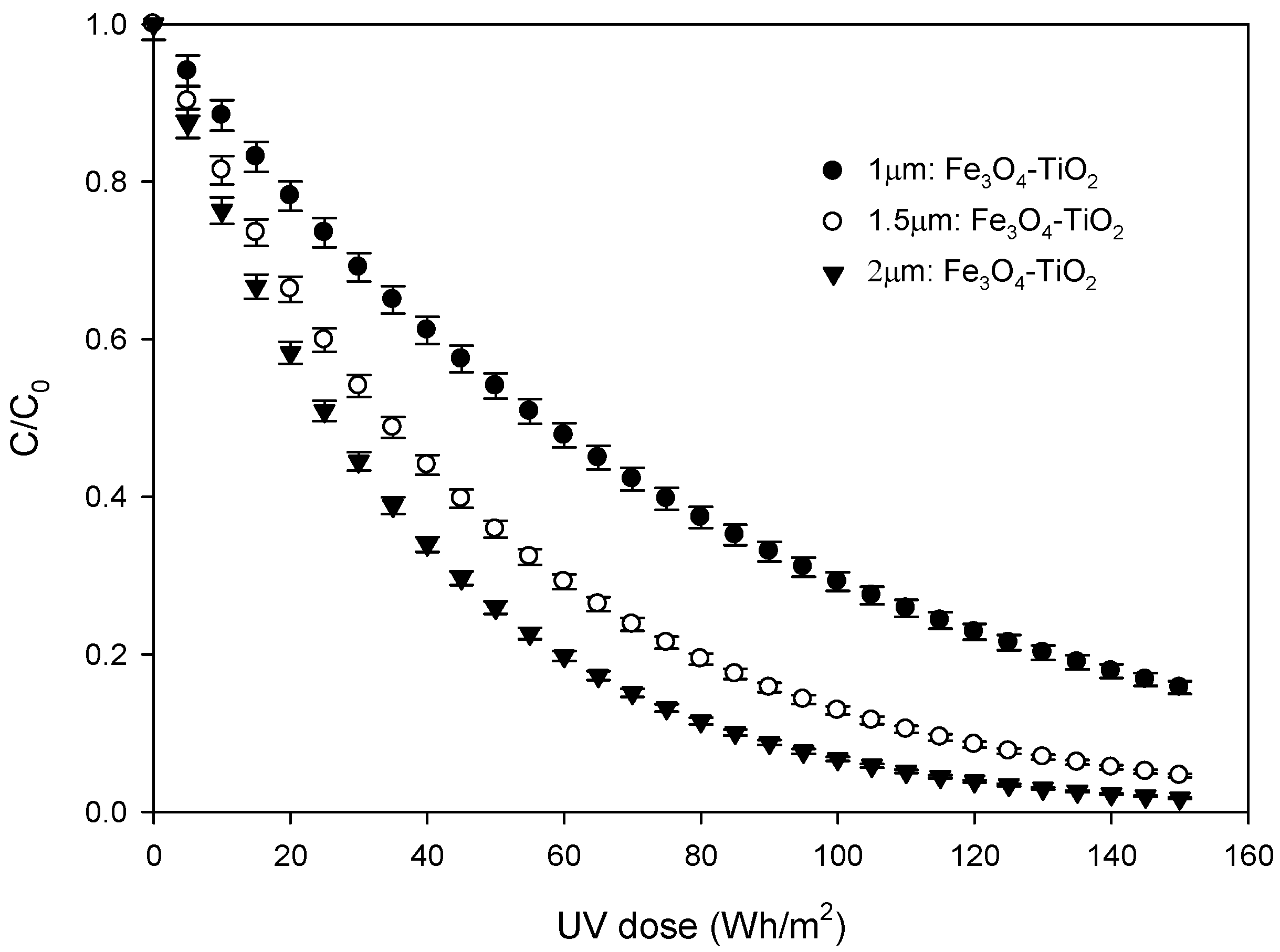
3.4. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, G.; Fu, Y.; Chu, X.; Chang, C.; Zhu, L. Highly Active Magnetic Bismuth Tungstate/Magnetite Composite under Visible Light Irradiation in the Presence of Hydrogen Peroxide. J. Colloid Interface Sci. 2015, 444, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior Antibacterial Activity of Fe3O4-TiO2 Nanosheets under Solar Light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef] [PubMed]
- Checa, M.; Figueredo, M.; Aguinaco, A.; Beltrán, F.J. Graphene Oxide/Titania Photocatalytic Ozonation of Primidone in a Visible LED Photoreactor. J. Hazard. Mater. 2019, 369, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Aguinaco, A.; Amaya, B.; Ramírez-del-Solar, M. Facile Fabrication of Fe-TiO2 Thin Film and Its Photocatalytic Activity. Environ. Sci. Pollut. Res. 2021, 29, 23292–23302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Wang, H.L.; Zi, L.Y.; Zhang, J.J.; Zhang, Y.S. Preparation and Photocatalytic Performance of Magnetic TiO2–Fe3O4/Graphene (RGO) Composites under VIS-Light Irradiation. Ceram. Int. 2015, 41, 10634–10643. [Google Scholar] [CrossRef]
- Mahadik, M.A.; Shinde, S.S.; Mohite, V.S.; Kumbhar, S.S.; Moholkar, A.V.; Rajpure, K.Y.; Ganesan, V.; Nayak, J.; Barman, S.R.; Bhosale, C.H. Visible Light Catalysis of Rhodamine B Using Nanostructured Fe2O3, TiO2 and TiO2/Fe2O3 Thin Films. J. Photochem. Photobiol. B Biol. 2014, 133, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Tarantola, M.; Schneider, D.; Sunnick, E.; Adam, H.; Pierrat, S.; Rosman, C.; Breus, V.; Sönnichsen, C.; Basché, T.; Wegener, J.; et al. Cytotoxicity of Metal and Semiconductor Nanoparticles Indicated by Cellular Micromotility. ACS Nano 2009, 3, 213–222. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanopartides. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Abbas, M.; Parvatheeswara Rao, B.; Reddy, V.; Kim, C. Fe3O4/TiO2 Core/Shell Nanocubes: Single-Batch Surfactantless Synthesis, Characterization and Efficient Catalysts for Methylene Blue Degradation. Ceram. Int. 2014, 40, 11177–11186. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. A Novel Magnetic Reusable Nanocomposite with Enhanced Photocatalytic Activities for Dye Degradation. Sep. Purif. Technol. 2014, 134, 210–219. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Toloman, D.; Popa, A.; Perhaita, I.; Senilă, M.; Marincas, O.; Barbu-Tudoran, L. Magnetic Recoverable Fe3O4-TiO2: Eu Composite Nanoparticles with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2016, 390, 248–259. [Google Scholar] [CrossRef]
