One of Nature’s Puzzles Is Assembled: Analog of the Earth’s Most Complex Mineral, Ewingite, Synthesized in a Laboratory
Abstract
1. Introduction
2. Materials and Methods
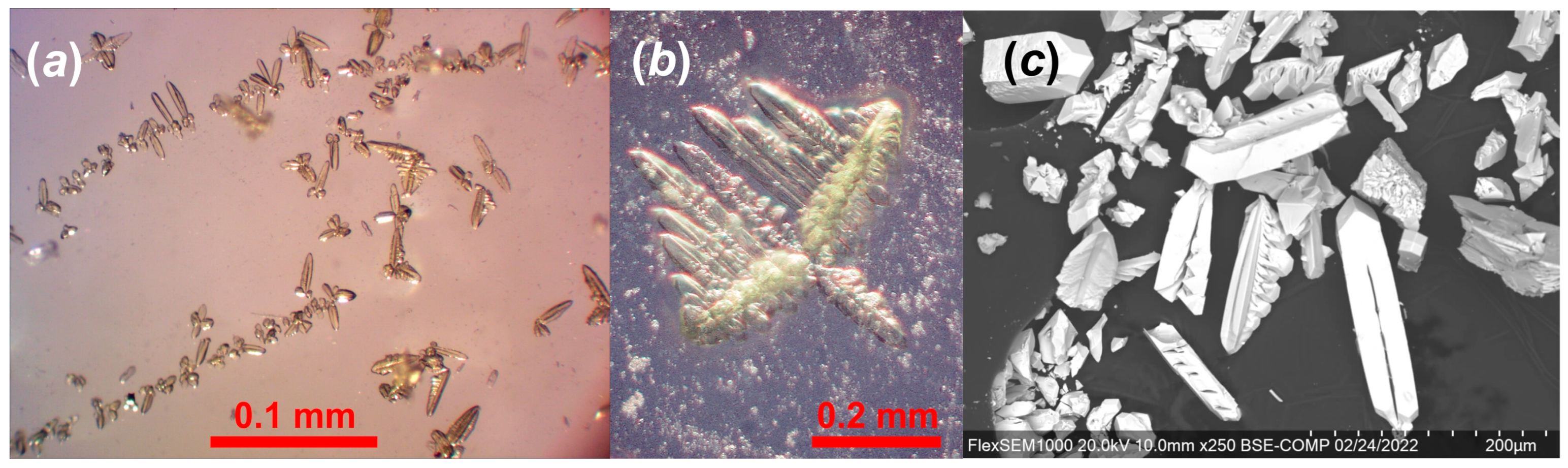
2.1. Synthesis
2.2. Chemical Composition
2.3. X-ray Diffraction Studies
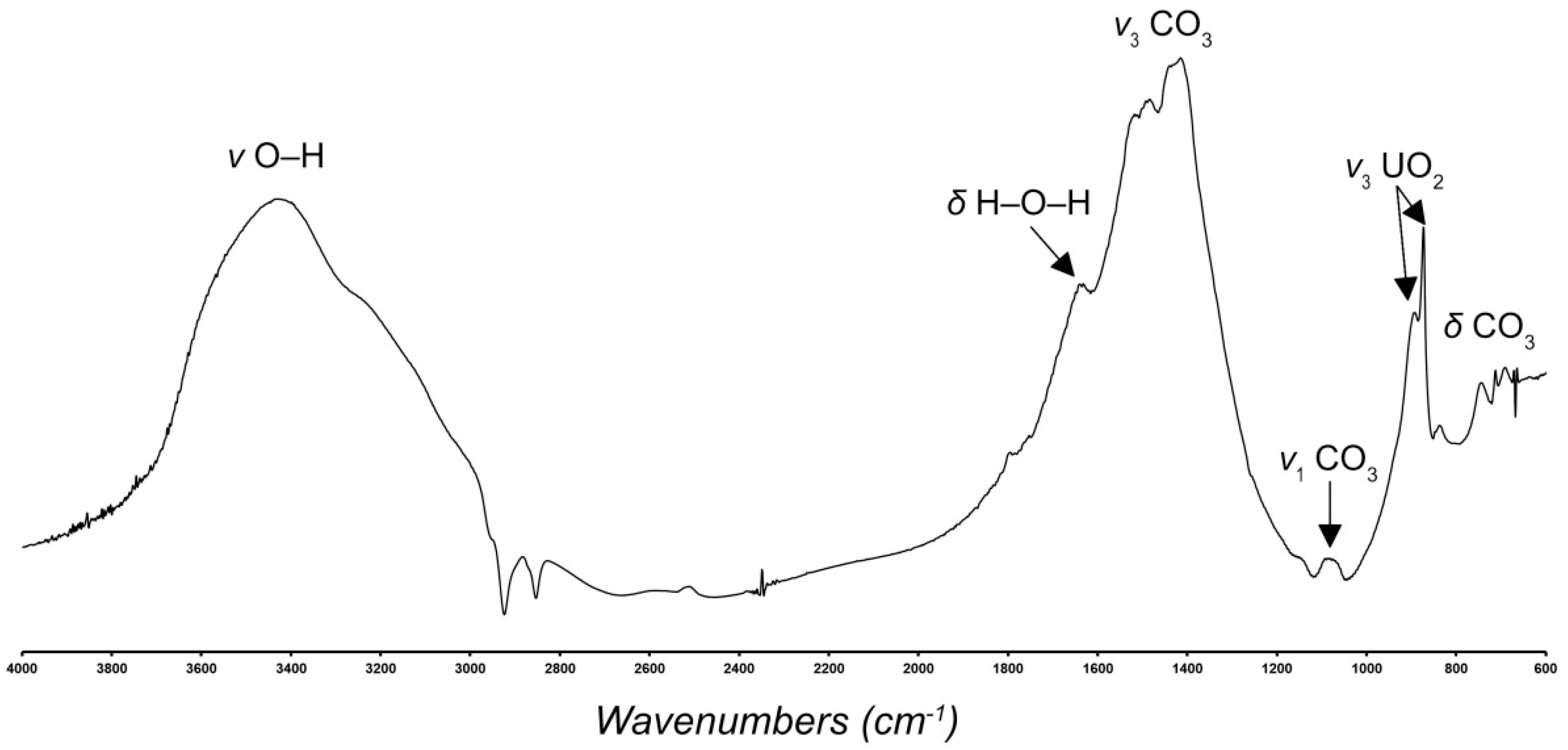
2.4. Infrared Spectroscopy
3. Results and Discussion
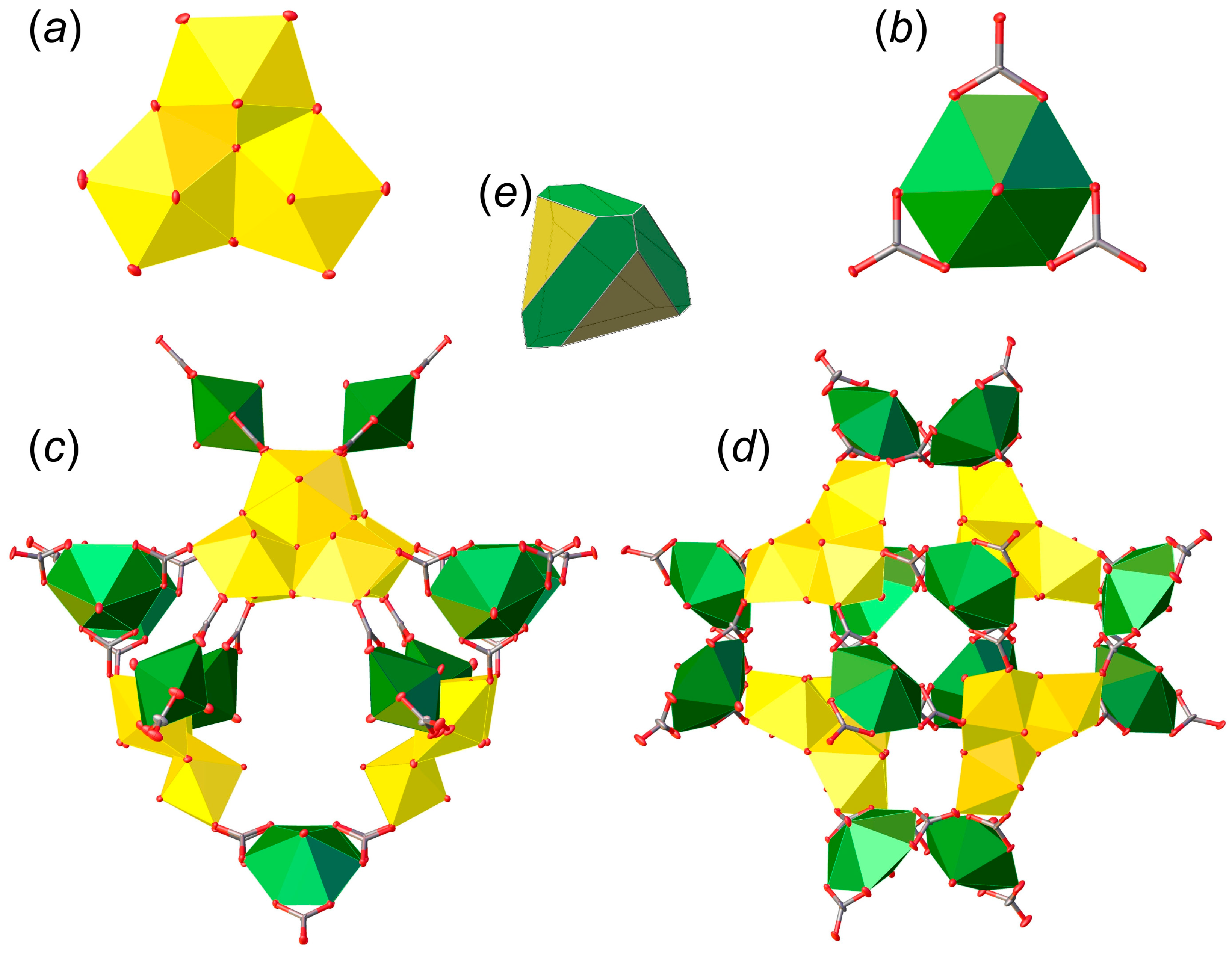
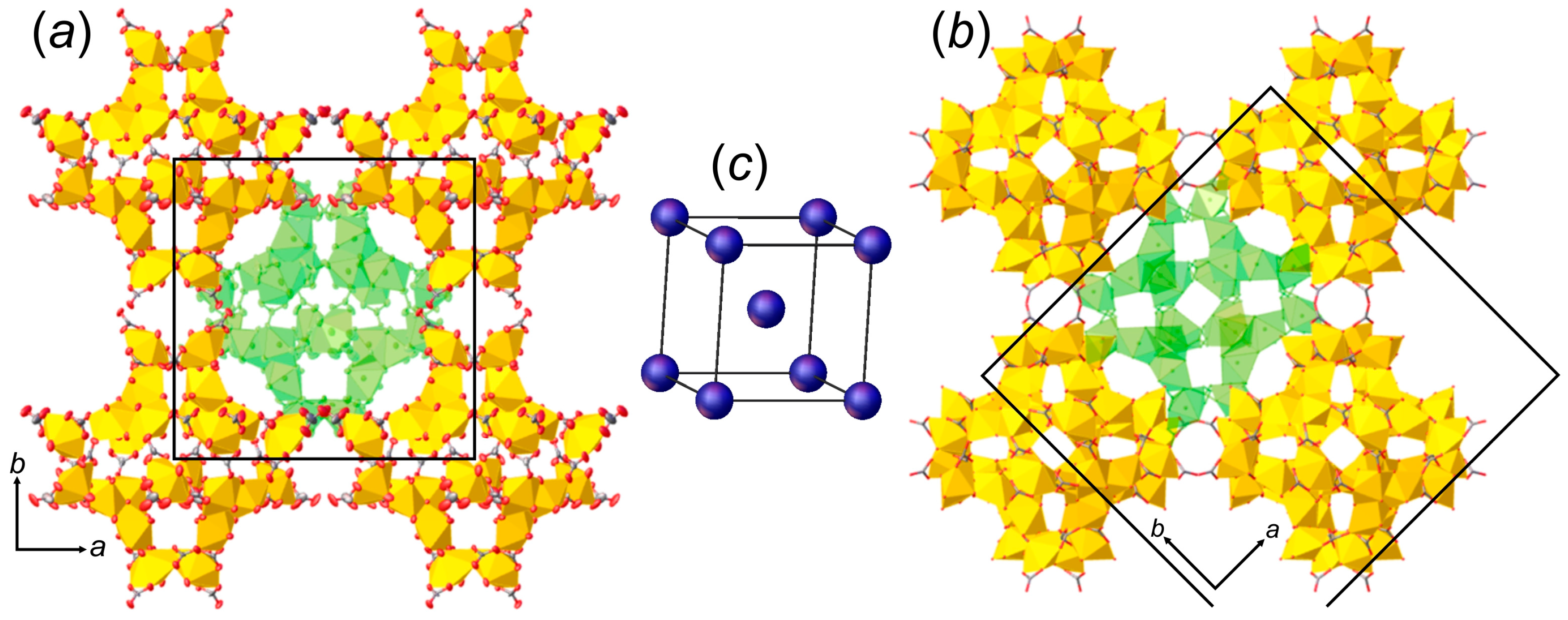
3.1. Structure Descriptions
3.2. Structural Complexity Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pauliš, P.; Babka, K.; Sejkora, J.; Škácha, P. Uranové Minerály České Republiky a Jejich Nejvýznamnější Naleziště; Kuttna: Kutná Hora, Czech Republic, 2016; 570p. (In Czech) [Google Scholar]
- Škácha, P.; Plášil, J.; Horák, V. Jáchymov Mineralogická Perla Krušnohoří; Academia: Prague, Czech Republic, 2019; 682p. (In Czech) [Google Scholar]
- Agricola, G. De Re Metallica, Translation from the 1st Latin edition of, 1556; Hoover, H.C., Hoover, L.H., Eds.; The Mining Magazine, Salisbury House: London, UK, 1912; 640p. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 86th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Plášil, J.; Mills, S.J.; Fejfarová, K.; Dušek, M.; Novák, M.; Škoda, R.; Čejka, J.; Sejkora, J. The crystal structure of natural zippeite, K1.85H+0.15[(UO2)4O2(SO4)2(OH)2](H2O)4, from Jáchymov, Czech Republic. Can. Mineral. 2011, 49, 1089–1103. [Google Scholar] [CrossRef]
