Performance of Cement Paste with Denitrified Fly Ash Containing NH4HSO4
Abstract
:1. Introduction
2. Raw Materials and Test Methods
2.1. Raw Materials
2.2. Methods
3. Results and Discussion
3.1. Properties of the Cement Paste
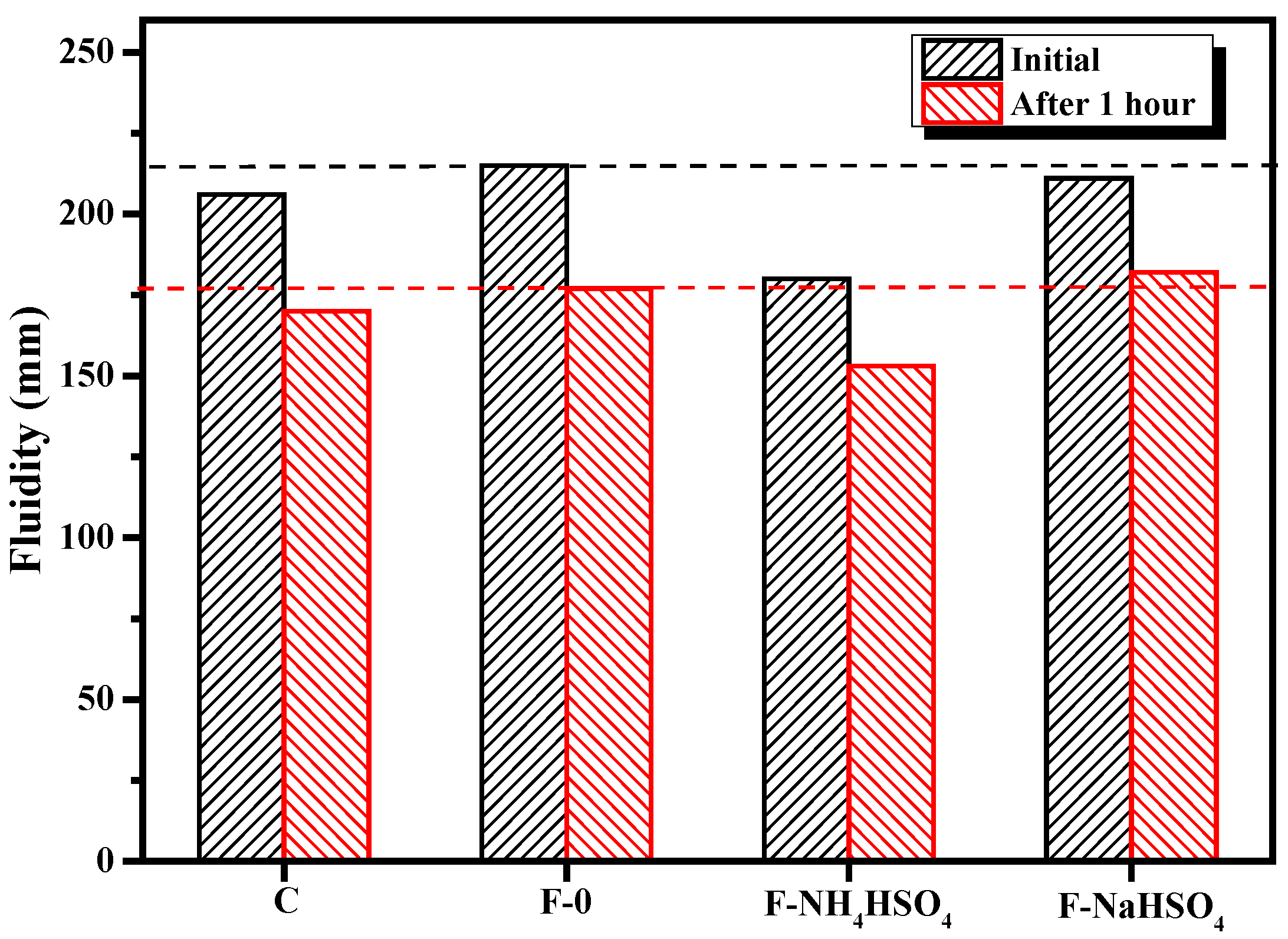
