Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
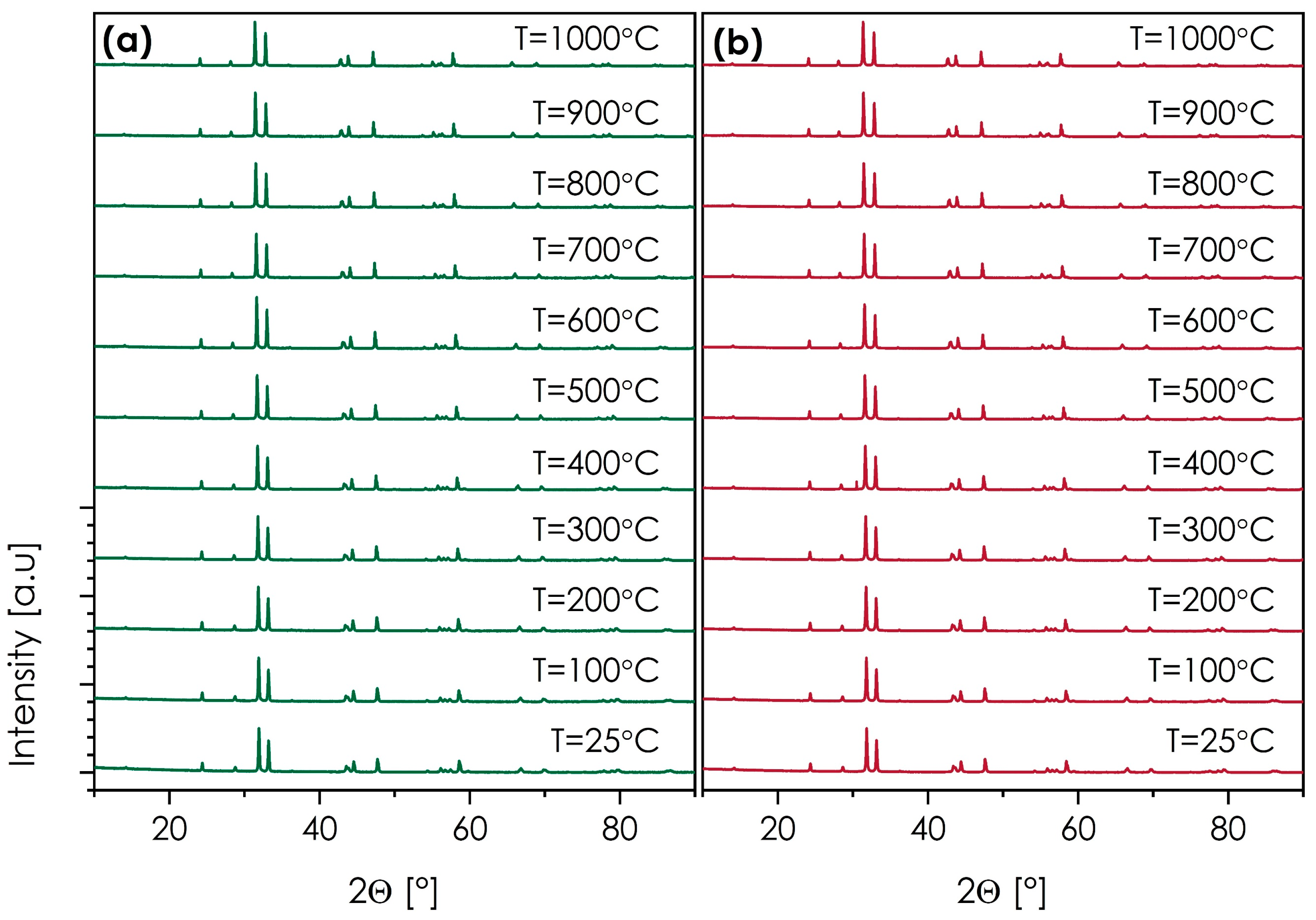
- All materials sintered at 1200 °C are characterized primarily by the I4/mmm, Ruddlesden–Popper (n = 1), T-type phase structure, with the secondary phases being observed only in the case of La- and Gd-based compositions (5.2 and 0.4 wt%, respectively). The expansion of the unit cell follows a linear trend across the series, with the exception of the La-based composition, for which the lattice parameters are slightly bigger than the trend in the rest of the series would suggest.
