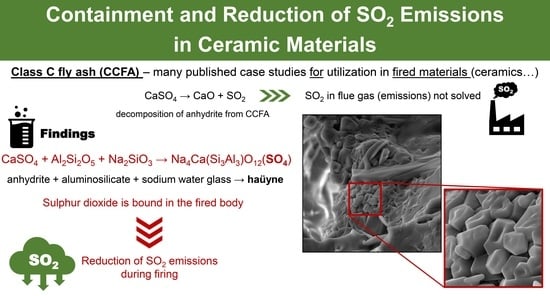
A Novel Process for the Containment of SO2 Emissions from Class C Fly Ash in the Fired Materials by Haüyne Formation
Abstract
1. Introduction
- Decomposition of limestone:
- 2.
- Formation of anhydrite:
2. Materials and Methods
3. Results of Experiments
3.1. Phase 1: Calculation of the Optimal Quantity and Molar Ratio of the Sodium Water Glass
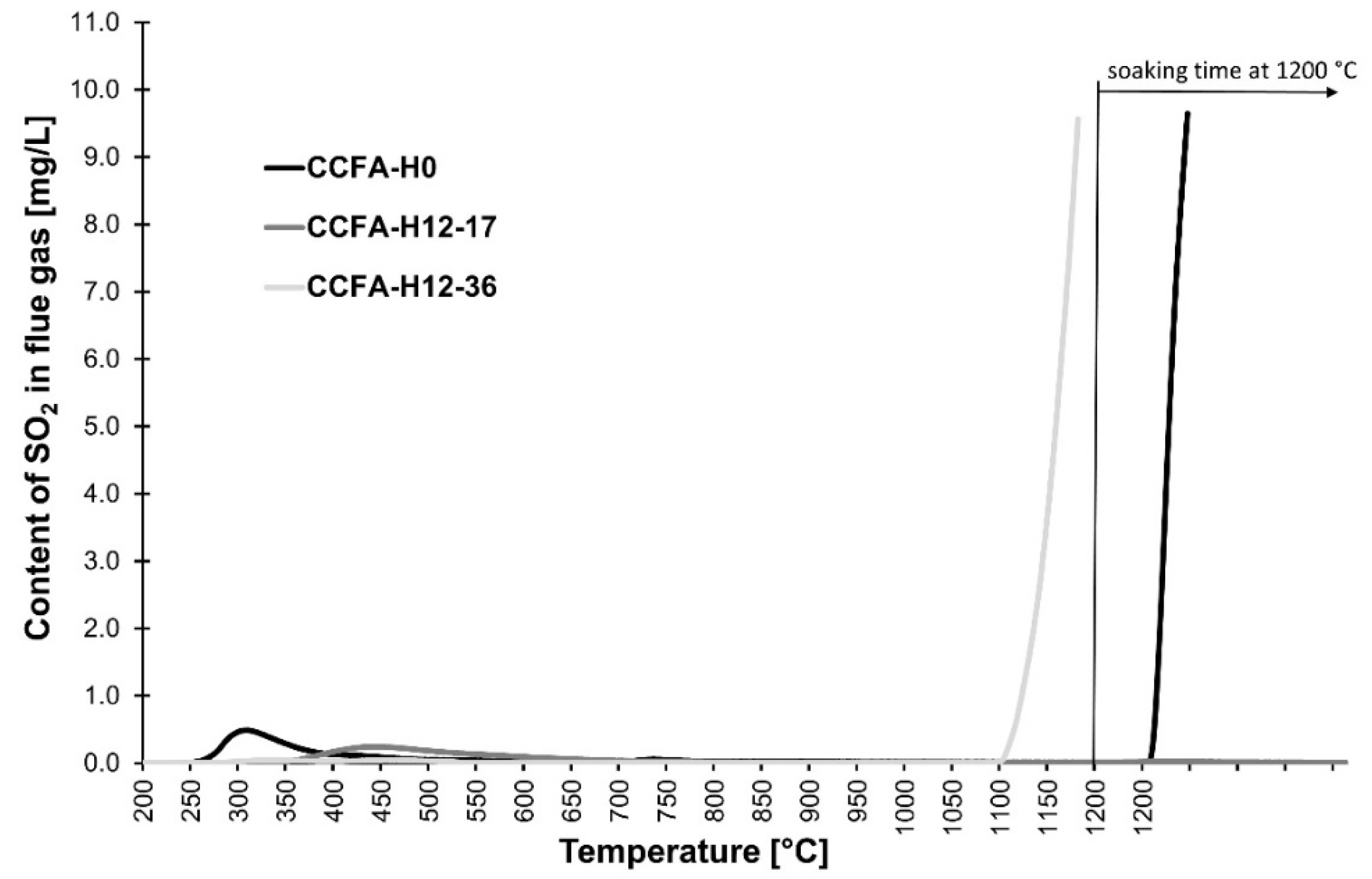
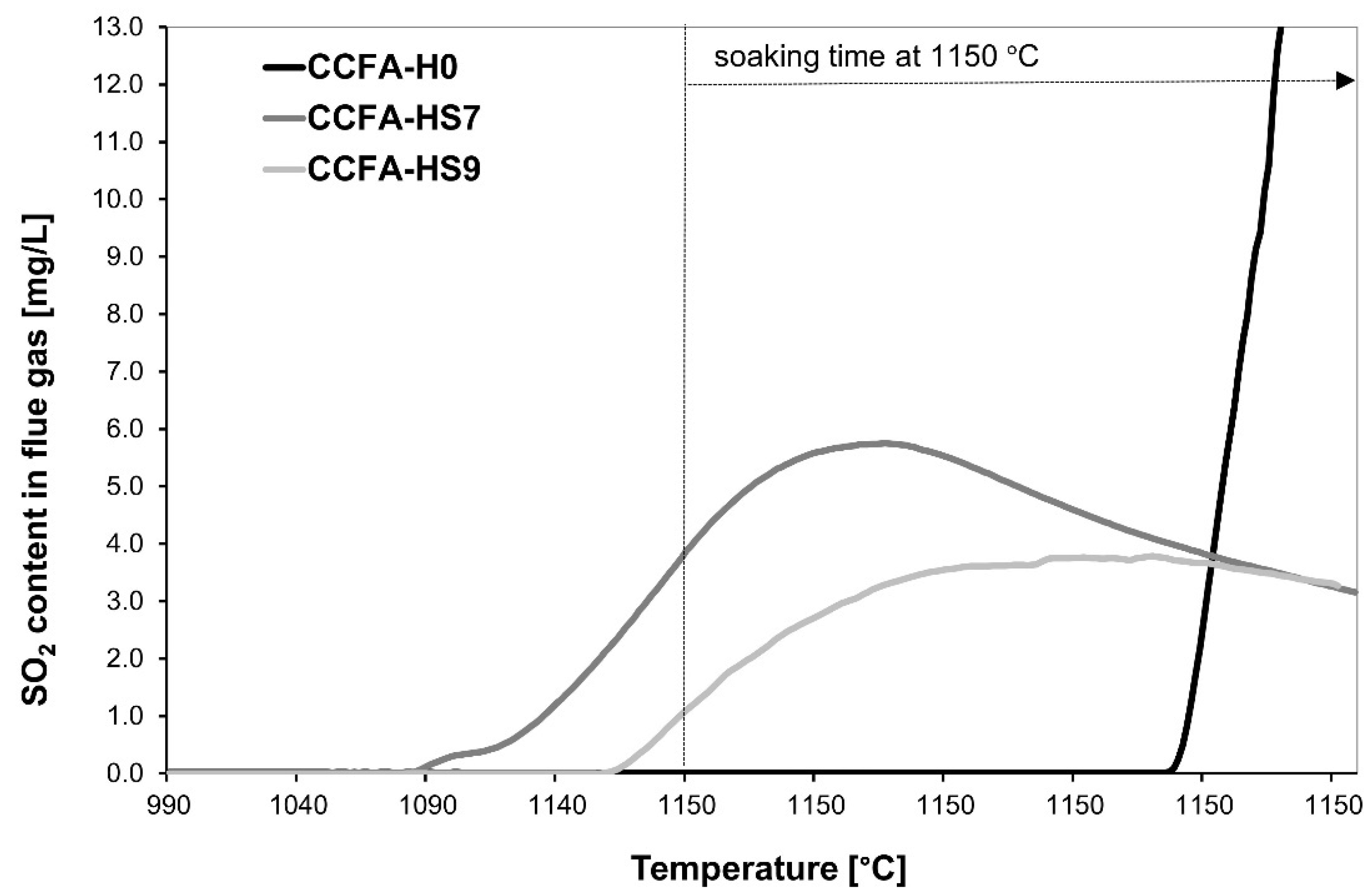
3.2. Phase 2: The Determination of the Soda/Soda-Water Glass Mixture Addition for the Reduction of SO2 Emissions
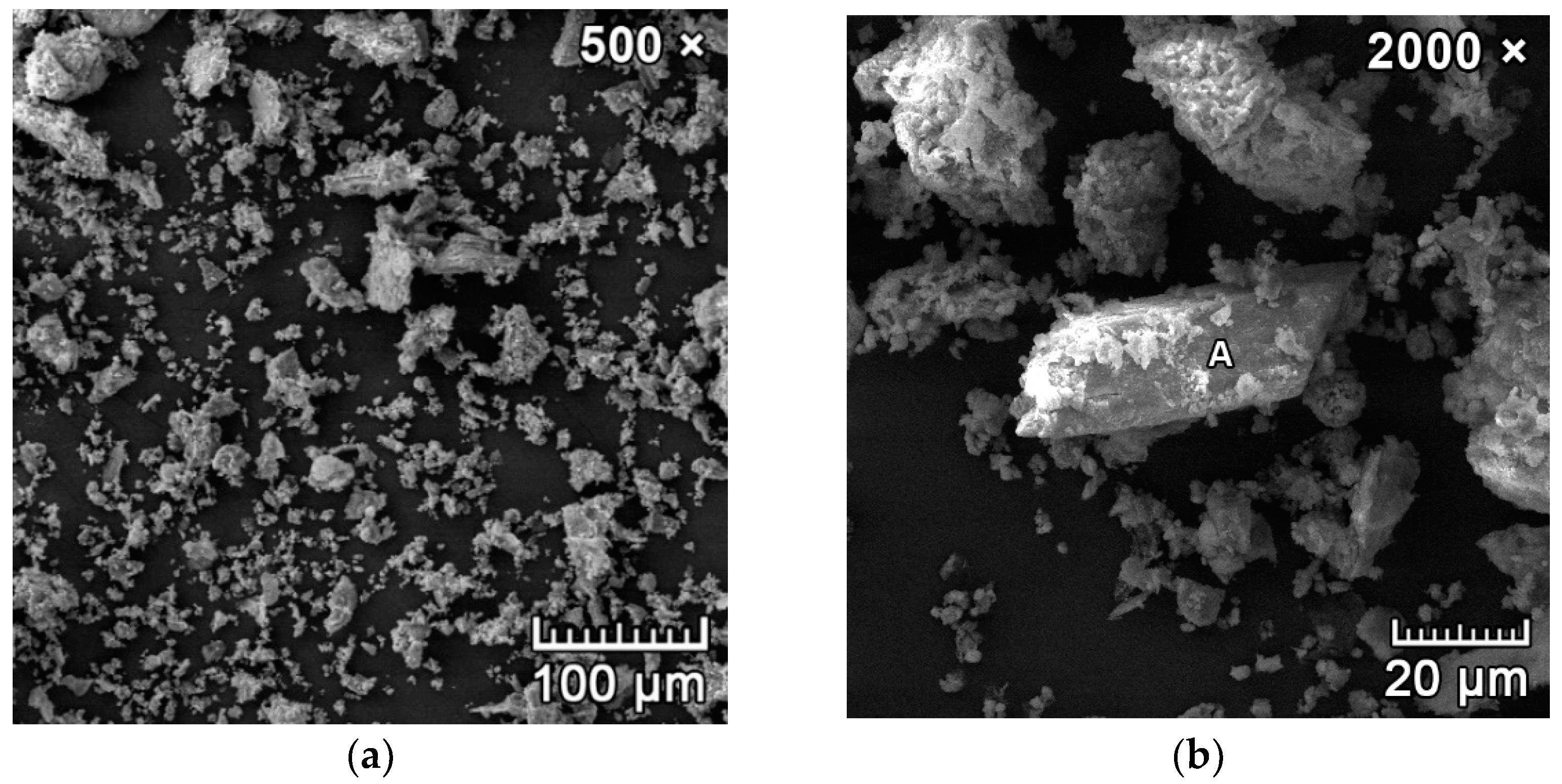
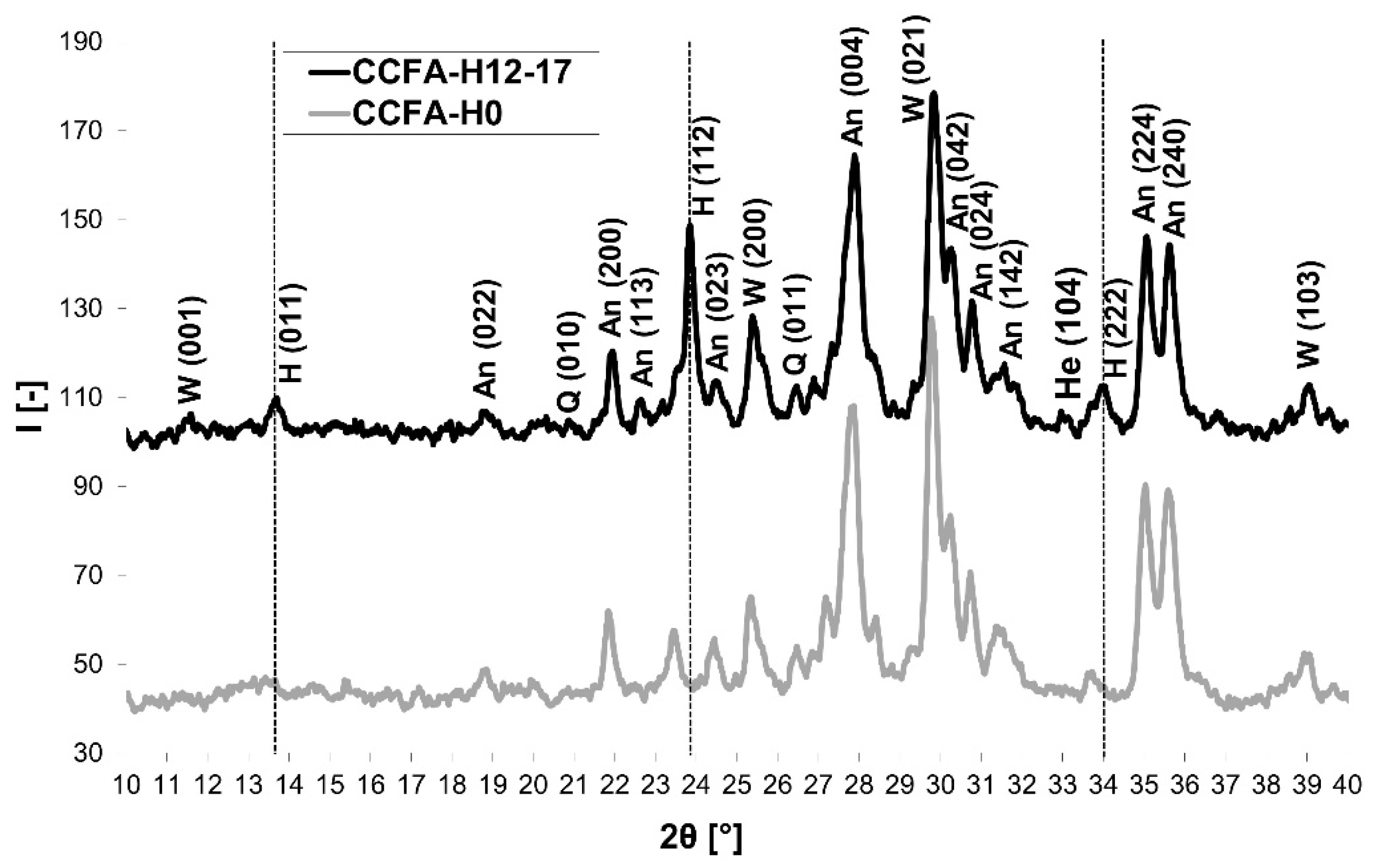
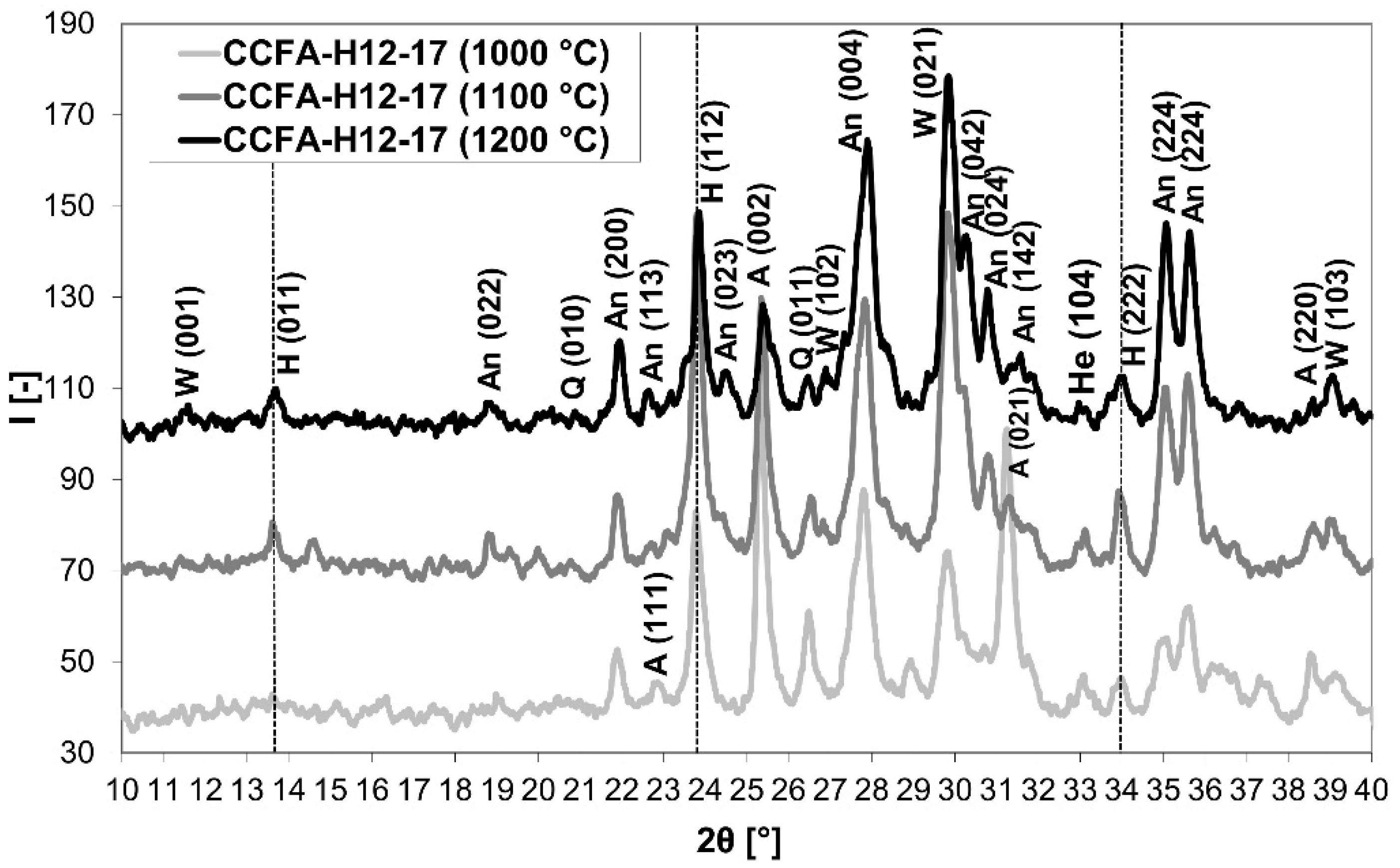
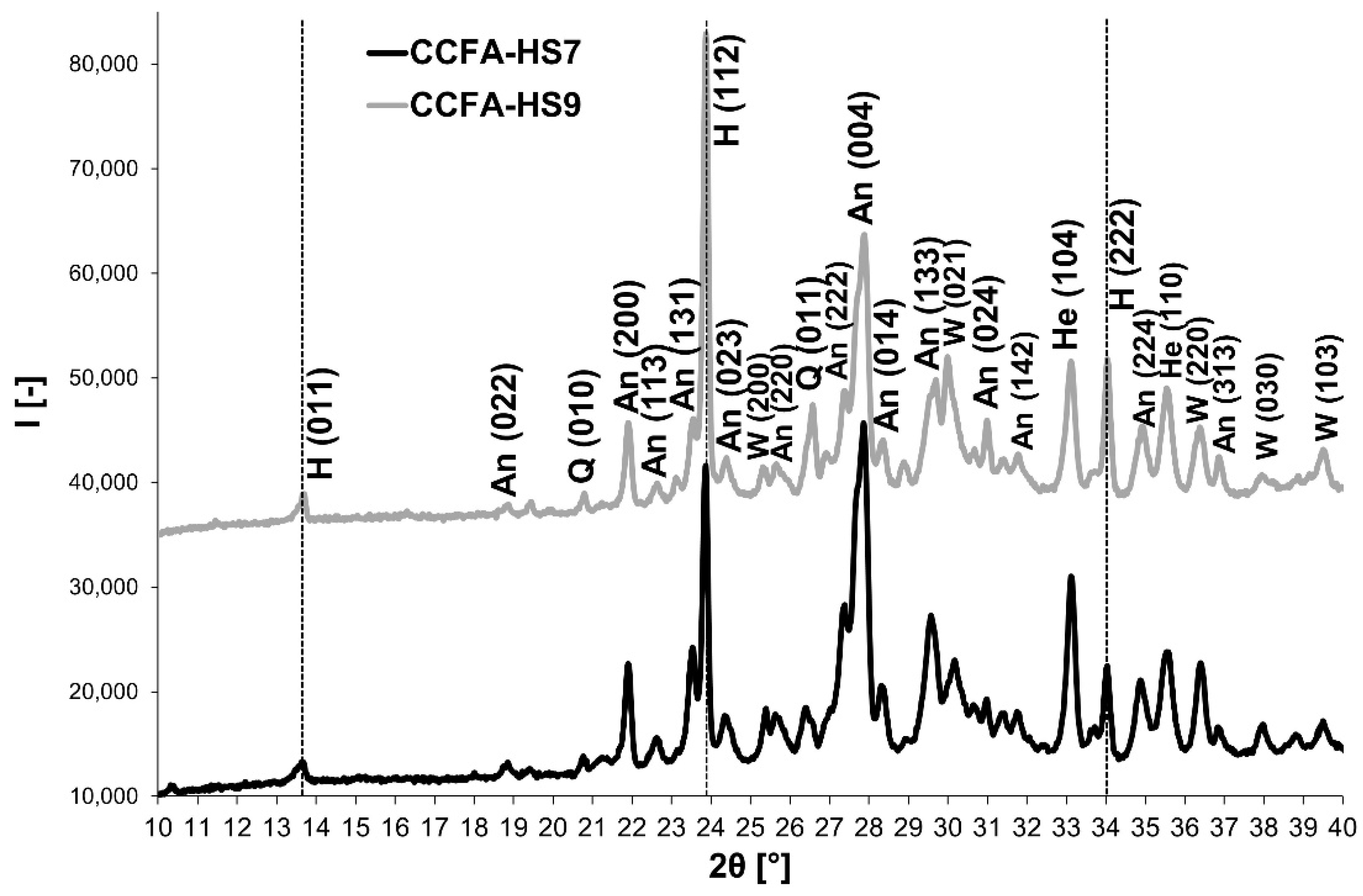
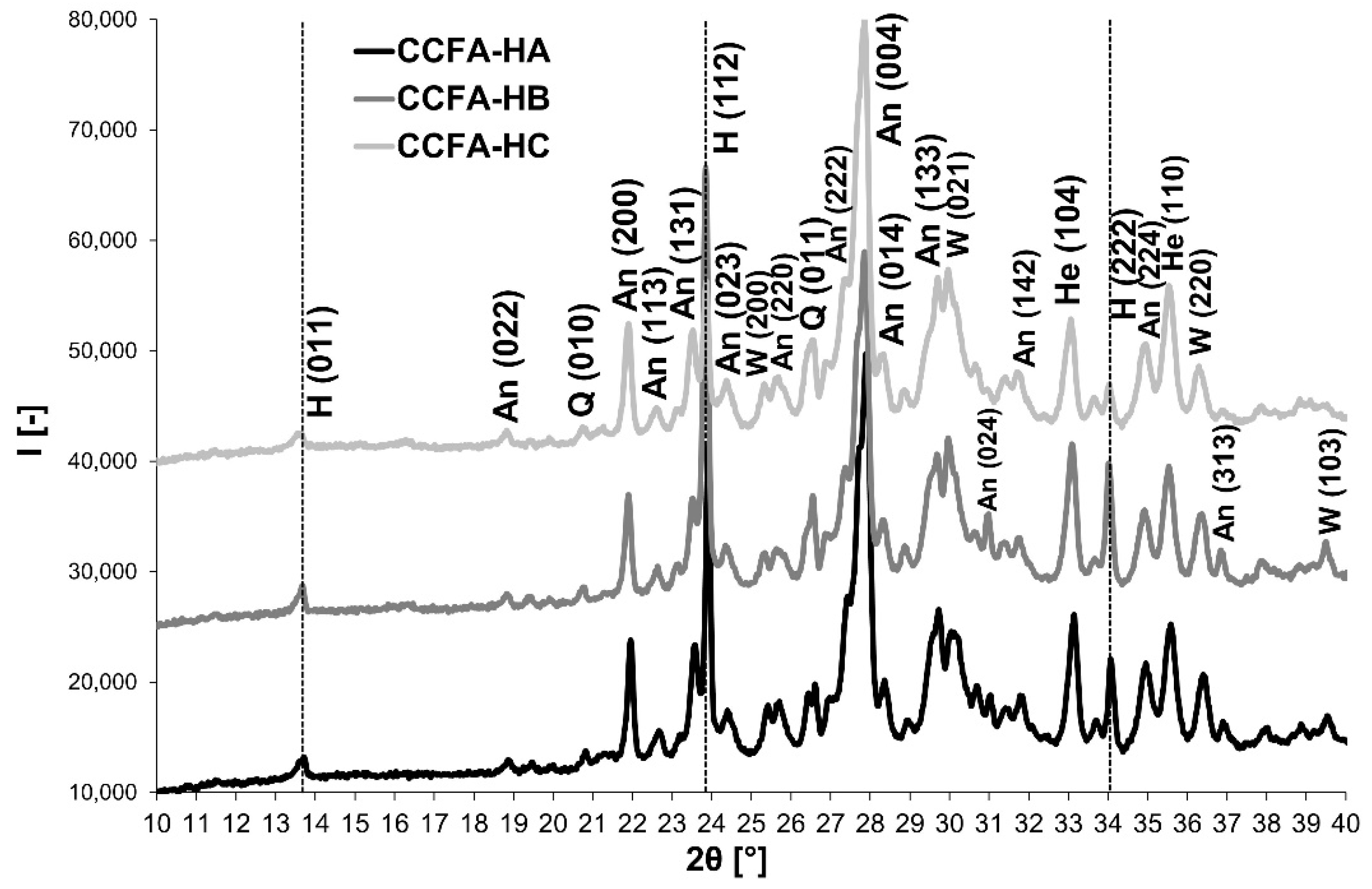
3.3. Phase 3: Identification of the Presence of Haüyne in the Microstructure of the Fired Body
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dayal, S.; Soundarapandian, N. Effect of fly-ash based geopolymer coated aggregate on bituminous mixtures. Građevinar 2018, 70, 187–199. [Google Scholar]