3.1.1. Fluidity
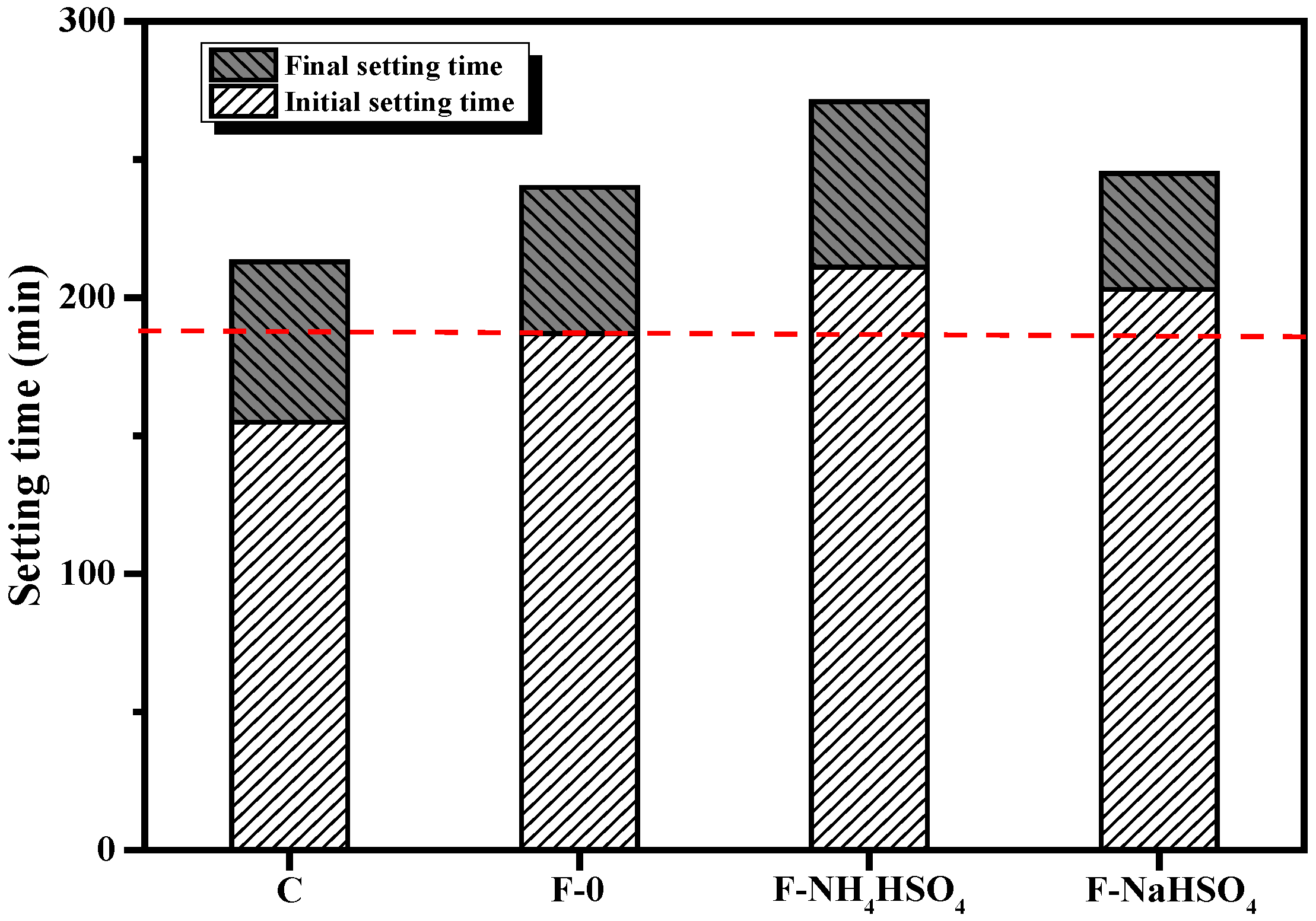
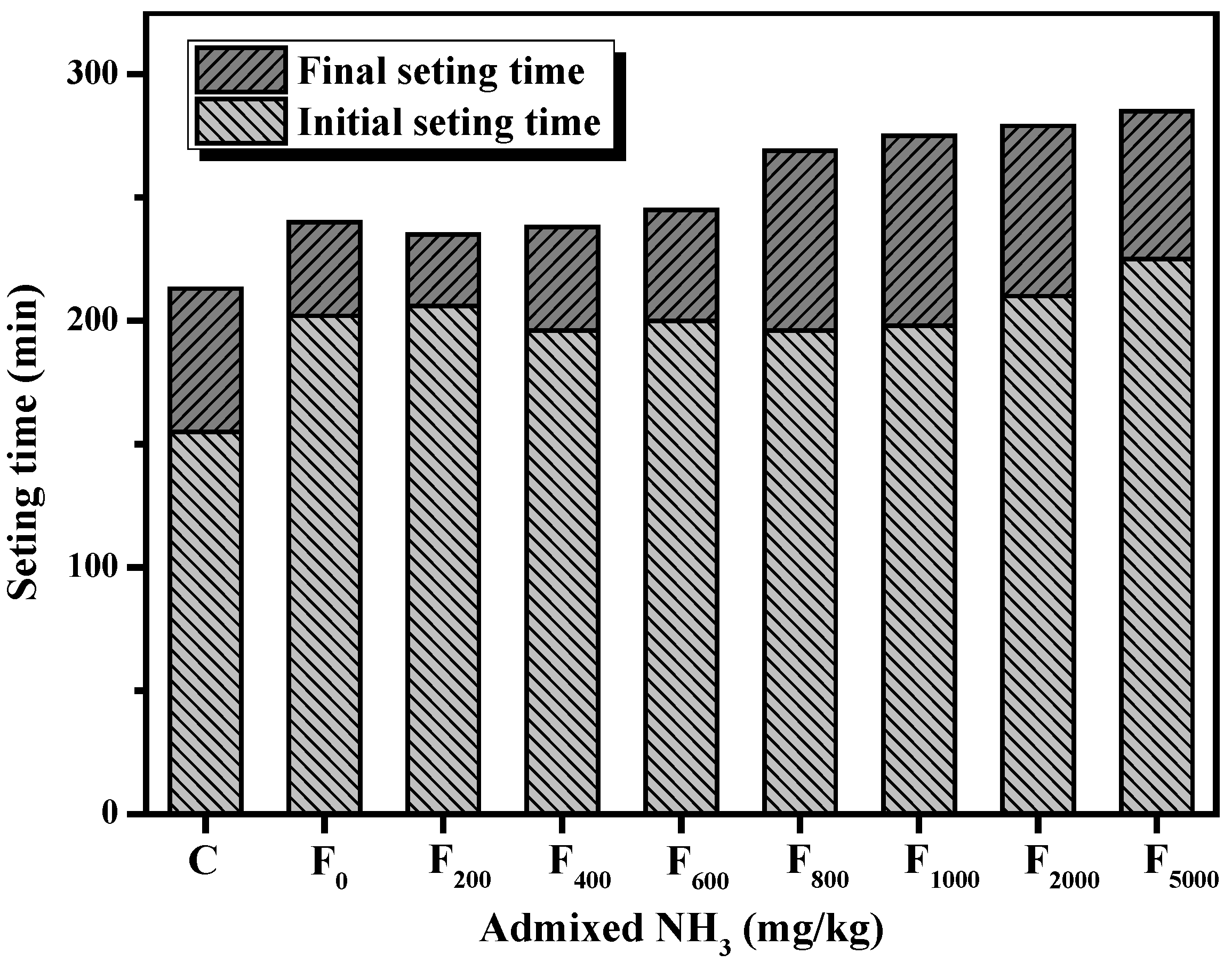
3.1.2. Setting Time
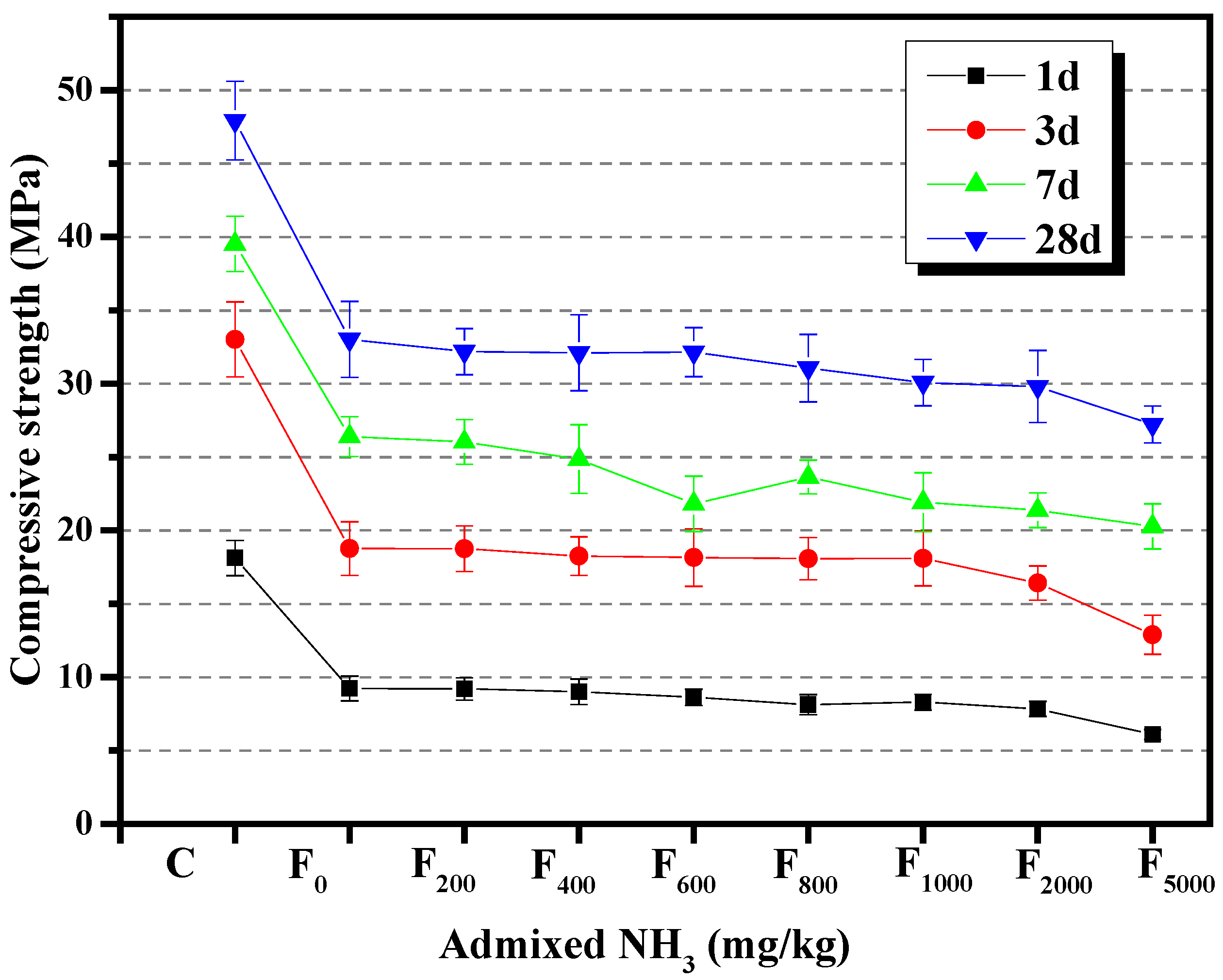
3.1.3. Compressive Strength
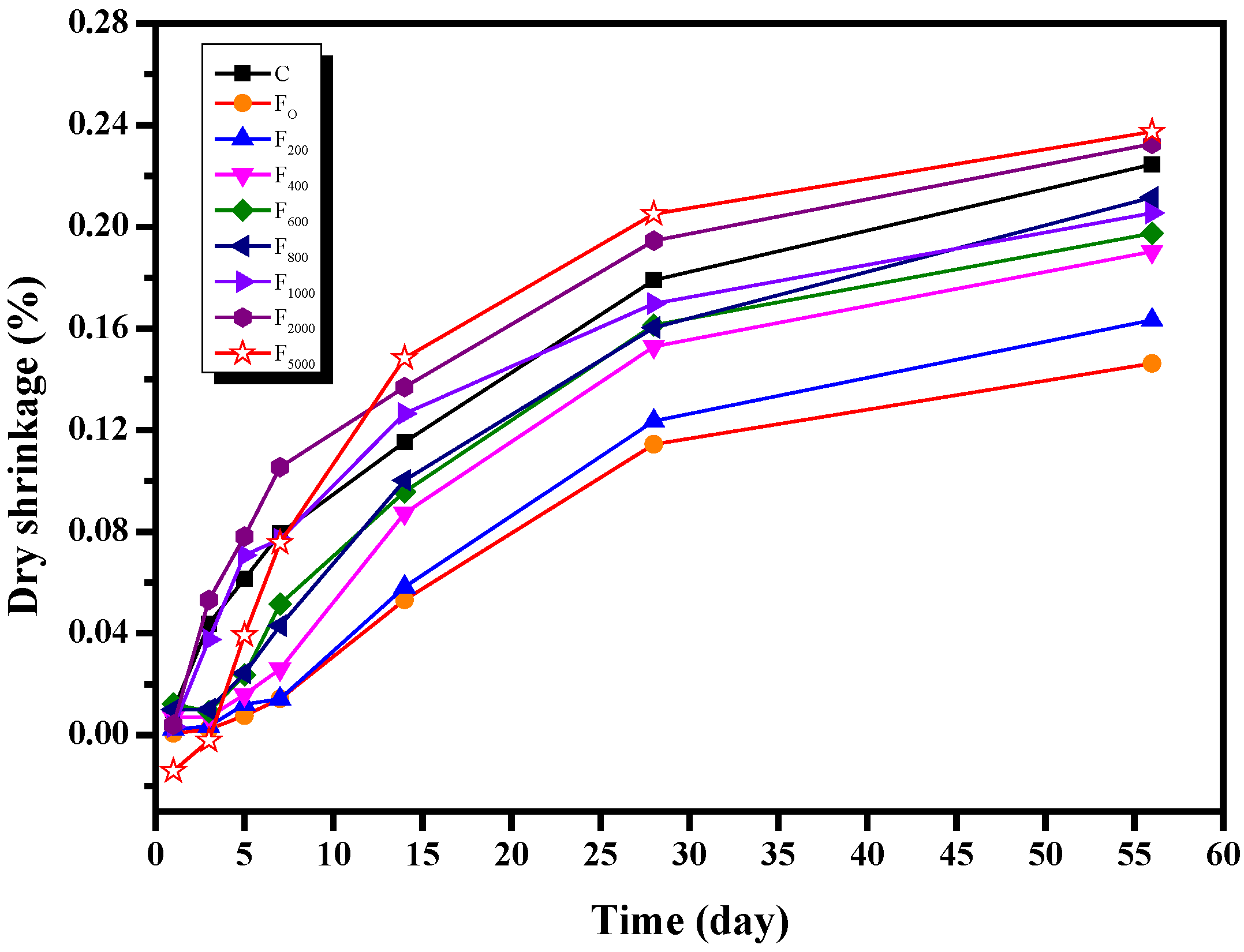
3.1.4. Drying Shrinkage
