Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Experiments and Methods
2.1. MD Simulation Models
2.1.1. Force Field Selection
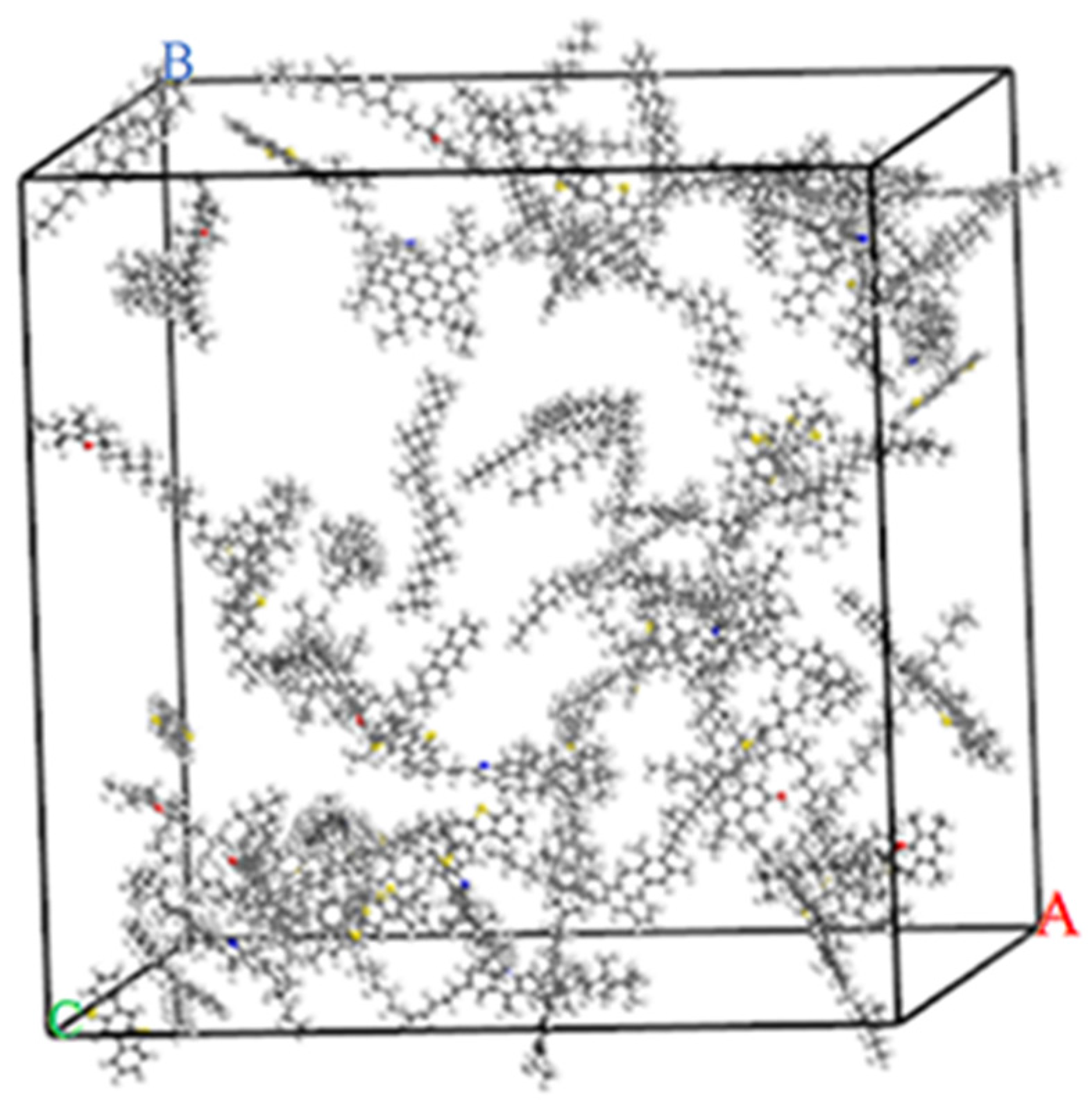
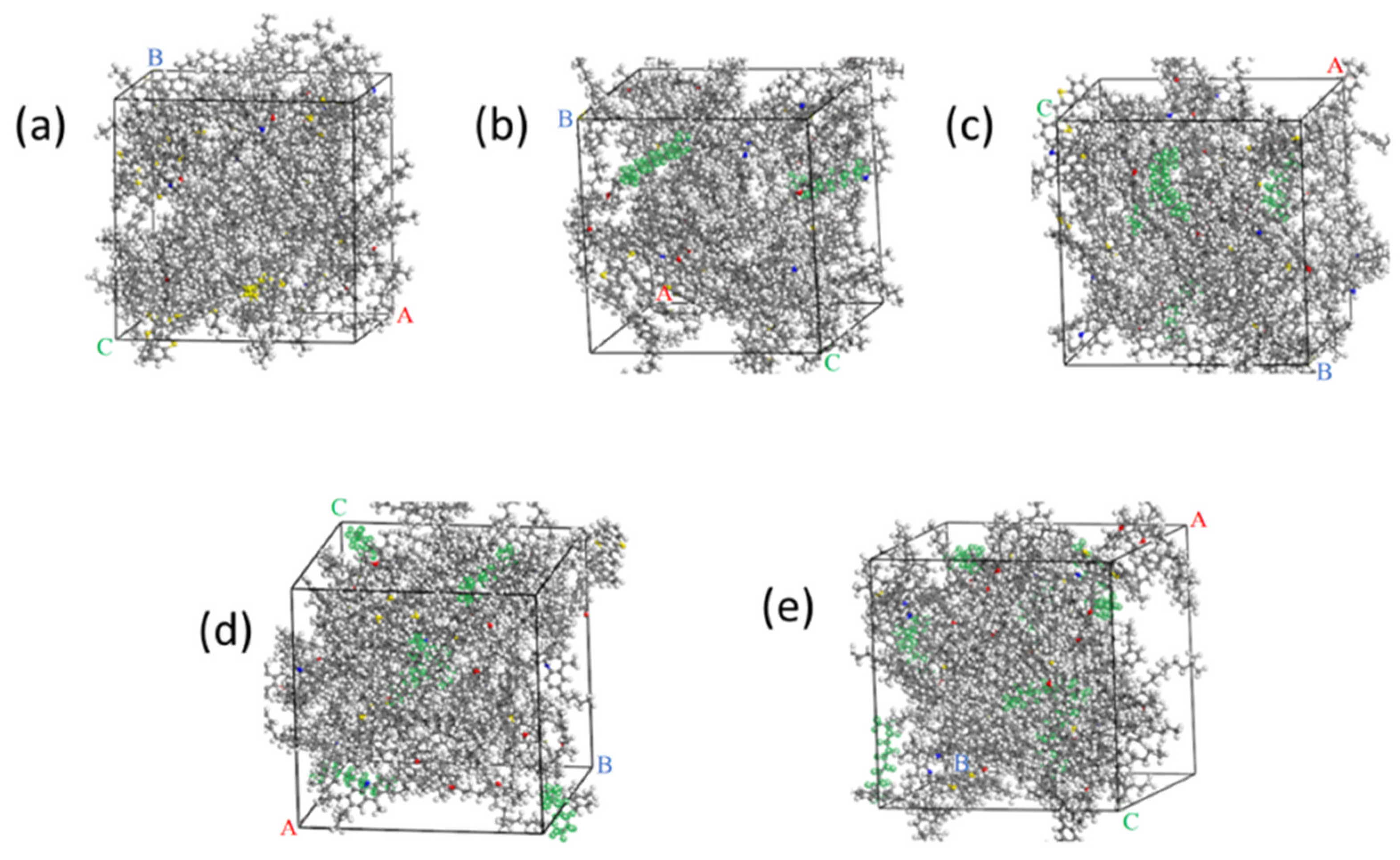
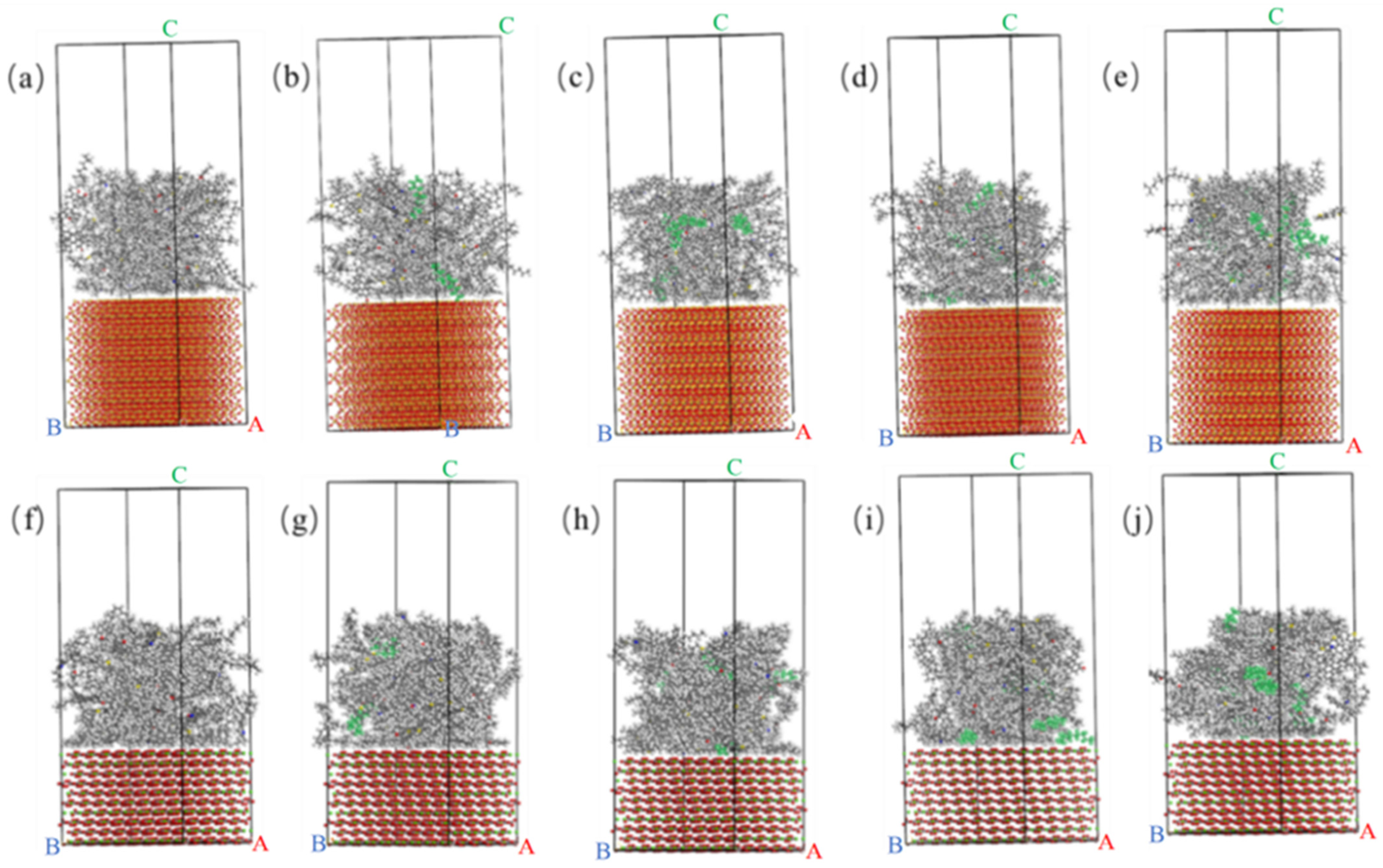
2.1.2. Matrix Asphalt Model
