Structural, Optical, Charge-Transport, and Dielectric Properties of Double-Perovskite La2Co1−zFezMnO6 (z = 0, 0.2–1.0)
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
3.1. Structural Properties
3.2. Optical Properties
3.3. Electrical Properties
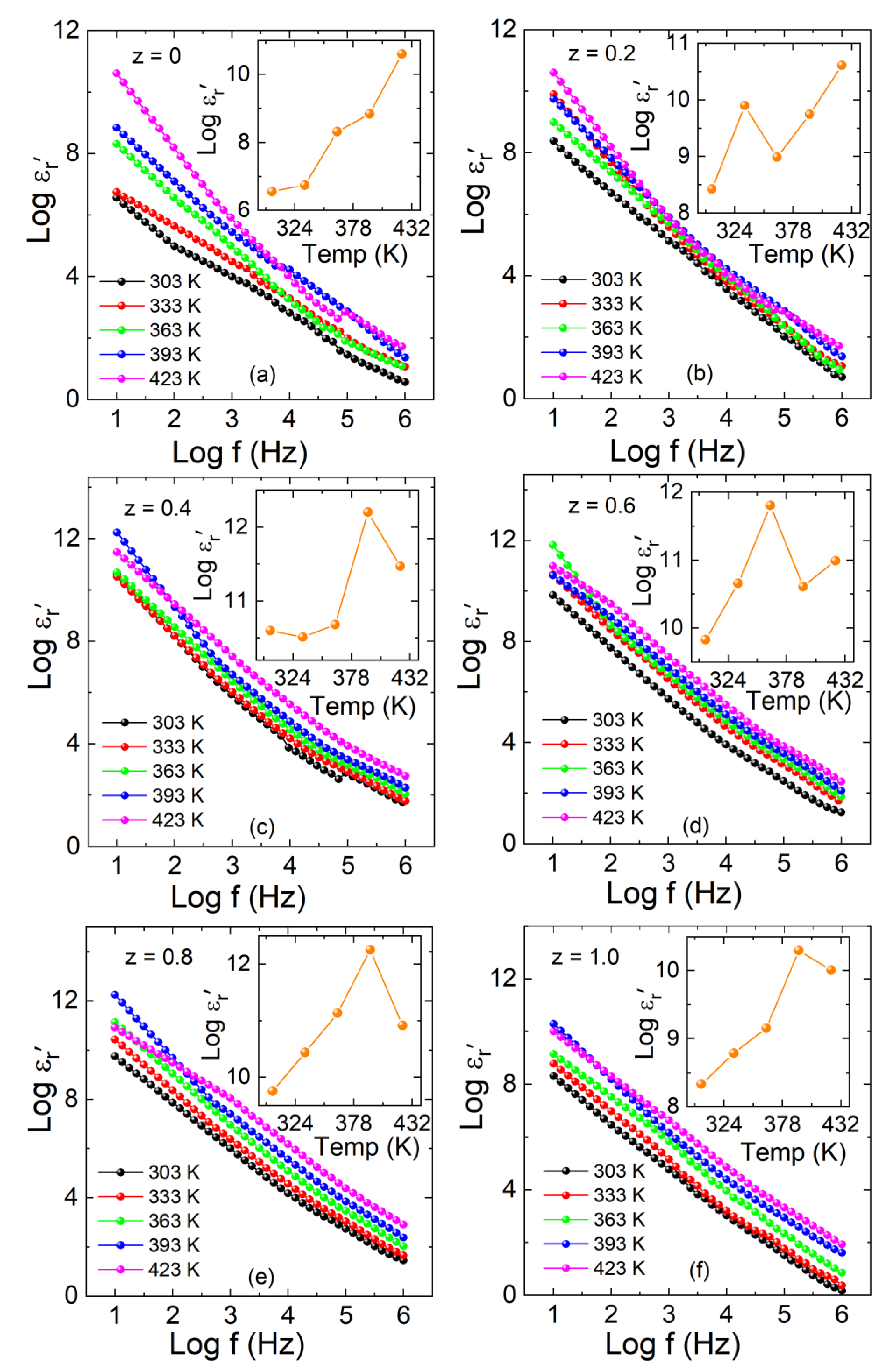
3.4. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiebig, M. Revival of the magnetoelectric effect. J. Phys. D 2005, 38, R123. [Google Scholar] [CrossRef]
- Tokura, Y. Multiferroics—toward strong coupling between magnetization and polarization in a solid. J. Magn. Magn. Mater. 2007, 310, 1145. [Google Scholar] [CrossRef]
- Monthoux, P.; Pines, D.; Lonzarich, G.G. Superconductivity without phonons. Nature 2007, 450, 1177. [Google Scholar] [CrossRef]
- Rawat, K.; Meenakshi; Mahato, R.N. Investigation of structural, magnetic, and magnetocaloric properties of nanocrystalline double perovskite Pr2CoMnO6. Mater. Res. Express 2018, 5, 066110. [Google Scholar] [CrossRef]
- Ascher, E.; Rieder, H.; Schmid, H.; Stossel, H. Some properties of ferromagnetoelectric nickel-iodine boracite Ni3B7O13I. J. Appl. Phys. 1966, 37, 1404–1405. [Google Scholar] [CrossRef]
- Nelmes, R.J. Structural studies of boracites. A review of the properties of boracites. J. Phys. C Solid State 1974, 7, 3840. [Google Scholar] [CrossRef]
- Dass, R.I.; Yan, J.Q.; Goodenough, J.B. Oxygen stoichiometry, ferromagnetism, and transport properties of La2− x NiMnO6+ δ. Phys. Rev. B 2003, 68, 064415. [Google Scholar] [CrossRef]
- Bull, C.L.; Mortimer, R.; Sankar, G.; Catlow, C.R.A.; Wood, I.G.; Price, G.D. Structural and physical properties of the binary transition metal-containing perovskite La2CoMnO6. Synth. Met. 2001, 121, 1464–1468. [Google Scholar] [CrossRef]
- Sánchez, M.C.; García, J.; Blasco, J.; Subías, G.; Perez-Cacho, J. Local electronic and geometrical structure of LaNi1−xMnxO3+δ perovskites determined by x-ray-absorption spectroscopy. Phys. Rev. B 2002, 65, 144409. [Google Scholar] [CrossRef]
- Maiti, R.P.; Dutta, S.; Mukherjee, M.; Mitra, M.K.; Chakravorty, D. Magnetic and dielectric properties of sol-gel derived nanoparticles of double perovskite Y2NiMnO6. J. Appl. Phys. 2012, 112, 044311. [Google Scholar] [CrossRef]
- Macedo Filho, R.B.; Barbosa, D.A.B.; Reichlova, H.; Marti, X.; Menezes, A.; Ayala, A.P.; Paschoal, C.W.A. Role of rare-earth ionic radii on the spin-phonon coupling in multiferroic ordered double perovskites. Mater. Res. Express 2015, 2, 075201. [Google Scholar] [CrossRef]