3.1.5. Heat of Hydration
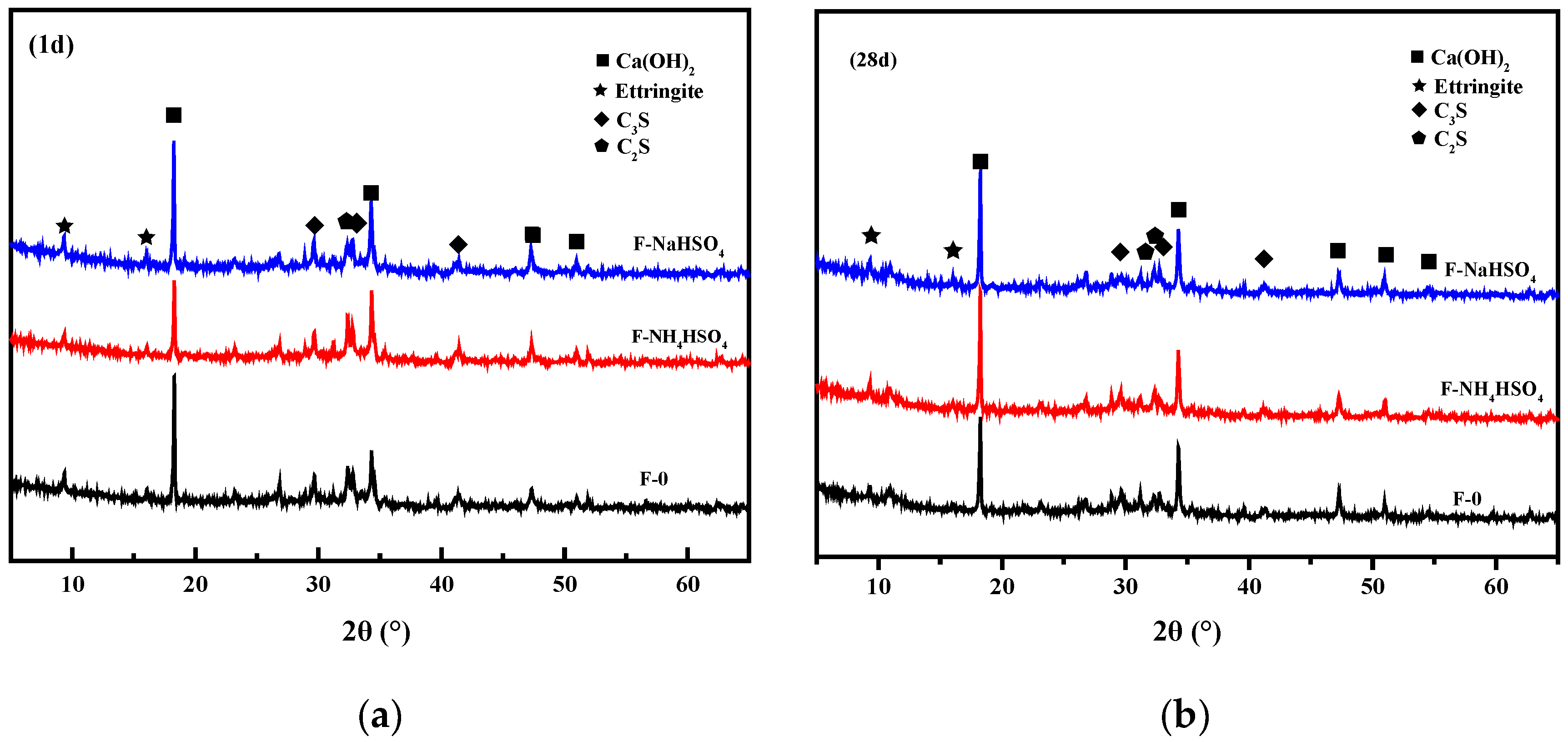
3.1.6. Hydration Products
3.2. Properties of the Fly Ash Cement Paste with Additional NH4+
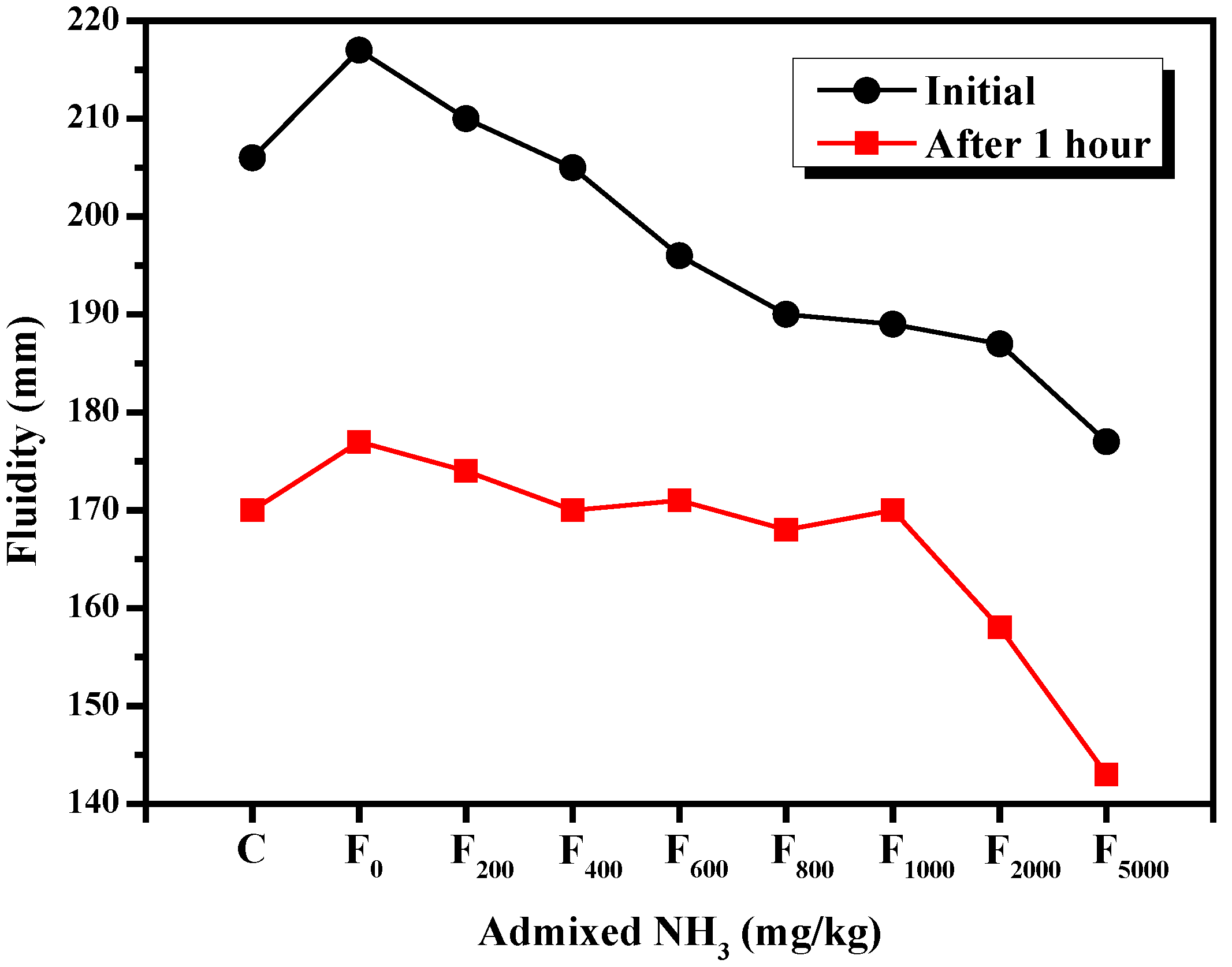
3.2.1. Fluidity
3.2.2. Setting time
3.2.3. Compressive Strength