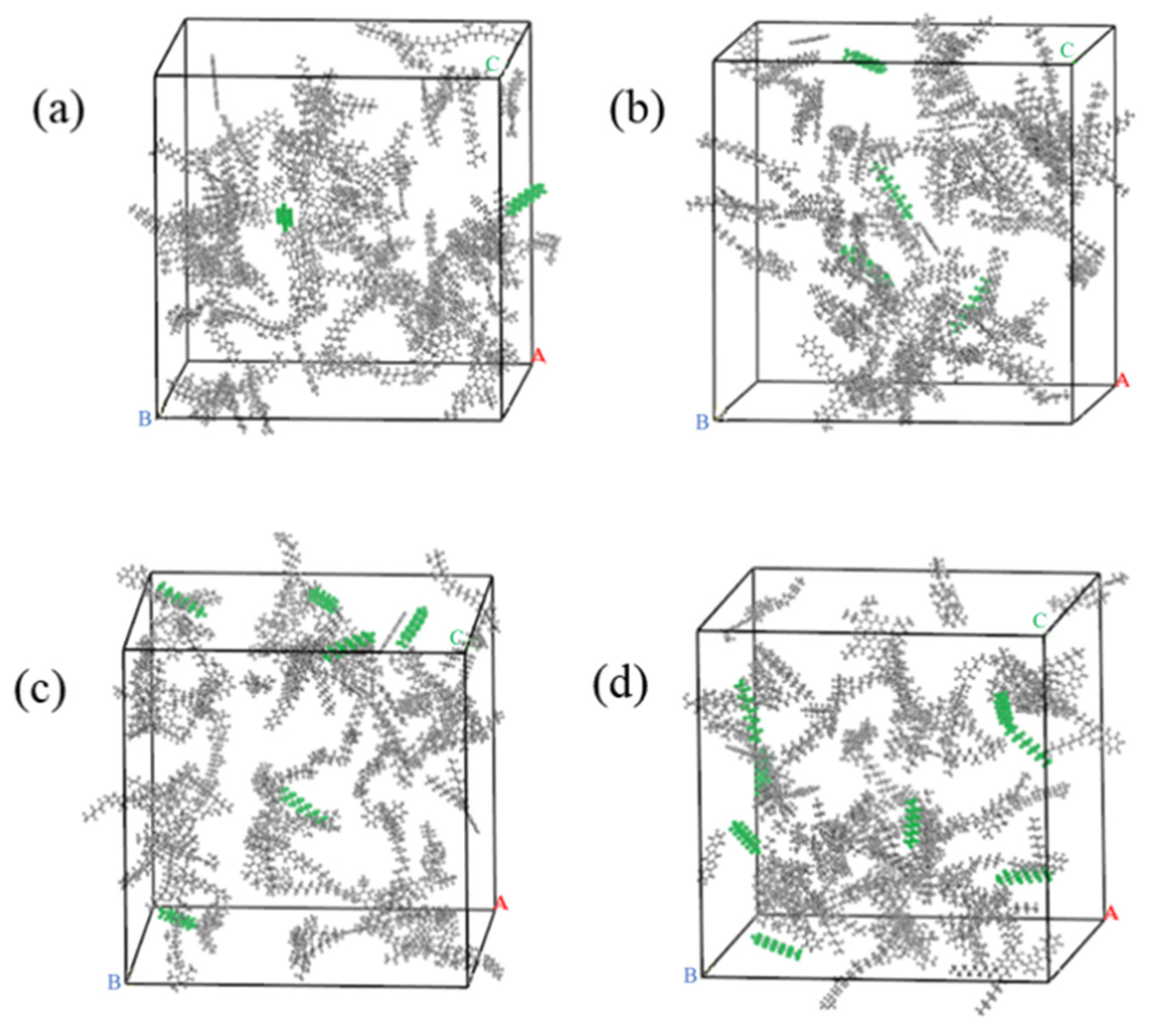
2.1.3. WWMA Models
2.1.4. Model Optimization
2.2. Adhesion Work
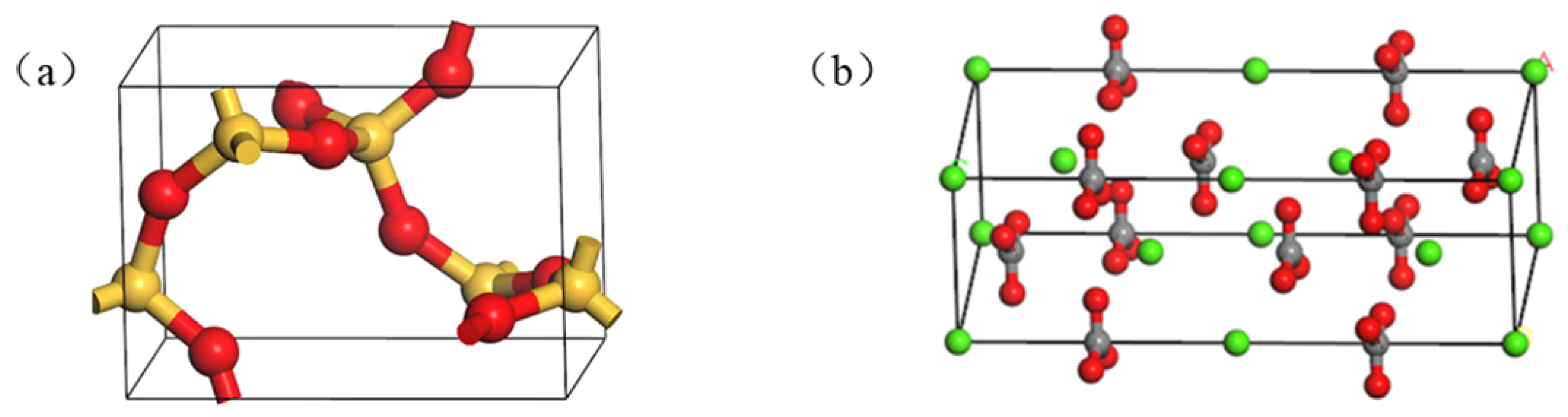