- Plášil, J.; Hloušek, J.; Veselovský, F.; Fejfarová, K.; Dušek, M.; Škoda, R.; Novák, M.; Čejka, J.; Sejkora, J.; Ondruš, P. Adolfpateraite, K(UO2)(SO4)(OH)(H2O), a new uranyl sulphate mineral from Jáchymov, Czech Republic. Am. Mineral. 2012, 97, 447–454. [Google Scholar] [CrossRef]
- Plášil, J.; Veselovský, F.; Hloušek, J.; Šák, M.; Sejkora, J.; Cejka, J.; Škácha, P.; Kasatkin, A.V. Mathesiusite, K5(UO2)4(SO4)4(VO5)(H2O)4, a new uranyl vanadate-sulfate from Jáchymov, Czech Republic. Am. Mineral. 2014, 99, 625–632. [Google Scholar] [CrossRef]
- Plášil, J.; Hloušek, J.; Kasatkin, A.V.; Škoda, R.; Novák, M.; Čejka, J. Geschieberite, K2(UO2)(SO4)2(H2O)2, a new uranyl sulfate mineral from Jáchymov. Mineral. Mag. 2015, 79, 205–216. [Google Scholar] [CrossRef]
- Plášil, J.; Hloušek, J.; Kasatkin, A.V.; Belakovskiy, D.I.; Čejka, J.; Chernyshov, D. Ježekite, Na8[(UO2)(CO3)3](SO4)2·3H2O, a new uranyl mineral from Jáchymov, Czech Republic. J. Geosci. 2015, 60, 259–267. [Google Scholar] [CrossRef]
- Plášil, J.; Hlousek, J.; Kasatkin, A.V.; Novak, M.; Cejka, J.; Lapcak, L. Svornostite, K2Mg[(UO2)(SO4)2]2∙8H2O, a new uranyl sulfate mineral from Jáchymov, Czech Republic. J. Geosci. 2015, 60, 113–121. [Google Scholar] [CrossRef]
