Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Object of Study
2.3. Biomass Characterization
2.4. Biosorption
2.5. Bioaccummulation
2.6. Biochemical Tests
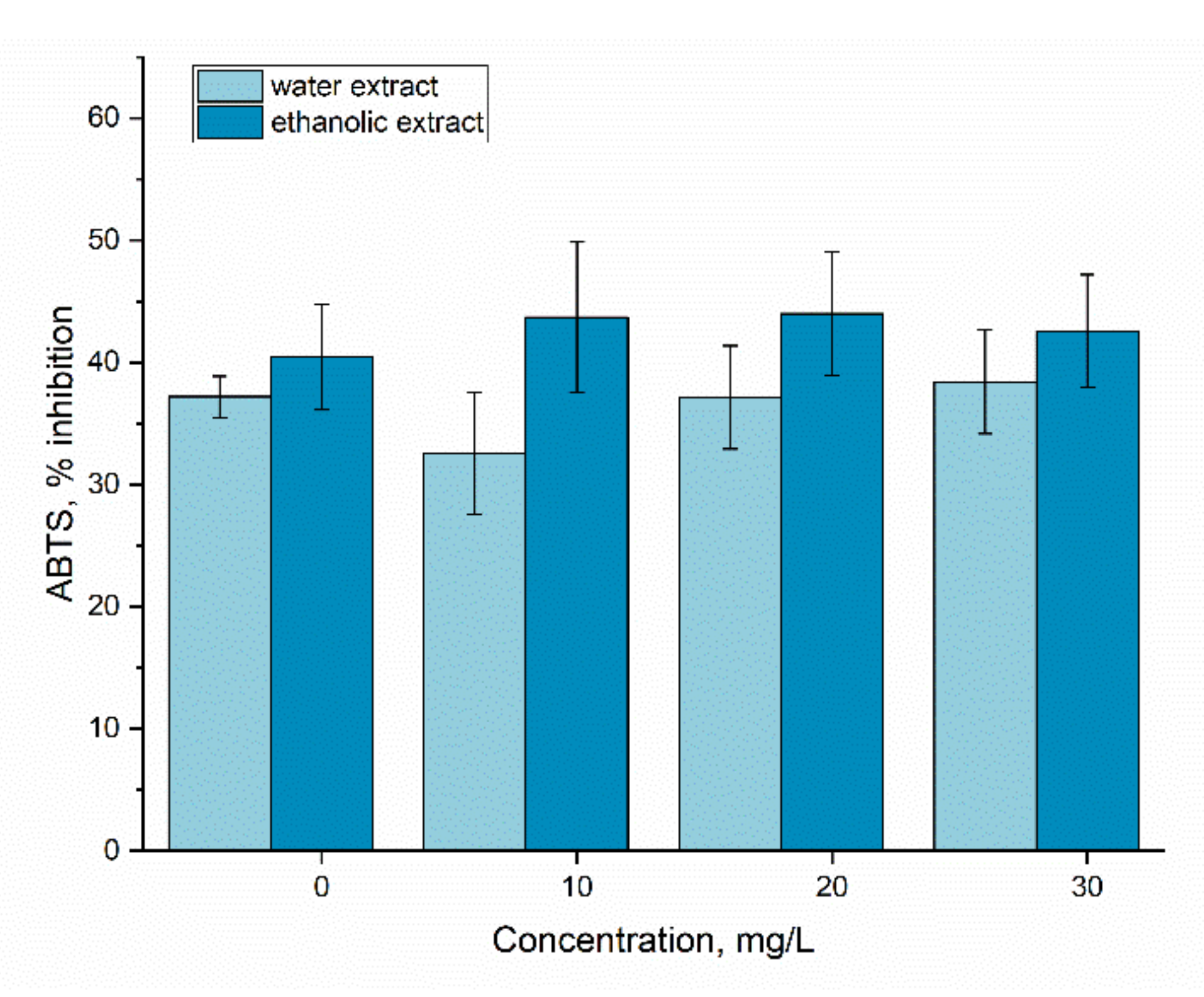
2.7. Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biosorbent Characterization
3.2. Effect of pH, Time, Temperature and Initial Er(III) Concentration on A. platensis Biosorption Capacity
3.3. Kinetics, Equlibrum and Thermodynamics of the Biosorption Process
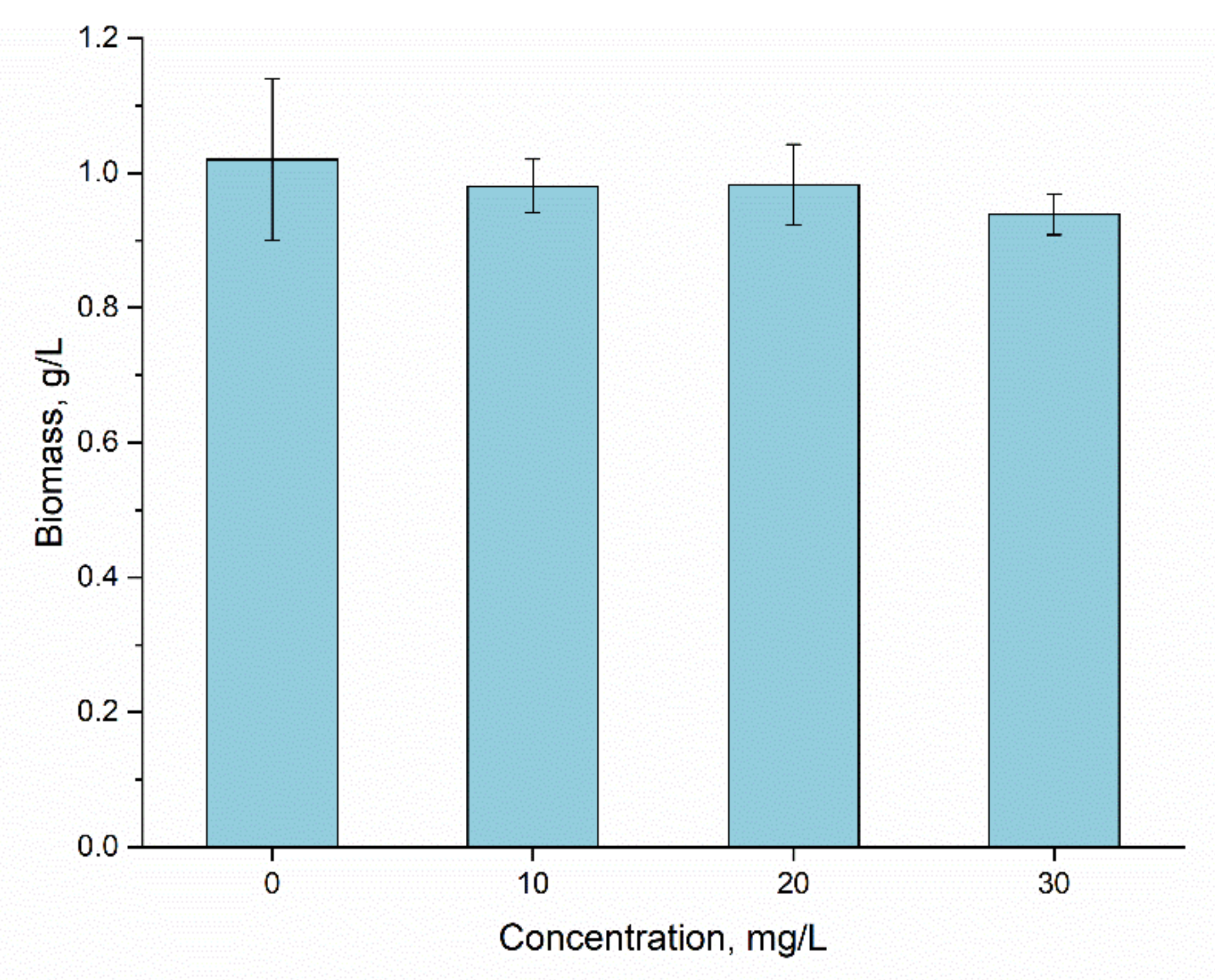
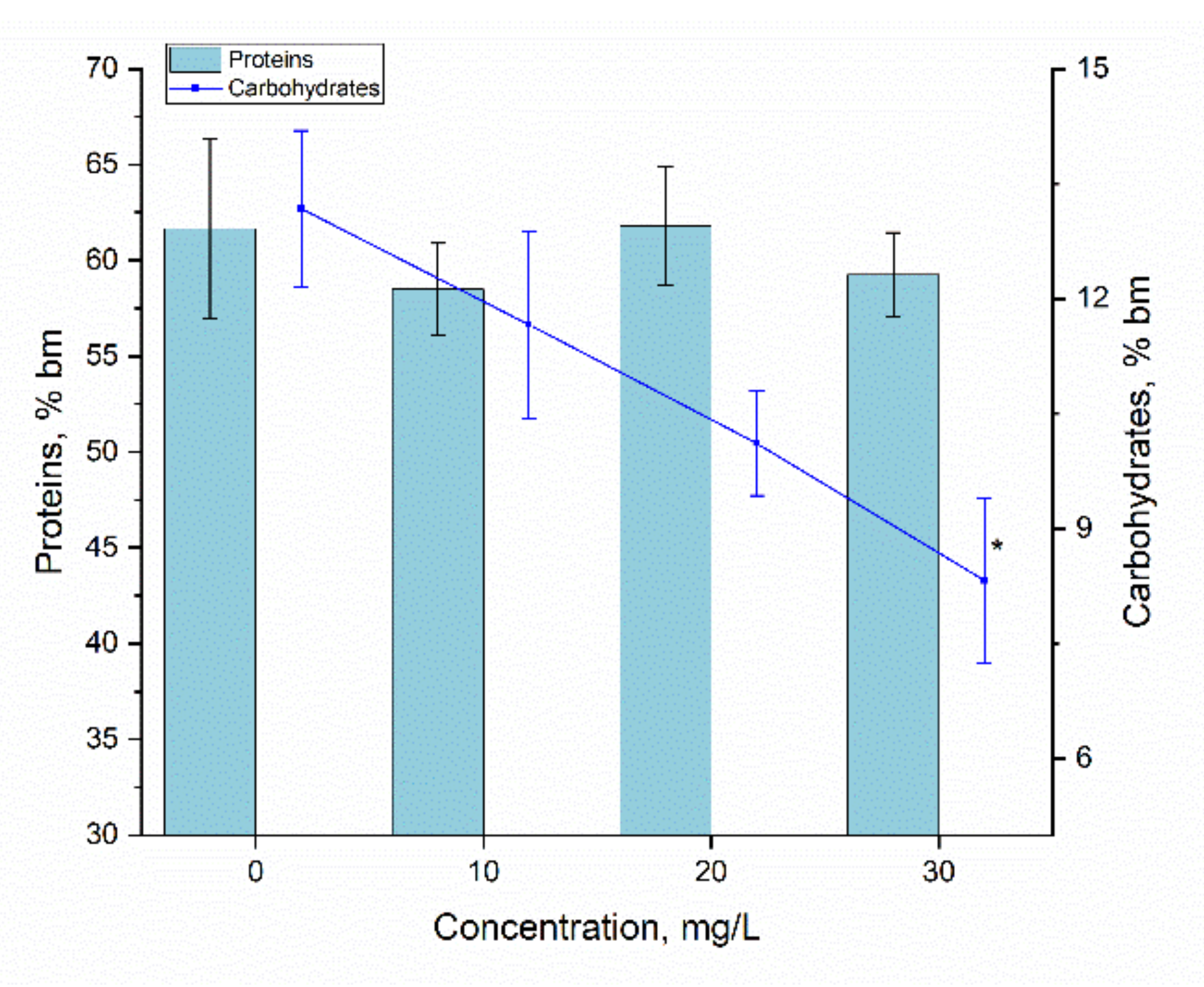
3.4. Bioaccumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awwad, N.S.; Gad, H.M.H.; Ahmad, M.I.; Aly, H.F. Sorption of lanthanum and erbium from aqueous solution by activated carbon prepared from rice husk. Colloids Surf. B Biointerfaces 2010, 81, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Anitha, M.; Kain, V. Development of solvent extraction process for erbium purification. Sep. Sci. Technol. 2017, 52, 2284–2290. [Google Scholar] [CrossRef]
- Pereao, O.; Bode-Aluko, C.; Fatoba, O.; Laatikainen, K.; Petrik, L. Rare earth elements removal techniques from water/wastewater: A review. Desalin. Water Treat. 2018, 130, 71–86. [Google Scholar] [CrossRef]