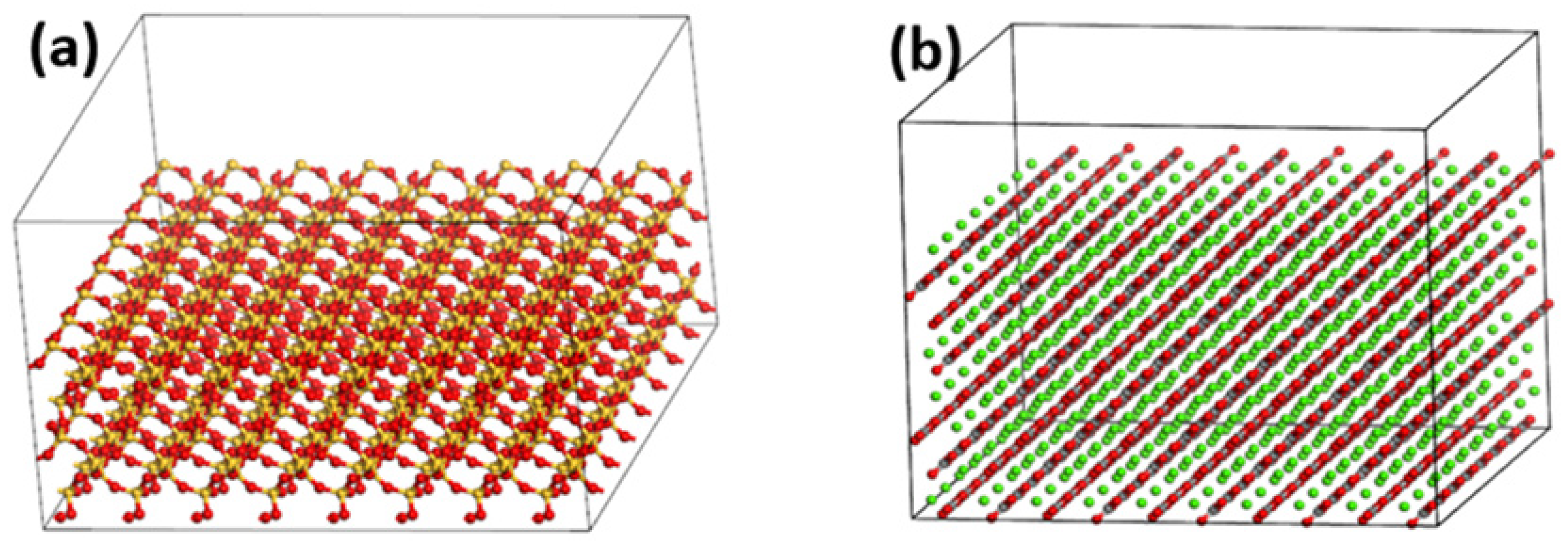
2.2.1. Single Cell Oxide Model
2.2.2. Oxide Supercell Model
2.2.3. Calculation of Adhesion Work
2.3. Macroscopic Adhesion