- Kubiak, A.; Kubacka, M.; Gabała, E.; Dobrowolska, A.; Synoradzki, K.; Siwińska-Ciesielczyk, K.; Czaczyk, K.; Jesionowski, T. Hydrothermally Assisted Fabrication of TiO2-Fe3O4 Composite Materials and Their Antibacterial Activity. Materials 2020, 13, 4715. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Wojciechowska, A.; Grzechulska-Damszel, J.; Narkiewicz, U.; Śniadecki, Z.; Idzikowski, B. Effective Processes of Phenol Degradation on Fe3O4–TiO2 Nanostructured Magnetic Photocatalyst. J. Phys. Chem. Solids 2020, 136, 109178. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In Situ Synthesis of Lead-Free Halide Perovskite-COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Li, X.; Le, Z.; Chen, X.; Li, Z.; Wang, W.; Liu, X.; Wu, A.; Xu, P.; Zhang, D. Graphene Oxide Enhanced Amine-Functionalized Titanium Metal Organic Framework for Visible-Light-Driven Photocatalytic Oxidation of Gaseous Pollutants. Appl. Catal. B Environ. 2018, 236, 501–508. [Google Scholar] [CrossRef]
- Wannapop, S.; Somdee, A.; Bovornratanaraks, T. Experimental Study of Thin Film Fe2O3/TiO2 for Photocatalytic Rhodamine B Degradation. Inorg. Chem. Commun. 2021, 128, 108585. [Google Scholar] [CrossRef]
- Wei, S.; Fu, Q.; Liu, Y.; Ji, Q.; Hou, Y.; Luo, L.; Li, W. Three-Phases Fe3O4@TiO2-P(VDF-HFP) Composite Films with High Energy Storage Density at Low Filler Fraction under Low Operating Electric Field. J. Phys. D Appl. Phys. 2020, 53, 055504. [Google Scholar] [CrossRef]
- Zhang, C.; Si, S.; Yang, Z. A Highly Selective Photoelectrochemical Biosensor for Uric Acid Based on Core-Shell Fe3O4@C Nanoparticle and Molecularly Imprinted TiO2. Biosens. Bioelectron. 2015, 65, 115–120. [Google Scholar] [CrossRef]
- Yeh, N.; Lee, Y.C.; Chang, C.Y.; Cheng, T.C. Anti-Fish Bacterial Pathogen Effect of Visible Light Responsive Fe3O4@TiO2 Nanoparticles Immobilized on Glass Using TiO2 Sol-Gel. Thin Solid Films 2013, 549, 93–97. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; Ramírez del Solar, M.; Levchuk, I. Photocatalytic Degradation of Pharmaceutically Active Compounds (PhACs) in Urban Wastewater Treatment Plants Effluents under Controlled and Natural Solar Irradiation Using Immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F.; Oropesa, A. Ozone and Photocatalytic Processes to Remove the Antibiotic Sulfamethoxazole from Water. Water Res. 2008, 42, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of Emerging Pollutants in Urban Wastewater and Their Removal through Biological Treatment Followed by Ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Mechanism and Kinetics of Sulfamethoxazole Photocatalytic Ozonation in Water. Water Res. 2009, 43, 1359–1369. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and Removal of Pharmaceuticals and Endocrine Disruptors in South Korean Surface, Drinking, and Waste Waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of Pharmaceuticals during Drinking Water Treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Hapeshi, E.; Achilleos, A.; Meric, S.; Gros, M.; Petrovic, M.; Barcelo, D. Existence of Pharmaceutical Compounds in Tertiary Treated Urban Wastewater That Is Utilized for Reuse Applications. Water Resour. Manag. 2011, 25, 1183–1193. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Katz, D.R.; Sullivan, J.C.; Shapley, D.; Lipscomb, J.; Epstein, J.; Juhl, A.R.; Knudson, C.; O’Mullan, G.D. Spatial Patterns of Pharmaceuticals and Wastewater Tracers in the Hudson River Estuary. Water Res. 2018, 137, 335–343. [Google Scholar] [CrossRef]
- Wöhler, L.; Niebaum, G.; Krol, M.; Hoekstra, A.Y. The Grey Water Footprint of Human and Veterinary Pharmaceuticals. Water Res. X 2020, 7, 100044. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, Distribution and Environmental Risk of Pharmaceutically Active Compounds (PhACs) in Coastal and Ocean Waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–Gel Synthesis of TiO2-Fe2O3 Systems: Effects of Fe2O3 Content and Their Photocatalytic Properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Bin Hamdan, M.R. Removal of Acetaminophen Using Fe2O3-TiO2 nanocomposites by Photocatalysis under Simulated Solar Irradiation: Optimization Study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
- Govindhan, P.; Pragathiswaran, C.; Chinnadurai, M. A Magnetic Fe3O4 Decorated TiO2 Nanoparticles Application for Photocatalytic Degradation of Methylene Blue (MB) under Direct Sunlight Irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 6458–6469. [Google Scholar] [CrossRef]
- Blanco, E.; González-Leal, J.M.; Ramírez-del Solar, M. Photocatalytic TiO2 Sol-Gel Thin Films: Optical and Morphological Characterization. Sol. Energy 2015, 122, 11–23. [Google Scholar] [CrossRef]
- Blanco, E.; Domínguez, M.; González-Leal, J.M.; Márquez, E.; Outón, J.; Ramírez-del-Solar, M. Insights into the Annealing Process of Sol-Gel TiO2 Films Leading to Anatase Development: The Interrelationship between Microstructure and Optical Properties. Appl. Surf. Sci. 2018, 439, 736–748. [Google Scholar] [CrossRef]