- Sahoo, R.C.; Das, S.; Nath, T.K. Role of Gd spin ordering on magnetocaloric effect and ferromagnetism in Sr substituted Gd2CoMnO6 double perovskite. J. Appl. Phys. 2018, 124, 103901. [Google Scholar] [CrossRef]
- Barbosa, D.A.B.; Lufaso, M.W.; Reichlova, H.; Marti, X.; Rezende, M.V.S.; Maciel, A.P.; Paschoal, C.W.A. Ba-doping effects on structural, magnetic and vibrational properties of disordered La2NiMnO6. J. Alloy. Compd. 2016, 663, 899–905. [Google Scholar] [CrossRef]
- Li, Q.; Xing, L.; Xu, M. Magnetic properties, resistivity and magnetoresistance effects of double perovskite La2Co1− xFexMnO6. J. Alloy. Compd. 2017, 710, 771–777. [Google Scholar] [CrossRef]
- Nasir, M.; Khan, M.; Bhatt, S.; Bera, A.K.; Furquan, M.; Kumar, S.; Yusuf, S.M.; Patra, N.; Bhattacharya, D.; Jha, S.N. Influence of cation order and valence states on magnetic ordering in La2Ni1−xMn1+xO6. Phys. Status Solidi 2019, 256, 1900019. [Google Scholar] [CrossRef]
- Murthy, J.K.; Chandrasekhar, K.D.; Venimadhav, A. Observation of Griffiths-like phase and its tunability in La2Ni1− xCoxMnO6 (0 ≤ x ≤ 1) nanoparticles. J. Magn. Magn. Mater. 2016, 418, 2–8. [Google Scholar] [CrossRef]
- Gan, H.; Wang, C.; Shen, Q. Improved magnetic performance of Co-doped La2NiMnO6 ceramics prepared at low temperature. J. Eur. Ceram. Soc. 2020, 5, 1909–1916. [Google Scholar] [CrossRef]
- Xin, Y.; Shi, L.; Zhao, J.; Yuan, X.; Zhou, S.; Hou, L.; Tong, R. Anomalous magnetism in Al doped La2CoMnO6 ceramics. J. Magn. Magn. Mater. 2020, 510, 166950. [Google Scholar] [CrossRef]
- Nissar, U.; Ahmad, J.; Bukhari, S.H.; Iqbal, Y. Impact of A-site rare earth substitution on structural, magnetic, optical and transport properties of double perovskites. Mater. Res. Bull. 2020, 127, 110844. [Google Scholar] [CrossRef]
- Silva, R.X.; Moreira, R.L.; Almeida, R.M.; Paniago, R.; Paschoal, C.W.A. Intrinsic dielectric properties of magnetodielectric La2CoMnO6. J. Appl. Phys. 2015, 117, 214105. [Google Scholar] [CrossRef] [Green Version]
- Patil, D.R.; Chougule, B.K. Effect of resistivity on magnetoelectric effect in (x) NiFe2O4–(1−x)Ba0.9Sr0.1TiO3 ME composites. J. Alloy. Compd. 2009, 470, 531–535. [Google Scholar] [CrossRef]
- Ryu, J.; Priya, S.; Uchino, K.; Kim, H.E. Magnetoelectric effect in composites of magnetostrictive and piezoelectric materials. J. Electroceramics 2002, 8, 107–119. [Google Scholar] [CrossRef]