Performance Experiment
2.3.1. Raw Materials
2.3.2. Preparation of WWMA
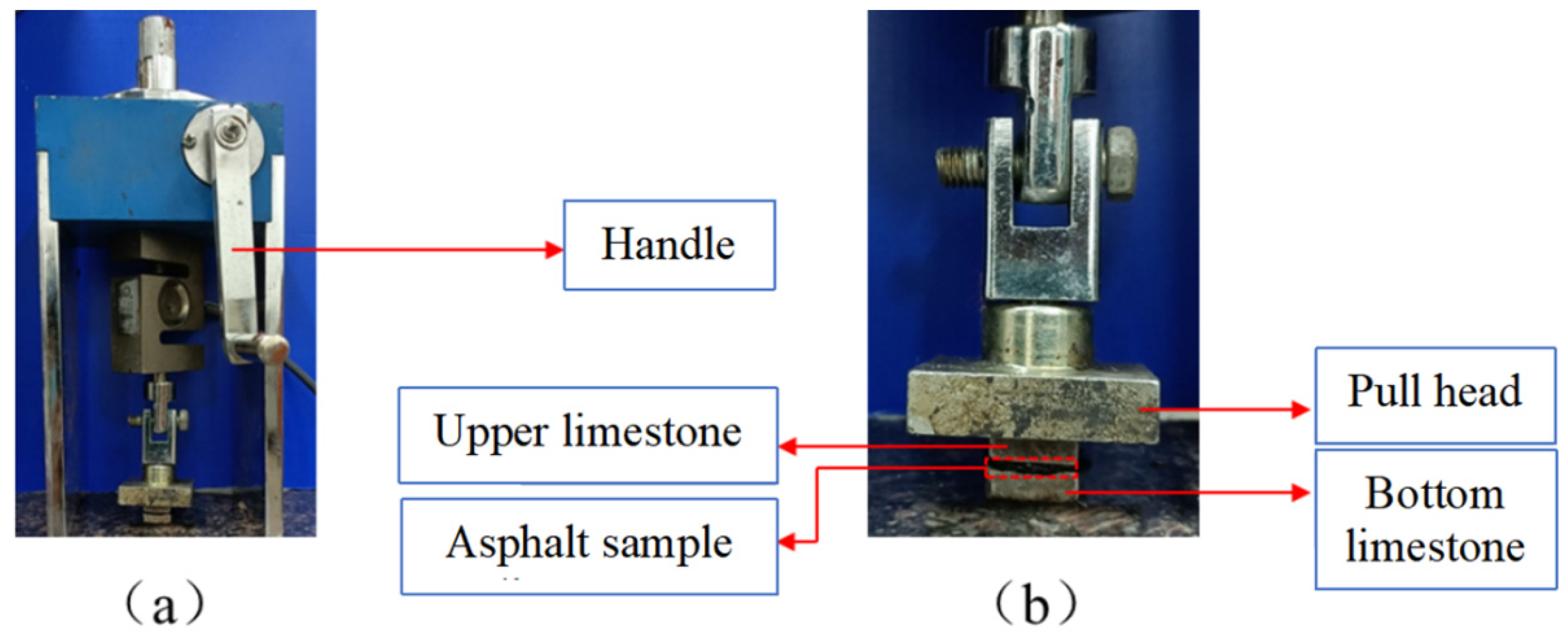
2.3.3. Pull-Off Test
