Biochar Nanocomposite as an Inexpensive and Highly Efficient Carbonaceous Adsorbent for Hexavalent Chromium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Method
2.3. Materials Characterization Methods
2.4. Cr(VI) Removal Experiments
2.5. Kinetic Models
3. Results
3.1. Sorbents Characterization
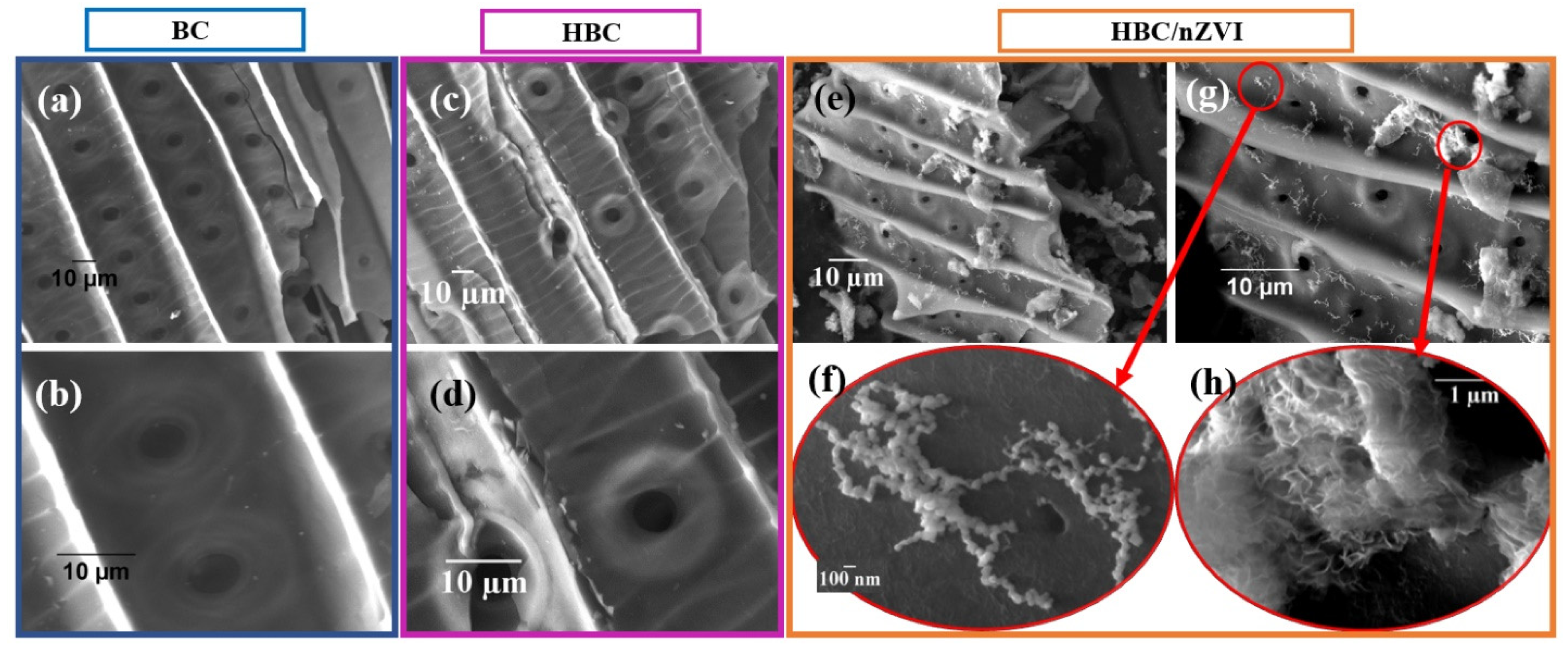
3.1.1. Surface Morphology
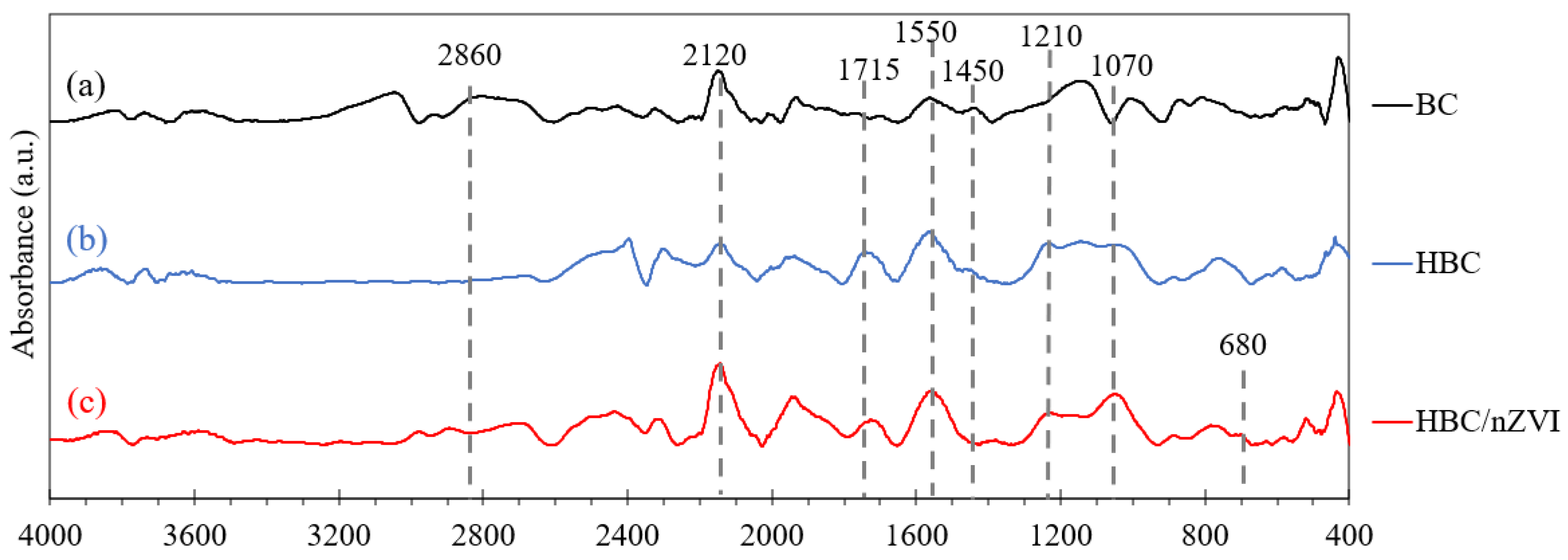
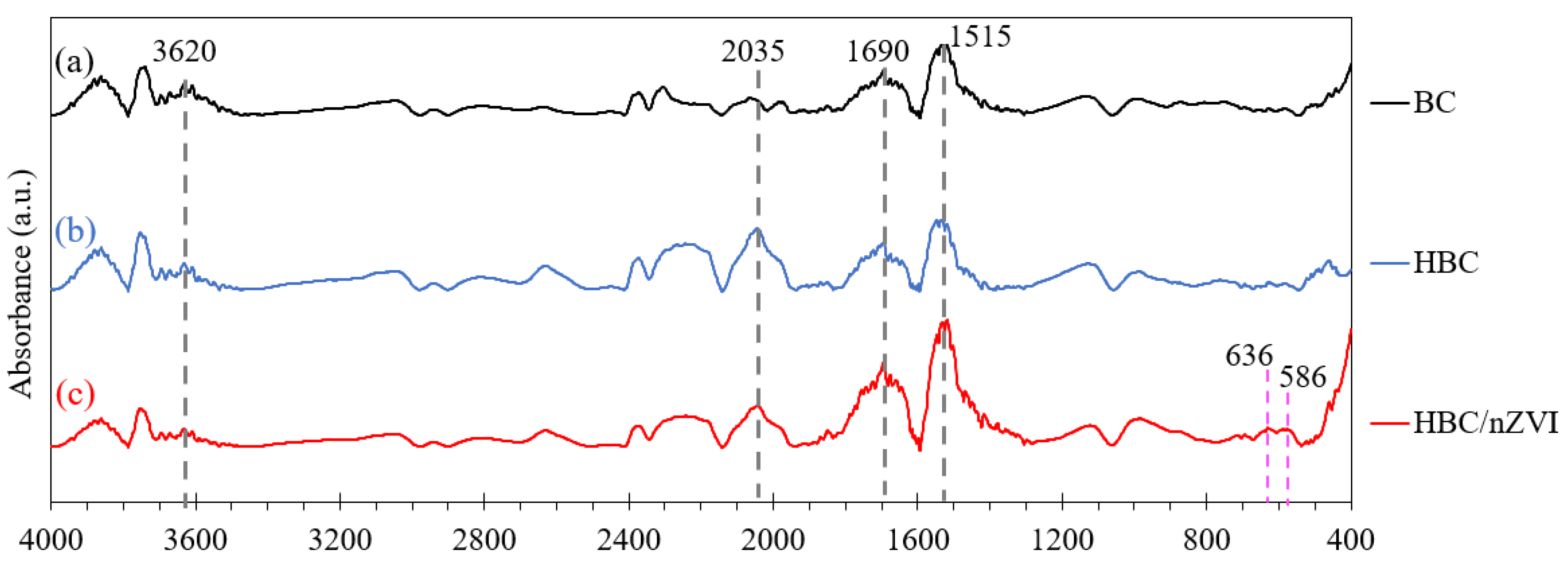
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
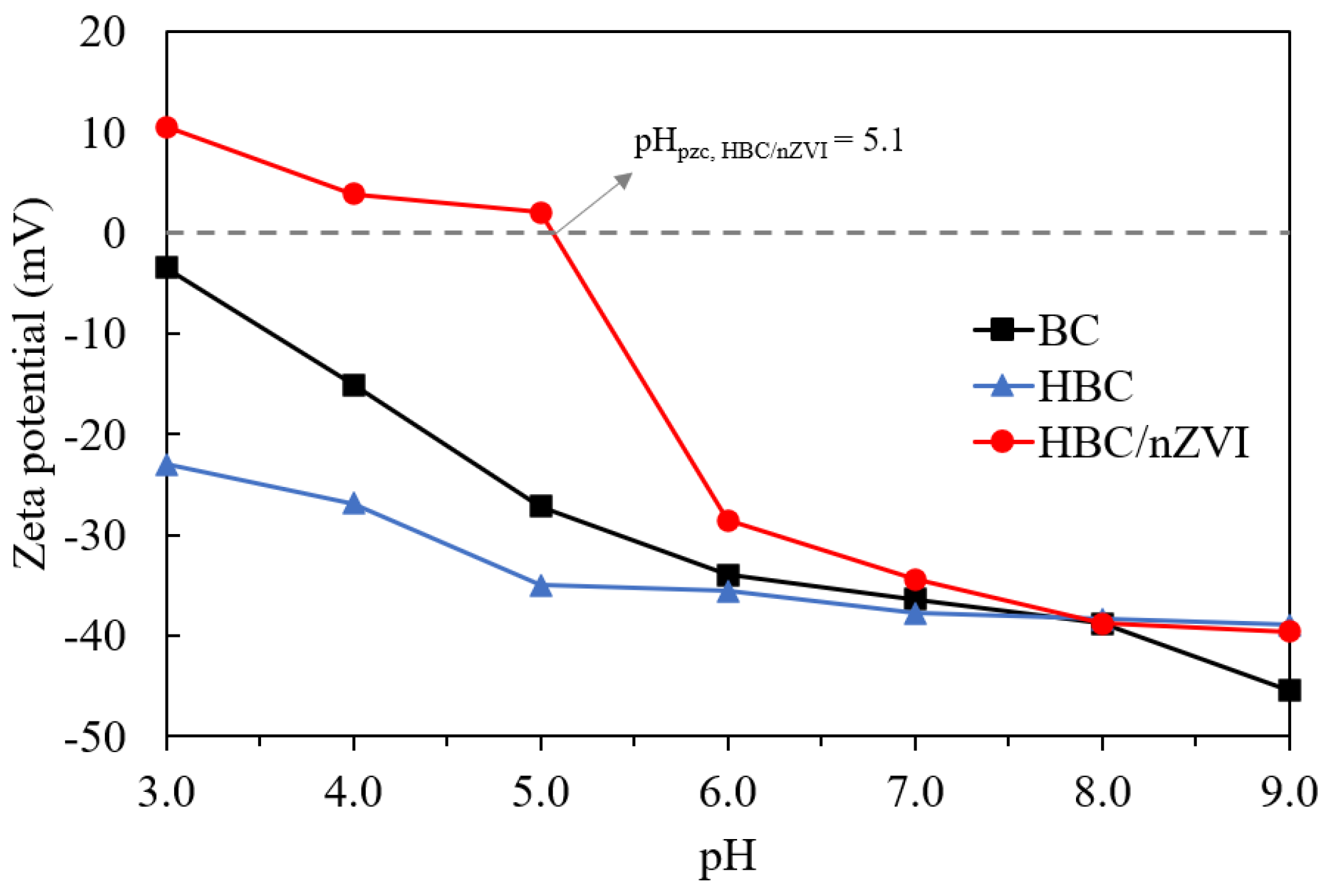
3.1.3. Zeta Potential Studies
3.2. Cr(VI) Removal
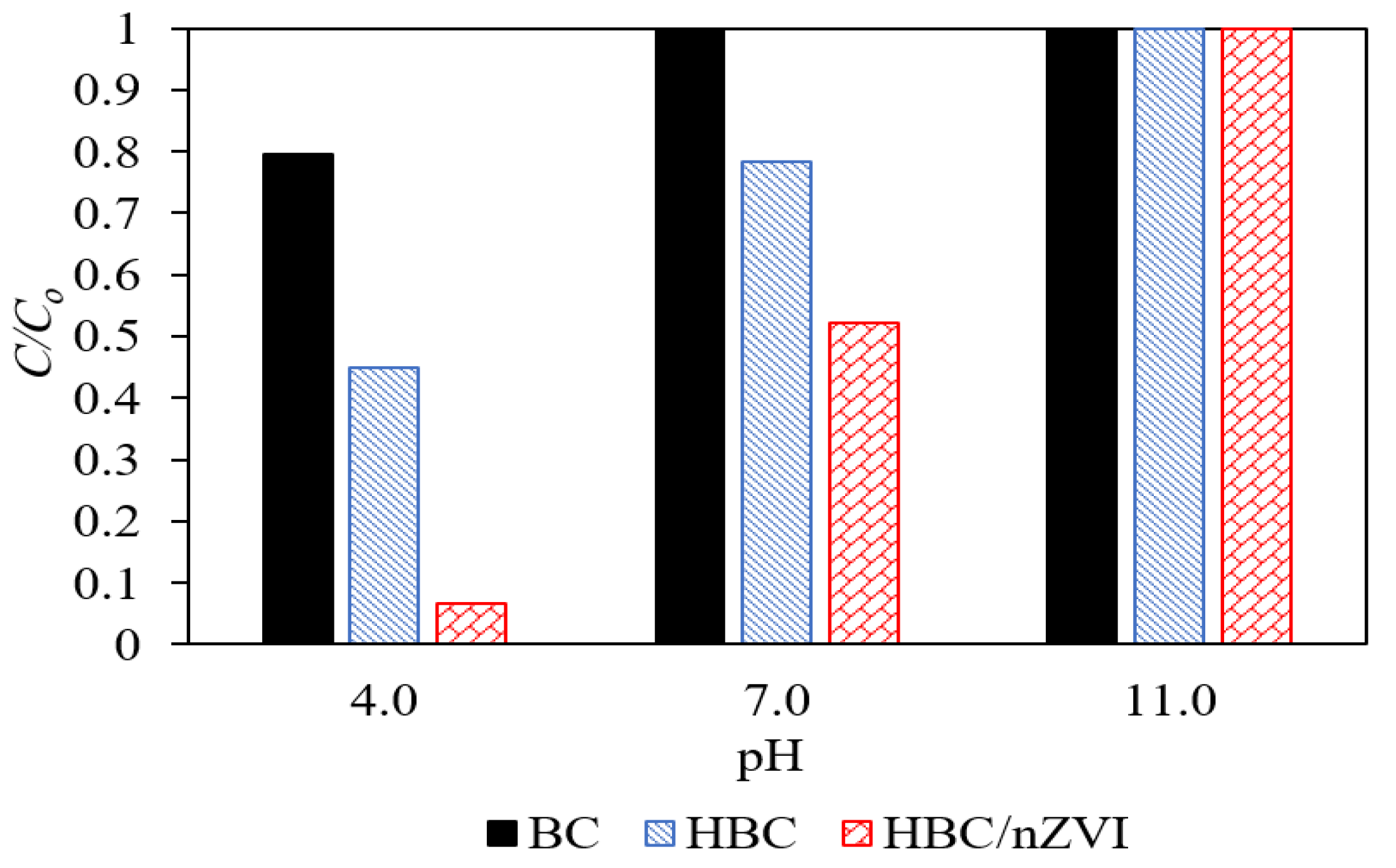
3.2.1. Effects of Solution pH on Cr(VI) Removal Performance
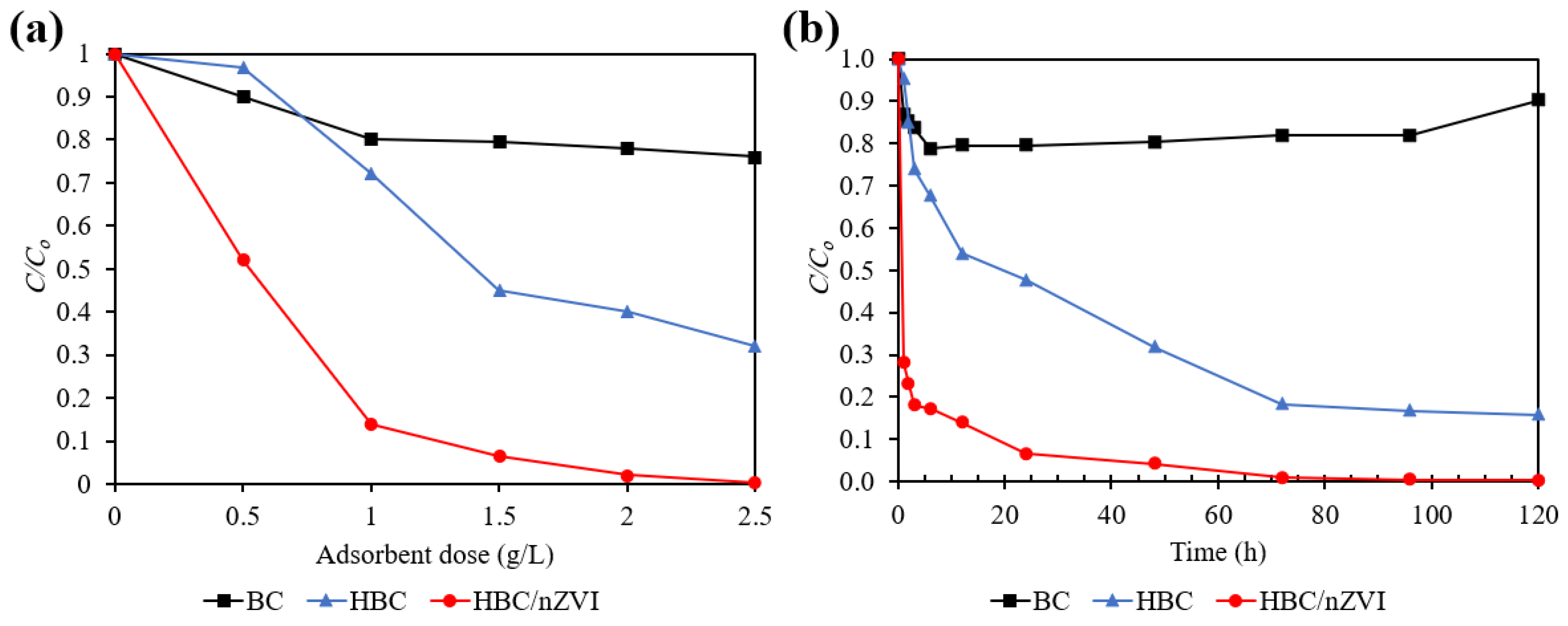
3.2.2. Cr(VI) Removal Efficiency Comparison between the BC, HBC, and HBC/nZVI Sorbents
3.2.3. Kinetics of Cr(VI) Removal Using BC, HBC, and HBC/nZVI