- The SEM + EDX data further supported the XRD results, showing a lack of secondary phase precipitates in all the studied materials, including LaSr(Co,Fe,Ga,Mn,Ni)O4+δ and GdSr(Co,Fe,Ga,Mn,Ni)O4+δ. Based on the EDX mappings, the materials are highly homogenous, although the point analysis data indicate slight deviations in the content of Ga; however, there are no signs of phase separation. The sinterability of the materials improves with the decreasing ionic radius of the lanthanide ions, which is in agreement with the relative density data obtained by the Archimedes method.
- The oxygen non-stoichiometry behavior reveals a very low, nearly identical level of oxygen excess, varying from δ equaling 0.06 to 0.015 for all materials, with no correlation to the size of the A-site lanthanide ion. Furthermore, the level of oxygen content is nearly temperature-independent for all single-phase materials. The observed behavior indicates a relatively high impact of B-site occupancy on the properties of the materials, in comparison to the influence of the A-site cations.
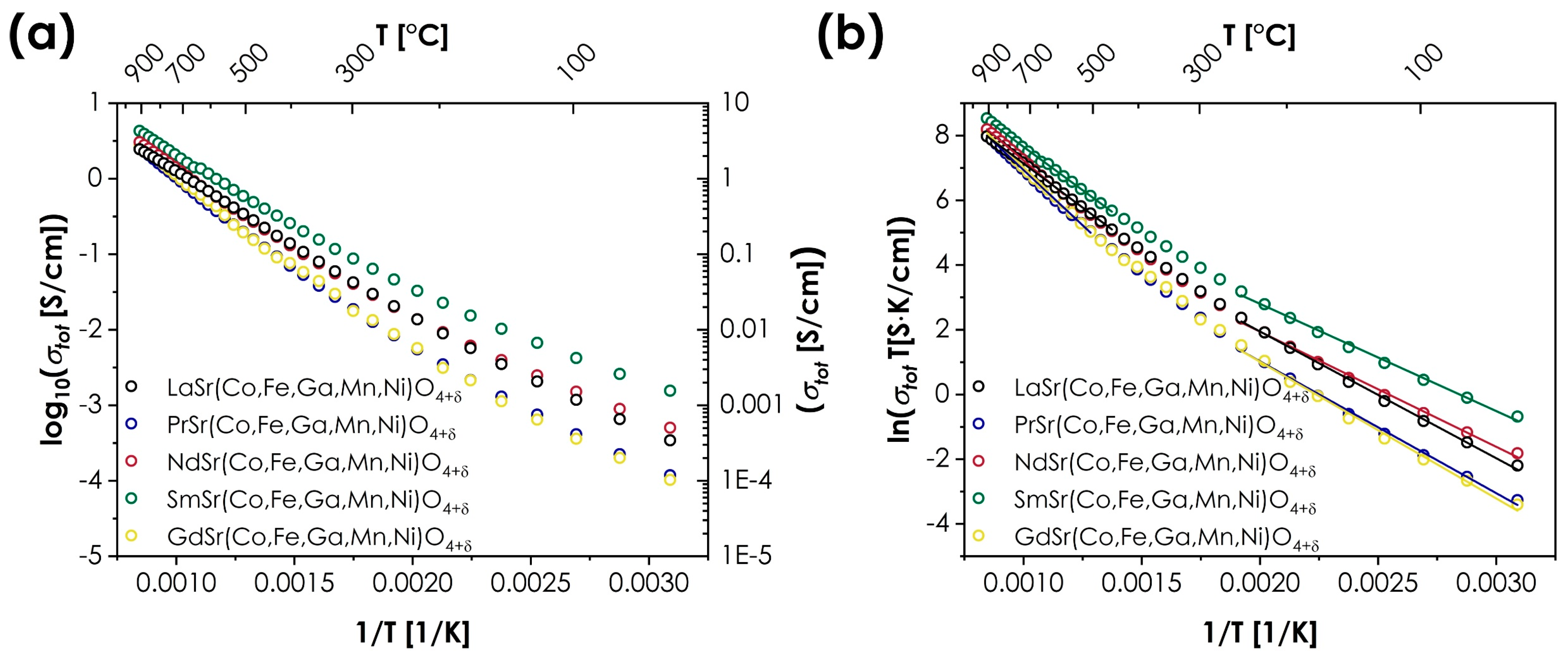
- The electrical behavior also appears to be highly unique for RP-type materials, with only small differences between the different materials in terms of both maximum total conductivity value (varying from 2.44 to 4.28 S/cm for GdSr(Co,Fe,Ga,Mn,Ni)O4+δ and SmSr(Co,Fe,Ga,Mn,Ni)O4+δ, respectively), and energies of activation. In all cases, the materials are characterized by a semiconducting behavior, with visible differences between low- and high-temperature ranges. This is contrary to the behavior reported for the conventional RP systems, in which the selection of the A-site lanthanide often has a profound impact on both the value and type of conductivity.
