3.1. Materials Characterization
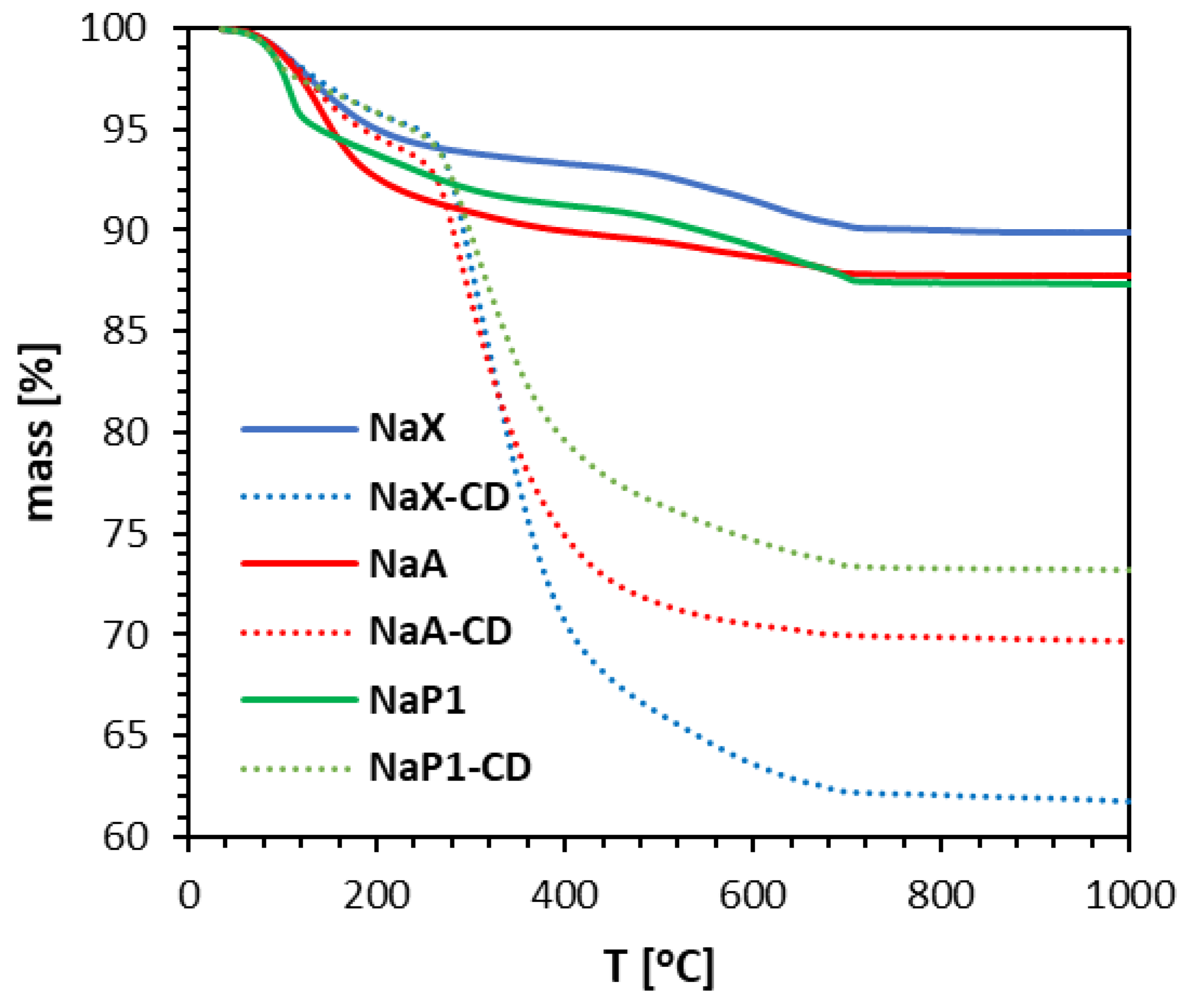
TG curves of zeolites before and after their modifications depicted the percentage mass losses due to the constant heating of the samples up to 1000 °C (
Figure 1). TG mass loss profiles for zeolites agreed well with the literature data [
42,
43]. The main mass loss occurred below 200 °C and is attributed to the release of adsorbed and zeolitic water [
32,
42,
44]. Further weight loss can be attributed to dehydroxylation processes [
45,
46]. The total mass loss was 10.02%, 12.24% and 12.65% for NaX, NaA and NaP1, respectively. TG curves of zeolites after modification indicate mass losses associated with water release (up to about 180 °C), as well as significant mass loss attributed to dehydration and decomposition of organic matter (in the range about 250–400 °C) as reported for zeolites modified with chitosan [
47,
48] and cyclodextrin [
36,
39] The total mass loss was 38.2%, 30.28% and 26.75% for NaX, NaA and NaP1, respectively. In summary, the mass loss associated with the presence of the modifying compound can be reflected as 28.18% for NaX-CD, 18.04% for NaA-CD, and 14.10% for NaP1-CD, respectively, indicating the highest modification efficacy for the zeolite NaX, and the lowest for NaP1.