- Plášil, J.; Škácha, P.; Sejkora, J.; Kampf, A.R.; Škoda, R.; Čejka, J.; Hloušek, J.; Kasatkin, A.V.; Pavlíček, R.; Babka, K. Plavnoite, a new K-Mn member of the zippeite group from Jáchymov, Czech Republic. Eur. J. Mineral. 2017, 29, 117–128. [Google Scholar] [CrossRef]
- Olds, T.; Plášil, J.; Kampf, A.; Dal Bo, F.; Burns, P. Paddlewheelite, a New Uranyl Carbonate from the Jáchymov District, Bohemia, Czech Republic. Minerals 2018, 8, 511. [Google Scholar] [CrossRef]
- Olds, T.; Plášil, J.; Kampf, A.; Simonetti, A.; Sadergaski, L.; Chen, Y.-S.; Burns, P. Ewingite: Earth’s most complex mineral. Geology 2017, 45, 1007–1010. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, Version 1.171.41.103a; Rigaku Oxford Diffraction: Oxford, UK, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-H…O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kalashnikova, S.A.; Kuporev, I.V.; Plášil, J. Crystal chemistry and structural complexity of the uranyl carbonate minerals and synthetic compounds. Crystals 2021, 11, 704. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals and mineral parageneses: Information and its evolution in the mineral world. In Highlights in Mineralogical Crystallography; Danisi, R., Armbruster, T., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2015; pp. 31–73. [Google Scholar]