- Tan, P.T.; Yen, P.H.; Nhat, H.N.; Hoa, N.Q. Structure and poperties of double perovskite system, La2Co1−xFexMnO6. Commun. Phys. 2014, 24, 80–84. [Google Scholar] [CrossRef]
- Idrees, M.; Nadeem, M.; Hassan, M.M. Investigation of conduction and relaxation phenomena in LaFe0.9Ni0.1O3 by impedance spectroscopy. J. Phys. D Appl. Phys. 2010, 43, 155401. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Sarkar, T.; Bazuev, G.V.; Kuznetsov, M.V.; Nordblad, P.; Mathieu, R. Modification of the structure and magnetic properties of ceramic La2CoMnO6 with Ru doping. J. Alloy. Compd. 2018, 752, 420–430. [Google Scholar] [CrossRef]
- De, K.; Ray, R.; Panda, R.N.; Giri, S.; Nakamura, H.; Kohara, T. The effect of Fe substitution on magnetic and transport properties of LaMnO3. J. Magn. Magn. Mater. 2005, 288, 339–346. [Google Scholar] [CrossRef]
- Baidya, S.; Saha-Dasgupta, T. Electronic structure and phonons in La2CoMnO6: A ferromagnetic insulator driven by coulomb-assisted spin-orbit coupling. Phys. Rev. B 2011, 84, 035131. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, Y.; Li, H.; Han, L.; Wang, Z.; Wu, X.; Lv, S.; Liu, X.; Meng, J. A-site-doping enhanced B-site ordering and correlated magnetic property in La2-xBixCoMnO6. J. Phys. Chem. C 2012, 116, 16841–16847. [Google Scholar] [CrossRef]
- Blasco, J.; Ritter, C.; Morellon, L.; Algarabel, P.A.; Teresa, J.M.D.; Serrate, D.; Garcia, J.; Ibarra, M.R. Structural, magnetic and transport properties of Sr2Fe1−xCrxMoO6−y. Solid State Sci. 2002, 4, 651. [Google Scholar] [CrossRef]
- Sayed, F.N.; Achary, S.N.; Deshpande, S.K.; Rajeswari, B.; Kadam, R.M.; Dwebedi, S.; Nigam, A.K.; Tyagi, A.K. Role of annealing atmosphere on structure, dielectric and magnetic properties of La2CoMnO6 and La2MgMnO6. Z. Anorg. Allg. Chem. 2014, 640, 1907–1921. [Google Scholar] [CrossRef]
- Yoshii, K.; Fukuda, T.; Akahama, H.; Kano, J.; Kambe, T.; Ikeda, N. Magnetic and dielectric study of Bi2CuO4. Phys. C 2011, 471, 766–769. [Google Scholar] [CrossRef]
- Konopka, J.; Wolff, I. Dielectric properties of CaNdAlO4 at microwave frequencies. J. Appl. Phys. 1992, 72, 218–223. [Google Scholar] [CrossRef]
- Singha, D.N.; Mahatoa, D.K.; Sinhab, T.P. Structural and electrical characterization of La2ZnMnO6 double perovskite. Phys. B Condens. Matter 2018, 550, 400–406. [Google Scholar] [CrossRef]
- Zhou, X.D.; Pederson, L.R.; Cai, Q.; Yang, J.; Scarfino, B.; Kim, M.; Yelon, W.; James, W.; Anderson, H.; Wang, C.J. Structural and magnetic properties of LaMn1− xFexO3 (0 < x < 1.0). J. Appl. Phys. 2006, 8, 08M918. [Google Scholar]