3.3. FTIR Analysis of Sorbents after Reacting with Cr(VI)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I. The quest for active carbon adsorbent substitutes: Inexpensive adsorbents for toxic metal ions removal from wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Mortazavian, S.; Jones-Lepp, T.; Bae, J.H.; Chun, D.; Bandala, E.R.; Moon, J. Heat-treated biochar impregnated with zero-valent iron nanoparticles for organic contaminants removal from aqueous phase: Material characterizations and kinetic studies. J. Ind. Eng. Chem. 2019, 76, 197–214. [Google Scholar] [CrossRef]
- Wu, L.; Liao, L.; Lv, G.; Qin, F.; He, Y.; Wang, X. Micro-electrolysis of Cr (VI) in the nanoscale zero-valent iron loaded activated carbon. J. Hazard. Mater. 2013, 254, 277–283. [Google Scholar] [CrossRef]
- Mortazavian, S.; Saber, A.; Hong, J.; Bae, J.H.; Chun, D.; Wong, N.; Gerrity, D.; Batista, J.; Kim, K.J.; Moon, J. Synthesis, characterization, and kinetic study of activated carbon modified by polysulfide rubber coating for aqueous hexavalent chromium removal. J. Ind. Eng. Chem. 2019, 69, 196–210. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, S.; Liu, T.; Deng, X. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Clean. Prod. 2018, 174, 184–190. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater. J. Clean. Prod. 2020, 267, 122009. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Yao, B.; Liu, M.; Gao, Y.; Liu, Y.; Cong, S.; Zou, D. Removal of hexavalent chromium in aqueous solution using organic iron-based composites synthesized and immobilized by natural dried willow leaves. J. Clean. Prod. 2020, 247, 119132. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.U.; Iqbal, M.M.; Imran, M.; Tariq, M.A.; Nadeem, M.; Kakar, M.Q.; Nawaz, M.; Amjad, M.; Qaisrani, S.A.; Rizwan, M.; et al. Potential of Fish Scale Biochar Nanocomposite with ZnO for Effective Sequestration of Cr (VI) from Water: Modeling and Kinetics. Int. J. Environ Res. 2022, 16, 1–13. [Google Scholar] [CrossRef]
- Fito, J.; Kefeni, K.K.; Nkambule, T.T. The potential of biochar-photocatalytic nanocomposites for removal of organic micropollutants from wastewater. Sci. Total Environ. 2022, 829, 154648. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Liu, Y.; Tan, X.; Wang, S.; Zeng, G.; Zheng, B.; Li, T.; Jiang, Z.; Liu, W. Effect of porous zinc-biochar nanocomposites on Cr(VI) adsorption from aqueous solution. RSC Adv. 2015, 5, 35107–35115. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shang, X.; Zhang, B.; Zhang, W.; Su, A.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lyu, H.; Tang, J.; Song, B.; Zhen, M.; Liu, X. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 2019, 217, 686–694. [Google Scholar] [CrossRef]
- Eugene, S.C.; Rodger, W.R.; Andrew, B.B.; Lenore, D.E. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Saber, A.; Tafazzoli, M.; Mortazavian, S.; James, D.E. Investigation of kinetics and absorption isotherm models for hydroponic phytoremediation of waters contaminated with sulfate. J. Environ. Manag. 2018, 207, 276–291. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, K.; Wang, Y.; Tsiakaras, P.; Song, S. In-situ electrosynthesis of hydrogen peroxide and wastewater treatment application: A novel strategy for graphite felt activation. Appl. Catal. B Environ. 2018, 237, 392–400. [Google Scholar] [CrossRef]
- Wei, X.; Wan, S.; Gao, S. Self-assembly-template engineering nitrogen-doped carbon aerogels for high-rate supercapacitors. Nano Energy 2016, 28, 206–215. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, X.; Wei, J.; Gao, S. Functional Groups and Pore Size Distribution Do Matter to Hierarchically Porous Carbons as High-Rate-Performance Supercapacitors. Chem. Mater. 2016, 28, 445–458. [Google Scholar] [CrossRef]
- Lu, Y.; Miller, J.D. Carboxyl stretching vibrations of spontaneously adsorbed and LB-transferred calcium carboxylates as determined by FTIR internal reflection spectroscopy. J. Colloid Interface Sci. 2002, 256, 41–52. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J. Use of three types of magnetic biochar in the removal of copper(II) ions from wastewaters. Sep. Sci. Technol. 2018, 53, 1045–1057. [Google Scholar] [CrossRef]
- Refractive Index of Graphite for Thin Film Thickness Measurement. Available online: https://www.filmetrics.com/refractive-index-database/Graphite# (accessed on 20 August 2020).