- Krivovichev, S.V.; Hawthorne, F.C.; Williams, P.A. Structural complexity and crystallization: The Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Struct. Chem. 2017, 28, 153–159. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskaya, O.V. Hydrated Calcium Oxalates: Crystal Structures, Thermal Stability and Phase Evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Plášil, J. Structural complexity of uranophane and uranophane-β: Implications for their formation and occurrence. Eur. J. Mineral. 2018, 30, 253–257. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Izatulina, A.R.; Krivovichev, S.V.; Tananaev, I.G. Chemically Induced Polytypic Phase Transitions in the Mg[(UO2)(TO4)2(H2O)](H2O)4 (T = S, Se) System. Inorg. Chem. 2019, 58, 14760−14768. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Ladders of information: What contributes to the structural complexity in inorganic crystals. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. 2019, B75, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tyumentseva, O.S.; Kornyakov, I.V.; Britvin, S.N.; Zolotarev, A.A.; Gurzhiy, V.V. Crystallographic Insights into Uranyl Sulfate Minerals Formation: Synthesis and Crystal Structures of Three Novel Cesium Uranyl Sulfates. Crystals 2019, 9, 660. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kuporev, I.V.; Kovrugin, V.M.; Murashko, M.N.; Kasatkin, A.V.; Plášil, J. Crystal chemistry and structural complexity of natural and synthetic uranyl selenites. Crystals 2019, 9, 639. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Selective Se-for-S substitution in Cs-bearing uranyl compounds. J. Solid State Chem. 2017, 248, 126–133. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Gurzhiy, V.V. Dimensional evolution in hydrated K+-bearing uranyl sulfates: From 2D-sheets to 3D frameworks. CrystEngComm. 2020, 22, 4621–4629. [Google Scholar] [CrossRef]
- Bruker AXS. Topas, V5.0: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker: Karlsruhe, Germany, 2014. [Google Scholar]
- Loopstra, B.O.; Rietveld, H.M. The structure of some alkaline-earth metal uranates. Acta Crystallogr. 1969, B25, 787–791. [Google Scholar] [CrossRef]


| Complexes That Contribute to Structural Complexity [27] | Ewingite [18] | SE | ||||
|---|---|---|---|---|---|---|
| υ, Atoms | IG,total, Bits/Cell (IG, Bits/Atom) | Contribution, % | υ | IG,total, Bits/Cell (IG, Bits/Atom) | Contribution, % | |
| Topological complexity of the cluster | 220 | 1271.820 (5.781) | 6.9 | 244 | 1447.100 (5.931) | 15.2 |
| Structural complexity of the cluster | 220 | 1271.820 | 0 | 244 | 1447.100 | 0 |
| Stacking of clusters | 880 | 3813.886 | 20.8 | 488 | 1506.180 | 15.8 |
| Interstitial structure | 588 | 4531.056 | 24.7 | 318 | 2499.190 | 26.3 |
| H-bonding | 1040 | 8717.650 | 47.6 | 484 | 4063.300 | 42.7 |