- Beato-López, J.J.; Domínguez, M.; Ramírez-del-Solar, M.; Litrán, R. Glutathione-Magnetite Nanoparticles: Synthesis and Physical Characterization for Application as MRI Contrast Agent. SN Appl. Sci. 2020, 2, 1202. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Cáceres, J.; Fernández-Alba, A.R.; Agüera, A.; Rodríguez, A. Photocatalytic Treatment of Water-Soluble Pesticides by Photo-Fenton and TiO2 Using Solar Energy. Catal. Today 2002, 76, 209–220. [Google Scholar] [CrossRef]
- Okamura, A.; Nakamura, S.; Tanaka, M.; Siratori, K. Mössbauer Study of the Impurity Effect of In3+ and Cr3+ in the High Temperature Phase of Fe3O4. J. Phys. Soc. Jpn. 1995, 64, 3484–3495. [Google Scholar] [CrossRef]
- Kim, D.W.; Enomoto, N.; Nakagawa, Z.E.; Kawamura, K. Molecular Dynamic Simulation in Titanium Dioxide Polymorphs: Rutile, Brookite, and Anatase. J. Am. Ceram. Soc. 1996, 79, 1095–1099. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Q.; Li, J.; Chou, X.; Zhang, W.; Ye, H.; Cui, Z.; Dobson, P.J. High Photocatalytic Activity of Fe3O4-SiO2-TiO2 Functional Particles with Core-Shell Structure. J. Nanomater. 2013, 2013, 762423. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and Characterization of TiO2 Doped Cobalt Ferrite Nanoparticles via Microwave Method: Investigation of Photocatalytic Performance of Congo Red Degradation Dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Charitidis, C.; Patsalas, P.; Logothetidis, S. Optical and Mechanical Performance of Nanostructured Cerium Oxides for Applications in Optical Devices. J. Phys. Conf. Ser. 2005, 10, 226–229. [Google Scholar] [CrossRef]
- Jiang, L.; Tinoco, M.; Fernández-García, S.; Sun, Y.; Traviankina, M.; Nan, P.; Xue, Q.; Pan, H.; Aguinaco, A.; González-Leal, J.M.; et al. Enhanced Artificial Enzyme Activities on the Reconstructed Sawtoothlike Nanofacets of Pure and Pr-Doped Ceria Nanocubes. ACS Appl. Mater. Interfaces 2021, 13, 38061–38073. [Google Scholar] [CrossRef]
- Yuan, L.D.; Deng, H.X.; Li, S.S.; Wei, S.H.; Luo, J.W. Unified Theory of Direct or Indirect Band-Gap Nature of Conventional Semiconductors. Phys. Rev. B 2018, 98, 245203. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, S.; Chen, J.; Shao, J.; Cao, S. A Practical Pathway for the Preparation of Fe2O3 Decorated TiO2 Photocatalyst with Enhanced Visible-Light Photoactivity. Mater. Chem. Phys. 2017, 190, 53–61. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface Modification and Enhanced Photocatalytic CO2 Reduction Performance of TiO2: A Review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Wei, X.N.; Wang, H.L. Preparation of Magnetic G-C3N4/Fe3O4/TiO2 Photocatalyst for Visible Light Photocatalytic Application. J. Alloys Compd. 