- Ramsey, J.D.; Xia, L.; Kendig, M.W.; McCreery, R.L. Raman spectroscopic analysis of the speciation of dilute chromate solutions. Corros. Sci. 2001, 43, 1557–1572. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Fang, Z.; Qiu, X.; Huang, R.; Qiu, X.; Li, M. Removal of chromium in electroplating wastewater by nanoscale zero-valent metal with synergistic effect of reduction and immobilization. Desalination 2011, 280, 224–231. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J. Environ. Manag. 2014, 146, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.J.; Zhang, N.; Li, Y.S.; Jiang, H.; Sheng, G.P. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour. Technol. 2014, 169, 403–408. [Google Scholar] [CrossRef]
- Mohamed, A.K.; Mahmoud, M.E. Nanoscale Pisum sativum pods biochar encapsulated starch hydrogel: A novel nanosorbent for efficient chromium (VI) ions and naproxen drug removal. Bioresour. Technol. 2020, 308, 123263. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Mortazavian, S.; Bandala, E.R.; Bae, J.; Chun, D.; Moon, J. Assessment of p-nitroso dimethylaniline (pNDA) suitability as a hydroxyl radical probe: Investigating bleaching mechanism using immobilized zero- valent iron nanoparticles. Chem. Eng. J. 2020, 385, 123748. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Li, J.; Wang, L. Degradation of nitrobenzene in groundwater by nanoscale zero-valent iron particles incorporated inside the channels of SBA-15 rods. J. Taiwan Inst. Chem. Eng. 2014, 45, 996–1000. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209. [Google Scholar] [CrossRef]
- Sheng, G.S.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Sheng, J.; Wang, X.; Li, H.; Huang, Y. Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation. Carbon N. Y. 2016, 99, 123–130. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Chromium Oxide. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C1308389&Mask=80 (accessed on 27 August 2020).

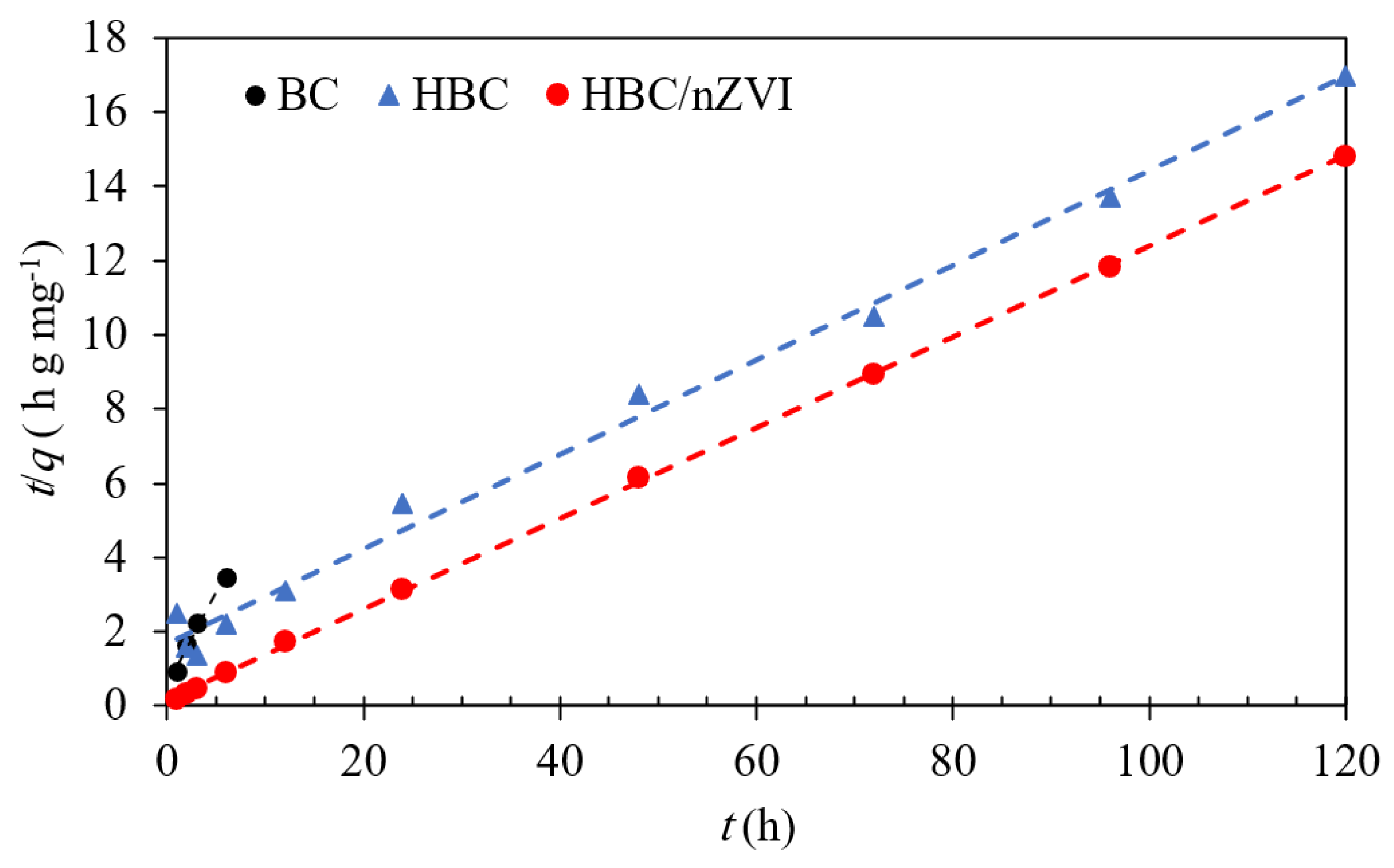
| k (g mg−1 h−1) | qe (mg g−1) | R2 | NRMSE | |
|---|---|---|---|---|
| BC | 0.39 | 2.05 | 0.98 | 0.08 |
| HBC | 0.01 | 7.87 | 0.99 | 0.09 |
| HBC/nZVI | 0.10 | 8.18 | >0.99 | 0.11 |
| (Equation (2)) | ||||
| Feedstock Materials | Modification Technique | pH of Cr(VI) Solution | Initial Cr(VI) Concentration (mg L−1) | Adsorbent Dose (g L−1) | Cr(VI) Removal Efficiency (%) | Ref. No. |