3.2.4. Drying Shrinkage
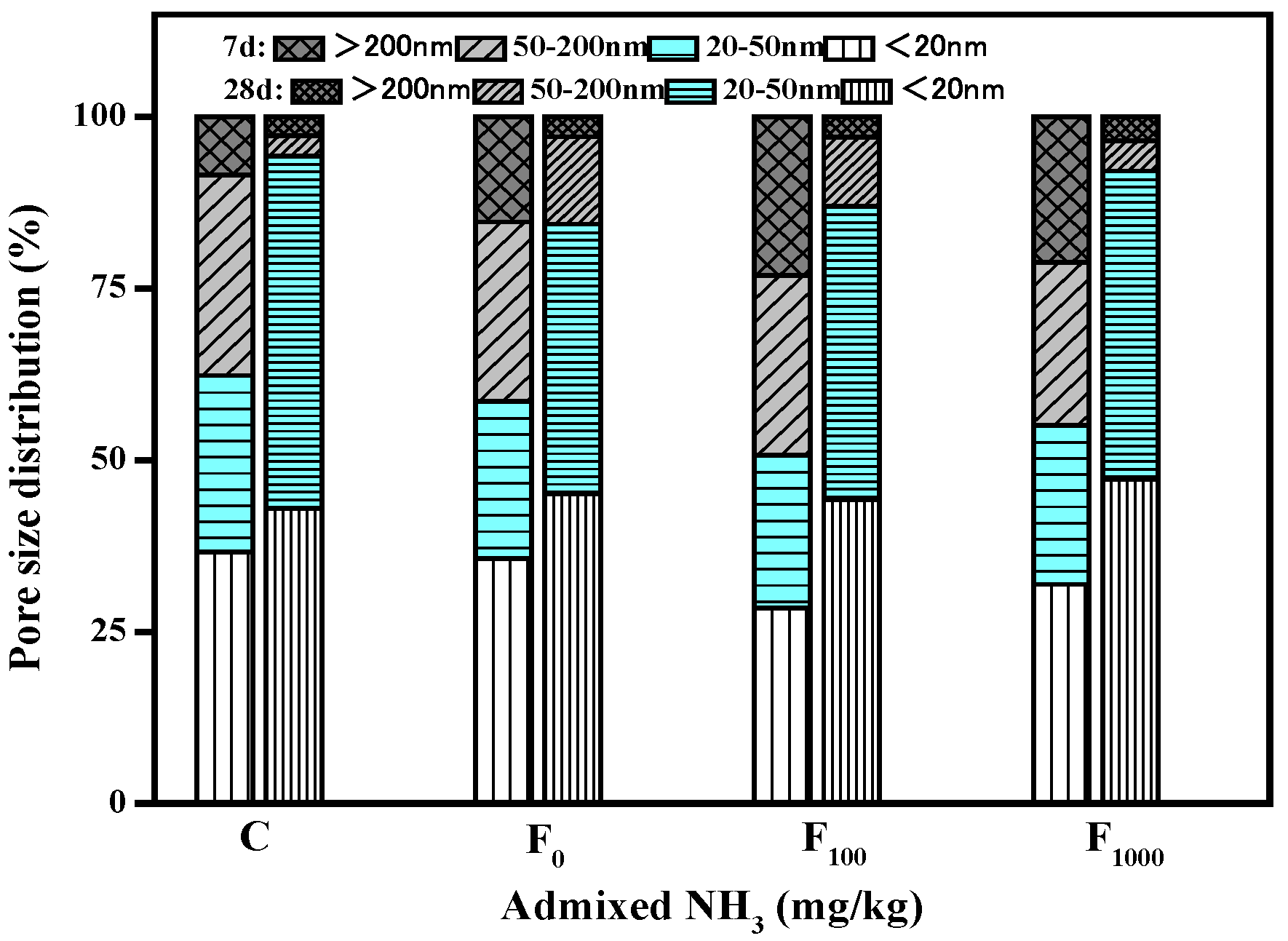
3.2.5. Pore Structure
4. Conclusions
- (1)
- Compared with cement slurry without NH4HSO4, when the NH4HSO4 content was 2%, the fluidity of the paste decreased by 17.0%, the initial setting time lengthened by 12.8%, and the compressive strength of 1 d decreased by 22.8%. When the NaHSO4 content was 2%, the fluidity of the paste decreased by 2.7% and initial setting time lengthened by 8.5%. The 1 d’s compressive strength increased by 1.3%, and it was found that the NH4+ in the by-product of denitrified fly ash was the main reason that affected the performance of the fly ash cement paste, while NH4+ obviously reduced the fluidity of the fly ash cement paste and retarded the setting time of the fly ash cement paste.