- Ni, S.; Chen, Q.; Gao, Y.; Guo, X.; Sun, X. Recovery of rare earths from industrial wastewater using extraction-precipitation strategy for resource and environmental concerns. Miner. Eng. 2020, 151, 106315. [Google Scholar] [CrossRef]
- XIONG, C.; MENG, Y.; YAO, C.; SHEN, C. Adsorption of erbium(III) on D113-III resin from aqueous solutions: Batch and column studies. J. Rare Earths 2009, 27, 923–931. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef]
- Arantza, S.J.; Hiram, M.R.; Erika, K.; Chávez-Avilés, M.N.; Valiente-Banuet, J.I.; Fierros-Romero, G. Bio- and phytoremediation: Plants and microbes to the rescue of heavy metal polluted soils. SN Appl. Sci. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Alfadaly, R.A.; Elsayed, A.; Hassan, R.Y.A.; Noureldeen, A.; Darwish, H.; Gebreil, A.S. Microbial sensing and removal of heavy metals: Bioelectrochemical detection and removal of chromium(vi) and cadmium(ii). Molecules 2021, 26, 2549. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, X.; Zhang, S.; Gao, J.; Xie, M.; Tao, L.; Sun, F.; Shen, C.; Hashmi, M.Z.; Su, X. Oxidative dehalogenation and mineralization of polychlorinated biphenyls by a resuscitated strain Streptococcus sp. SPC0. Environ. Res. 2022, 207, 112648. [Google Scholar] [CrossRef]
- Dhar, S.; Das, A.; Bhattacharjee, P. Heavy metal removal using microbial bioremediation techniques. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Elsevier: Amsterdam, The Netherlands, 2021; pp. 649–673. ISBN 9780128229651. [Google Scholar]
- Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V. A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zabochnicka-Światek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014, 23, 551–561. [Google Scholar]
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Osman, G.E.H.; Abulreesh, H.H.; Elbanna, K.; Shaaban, M.R.; Samreen; Ahmad, I. Recent progress in metal-microbe interactions: Prospects in bioremediation. J. Pure Appl. Microbiol. 2019, 13, 13–26. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Biosorption and Bioaccumulation Capacity of Arthospiraplatensis toward Europium Ions. Water 2022, 14, 2128. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Miscu, V.; Djur, S.; Strelkova, L.; Grozdov, D. Spirulina platensis as renewable accumulator for heavy metals accumulation from multi-element synthetic effluents. Environ. Sci. Pollut. Res. 2020, 27, 31793–31811. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Djur, S.; Yushin, N.; Grozdov, D. Assessment of Metal Accumulation by Arthrospira platensis and Its Adaptation to Iterative Action of Nickel Mono- and Polymetallic Synthetic Effluents. Microorganisms 2022, 10, 1041. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Miscu, V.; Djur, S.; Strelkova, L.; Vergel, K.; Nekhoroshkov, P. Growth and heavy metals accumulation by Spirulina platensis biomass from multicomponent copper containing synthetic effluents during repeated cultivation cycles. Ecol. Eng. 2020, 142, 105637. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Park, J.; Jeong, H.J.; Yoon, E.Y.; Moon, S.J. Easy and rapid quantification of lipid contents of marine dinoflagellates using the sulpho-phospho-vanillin method. Algae 2016, 31, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Carra, P.O. Algal biliproteins. Biochem. J. 1970, 119, 2P. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ching, S.L.; Yusoff, M.S.; Aziz, H.A.; Umar, M. Influence of impregnation ratio on coffee ground activated carbon as landfill leachate adsorbent for removal of total iron and orthophosphate. Desalination 2011, 279, 225–234. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Morsy, H. Biochar of spent coffee grounds as per se and impregnated with tio2: Promising waste-derived adsorbents for balofloxacin. Molecules 2021, 26, 2295. [Google Scholar] [CrossRef]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Salem, N.A.; Yakout, S.M. Sorpcija erbijuma iz vodenih rastvora na sol-gel dobijenom cirkonijum-oksidu. Hem. Ind. 2016, 70, 383–390. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A.; Adebowale, K.O. Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex. Eng. J. 2015, 54, 757–767. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Gundorina, S.; Demčák, Š.; Frontasyeva, M.; Kamanina, I. Biosorption of nickel from model solutions and electroplating industrial effluenusing cyanobacterium arthrospira platensis. Desalin. Water Treat. 2018, 120, 158–165. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Shvetsova, M.; Frontasyeva, M. Zinc removal from model solution and wastewater by Arthrospira (Spirulina) Platensis biomass. Int. J. Phytoremed. 2018, 20, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, B.; Zhao, Q.; Liu, F.; Zhang, P.; Du, Q.; Wang, D.; Li, D.; Wang, Z.; Xia, Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Grozdov, D.; Pavlov, S.; Djur, S. Accumulation of dysprosium, samarium, terbium, lanthanum, neodymium and ytterbium by Arthrospira platensis and their effects on biomass biochemical composition. J. Rare Earths 2021, 39, 1133–1143. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Deng, S.; Chen, Y.; Yu, Y. Effects of neodymium on growth and physiological characteristics of Microcystis aeruginosa. J. Rare Earths 2011, 29, 388–395. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Lü, Y.; Jin, H.; Deng, S.; Zeng, Y. Effects of cerium on growth and physiological characteristics of Anabaena flosaquae. J. Rare Earths 2012, 30, 1287–1292. [Google Scholar] [CrossRef]
- Wenhua, L.; Ruming, Z.; Zhixiong, X.; Xiangdong, C.; Ping, S. Effects of La3+ on growth, transformation, and gene expression of Escherichia coli. Biol. Trace Elem. Res. 2003, 94, 167–177. [Google Scholar] [CrossRef]
- Jin, X.; Chu, Z.; Yan, F.; Zeng, Q. Effects of lanthanum(III) and EDTA on the growth and competition of Microcystis aeruginosa and Scenedesmus quadricauda. Limnologica 2009, 39, 86–93. [Google Scholar] [CrossRef] [Green Version]

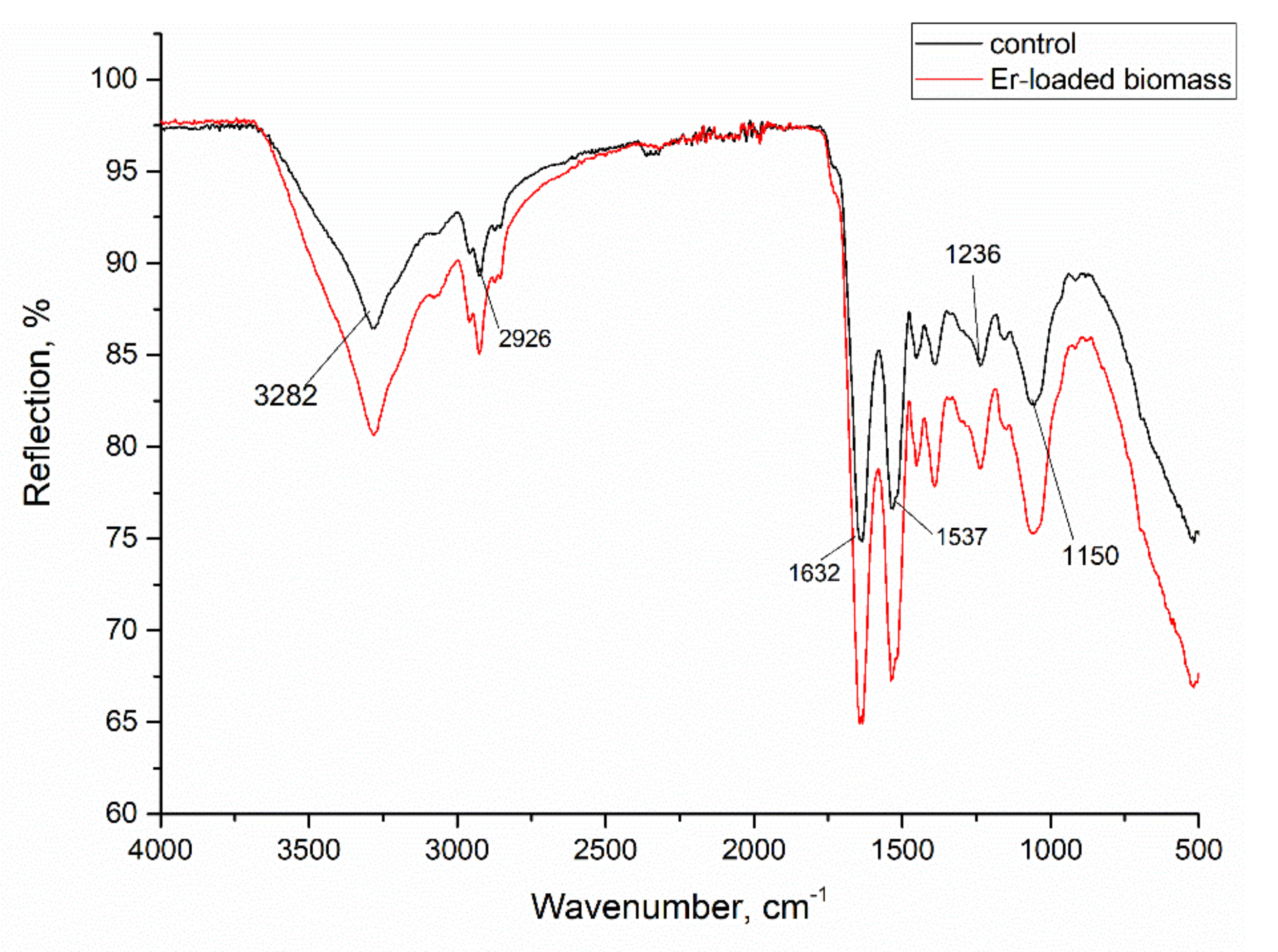
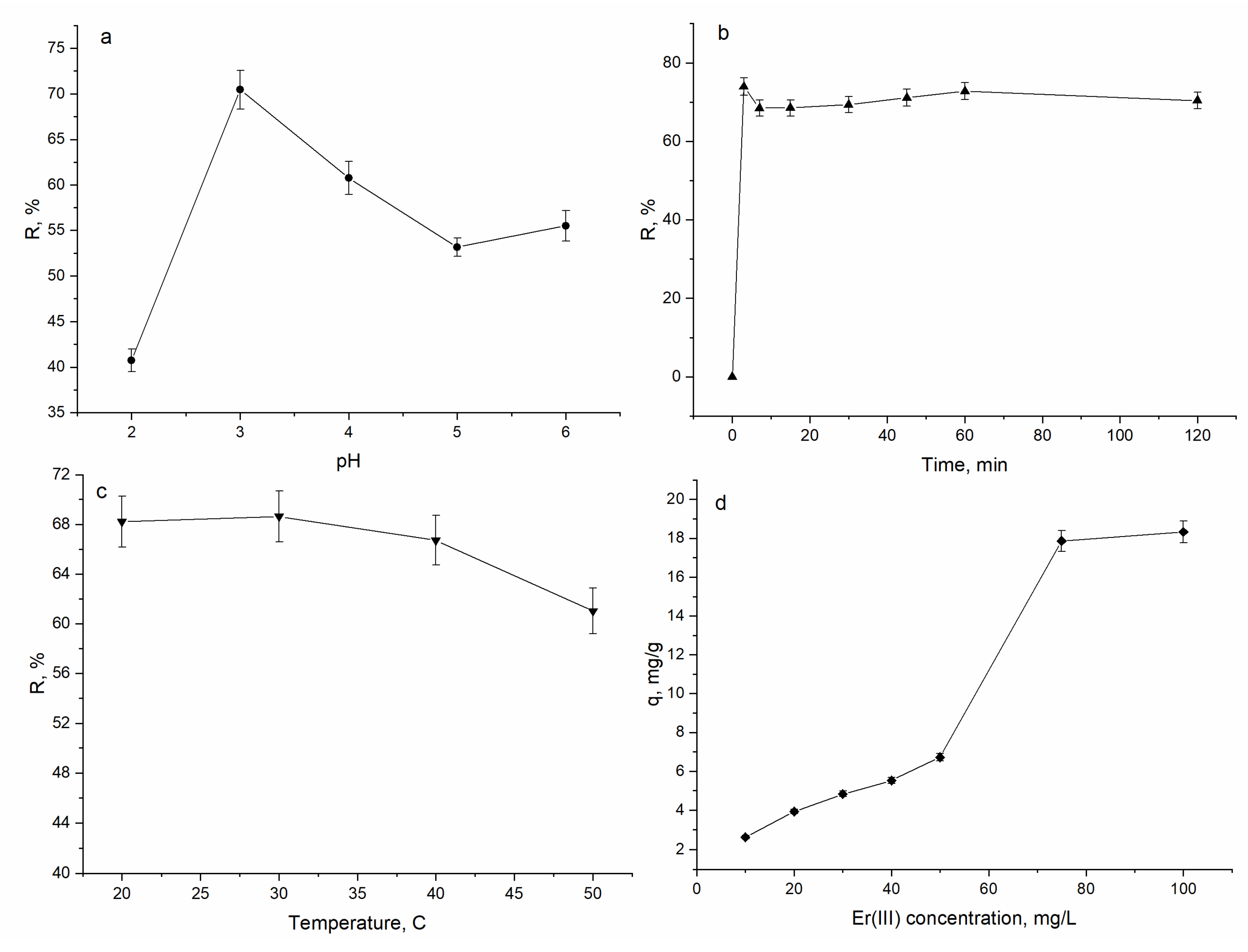
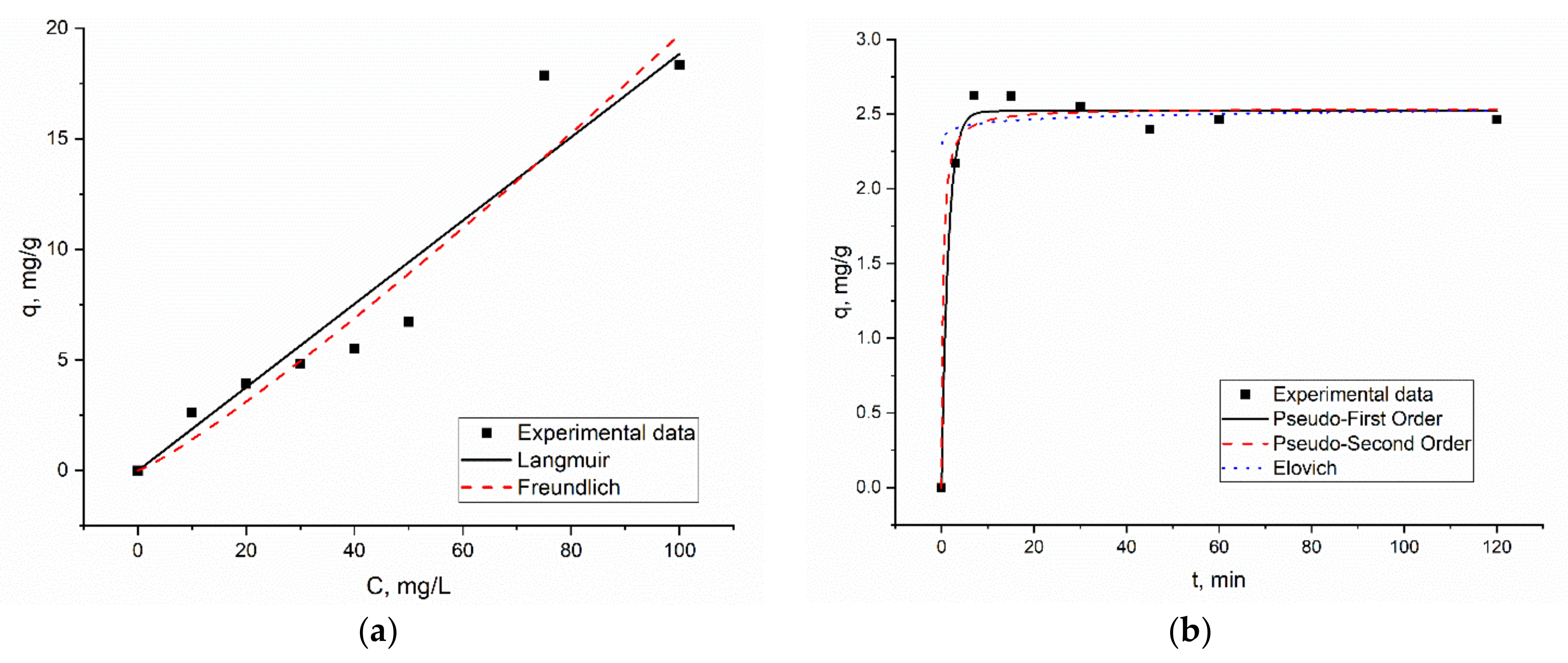
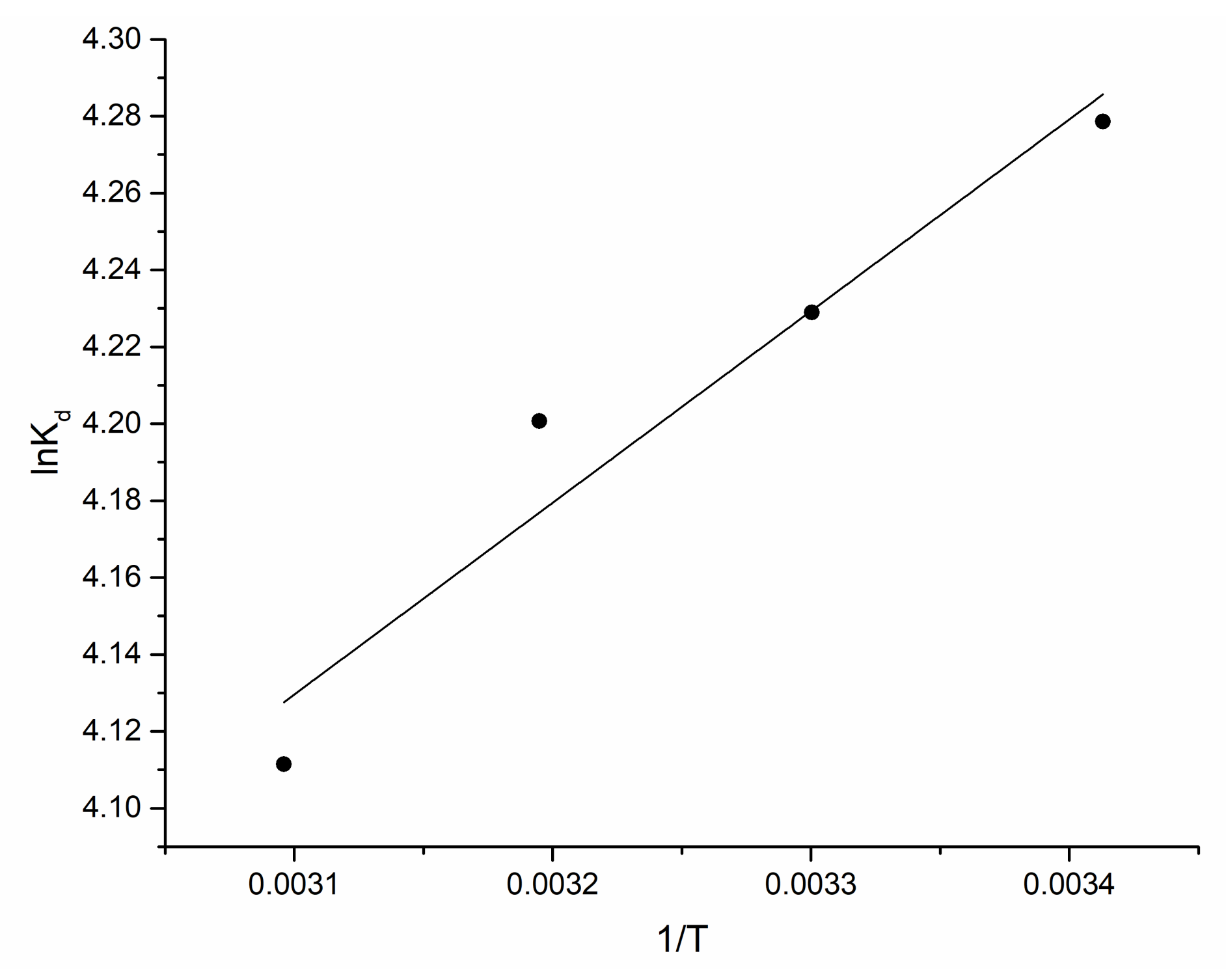


| Element | Content, mg/kg | Element | Content, mg/kg |
|---|---|---|---|
| Na | 10,600 ± 420 | Co | 0.12 ± 0.01 |
| Mg | 5380 ± 270 | Zn | 34 ± 2.7 |
| Al | 130 ± 6 | As | 0.44 ± 0.02 |
| Cl | 6430 ± 385 | Se | 0.12 ± 0.03 |
| K | 18,600 ± 1670 | Br | 1.9 ± 0.2 |
| Ca | 21,100 ± 2300 | Rb | 0.32 ± 0.07 |
| Sc | 0.01 ± 0.002 | Sb | 0.06 ± 0.003 |
| Cr | 8.9 ± 0.9 | I | 4 ± 0.7 |
| Mn | 117 ± 4.5 | Ba | 25 ± 1.3 |
| Fe | 4610 ± 230 | Cs | 0.009 ± 0.002 |
| Ni | 4.4 ± 0.4 | U | 0.04 ± 0.003 |
| Isotherms | ||||||
|---|---|---|---|---|---|---|
| Model | Langmuir | Freundlich | ||||
| Parameters | qm | b | R2 | KF | n | R2 |