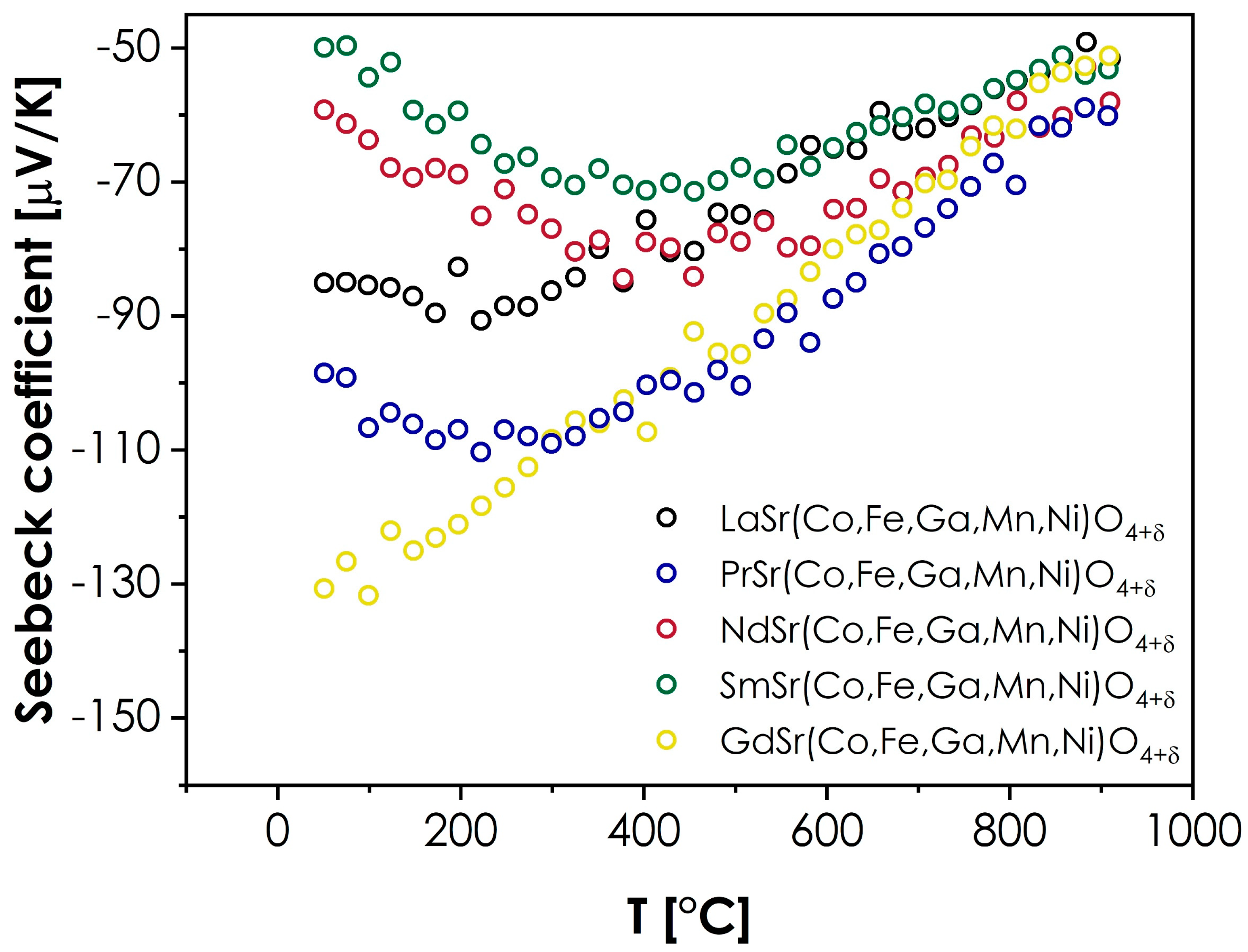
- The values of the Seebeck coefficient for all materials are negative within the whole investigated temperature range, varying from −50 to −130 μV/K. Despite the likely p-type character of the studied materials, such results can be explained based on the theories developed for conventional, RP-type oxides. It can be assumed that the determined, negative values of the Seebeck coefficient can be related to the effective masses of electron and hole charge carriers, which in turn, impact the sign of the Seebeck coefficient. Interestingly, a direct correlation between the total electrical conductivity and RT value of the Seebeck coefficient value can be observed, with the sequence in both cases being Sm > Nd > La > Pr > Gd.