|---|---|---|---|---|---|---|
| Pristine Biochar | ||||||
| Rice Husk | None | 7 to 9.5 | 0.19 | 16 | 18% | [34] |
| Municipal solid waste | None | 7 to 9.5 | 0.19 | 16 | 44% | [34] |
| Sugar beet tailing | None | 4.0 | 100 | 2 | 55% | [36] |
| Oak wood | None | 4.0 | 10 | 10 | 19% | [37] |
| Oak bark | None | 4.0 | 10 | 10 | 10% | [37] |
| Municipal sewage sludge | None | 5.0 | 50 | 2 | 10% | [38] |
| Pomelo peel | None | 4.0 | 200 | 1 | 5% | [39] |
| Pine tree residues | None | 4.0 | 10 | 1.5 | 20.5% | This study |
| Modified biochar | ||||||
| Rice husk | Fe0 impregnation | 7.0 | 0.85 | 16 | 24% | [35] |
| Rice husk | Fe3+ impregnation | 7.0 | 0.85 | 1 | 35% | [35] |
| Municipal solid waste | Fe0 impregnation | 7.0 | 0.85 | 16 | 14% | [35] |
| Municipal solid waste | Fe3+ impregnation | 7.0 | 0.85 | 1 | 89% | [35] |
| Rice husk | KOH and Polyethylenimine surface treatments | 4.0 | 100 | 1 | 98% | [40] |
| Pisum sativum | Encapsulation of starch hydrogel | 4.0 | 50 | 2 | 70% | [41] |
| Pomelo peel | K2FeO4-promoted Pyrolysis | 4.0 | 200 | 1 | 8% | [39] |
| Wheat straw | carboxymethyl cellulose stabilization and FeS deposition | 4.0 | 100 | 0.72 | 85% | [42] |
| Pine tree residues | Heat treatment at 300 °C | 4.0 | 10 | 1.5 | 55% | This study |
| Pine tree residues | Heat treatment and Fe0 impregnation | 4.0 | 10 | 1.5 | 93% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavian, S.; Murph, S.E.H.; Moon, J. Biochar Nanocomposite as an Inexpensive and Highly Efficient Carbonaceous Adsorbent for Hexavalent Chromium Removal. Materials 2022, 15, 6055. https://doi.org/10.3390/ma15176055
Mortazavian S, Murph SEH, Moon J. Biochar Nanocomposite as an Inexpensive and Highly Efficient Carbonaceous Adsorbent for Hexavalent Chromium Removal. Materials. 2022; 15(17):6055. https://doi.org/10.3390/ma15176055
Chicago/Turabian StyleMortazavian, Soroosh, Simona E. Hunyadi Murph, and Jaeyun Moon. 2022. "Biochar Nanocomposite as an Inexpensive and Highly Efficient Carbonaceous Adsorbent for Hexavalent Chromium Removal" Materials 15, no. 17: 6055. https://doi.org/10.3390/ma15176055
APA StyleMortazavian, S., Murph, S. E. H., & Moon, J. (2022). Biochar Nanocomposite as an Inexpensive and Highly Efficient Carbonaceous Adsorbent for Hexavalent Chromium Removal. Materials, 15(17), 6055. https://doi.org/10.3390/ma15176055