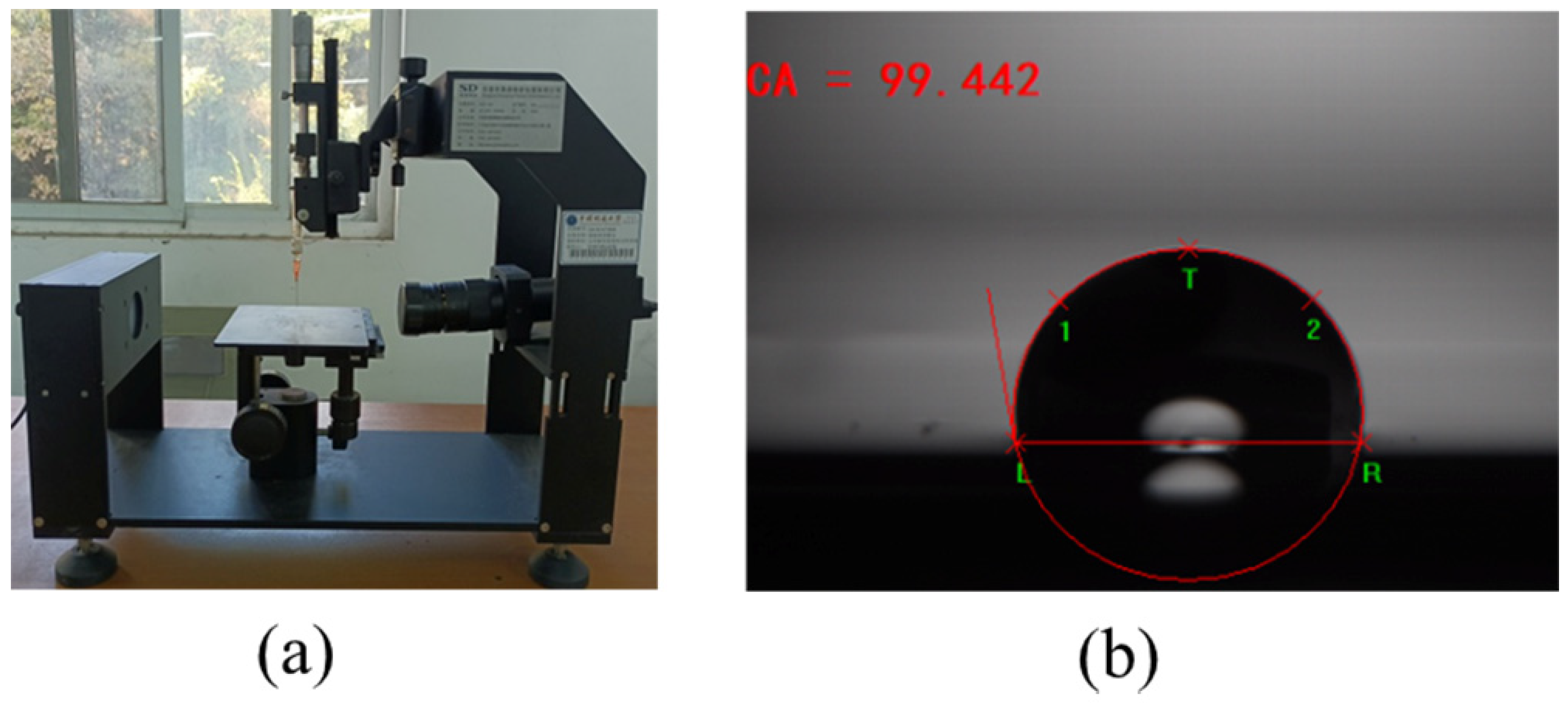
2.3.4. Contact Angle Experiment
2.3.5. FTIR Experiment
3. Results and Discussion
3.1. The Rationality Verification of the Asphalt Model