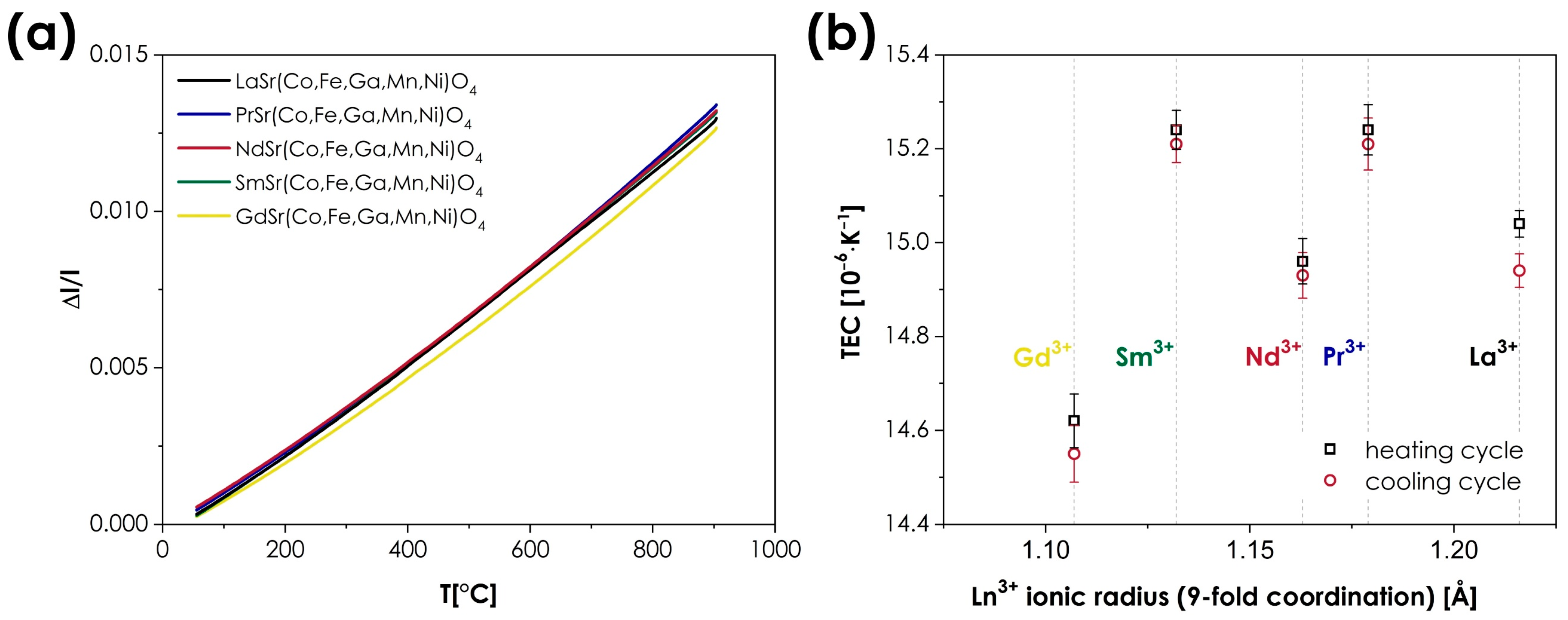
- The thermal expansion behavior is, again, remarkably similar across the whole series, with the thermal expansion coefficient varying from 14.55 to 15.21·10−6 K−1. The observed values, while relatively high for the n = 1 RP materials, in general, show similar behavior to that observed in conventional systems, in which a relatively small influence of the lanthanide type on the TEC values is typically reported.