| Structural complexity of the entire structure | 2508 | 18,335.988 (7.311) | 100 | 1290 | 9515.77 (7.377) | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyumentseva, O.S.; Kornyakov, I.V.; Kasatkin, A.V.; Plášil, J.; Krzhizhanovskaya, M.G.; Krivovichev, S.V.; Burns, P.C.; Gurzhiy, V.V. One of Nature’s Puzzles Is Assembled: Analog of the Earth’s Most Complex Mineral, Ewingite, Synthesized in a Laboratory. Materials 2022, 15, 6643. https://doi.org/10.3390/ma15196643
Tyumentseva OS, Kornyakov IV, Kasatkin AV, Plášil J, Krzhizhanovskaya MG, Krivovichev SV, Burns PC, Gurzhiy VV. One of Nature’s Puzzles Is Assembled: Analog of the Earth’s Most Complex Mineral, Ewingite, Synthesized in a Laboratory. Materials. 2022; 15(19):6643. https://doi.org/10.3390/ma15196643
Chicago/Turabian StyleTyumentseva, Olga S., Ilya V. Kornyakov, Anatoly V. Kasatkin, Jakub Plášil, Maria G. Krzhizhanovskaya, Sergey V. Krivovichev, Peter C. Burns, and Vladislav V. Gurzhiy. 2022. "One of Nature’s Puzzles Is Assembled: Analog of the Earth’s Most Complex Mineral, Ewingite, Synthesized in a Laboratory" Materials 15, no. 19: 6643. https://doi.org/10.3390/ma15196643
APA StyleTyumentseva, O. S., Kornyakov, I. V., Kasatkin, A. V., Plášil, J., Krzhizhanovskaya, M. G., Krivovichev, S. V., Burns, P. C., & Gurzhiy, V. V. (2022). One of Nature’s Puzzles Is Assembled: Analog of the Earth’s Most Complex Mineral, Ewingite, Synthesized in a Laboratory. Materials, 15(19), 6643. https://doi.org/10.3390/ma15196643