- (2)
- Through hydration heat and TG analysis, NH4+ will reduce the peak and total heat release of the fly ash cement paste during hydration and affect the calcium hydroxide hydrate content.
- (3)
- When the content of NH4HSO4 was 1000 mg/kg, the number of large pores in the net paste of the fly ash cement increased by 5.9% and 0.6% at 7d and 28d, respectively, compared with that of the net paste of the fly ash cement with the content of NH4HSO4 being 0 mg/kg. Therefore, NH4+ will increase the porosity of the cement stone and the number of macropores in the fly ash cement paste, thereby reducing the strength of the fly ash cement paste and increasing the drying shrinkage of the fly ash cement paste. In practical engineering, ammonia content in denitrified fly ash should be strictly controlled.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.Y.; Wang, S.X.; Xing, J.; Zhao, Y.; Hao, J.M. Current status and future projections of NOx emissions from energy related industries in China. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2008, 28, 2470–2479. [Google Scholar]
- Li, X.; Zhu, P.; Wang, F.; Wang, L.; Tsang, S.C. Simultaneous Catalytic Reductions of NO and SO2 by H2 over Nickel-Tungsten Catalysts. J. Phys. Chem. C 2008, 112, 3376–3382. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Fang, Z. Flue Gas Denttrification Technologies and Analysis of Their Chemical Reactions. Therm. Power Gener. 2006, 11, 59,60,64,67. [Google Scholar]
- Larrimore, L.; Monroe, L.; Dodgen, D. Characterization of Ammonia Effects on Ash and Evaluation of Removal Methods; American Coal Ash Symposium: Orlando, FL, USA, 1999. [Google Scholar]
- Brendel, G.F.; Bonetti, J.E.; Rathbone, R.F.; Frey, R.N., Jr. Investigation of Ammonia Adsorption on Fly Ash Due to Installation of Selective Catalytic Reduction Systems; Technical Reports; Office of Scientific & Technical Information: Oak Ridge, TN, USA, 2000.
- Shou, L.; Hayes, J.; Cheng, W.; Wu, C.Y.; Townsend, T.; Vinson, T.; Schert, J. Characterization of ammonia gas release from concrete added with ammoniated fly ash. Air Qual. Atmos. Health 2014, 7, 505–513. [Google Scholar] [CrossRef]
- Schert, J.; Townsend, T.; Wu, C.Y. Identification of Potential Concerns Associated with FDOT Use of Ammoniated Fly Ash; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- Duan, F.; Chyang, C.S.; Zhang, L.H.; Chi, Y.T. Effect of the molecular structure of nitrogen compounds on the pollutant formation in a bubbling fluidized-bed combustor. Energy 2015, 83, 394–402. [Google Scholar] [CrossRef]
- Chyliński, F.; Goljan, A.; Michalik, A. Fly ash with ammonia: Properties and emission of ammonia from cement composites. Materials 2021, 14, 707. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. Analysis of Concrete Abnormal Phenomena Caused by Off-Grade Fly Ash. Coal Ash 2009, 21, 42. [Google Scholar]
- He, Y. Effects of Ammonia Release and Retention of Ammoniated Fly Ash on the Performance of Cement Concrete. Master’s Thesis, Chongqing University, Chongqing, China, 2018. [Google Scholar]
- Tan, X.; Yang, L.; Rui, O.; Lin, M.; Kang, M. Research on Application of Denitrated Fly Ash in Cement and Concrete. Coal Ash 2016, 28, 7,9,12. [Google Scholar]
- Liu, Y.; Kong, X.; Wu, K.; Zhang, S.; Ji, G.; Liu, C. Effect of the Ammonia Present in Fly Ash on the Properties of Fly Ash and Mortar. Water Power 2022, 48, 125–131. [Google Scholar]
- Zheng, X.; Kong, X.; Liu, C.; Ji, G.; Zhu, Z.; Kang, X.; Wang, H.; Pei, X. Effect of Ammonia Content in Denitrification Fly Ash on the Properties of Fly Ash and Mortar. Cement 2020, 1, 10–16. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Guo, H.; Wu, H.; Wang, H. Experimental Study on the Effect of Ammonia in Fly Ash on the Filling Paste Performance. Coal Eng. 2020, 52, 149–153. [Google Scholar]