- Luga, E.; Atis, C.D.; Karahan, O.; Ilkentapar, S.; Gorur, E.B. Strength properties of slag/fly ash blends activated with sodium metasilicate. Građevinar 2017, 69, 199–205. [Google Scholar] [CrossRef]
- Šešlija, M.; Radovič, N.; Vasič, M.; Dogo, M.; Jotič, M. Physico-mechanical properties of fly ash applicable in road construction. Građevinar 2017, 69, 923–932. [Google Scholar]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Characterization of coal fly ash for possible utilization in glass production. Fuel 2007, 86, 706–714. [Google Scholar] [CrossRef]
- Fernández-Pereira, C.; De La Casa, J.A.; Gómez-Barea, A.; Arroyo, F.; Leiva, C.; Luna, Y. Application of biomass gasification fly ash for brick manufacturing. Fuel 2011, 90, 220–232. [Google Scholar] [CrossRef]
- Sokolar, R.; Smetanova, L. Dry pressed ceramic tiles based on fly ash–clay body: Influence of fly ash granulometry and pentasodium triphosphate addition. Ceram. Int. 2010, 36, 215–221. [Google Scholar] [CrossRef]
- Queralt, I.; Querol, X.; López-Soler, A.; Plana, F. Use of coal fly ash for ceramics: A case study for a large Spanish power station. Fuel 1997, 76, 87–791. [Google Scholar] [CrossRef]
- Yuan, Q.; Robert, D.; Mohajerani, A.; Tran, P.; Pramanik, B.K. Utilisation of waste-to-energy fly ash in ceramic tiles. Constr. Build. Mater. 2022, 347, 128475. [Google Scholar] [CrossRef]
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manag. 2007, 27, 59–68. [Google Scholar] [CrossRef]
- Haiying, Z.; Youcai, Z.; Jingyu, Q. Study on use of MSWI fly ash in ceramic tile. J. Hazard. Mater. 2007, 141, 106–114. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.H.; Wang, X. Ceramic tiles derived from coal fly ash: Preparation and mechanical characterization. Ceram. Int. 2017, 43, 11953–11966. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Gao, J.; Gao, Y.; Cheng, F. Synthesis and characterization of geopolymer prepared from circulating fluidized bed-derived fly ash. Ceram. Int. 2022, 48, 11820–11829. [Google Scholar] [CrossRef]