Mineral composition of the materials is presented in
Figure 2 (left column). All the zeolites were composed of mineral phases attributed to NaX, NaA and NaP1 types, accompanied by some traces of mullite, quartz and amorphous glassy phase recognized by the characteristic rise in the background line in the angular range 15–35 °2θ.
The lower contribution of the amorphous phase is attributed to the NaX, suggesting the highest degree of crystallinity. The peaks characteristic for the zeolite phases can be recognized by the strongest reflections at the following dhkl values: 14.45; 8.85; 3.34; 2.89, 2.79 Å for NaX; 12.23; 8.65; 3.70; 3.28; 2.98 Å for NaA; 3.17; 7.10; 4.10; 2.68; 5.02 Å for NaP1. It was in good agreement with the standards collected in the PDF2-2022 database. According to the database, the reference numbers are 00–038–0237 for NaX, 00–038–0241 for NaA, and 00–025–0778 for NaP1.
The reflections attributed to the zeolites are also present in the CD-modified materials, confirming that the modification process does not affect the zeolites’ mineral structure. Moreover, a characteristic ‘hump’ in the angular range from 15 to 25 °2θ can be identified, related to the occurrence of organic matter. A preliminary analysis of the diffractograms allows one to conclude that NaP1 was the least susceptible to modification with CD, which is in good agreement with TG results.
According to the IUPAC classification [
49], the isotherms of the materials studied are a combination of types I and II (
Figure 3). The hysteresis shapes can be attributed to the type H4, which is typical of aggregated zeolite crystals or mesoporous zeolites. All materials revealed narrow hysteresis loops, probably due to the tensile strength effect [
50]. The rise in the nitrogen adsorption at low p/p
0 values, related to micropore filling, is the highest for NaX, and higher than for NaA, thus revealing their microporous character.
The modification with β-CD caused a dramatical decrease in the nitrogen adsorbed volume indicating the partial blocking of materials’ pores by β-CD. As a consequence, their volume decreased significantly.
Table 3 shows textural parameters of the materials.
The most developed specific surface area and total volume of pores was noted for NaX. After the modification, a significant decrease in the SBET of the hybrid materials compared to the corresponding zeolites was observed, while this decrease was the largest for the zeolite NaP1 (by about 83%), than similar for NaA (76%), and for NaX (75%). The total volume of pores Vtot decreased by about 78% for NaX, 75% for NaA, and 76% for NaP1. These textural changes suggest, that regardless of the structure type, Vtot of zeolites after modification decreased similarly (in %). On the other hand, the specific surface of NaP1 decreased the most after the modification. That may suggest the modification mostly occurs on the external surface of materials.