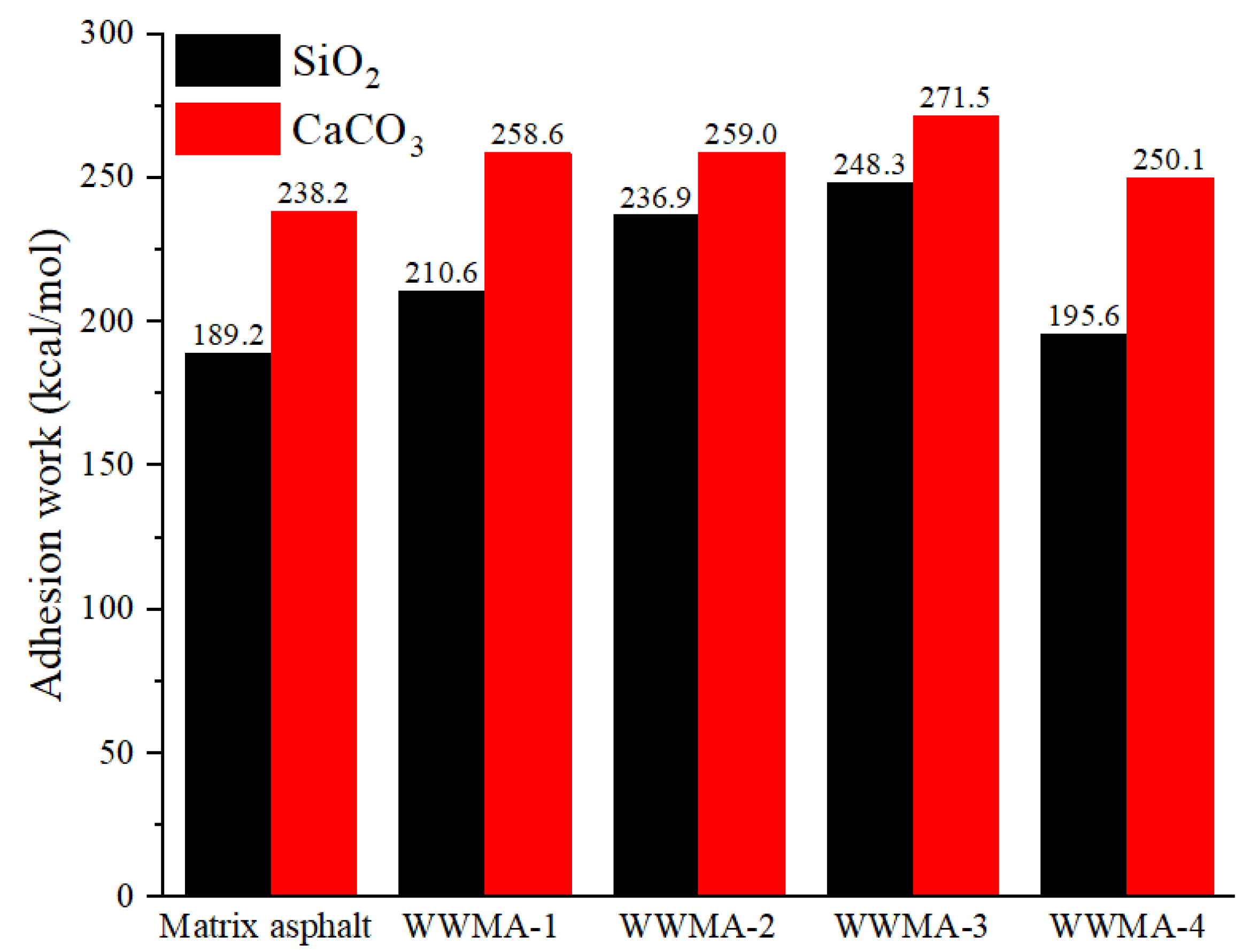
3.2. Adhesion Work between Asphalt and Aggregate Oxide
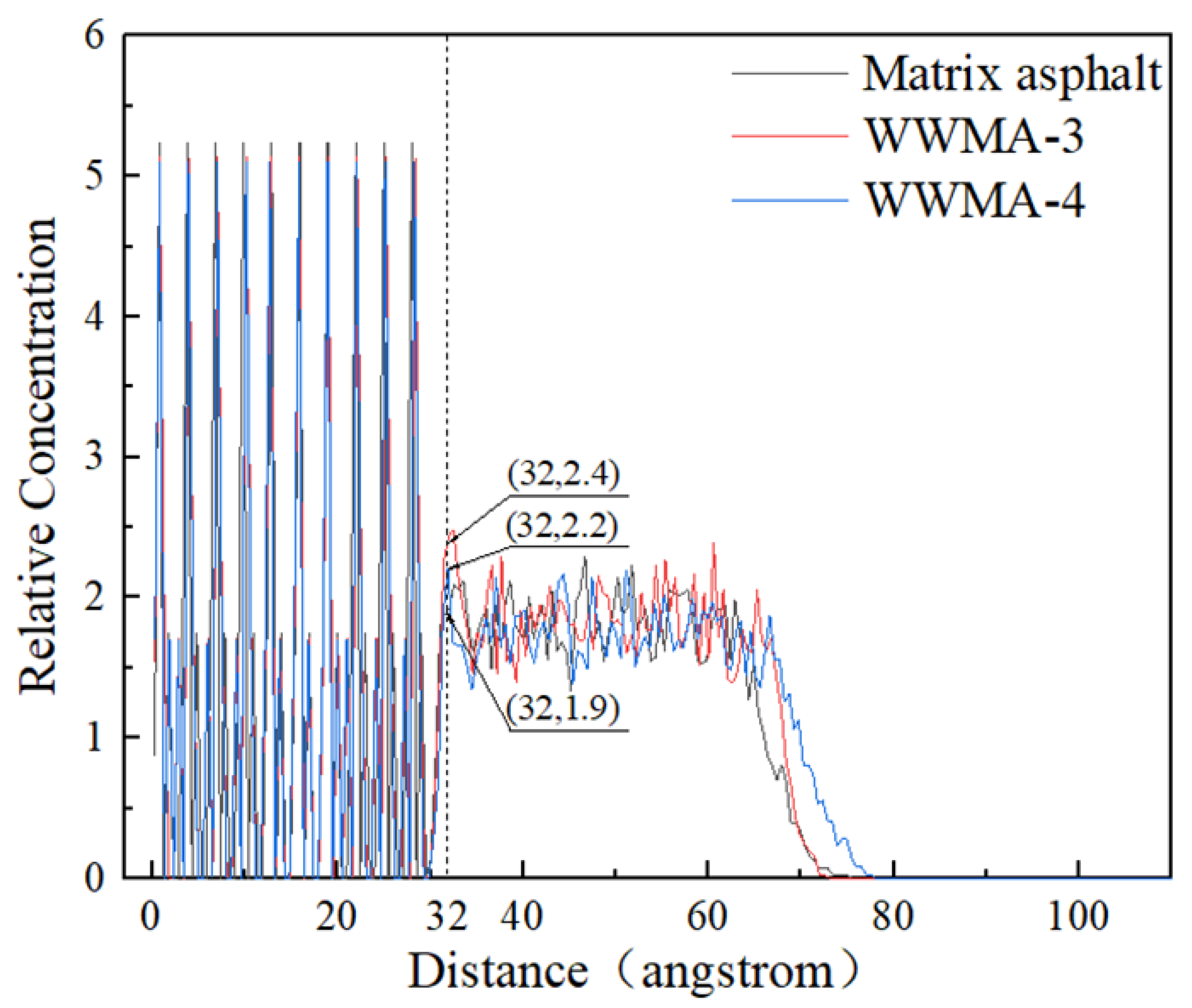