- Erol, M.; Genç, A.; Öveçolu, M.L.; Yücelen, E.; Küçükbayrak, S.; Taptik, Y. Characterization of a glass-ceramic produced from thermal power plant fly ashes. J. Eur. Ceram. Soc. 2000, 20, 2209–2214. [Google Scholar] [CrossRef]
- Barbieri, L.; Lancellotti, I.; Manfredini, T.; Queralt, I.; Rincon, J.M.; Romero, M. Design, obtainment and properties of glasses and glass–ceramics from coal fly ash. Fuel 1999, 78, 271–276. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Liu, L.; Ma, J.; Shen, B. Preparation of additive-free glass-ceramics from MSW incineration bottom ash and coal fly ash. Constr. Build. Mater. 2020, 254, 119345. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Liu, L.; Shen, B. Preparation and characterization of glass-ceramics via co-sintering of coal fly ash and oil shale ash-derived amorphous slag. Ceram. Int. 2019, 45, 20058–20065. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Jiang, H.; Zhang, W.; Mao, L. Recycling municipal solid waste incineration fly ash in fired bricks: An evaluation of physical-mechanical and environmental properties. Constr. Build. Mater. 2021, 294, 1–12. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Das, V.; Jhatial, A.A.; Hussain Ali, T. Assessing the structural efficiency and durability of burnt clay bricks incorporating fly ash and silica fume as additives. Constr. Build. Mater. 2021, 310, 1–13. [Google Scholar] [CrossRef]
- Kirkelund, G.M.; Skevi, L.; Ottosen, L.M. Electrodialytically treated MSWI fly ash use in clay bricks. Constr. Build. Mater. 2020, 254, 119286. [Google Scholar] [CrossRef]
- Ilic, M.; Cheeseman, C.; Sollars, C.; Knight, J. Mineralogy and microstructure of sintered lignite coal fly ash. Fuel 2003, 82, 331–336. [Google Scholar] [CrossRef]
- Sokolar, R.; Vodova, L. The effect of fluidized fly ash on the properties of dry-pressed ceramic tiles based on fly ash—Clay body. Ceram. Int. 2011, 37, 2879–2885. [Google Scholar] [CrossRef]
- Swift, W.H.; Panek, A.F.; Smith, G.W.; Vogel, G.J.; Jonke, A.A. Decomposition of Calcium Sulfate: A Review of the Literature, 1st ed.; Argonne National Laboratory: Lemont, IL, USA, 1976.
- Labus, M. Pyrite thermal decomposition in source rocks. Fuel 2021, 287, 119529. [Google Scholar] [CrossRef]
- Sokolar, R.; Nguyen, M. Sulphur dioxide emissions during the firing of ceramic bodies based on class C fly ash. Solid State Phenom. 2019, 296, 149–154. [Google Scholar] [CrossRef]
- Sokolar, R.; Nguyen, M. The fly ash of Class C for ceramic technology. IOP Conf. Ser. Mater. Sci. Eng. 2018, 385, 012053. [Google Scholar] [CrossRef]
- Hassan, I.; Grundy, H.D. The crystal structure of haüyne at 293 and 153 K. Can. Mineral. 1991, 29, 123–130. [Google Scholar]
- The Hudson Institute of Mineralogy. Mineralogy Database. Available online: https://www.mindat.org/min-1833.html (accessed on 2 May 2022).
- Database of Raman Spectra, X-ray Diffraction and Chemistry Data for Minerals. RRUFF. Available online: https://rruff.info/hauyne/display=default (accessed on 10 May 2022).
- Barthelemy, D. Mineralogy Database. Available online: http://webmineral.com/data/Hauyne.shtml#.YNWGhOgzYuU (accessed on 24 April 2022).