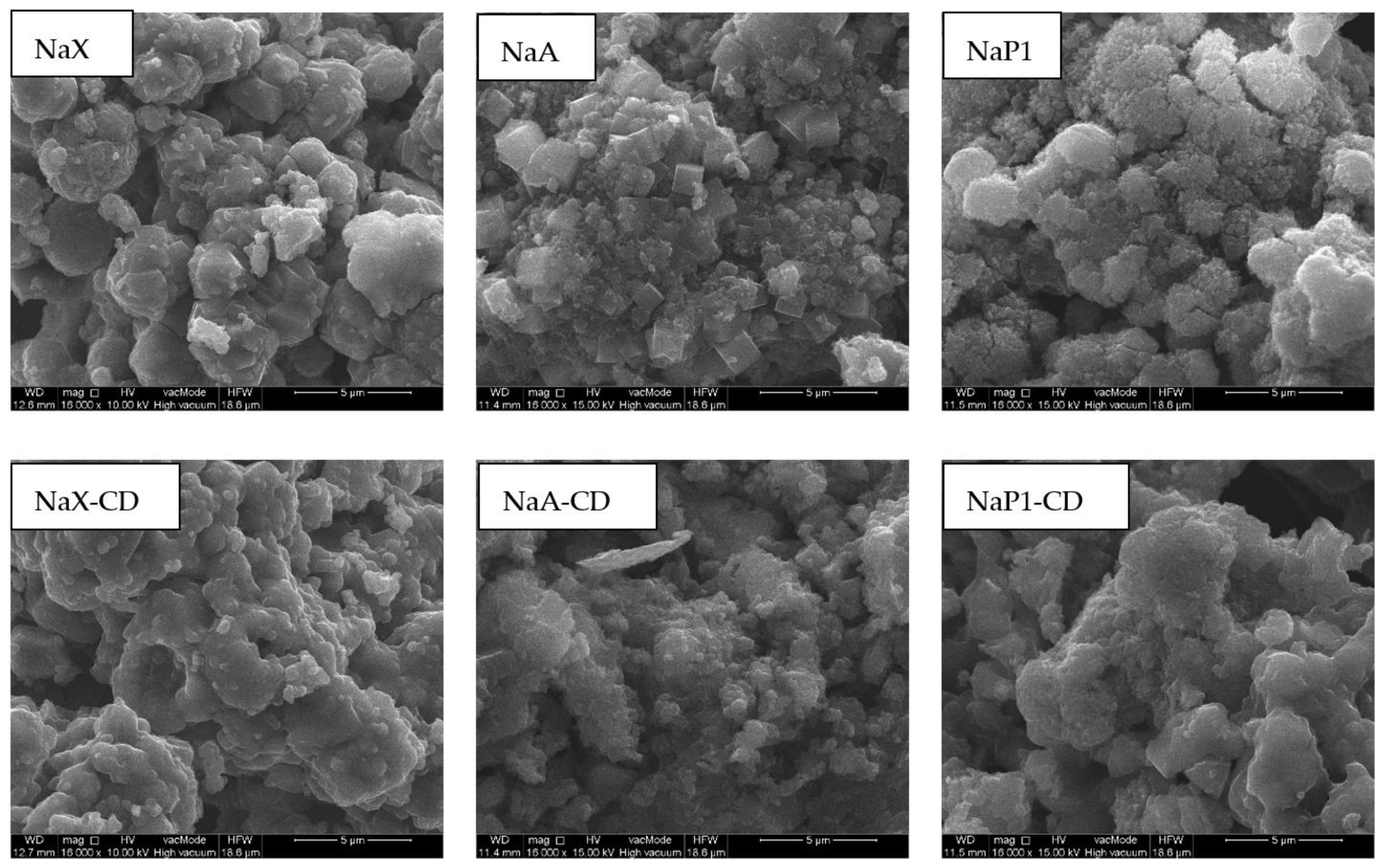
Surface morphology of the zeolites before and after modification with β-CD was investigated via SEM (
Figure 4). NaX occurs in a form of regular, isometric cubic crystals with the sizes of 2–6 µm, overlapping each other randomly. NaA has well-shaped cubic crystals with a size of 1–2 µm. Na-P1 occurs in the form of rosette-like adhesions composed of fine pillars. The sizes of aggregates usually exceed 10 µm in size. Significant changes in the morphological habit of the zeolites after the modification occurred. crystalic and well-shaped structures have been covered by the amorphous organic substance. The CD formed a specific layer that occluded the mineral material. This layer enveloped the crystalline forms and the spaces between them creating more rounded, softer shapes and globular structures. Similar forms were observed by us in previous work [
36] as well as by Lv et al. [
51] for pure CD, and for β-CD/Al(OH)
3 composites [
52].