- Qin, H.; Liu, Z.; Jia, X.; Wang, Z. Effect and Mechanism of Denitrification Fly Ash on Performance of Cement Concrete. Fly Ash Compr. Util. 2020, 34, 50–54. [Google Scholar]
- Zhang, Y. Study on the Effect of Selective Catalytic Reduction Denitration (SCR) on the Properties of Fly Ash. Master’s Thesis, Chongqing University, Chongqing, China, 2016. [Google Scholar]
- Wang, L. Study on the Mechanism and Control Technology of Denitrification and Desulphurization Fly Ash on Cement Performance. Master’s Thesis, University of Jinan, Jinan, China, 2017. [Google Scholar]
- Qin, L.; Gao, X.; Li, Q. Influences of coal fly ash containing ammonium salts on properties of cement paste. J. Environ. Manag. 2019, 249, 109374. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.S. Characteristics of Fly Ash and Fly Ash Concrete; Science Press: Beijing, China, 2002; pp. 21–83. [Google Scholar]
- Wang, Z.; Wang, Z.; Sun, H.; Zhang, Y.; Wen, C.; Zhang, Y.; Tang, Z. Effect of Denitration on Properties of Fly Ash as Mineral Admixtures. Bull. Chin. Ceram. Soc. 2016, 35, 884–890. [Google Scholar]
- Sun, H.; Qian, J.; Yang, S.; Xiong, Q.; Chen, X. Effect of Alkali Sulfates in Clinker on Early Setting Behavior of Portland Cement. J. Chin. Ceram. Soc. 2017, 45, 690–696. [Google Scholar]
- Nguyen, T.H.Y.; Tran, V.M.; Pansuk, W.; Cao, N.T. Electrochemical chloride extraction on reinforced concrete contaminated external chloride: Efficiencies of intermittent applications and impacts on hydration products. Cem. Concr. Compos. 2021, 121, 104076. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Wang, Z. Mineral composition of fly ash (I). Fly Ash Compr. Util. 2001, 1, 26–31. [Google Scholar]




| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | TiO2 | K2O | Na2O | MgO | LOI | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash | 49.16 | 27.84 | 7.26 | 4.97 | 2.57 | 1.72 | 1.66 | 1.33 | - | 4.32 | 3.47 |
| Cement | 23.22 | 5.16 | 2.53 | 61.32 | 2.95 | - | - | - | 2.19 | 2.92 | 2.63 |
| Fly Ash (g) | NH4HSO4 (g) | NaHSO4 (g) | |
|---|---|---|---|
| F-0 | 300 | 0.00 | 0.00 |
| F-NH4HSO4 | 300 | 6.00 | 0.00 |
| F-NaHSO4 | 300 | 0.00 | 6.26 |
| Cement (g) | Fly Ash (g) | Water (g) | Additional NH4HSO4 (mg/kg) | Total Ammonia (mg/kg) | |
|---|---|---|---|---|---|
| C | 500 | 0 | 200 | 0 | 0 |
| F0 | 350 | 150 | 200 | 0 | 105 |
| F200 | 350 | 150 | 200 | 200 | 305 |
| F400 | 350 | 150 | 200 | 400 | 505 |
| F600 | 350 | 150 | 200 | 600 | 705 |
| F800 | 350 | 150 | 200 | 800 | 905 |
| F1000 | 350 | 150 | 200 | 1000 | 1105 |
| F2000 | 350 | 150 | 200 | 2000 | 2105 |
| F5000 | 350 | 150 | 200 | 5000 | 5105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Qin, H.; Jiang, L.; Niu, J.; Liu, Z. Performance of Cement Paste with Denitrified Fly Ash Containing NH4HSO4. Materials 2022, 15, 6083. https://doi.org/10.3390/ma15176083
Wang Y, Wang Z, Qin H, Jiang L, Niu J, Liu Z. Performance of Cement Paste with Denitrified Fly Ash Containing NH4HSO4. Materials. 2022; 15(17):6083. https://doi.org/10.3390/ma15176083
Chicago/Turabian StyleWang, Yuan, Zhi Wang, Hongyi Qin, Linbo Jiang, Jinghang Niu, and Zhenhua Liu. 2022. "Performance of Cement Paste with Denitrified Fly Ash Containing NH4HSO4" Materials 15, no. 17: 6083. https://doi.org/10.3390/ma15176083
APA StyleWang, Y., Wang, Z., Qin, H., Jiang, L., Niu, J., & Liu, Z. (2022). Performance of Cement Paste with Denitrified Fly Ash Containing NH4HSO4. Materials, 15(17), 6083. https://doi.org/10.3390/ma15176083