2018, 763, 844–853. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. Role of Silica Mid-Layer in Thermal and Chemical Stability of Hierarchical Fe3O4-SiO2-TiO2 Nanoparticles for Improvement of Lead Adsorption: Kinetics, Thermodynamic and Deep XPS Investigation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114690. [Google Scholar] [CrossRef]
- Henderson, M.A. Structural Sensitivity in the Dissociation of Water on TiO2 Single-Crystal Surfaces. Langmuir 1996, 12, 5093–5098. [Google Scholar] [CrossRef]
- Scott, T.B.; Allen, G.; Heard, P.J.; Randell, M.G. Reduction of U(VI) to U(IV) on the Surface of Magnetite. Geochim. Cosmochim. Acta 2005, 69, 5639–5646. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Wang, F.; Li, M.; Yu, L.; Sun, F.; Wang, Z.; Zhang, L.; Zeng, H.; Xu, X. Corn-like, Recoverable γ-Fe2O3@SiO2@TiO2 Photocatalyst Induced by Magnetic Dipole Interactions. Sci. Rep. 2017, 7, 2–11. [Google Scholar] [CrossRef]
- Ran, F.Y.; Tsunemaru, Y.; Hasegawa, T.; Takeichi, Y.; Harasawa, A.; Yaji, K.; Kim, S.; Kakizaki, A. Angle-Resolved Photoemission Study of Fe3O4(001) Films across Verwey Transition. J. Phys. D Appl. Phys. 2012, 45, 275002. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Güzelçimen, F.; Tanören, B.; Çetinkaya, Ç.; Kaya, M.D.; Efkere, H.İ.; Özen, Y.; Bingöl, D.; Sirkeci, M.; Kınacı, B.; Ünlü, M.B.; et al. The Effect of Thickness on Surface Structure of Rf Sputtered TiO2 Thin Films by XPS, SEM/EDS, AFM and SAM. Vacuum 2020, 182, 109766. [Google Scholar] [CrossRef]
- Jiang, L.; Fernandez-Garcia, S.; Tinoco, M.; Yan, Z.; Xue, Q.; Blanco, G.; Calvino, J.J.; Hungria, A.B.; Chen, X. Improved Oxidase Mimetic Activity by Praseodymium Incorporation into Ceria Nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 18595–18608. [Google Scholar] [CrossRef]
- Ropero Vega, J.L.; Flórez-Castillo, J.M. Desinfección de Aguas Por Fotocatálisis Heterogénea Usando Semiconductores Acoplados de WO3-TiO2. 2015; 1–5. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. Using One-Step Facile and Solvent-Free Mechanochemical Process to Synthesize Photoactive Fe2O3-TiO2for Treating Industrial Wastewater. J. Alloys Compd. 2017, 695, 496–507. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, L. Core-Shell Structured α-Fe2O3@TiO2 Nanocomposites with Improved Photocatalytic Activity in the Visible Light Region. Phys. Chem. Chem. Phys. 2013, 15, 18627–18634. [Google Scholar] [CrossRef]