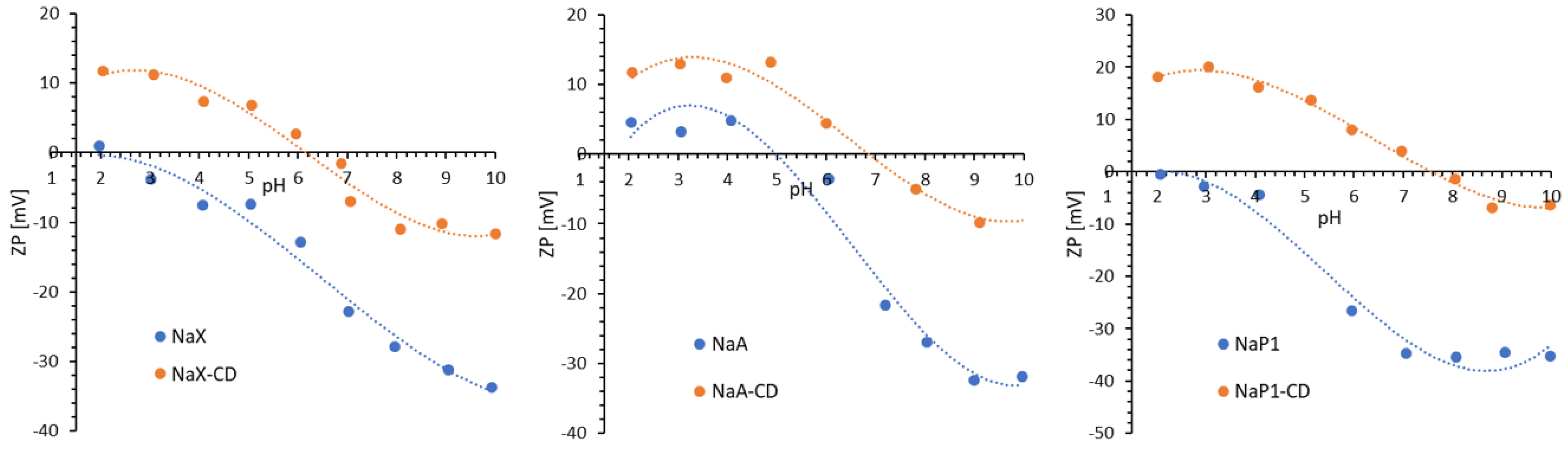
Electrokinetic properties of solid particles dispersed in aqueous media provides information on the solid surface chemical nature. The charge density of the diffusion layer within the electric double layer (EDL) formed on the surface of a solid particle depends on the value of the zeta potential (ζ). The charge of the diffusion layer increases when a displacement of counter ions perpendicular to the surface takes place, which causes an increase in the ζ-potential [
53]. In the case of the zeolites studied, the attachment of β-CD molecules to their surface (via the linker GLYMO), which requires the formation of two bonds, probably results in the removal of divalent cations present in the zeolite structure. This displacement of counter ions in the direction perpendicular to the zeolite surface is therefore the result of the active sites blocking on the solid surface by the adsorbed modifier molecules.
Figure 5 shows ζ-potential for the zeolites before and after modification. An increase in the ζ-potential was observed over the entire pH range after CD modification, as well as a significant shift in the position of their isoelectric points (IEP) towards higher pH values—the highest for the zeolite NaP1 (pH
IEP increased from a value of about 2 for the initial NaP1 to a value of about 8 for the modified NaP1-CD). Similar behavior was observed by other authors who studied the interactions of dextrins with the surface of various solids [
54] and sepiolite treated with organosilanes [
55].