- The selected SmSr(Co,Fe,Ga,Mn,Ni)O4+δ and NdSr(Co,Fe,Ga,Mn,Ni)O4+δ materials, based on the performed HT-XRD measurements, are characterized by high thermal stability within the RT–1000 °C range.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kundu, A.K. Magnetic Perovskites; Springer: New Delhi, India, 2016. [Google Scholar]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2021, 11, 2000459. [Google Scholar] [CrossRef]
- Das, A.; Xhafa, E.; Nikolla, E. Electro- and thermal-catalysis by layered, first series Ruddlesden-Popper oxides. Catal. Today 2016, 277, 214–226. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden–Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Sharma, I.B.; Singh, D. Solid state chemistry of Ruddlesden-Popper type complex oxides. Bull. Mater. Sci. 1998, 21, 363–374. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Sun, L. Ln2MO4 cathode materials for solid oxide fuel cells. Sci. China Chem. 2011, 54, 898–910. [Google Scholar] [CrossRef]
- Zheng, K.; Gorzkowska-Sobaś, A.; Świerczek, K. Evaluation of Ln2CuO4 (Ln: La, Pr, Nd) oxides as cathode materials for IT-SOFCs. Mater. Res. Bull. 2012, 47, 4089–4095. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Marlowe, C.A.; Lee, K.T. Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 2012, 5, 5498–5509. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Sreedhar, I.; Agarwal, B.; Goyal, P.; Agarwal, A. An overview of degradation in solid oxide fuel cells-potential clean power sources. J. Solid State Electrochem. 2020, 24, 1239–1270. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Gu, X.-K.; Nikolla, E. Design of Ruddlesden–Popper Oxides with Optimal Surface Oxygen Exchange Properties for Oxygen Reduction and Evolution. ACS Catal. 2017, 7, 5912–5920. [Google Scholar] [CrossRef]
- Huan, D.; Shi, N.; Xie, Y.; Li, X.; Wang, W.; Xue, S.; Peng, R.; Lu, Y. Chapter 8—Cathode materials for proton-conducting solid oxide fuel cells. In Intermediate Temperature Solid Oxide Fuel Cells; Kaur, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–314. [Google Scholar]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; da Costa, J.C.D. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Drioli, E.; Barbieri, G.; Brunetti, A. Membrane Engineering for the Treatment of Gases; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Smart, S.; Liu, S.; Serra, J.M.; Basile, A.; da Costa, J.C.D. Chapter 7—Perovskite membrane reactors: Fundamentals and applications for oxygen production, syngas production and hydrogen processing. In Membranes for Clean and Renewable Power Applications; Gugliuzza, A., Basile, A., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 182–217. [Google Scholar]