- Liu, X.J.; Li, Z.Q.; Yu, A.; Liu, M.L.; Li, W.R.; Li, B.L.; Wu, P.; Bai, H.L.; Jiang, E.Y. Magnetic, electrical transport and electron spin resonance studies of Fe-doped manganite LaMn0.7Fe0.3O3+δ. J. Magn. Magn. Mater. 2007, 2, 354–360. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Chen, X.M. Dielectric relaxation and polaronic conduction in double perovskite La2MgMnO6. Appl. Phys. Lett. 2010, 96, 142902. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Chen, X.M.; Liu, X.Q. Relaxer-like dielectric behavior in La2NiMnO6 double perovskite ceramics. Solid State Commun. 2009, 149, 784–787. [Google Scholar] [CrossRef]
- Zangina, T.; Hassan, J.; Matori, K.A.; Azis, R.S.; Ahmadu, U.; See, A. Sintering behavior, ac conductivity and dielectric relaxation of Li1.3Ti1.7Al0.3(PO4)3 NASICON compound. Results Phys. 2016, 6, 719–725. [Google Scholar] [CrossRef] [Green Version]










| Material | Z = 0 | Z = 0.2 | Z = 0.4 | Z = 0.6 | Z = 0.8 | Z = 1.0 |
|---|---|---|---|---|---|---|
| S. G | P21/n | P21/n | P21/n | P21/n | P21/n | P21/n |
| a (Å) | 5.5235 | 5.5225 | 5.5166 | 5.5445 | 5.5473 | 5.5424 |
| b (Å) | 5.4961 | 5.4951 | 5.4911 | 5.5147 | 5.5226 | 5.5189 |
| c (Å) | 7.6830 | 7.6907 | 7.7424 | 7.6944 | 7.7254 | 7.7165 |
| V (A)3 | 233.2 | 233.4 | 235.3 | 235.3 | 236.7 | 236.3 |
| β | 90.30 | 89.78 | 90.62 | 89.70 | 90.40 | 90.31 |
| Rp | 6.34 | 10.40 | 7.28 | 8.77 | 10.83 | 8.42 |
| Rwp | 8.03 | 13.27 | 9.30 | 11.28 | 13.74 | 10.67 |
| GOF | 0.91 | 0.86 | 0.92 | 0.91 | 0.86 | 0.87 |
| Grain size (nm) | 83 | 84 | 81 | 82 | 81 | 80 |
| ωTO (cm−1) | Z = 0 | Z = 0.2 | Z = 0.4 | Z = 0.6 | Z = 0.8 | Z = 1.0 |
|---|---|---|---|---|---|---|
| ωTO1 | 127.10 | 124.83 | 130.83 | 129.10 | 128.75 | 127.40 |
| ωTO2 | 172.30 | 172.06 | 173.55 | 172.90 | 168.10 | 168.90 |
| ωTO3 | 183.10 | 182.94 | 181.82 | 182.56 | 178.61 | 182.24 |
| ωTO4 | 240.44 | 241.90 | 231.07 | 246.40 | - | - |
| ωTO5 | 268.82 | 264.81 | 263.92 | 257.54 | 257.82 | 279.35 |
| ωTO6 | 283.72 | 285.78 | 278.33 | 279.33 | 286.44 | 293.97 |
| ωTO7 | 366.8 | 374.52 | 369.39 | 371.78 | 374.31 | 370.05 |
| ωTO8 | 413.41 | 414.79 | 412.96 | 414.76 | 419.40 | 422.03 |
| ωTO9 | 437.57 | 438.48 | 430.12 | 430.9 | 435.95 | - |
| ωTO10 | 465.09 | 469.81 | 467.49 | 466.6 | 472.02 | 472.88 |
| ωTO11 | - | - | 563.29 | 568.21 | 569.76 | 562.23 |
| ωTO11 | 589.91 | 583.29 | 593.51 | 571.75 | 583.47 | 594.84 |