The FTIR spectra of the zeolite materials NaX, NaA and NaP1 (
Figure 2, right column) are characterized by the presence of bands typical for zeolite structures of the faujasite (FAU), zeolite A (LTA) and gismondite (GIS) groups, respectively [
42,
56,
57]. The spectra of the hybrid materials (NaX-CD, NaA-CD and NaP1-CD) confirm the effectiveness of the modifications using β-CD, revealing the presence of bands characteristic of zeolites and β-CD. The bands with a maximum in the range of 3800–3000 cm
−1 are attributed to the stretching vibrations of the O-H groups present on the materials’ surface. In the case of the hybrid materials, two characteristic bands originating from asymmetric vibrations of -CH
2- groups and symmetric stretching vibrations of -CH
3 and -CH
2- groups can be distinguished, located at 2936 and 2866 cm
−1 for NaX-CD, 2938 and 2870 cm
−1 for NaA-CD, and 2939 and 2869 cm
−1 for NaP1-CD. These bands are indicative of the presence of the hydrocarbon chain and methyl group of the linker molecule, and may also be related to the vibration of the hydrocarbon groups present in β-CD. Bands at wavenumbers in the range of 1650–1620 cm
−1 are associated with bending vibrations of the O-H groups of the so-called zeolite water. The band at about 1450 cm
−1, present for all zeolite materials, indicates the presence of carbonates. On the spectra of the hybrid materials, significant changes are observed in the wavenumber range of 1420–1350 cm
−1. In this region, vibrations originating from the glucose units that build the CD cup are present, and the visible changes indicate the effectiveness of the modification performed. Furthermore, the presence of a band at about 1200 cm
−1 on the spectra of the hybrid materials indicates the chemical bonding of the linker molecule (GLYMO) to the surface of the zeolite materials. For all zeolite materials, we observe the presence of a strong band in the range of 1090–900 cm
−1, which is attributed to the symmetric and asymmetric stretching vibrations of the Si-O(Al) and Si-O(Si) bridge bonds. Due to the modification process, the intensity of this band decrease and its shape change which is related to the presence of glucosidic -O-C-C stretching vibrations of β-CD units. In zeolite-CD-TC complex, slight changes in the bond shapes at the wavelength range 1200–1700 cm
−1 can be noticed that may indicate the occurrence of new oxygen-containing groups and/or changes in the surface functional groups of the material after TC adsorption. These observation is consistent with the spectra obtained for core–shell TiO2@C ultralong nanotubes TC adsorption [