- Rost, C.M.; Schaet, R.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, D. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy charcterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2016, 5, 102–109. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtosov, D.A.; Kirsanova, A.A.; Habner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, J.; Zajusz, M.; Moździerz, M.; Berent, K.; Mikuła, A.; Stępień, A.; Świerczek, K. Structure and transport properties of the novel (Dy,Er,Gd,Ho,Y)3Fe5O12 and (Dy,Gd,Ho,Sm,Y)3Fe5O12 high entropy garnets. J. Eur. Ceram. Soc. 2021, 41, 3844–3849. [Google Scholar] [CrossRef]
- Zhang, W.; Mazza, A.R.; Skoropata, E.; Mukherjee, D.; Musico, B.; Zhang, J.; Keppens, V.M.; Zhang, L.; Kisslinger, K.; Stavitski, E.; et al. Applying Configurational Complexity to the 2D Ruddlesden–Popper Crystal Structure. ACS Nano 2020, 14, 13030–13037. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, F.; Lu, Y.; Chen, L.; Hu, Y.S. High-Entropy Layered Oxide Cathodes for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 264–269. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Basi, L.; Kubel, C.; Brezesisnki, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Schwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An innovative approach to design SOFC air electrode materials: High entropy La1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (x = 0, 0.1, 0.2, 0.3) perovskites synthesized by the sol–gel method. J. Mater. Chem. A 2020, 8, 24455–24468. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, H.; Ni, H.; Ou, X.; Wang, S.; Lin, B.; Feng, P.; Ling, Y. A novel facile strategy to suppress Sr segregation for high-entropy stabilized La0·8Sr0·2MnO3-δ cathode. J. Power Sources 2021, 482, 228959. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Musicó, B.L.; Wright, Q.; Delzer, C.; Ward, T.Z.; Rawn, C.J.; Mandrus, D.G.; Keppens, V. Synthesis method comparison of compositionally complex rare earth-based Ruddlesden–Popper n = 1 T′-type cuprates. J. Am. Ceram. Soc. 2021, 104, 3750–3759. [Google Scholar] [CrossRef]
- Mazza, A.R.; Gao, X.; Rossi, D.J.; Musico, B.L.; Valentine, T.W.; Kennedy, Z.; Zhang, J.; Lapano, J.; Keppens, V.; Moore, R.G.; et al. Searching for superconductivity in high entropy oxide Ruddlesden–Popper cuprate films. J. Vac. Sci. Technol. A 2021, 40, 013404. [Google Scholar] [CrossRef]
- Shijie, Z.; Na, L.; Liping, S.; Qiang, L.; Lihua, H.; Hui, Z. A novel high-entropy cathode with the A2BO4-type structure for solid oxide fuel cells. J. Alloys Compd. 2022, 895, 162548. [Google Scholar] [CrossRef]
- Salame, P.H. Structural and electrical properties of T′-type Ln2CuO4 (Ln = Pr, Nd, Sm, Eu and Gd) ceramics. AIP Conf. Proc. 2016, 1731, 120005. [Google Scholar]