- Skvara, F.; Sulc, R.; Snop, R.; Zlamalova-Cilova, Z.; Peterova, A.; Kopecky, L.; Formacek, P. Czech fluid sulfocalcic ash and fly ash. Ceram. Silik. 2016, 60, 344–352. [Google Scholar] [CrossRef]


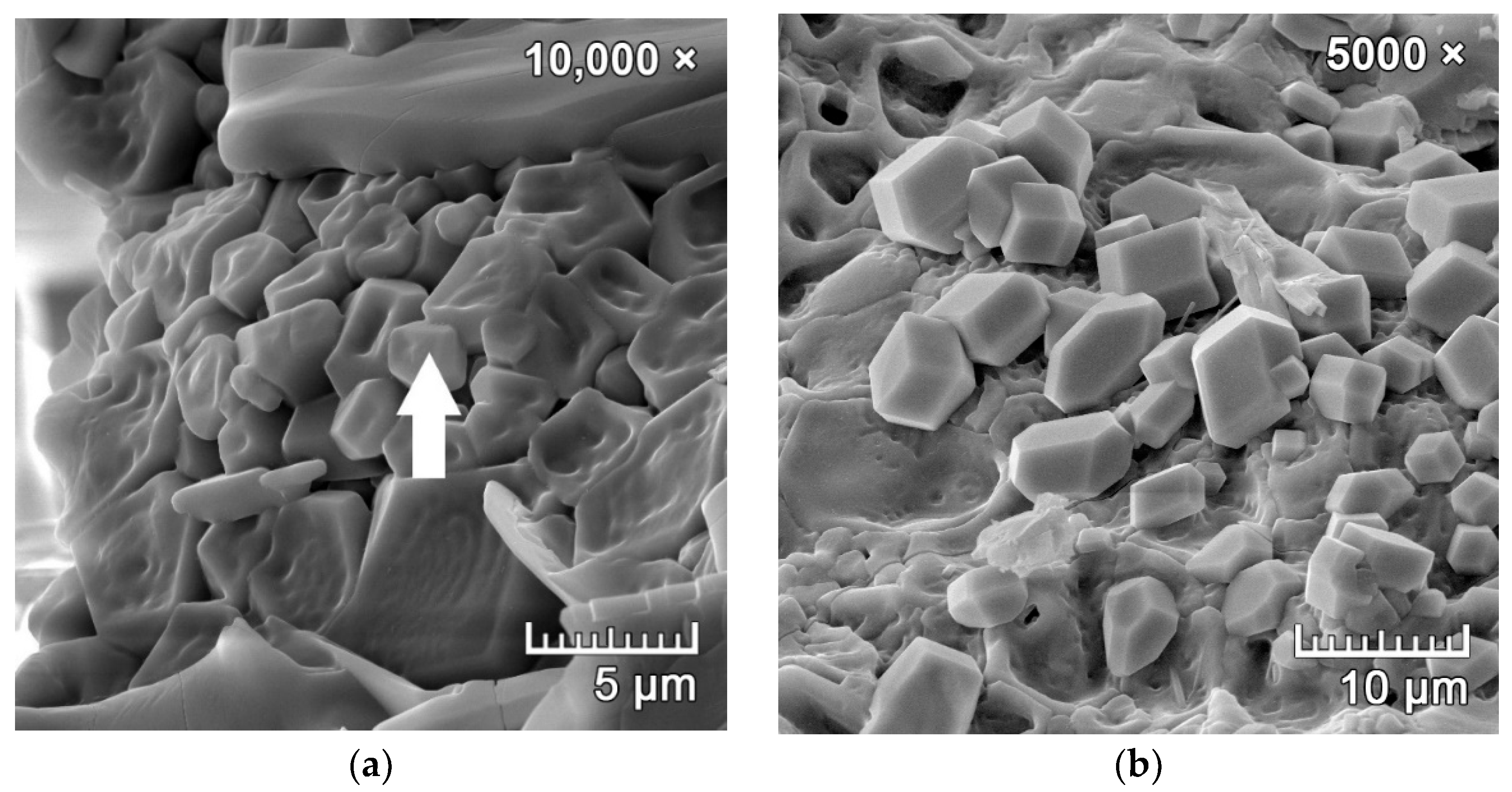
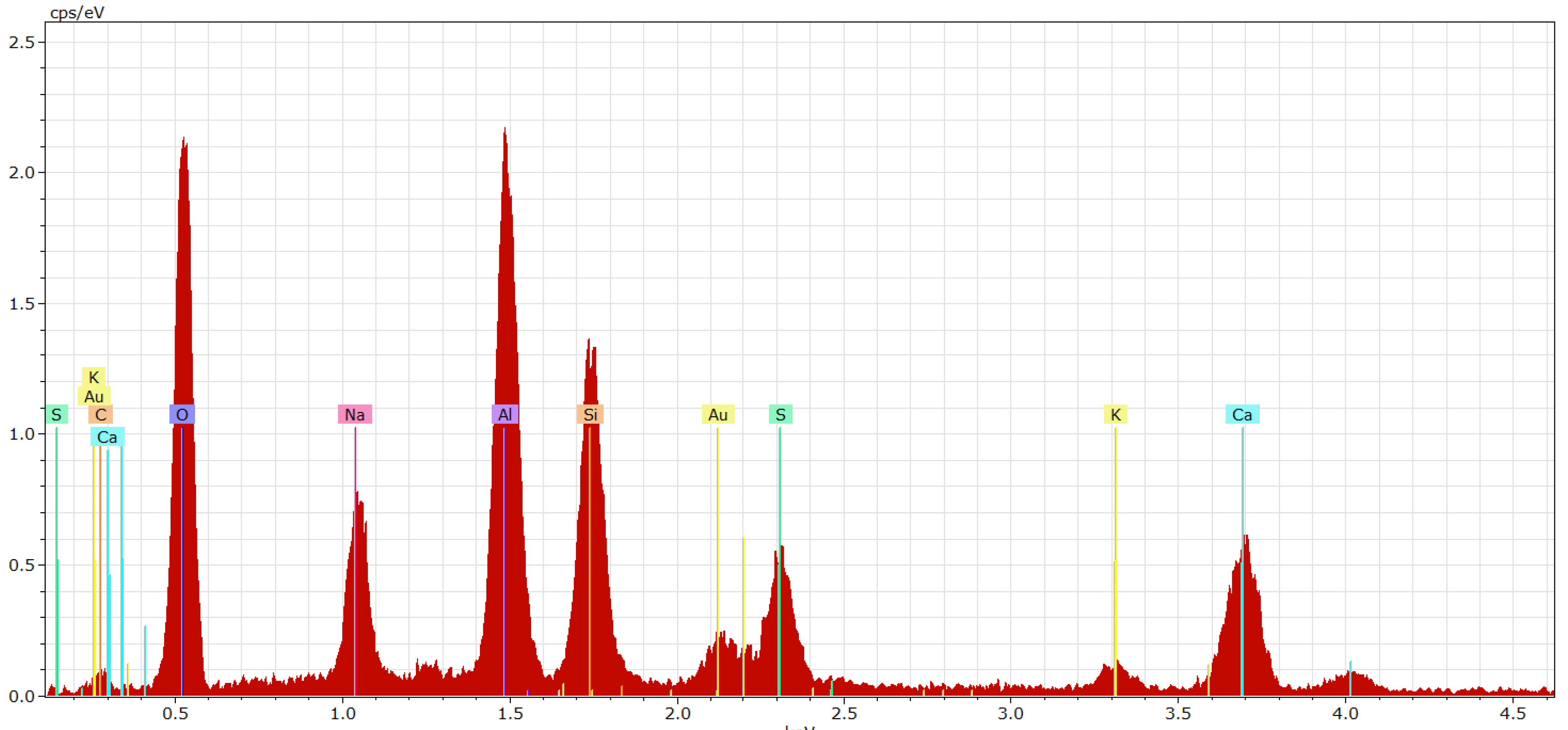
| Fly Ash | Σ(SiO2 + Al2O3 + Fe2O3) | SO3 Content | Loss on Ignition |
|---|---|---|---|
| Class C | ≥50% | ≤5% | ≤6% |
| Class F | ≥70% | ≤3% | ≤6% |
| Mineral | SiO2 | Al2O3 | CaO | Na2O | K2O | MnO | SO3 |
|---|---|---|---|---|---|---|---|
| Haüyne | 34.28 | 28.70 | 7.11 | 18.04 | 0.09 | 0.03 | 12.54 |
| Nosean | 34.95 | 24.41 | 4.40 | 19.01 | 0.33 | 0.00 | 8.11 |
| Oxide | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | MnO | K2O | Na2O | SO3 | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content [%] | 32.45 | 16.08 | 6.67 | 0.60 | 24.52 | 3.41 | 0.00 | 0.63 | 0.10 | 4.04 | 4.43 |
| Mineral | Crystal System | Lattice Parameters [Å; °] | Cell Volume [Å3] | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | α | β | γ | |||
| Quartz | Hexagonal | 4.912 | 4.912 | 5.404 | 90 | 90 | 120 | 112.92 |
| Anhydrite | Orthorhombic | 6.995 | 6.245 | 6.993 | 90 | 90 | 90 | 305.48 |
| Calcite | Hexagonal | 4.984 | 4.984 | 17.121 | 90 | 90 | 120 | 368.31 |
| Lime (CaO) | Cubic | 4.937 | 4.937 | 4.937 | 90 | 90 | 90 | 120.33 |
| Hematite | Hexagonal | 5.0288 | 5.0288 | 13.730 | 90 | 90 | 120 | 300.70 |
| CCFA-H0 | CCFA-H6-17 CCFA-H6-35 | CCFA-H9-17 CCFA-H9-35 | CCFA-H12-17 CCFA-H12-35 | |
|---|---|---|---|---|
| Water | 18% | 12% | 9% | 6% |
| Water glass (1.7–3.5) | 0% | 6% | 9% | 12% |
| Mineral | Crystal System | Lattice Parameters [Å; °] | Cell Volume [Å3] | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | α | β | γ | |||
| Haüyne | Cubic | 9.100 | 9.100 | 9.100 | 90 | 90 | 90 | 753.57 |