58].
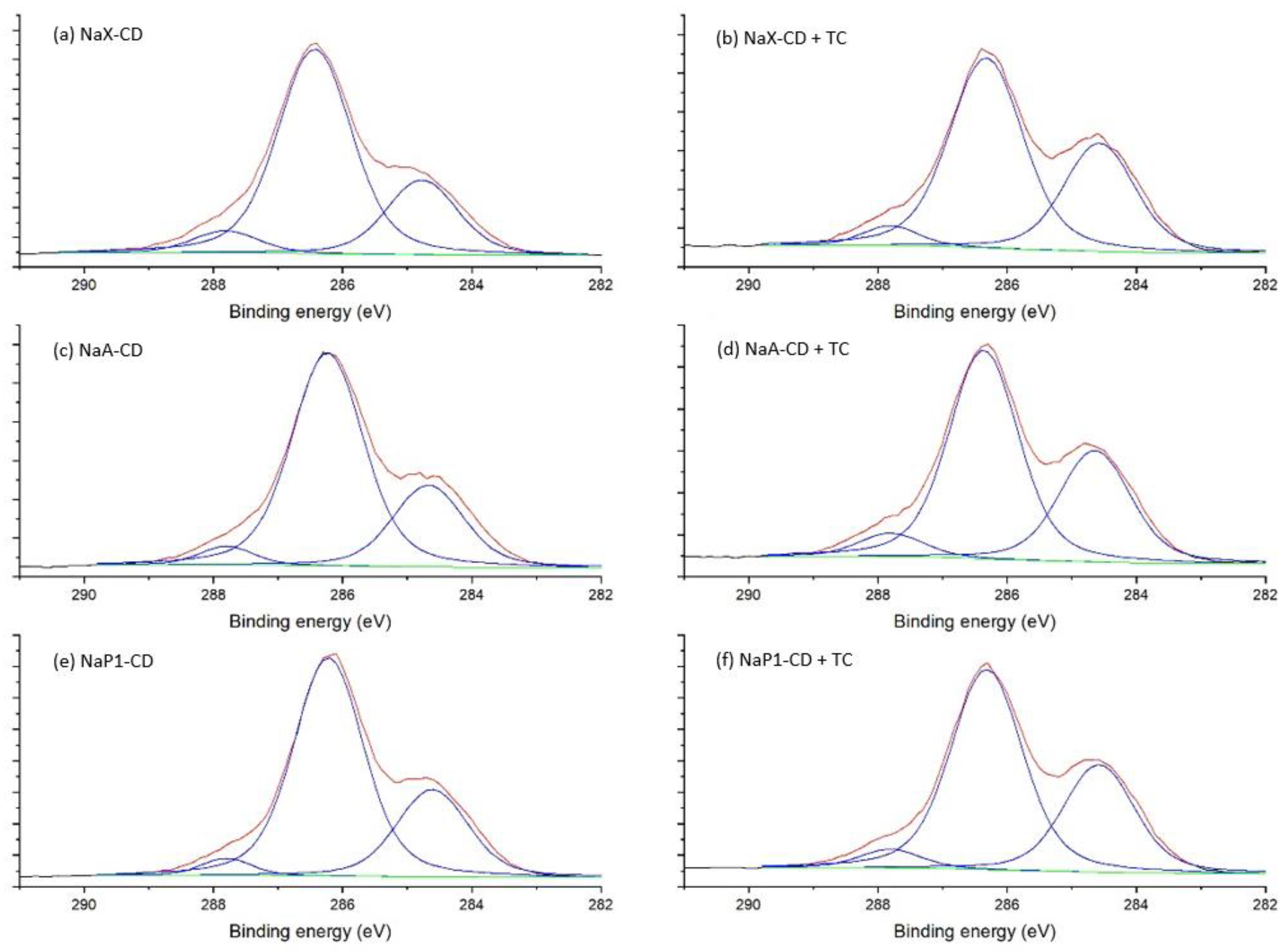
XPS was employed to explore the chemical composition of the materials’ surface and binding energies of adsorbents before and after adsorption of TC (
Figure 6). As can be seen in
Table 4, the surface of fly ash zeolites is mainly composed of oxygen (58.8–62.0%), silicon (14.4–19.0%) and aluminum (5.6–9.4%) atoms. The basic chemical composition is complemented by mono- and divalent metal atoms such as sodium (3.6–6.7%), potassium (0.5–0.8%, magnesium (1.6–6.3%) and calcium (2.5–3.5%). Carbon (0.8–1.3%) and iron (0.3–1.3%) were found in much smaller amounts on the surface of zeolites. After modification with β-CD, the chemical composition of the materials’ surface changed significantly. The vast majority of atoms were oxygen (37.6–41.0%) and carbon (48.2–53.7%), associated with molecules of the organic modifier. Other elements, such as silicon and aluminum, were present in small amounts of 6.4–7.1%, which is due to the deposition of the modifier molecules on the surface of the materials. There was also a significant decrease in mono- and divalent metal atoms which play the role of exchangeable cations.
The small amount of nitrogen in zeolites after β-CD modification was originating from the DMF used during the synthesis while N1s peak was not observed in bare zeolites. The N1s spectra of the NaX-CD, NaA-CD and NaP1-CD showed two peaks at around 399.4 eV and 402.5 eV, which were assigned to the amide and protonated N, respectively (data not shown). The proportion of N1s slightly increased after TC adsorption and the peaks were found at similar binding energies (BEs) with no significant changes in their proportions. The peak at 399.4 eV included tetracycline’s amine and amide nitrogen.
The C1s spectra of the hybrid materials NaX-CD, NaA-CD and NaP1-CD were fitted with three peak components (
Figure 7). The first component with (BEs) at 284.6–284.8 eV belongs to the C–C/C–H bonds. The middle component centered at 286.2–286.4 eV was associated with C–N (from DMF) and C–O bonds. The third component at 287.8 eV was associated with O–C–O bonds of the β-CD structure [
59] and N–C=O of the DMF. After TC adsorption, the BEs of these components were similar (±0.1 eV), while the proportion of the first component clearly increased (from 24–28% to 33–34%). This was due to introducing a higher proportion of C–C/C–H bonds and, additionally, aromatic carbon by the TC.