- Olszewska, A.; Du, Z.; Świerczek, K.; Zhao, H.; Dąbrowski, B. Novel ReBaCo1.5Mn0.5O5+δ (Re: La, Pr, Nd, Sm, Gd and Y) perovskite oxide: Influence of manganese doping on the crystal structure, oxygen nonstoichiometry, thermal expansion, transport properties, and application as a cathode material in solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 13271–13285. [Google Scholar]
- Xu, S.; Jacobs, R.; Morgan, D. Factors Controlling Oxygen Interstitial Diffusion in the Ruddlesden–Popper Oxide La2–xSrxNiO4+δ. Chem. Mater. 2018, 30, 7166–7177. [Google Scholar] [CrossRef]
- Al Daroukh, M.; Vashook, V.V.; Ullmann, H.; Tietz, F.; Arual Raj, I. Oxides of the AMO3 and A2MO4-type: Structural stability, electrical conductivity and thermal expansion. Solid State Ion. 2003, 158, 141–150. [Google Scholar] [CrossRef]
- Vashook, V.V.; Ullmann, H.; Olshevskaya, O.P.; Kulik, V.P.; Lukashevich, V.E.; Kokhanovskij, L.V. Composition and electrical conductivity of some cobaltates of the type La2−xSrxCoO4.5−x/2±δ. Solid State Ion. 2000, 138, 99–104. [Google Scholar] [CrossRef]
- Han, J.; Zheng, K.U.N.; Świerczek, K. Nickel-based layered perovskite cathode materials for application in intermediate-temperature solid oxide fuel cells. Funct. Mater. Lett. 2011, 4, 151–155. [Google Scholar] [CrossRef]
- Vashook, V.V.; Yushkevich, I.I.; Kokhanovsky, L.V.; Makhnach, L.V.; Tolochko, S.P.; Kononyuk, I.F.; Ullmann, H.; Altenburg, H. Composition and conductivity of some nickelates. Solid State Ion. 1999, 119, 23–30. [Google Scholar] [CrossRef]
- Goodenough, J.B. Interpretation of the transport properties of Ln2NiO4 and Ln2CuO4 compounds. Mater. Res. Bull. 1973, 8, 423–431. [Google Scholar] [CrossRef]
- Nikonov, A.V.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Pavzderin, N.B. A brief review of conductivity and thermal expansion of perovskite-related oxides for SOFC cathode. Eurasian J. Phys. Funct. Mater. 2018, 2, 274–292. [Google Scholar] [CrossRef]
- Kim, J.H.; Manthiram, A. Layered LnBaCo2O5+d perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195. [Google Scholar] [CrossRef]
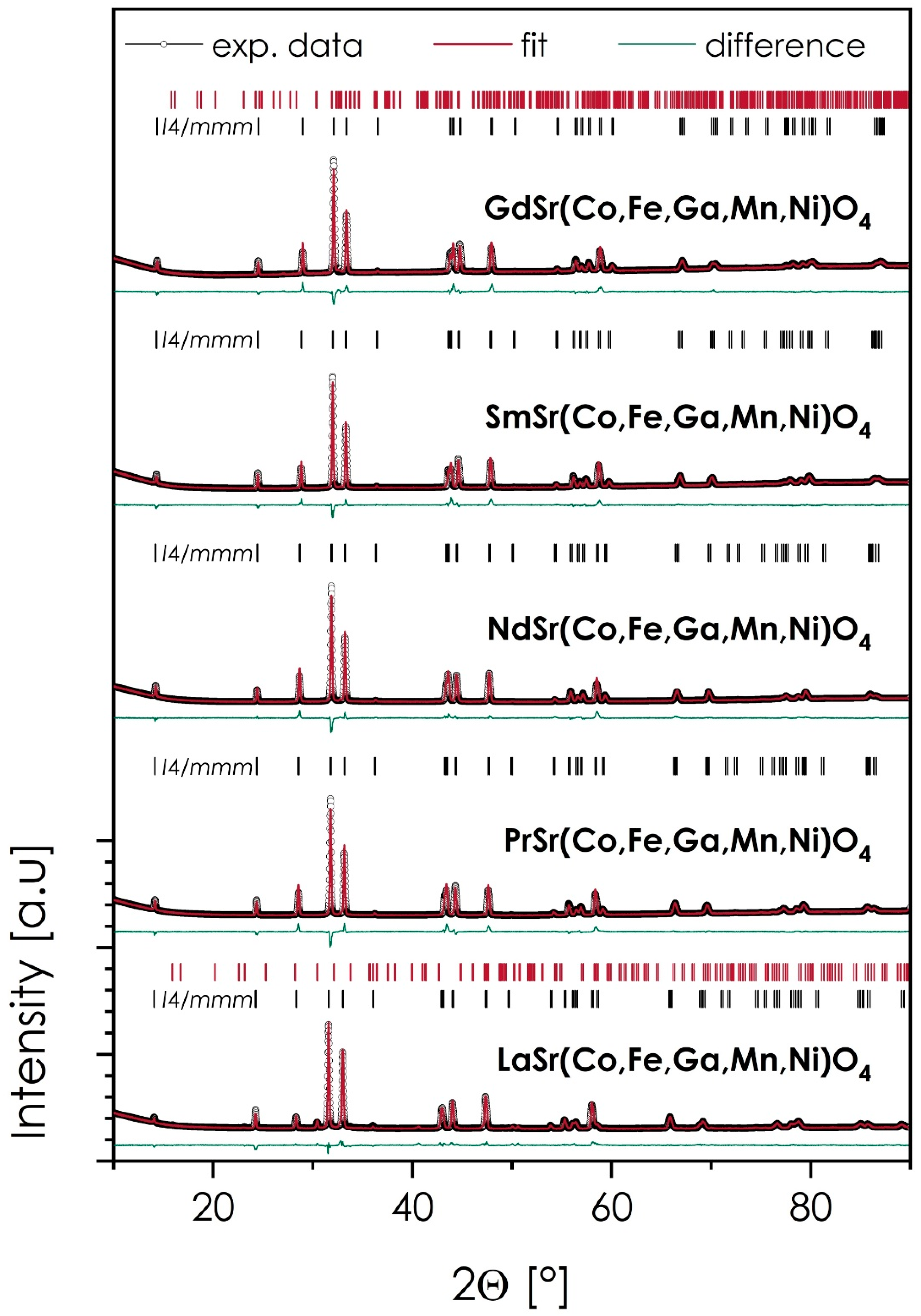


| Space Group | Content (wt%) | a [Å] | b [Å] | c [Å] | V [Å3] | a0 [Å] | GoF | Rwp | δthe (g/cm3) | δexp (g/cm3) | δrel (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LaSr(Co,Fe,Ga,Mn,Ni)O4 | I4/mmm | 94.8 | 3.8393(1) | 3.8393(1) | 12.6075(3) | 185.834(1) | 5.7066(1) | 5.25 | 4.96 | 6.26 | - | - |
| P-421 m | 5.2 | 7.885(1) | 7.885(1) | 5.313(2) | - | - | - | - | - | |||
| PrSr(Co,Fe,Ga,Mn,Ni)O4 | I4/mmm | 100 | 3.8164(1) | 3.8164(1) | 12.4914(3) | 181.939(1) | 5.6664(1) | 10.78 | 4.77 | 6.43 | 5.76 | 89.6 |
| NdSr(Co,Fe,Ga,Mn,Ni)O4 | I4/mmm | 100 | 3.8101(1) | 3.8101(1) | 12.4470(3) | 180.692(1) | 5.6534(1) | 12.09 | 4.93 | 6.53 | 5.75 | 88.1 |