- Hassan, M.E.; Chen, Y.; Liu, G.; Zhu, D.; Cai, J. Heterogeneous Photo-Fenton Degradation of Methyl Orange by Fe2O3/TiO2 Nanoparticles under Visible Light. J. Water Process Eng. 2016, 12, 52–57. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. Photocatalytic Degradation of Industrial Pulp and Paper Mill Effluent Using Synthesized Magnetic Fe2O3-TiO2: Treatment Efficiency and Characterizations of Reused Photocatalyst. J. Environ. Manag. 2017, 187, 298–310. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Shekofteh-Gohari, M. Novel Magnetic Fe3O4/ZnO/NiWO4 Nanocomposites: Enhanced Visible-Light Photocatalytic Performance through p-n Heterojunctions. Sep. Purif. Technol. 2017, 184, 334–346. [Google Scholar] [CrossRef]
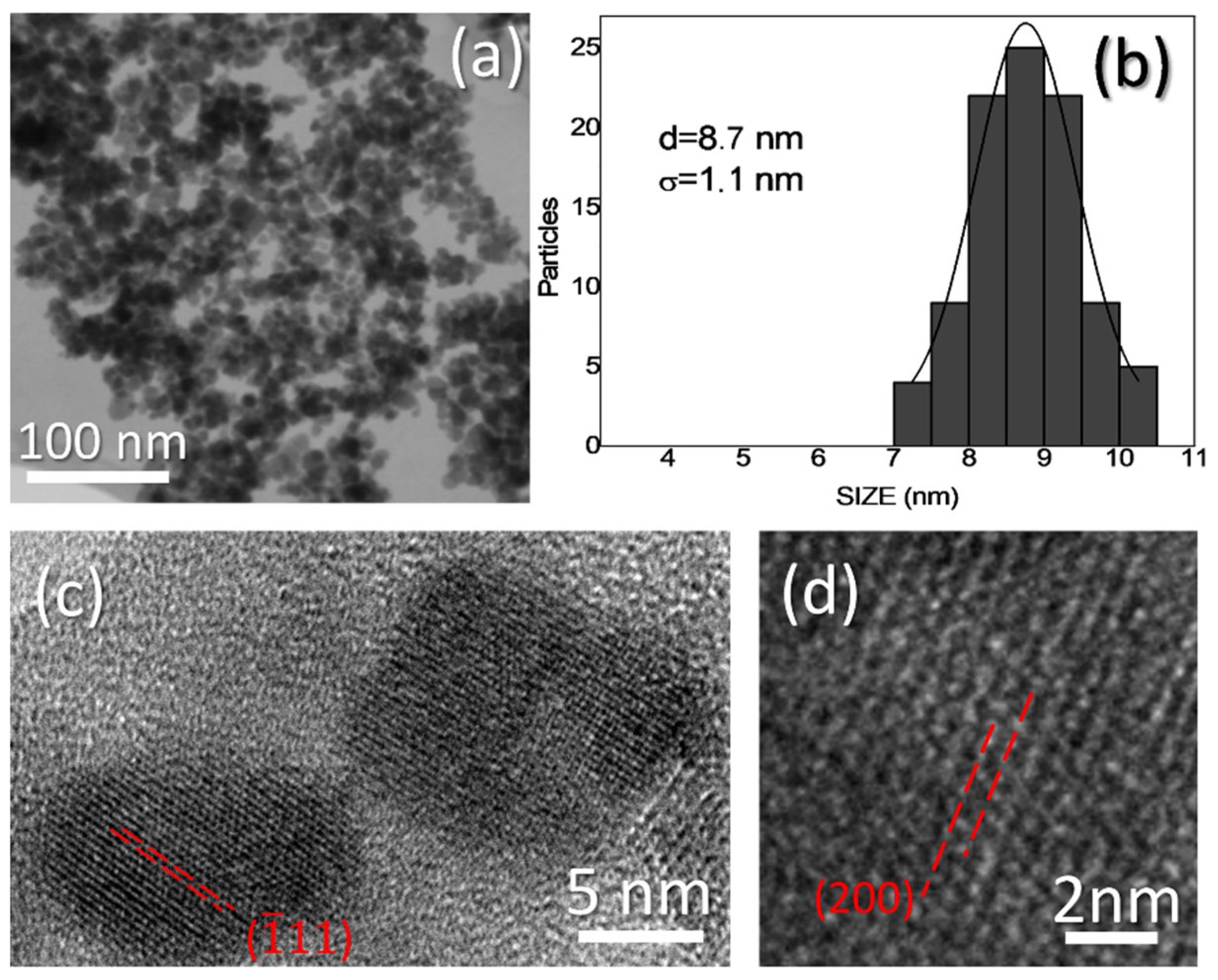



| DLS Size (nm) | PDI | Z Potential (mV) |
|---|---|---|
| 68.7 | 0.3 | 23.7 |
| Material | Indirect Eg (eV) | Direct Eg (eV) |
|---|---|---|
| TiO2 | 3.19 ± 0.05 | 3.61 ± 0.06 |
| Fe3O4-TiO2 | 2.79 ± 0.05 | 3.31 ± 0.04 |
| Fe3O4 | 0.93 ± 0.01 | 2.85 ± 0.06 |
| Sample | Measurement | RMS (nm) |
|---|---|---|
| 1 µm:Fe3O4-TiO2 | 1 | 25.6 |
| 2 | 23.4 | |
| 3 | 21.5 | |
| Mean ± s.d | 23.5 ± 2.1 | |
| 1.5 µm:Fe3O4-TiO2 | 1 | 20.2 |
| 2 | 18.8 | |
| 3 | 15.9 | |
| Mean ± s.d. | 18.3 ± 2.2 | |
| 2 µm:Fe3O4-TiO2 | 1 | 20.6 |
| 2 | 24.8 | |
| 3 | 20.2 | |
| Mean ± s.d. | 21.9 ± 2.5 |
| Sample | Thickness (µm) | k (10−2 min−1) |
|---|---|---|
| 1 µm:Fe3O4-TiO2 | 0.97 ± 0.03 | 0.62 ± 0.004 |
| 1.5 µm:Fe3O4-TiO2 | 1.51 ± 0.04 | 1.03 ± 0.004 |
| 2 µm:Fe3O4-TiO2 | 1.99 ± 0.02 | 1.35 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguinaco, A.; Mánuel, J.M.; Blanco, E.; Domínguez, M.; Litrán, R.; Delgado, J.J.; Ramírez-del-Solar, M. Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes. Materials 2022, 15, 6718. https://doi.org/10.3390/ma15196718
Aguinaco A, Mánuel JM, Blanco E, Domínguez M, Litrán R, Delgado JJ, Ramírez-del-Solar M. Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes. Materials. 2022; 15(19):6718. https://doi.org/10.3390/ma15196718
Chicago/Turabian StyleAguinaco, Almudena, José M. Mánuel, Eduardo Blanco, Manuel Domínguez, Rocío Litrán, Juan J. Delgado, and Milagrosa Ramírez-del-Solar. 2022. "Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes" Materials 15, no. 19: 6718. https://doi.org/10.3390/ma15196718
APA StyleAguinaco, A., Mánuel, J. M., Blanco, E., Domínguez, M., Litrán, R., Delgado, J. J., & Ramírez-del-Solar, M. (2022). Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes. Materials, 15(19), 6718. https://doi.org/10.3390/ma15196718