| Anorthite | Triclinic | 8.194 | 12.897 | 12.931 | 86.00 | 81.04 | 88.85 | 1345.52 |
| Wollastonite | Triclinic | 7.065 | 7.320 | 7.926 | 103.43 | 95.22 | 90.06 | 396.94 |
| Quartz | Hexagonal | 4.912 | 4.912 | 5.404 | 90 | 90 | 120 | 112.92 |
| Anhydrite | Orthorhombic | 6.995 | 6.245 | 6.993 | 90 | 90 | 90 | 305.48 |
| Hematite | Hexagonal | 5.0288 | 5.0288 | 13.730 | 90 | 90 | 120 | 300.70 |
| Indication | Admixture | Content/Ratio |
|---|---|---|
| CCFA-HS7 | Sodium carbonate | 7% |
| CCFA-HS9 | 9% | |
| CCFA-HA | Sodium carbonate: sodium water glass (1.7) | 10% (1:1) |
| CCFA-HB | 10% (2:1) | |
| CCFA-HC | 10% (1:2) |
| Batch | Ts [°C] | SO2-max [mg/L] |
|---|---|---|
| CCFA-H0 | 1150 | >13.0 |
| CCFA-HS7 | 1078 | 6.39 |
| CCFA-HS9 | 1145 | 5.75 |
| CCFA-HA | 1107 | 6.90 |
| CCFA-HB | 1100 | 2.31 |
| CCFA-HC | 1125 | 8.86 |
| CCFA-H0 | CCFA-H12-17 | |
|---|---|---|
| Total sulfur content as SO3 [%] | 1.89 | 3.42 |
| Total sulfur content [%] | 0.76 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolar, R.; Nguyen, M. A Novel Process for the Containment of SO2 Emissions from Class C Fly Ash in the Fired Materials by Haüyne Formation. Materials 2022, 15, 6701. https://doi.org/10.3390/ma15196701
Sokolar R, Nguyen M. A Novel Process for the Containment of SO2 Emissions from Class C Fly Ash in the Fired Materials by Haüyne Formation. Materials. 2022; 15(19):6701. https://doi.org/10.3390/ma15196701
Chicago/Turabian StyleSokolar, Radomir, and Martin Nguyen. 2022. "A Novel Process for the Containment of SO2 Emissions from Class C Fly Ash in the Fired Materials by Haüyne Formation" Materials 15, no. 19: 6701. https://doi.org/10.3390/ma15196701
APA StyleSokolar, R., & Nguyen, M. (2022). A Novel Process for the Containment of SO2 Emissions from Class C Fly Ash in the Fired Materials by Haüyne Formation. Materials, 15(19), 6701. https://doi.org/10.3390/ma15196701