| Er(III) | 30 | 0.006 | 0.90 | 0.1 | 0.87 | 0.98 |
| Kinetics | ||||||
| Model | Pseudo-first order | Pseudo-second order | ||||
| Parameters | qe | k1 | R2 | qe | k2 | R2 |
| Er(III) | 2.5 | 0.67 | 0.99 | 2.5 | 1.18 | 0.98 |
| Temperature, K | ∆G0, kJ/mol | ∆H0, kJ/mol | ∆S0, J/mol·K |
|---|---|---|---|
| 293 | −10.4 | −4.1 | 21.5 |
| 303 | −10.6 | ||
| 313 | −10.9 | ||
| 323 | −11.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials 2022, 15, 6101. https://doi.org/10.3390/ma15176101
Yushin N, Zinicovscaia I, Cepoi L, Chiriac T, Rudi L, Grozdov D. Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials. 2022; 15(17):6101. https://doi.org/10.3390/ma15176101
Chicago/Turabian StyleYushin, Nikita, Inga Zinicovscaia, Liliana Cepoi, Tatiana Chiriac, Ludmila Rudi, and Dmitrii Grozdov. 2022. "Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater" Materials 15, no. 17: 6101. https://doi.org/10.3390/ma15176101
APA StyleYushin, N., Zinicovscaia, I., Cepoi, L., Chiriac, T., Rudi, L., & Grozdov, D. (2022). Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials, 15(17), 6101. https://doi.org/10.3390/ma15176101