| ωTO12 | 641.22 | 609.61 | 642.18 | 637 | 615.79 | 612.06 |
| Z | Z = 0 | Z = 0.2 | Z = 0.4 | Z = 0.6 | Z = 0.8 | Z = 1.0 |
|---|---|---|---|---|---|---|
| S1 | 0.061 | 0.540 | 1.250 | 0.016 | 0.127 | 0.257 |
| S2 | 0.680 | 0.373 | 0.585 | 0.741 | 0.622 | 1.771 |
| S3 | 0.426 | 0.105 | 0.327 | 0.455 | 0.649 | 0.258 |
| S4 | 0.273 | 0.173 | 0.134 | 0.139 | 0.135 | - |
| S5 | 0.197 | 0.345 | 0.222 | 0.241 | 0.038 | 0.517 |
| S6 | 0.115 | 0.712 | 0.125 | 0.626 | 1.012 | 0.0.58 |
| S7 | 0.015 | 0.005 | 0.383 | 0.185 | 0.011 | 0.017 |
| S8 | 0.339 | 0.283 | 0.133 | 0.169 | 0.162 | 0.147 |
| S9 | 0.025 | 0.065 | 0.111 | 0.184 | 0.107 | - |
| S10 | 0.053 | 0.067 | 0.137 | 0.122 | 0.075 | 0.025 |
| S/11 | - | - | 0.377 | 0.142 | 0.158 | 0.069 |
| S11 | 0.011 | 0.141 | 0.123 | 0.650 | 0.151 | 0.540 |
| S12 | 0.013 | 0.233 | 0.015 | 1.760 | 0.223 | 0.740 |
| Z | z = 0 | z = 0.2 | z = 0.4 | z = 0.6 | z = 0.8 | z = 1.0 |
|---|---|---|---|---|---|---|
| γ1 | 9 | 45 | 41 | 34 | 30 | 26 |
| γ2 | 08 | 16 | 23 | 10 | 14 | 18 |
| γ3 | 15 | 12 | 20 | 16 | 28 | 16 |
| γ4 | 10 | 13 | 30 | 11 | 16 | - |
| γ5 | 25 | 17 | 25 | 11 | 17 | 21 |
| γ6 | 30 | 65 | 26 | 23 | 82 | 35 |
| γ7 | 17 | 7 | 53 | 25 | 09 | 11 |
| γ8 | 44 | 44 | 28 | 29 | 41 | 45 |
| γ9 | 15 | 26 | 32 | 36 | 36 | |
| γ10 | 38 | 45 | 68 | 50 | 49 | 34 |
| γ/11 | - | - | 73 | 44 | 51 | 34 |
| γ11 | 46 | 78 | 71 | 88 | 61 | 83 |
| γ12 | 35 | 27 | 57 | 61 | 70 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, G.; Batool, S.; Zheng, Y.; Li, S.; Wang, X. Structural, Optical, Charge-Transport, and Dielectric Properties of Double-Perovskite La2Co1−zFezMnO6 (z = 0, 0.2–1.0). Materials 2022, 15, 6249. https://doi.org/10.3390/ma15186249
Hussain G, Batool S, Zheng Y, Li S, Wang X. Structural, Optical, Charge-Transport, and Dielectric Properties of Double-Perovskite La2Co1−zFezMnO6 (z = 0, 0.2–1.0). Materials. 2022; 15(18):6249. https://doi.org/10.3390/ma15186249
Chicago/Turabian StyleHussain, Ghulam, Shanta Batool, Yuruo Zheng, Shuyi Li, and Xiawa Wang. 2022. "Structural, Optical, Charge-Transport, and Dielectric Properties of Double-Perovskite La2Co1−zFezMnO6 (z = 0, 0.2–1.0)" Materials 15, no. 18: 6249. https://doi.org/10.3390/ma15186249
APA StyleHussain, G., Batool, S., Zheng, Y., Li, S., & Wang, X. (2022). Structural, Optical, Charge-Transport, and Dielectric Properties of Double-Perovskite La2Co1−zFezMnO6 (z = 0, 0.2–1.0). Materials, 15(18), 6249. https://doi.org/10.3390/ma15186249