| SmSr(Co,Fe,Ga,Mn,Ni)O4 | I4/mmm | 100 | 3.8003(1) | 3.8003(1) | 12.3757(3) | 178.734(1) | 5.6329(1) | 7.51 | 3.66 | 6.72 | 6.30 | 93.8 |
| GdSr(Co,Fe,Ga,Mn,Ni)O4 | I4/mmm | 99.6 | 3.7946(1) | 3.7946(1) | 12.3157(4) | 177.337(1) | 5.6182(1) | 7.50 | 3.27 | 6.90 | 6.52 | 94.6 |
| Iba | 0.4 | 11.214(1) | 18.883(1) | 5.527(1) | - | - | - |
| Ln | Sr | Co | Fe | Ga | Mn | Ni | O (at. %) | |
|---|---|---|---|---|---|---|---|---|
| LaSr(Co,Fe,Ga,Mn,Ni)O4 | 17.6(1) | 14.1(1) | 3.5(4) | 3.5(2) | 4.2(2) | 3.3(2) | 3.5(1) | 50.4(3) |
| PrSr(Co,Fe,Ga,Mn,Ni)O4 | 17.0(1) | 14.4(1) | 3.3(1) | 3.1(1) | 4.2(5) | 3.4(1) | 3.4(3) | 51.2(2) |
| NdSr(Co,Fe,Ga,Mn,Ni)O4 | 17.1(2) | 14.3(1) | 3.2(1) | 3.2(2) | 4.2(4) | 3.4(1) | 3.4(2) | 51.4(2) |
| SmSr(Co,Fe,Ga,Mn,Ni)O4 | 17.2(2) | 14.3(1) | 3.3(3) | 3.4(1) | 3.9(4) | 3.2(1) | 3.2(1) | 51.7(4) |
| GdSr(Co,Fe,Ga,Mn,Ni)O4 | 16.6(2) | 14.1(1) | 2.9(2) | 3.4(1) | 4.0(5) | 3.2(1) | 3.2(1) | 52.6(3) |
| Ea (eV) | T (°C) | σmax (S/cm) | |
|---|---|---|---|
| LaSr(Co,Fe,Ga,Mn,Ni)O4+δ | 0.34(1) | 50–250 | 2.45 |
| 0.47(1) | 450–900 | ||
| PrSr(Co,Fe,Ga,Mn,Ni)O4+δ | 0.35(1) | 50–250 | 2.54 |
| 0.59(1) | 500–900 | ||
| NdSr(Co,Fe,Ga,Mn,Ni)O4+δ | 0.31(1) | 50–250 | 3.05 |
| 0.53(1) | 575–900 | ||
| SmSr(Co,Fe,Ga,Mn,Ni)O4+δ | 0.29(1) | 50–250 | 4.28 |
| 0.47(1) | 450–900 | ||
| GdSr(Co,Fe,Ga,Mn,Ni)O4+δ | 0.36(1) | 50–250 | 2.74 |
| 0.60(1) | 625–900 |
| TEC (10−6·K−1) | ||
|---|---|---|
| Heating | Cooling | |
| LaSr(Co,Fe,Ga,Mn,Ni)O4+δ | 15.04 ± 0.03 | 14.94 ± 0.04 |
| PrSr(Co,Fe,Ga,Mn,Ni)O4+δ | 15.24 ± 0.05 | 15.21 ± 0.06 |
| NdSr(Co,Fe,Ga,Mn,Ni)O4+δ | 14.96 ± 0.05 | 14.94 ± 0.05 |
| SmSr(Co,Fe,Ga,Mn,Ni)O4+δ | 15.24 ± 0.04 | 15.21 ± 0.04 |
| GdSr(Co,Fe,Ga,Mn,Ni)O4+δ | 14.62 ± 0.06 | 14.55 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowa, J.; Adamczyk, J.; Stępień, A.; Zajusz, M.; Bar, K.; Berent, K.; Świerczek, K. Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement. Materials 2022, 15, 6500. https://doi.org/10.3390/ma15186500
Dąbrowa J, Adamczyk J, Stępień A, Zajusz M, Bar K, Berent K, Świerczek K. Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement. Materials. 2022; 15(18):6500. https://doi.org/10.3390/ma15186500
Chicago/Turabian StyleDąbrowa, Juliusz, Jan Adamczyk, Anna Stępień, Marek Zajusz, Karolina Bar, Katarzyna Berent, and Konrad Świerczek. 2022. "Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement" Materials 15, no. 18: 6500. https://doi.org/10.3390/ma15186500
APA StyleDąbrowa, J., Adamczyk, J., Stępień, A., Zajusz, M., Bar, K., Berent, K., & Świerczek, K. (2022). Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement. Materials, 15(18), 6500. https://doi.org/10.3390/ma15186500