3.3. Relative Concentration Analysis
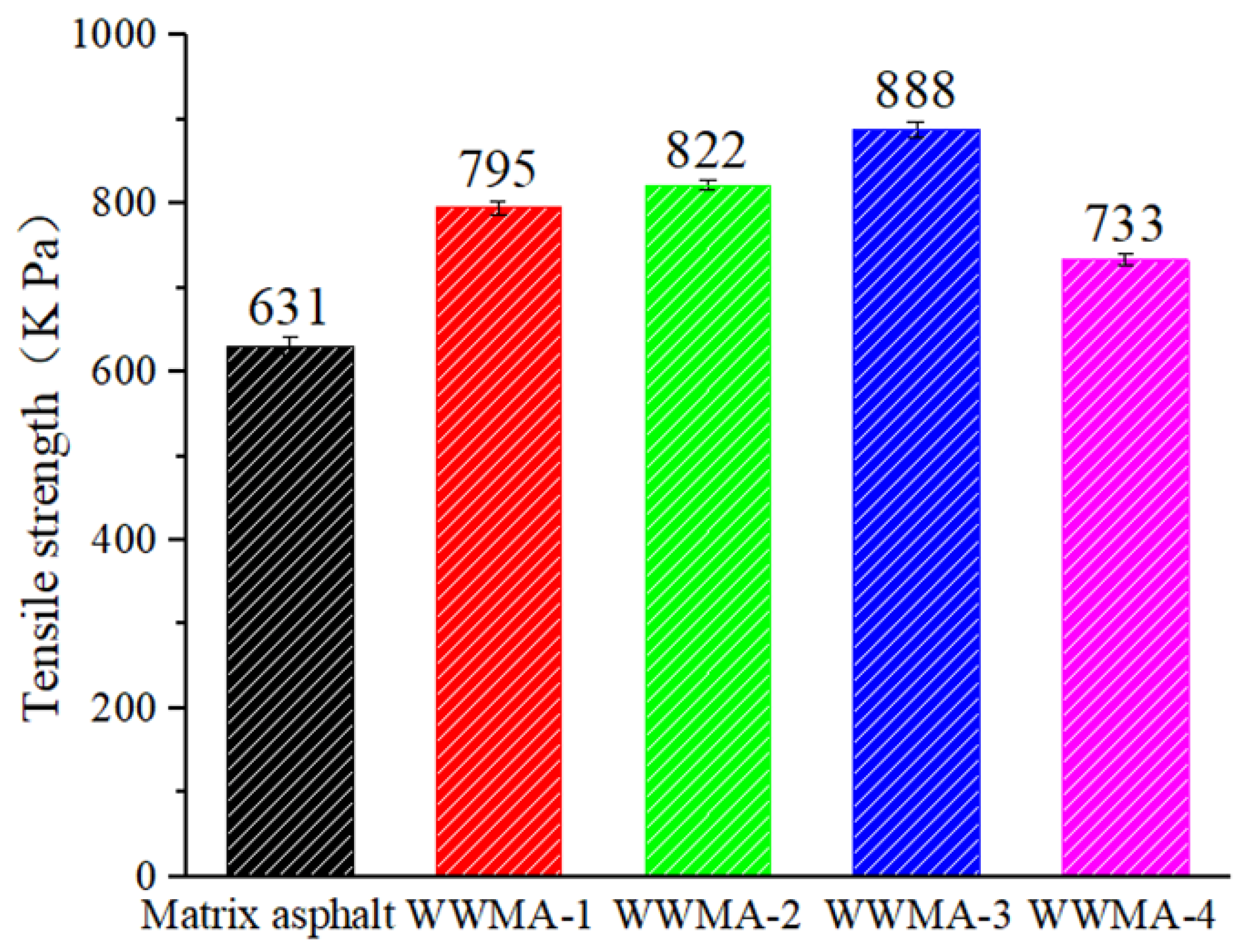
3.4. Pull-Off Test Results
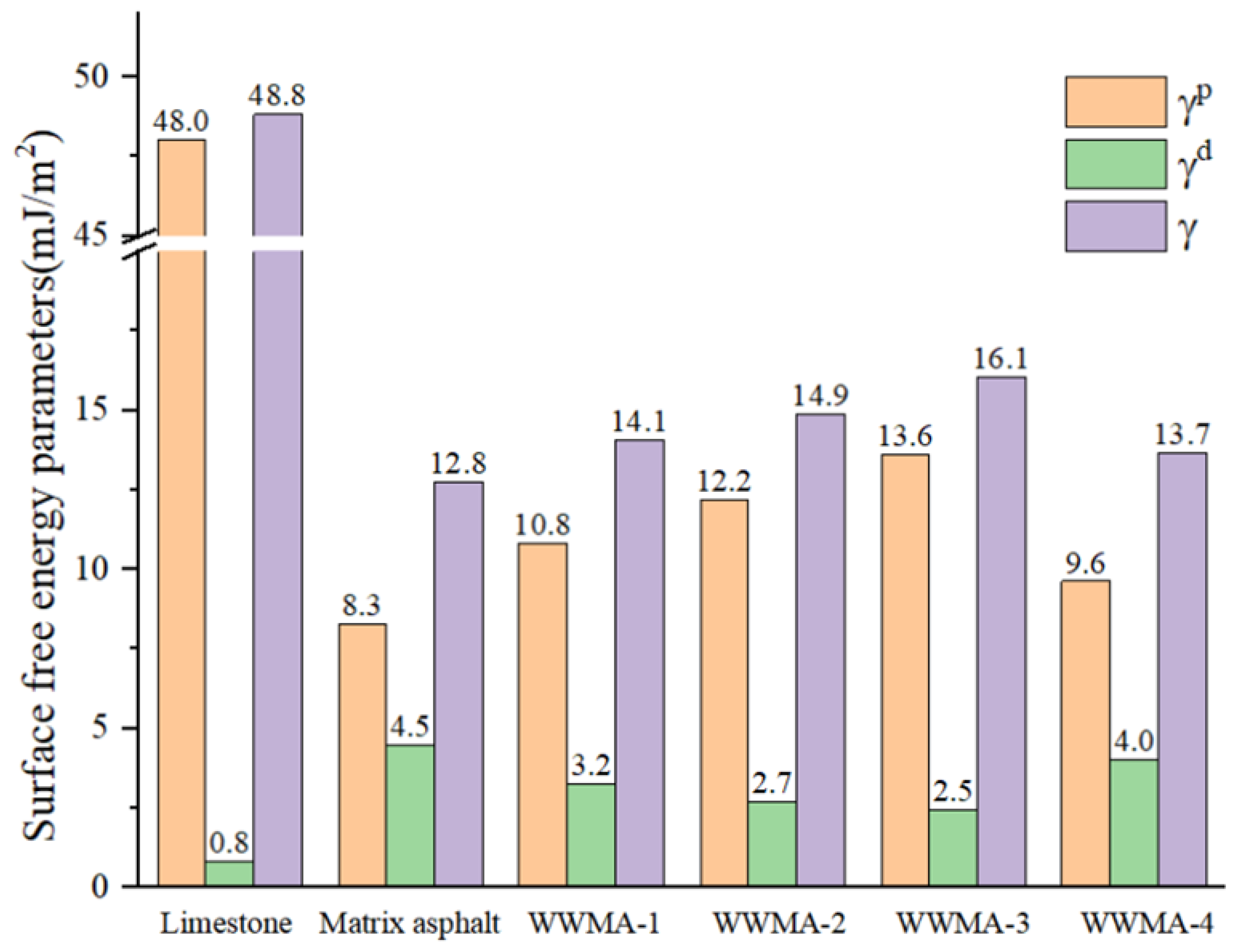
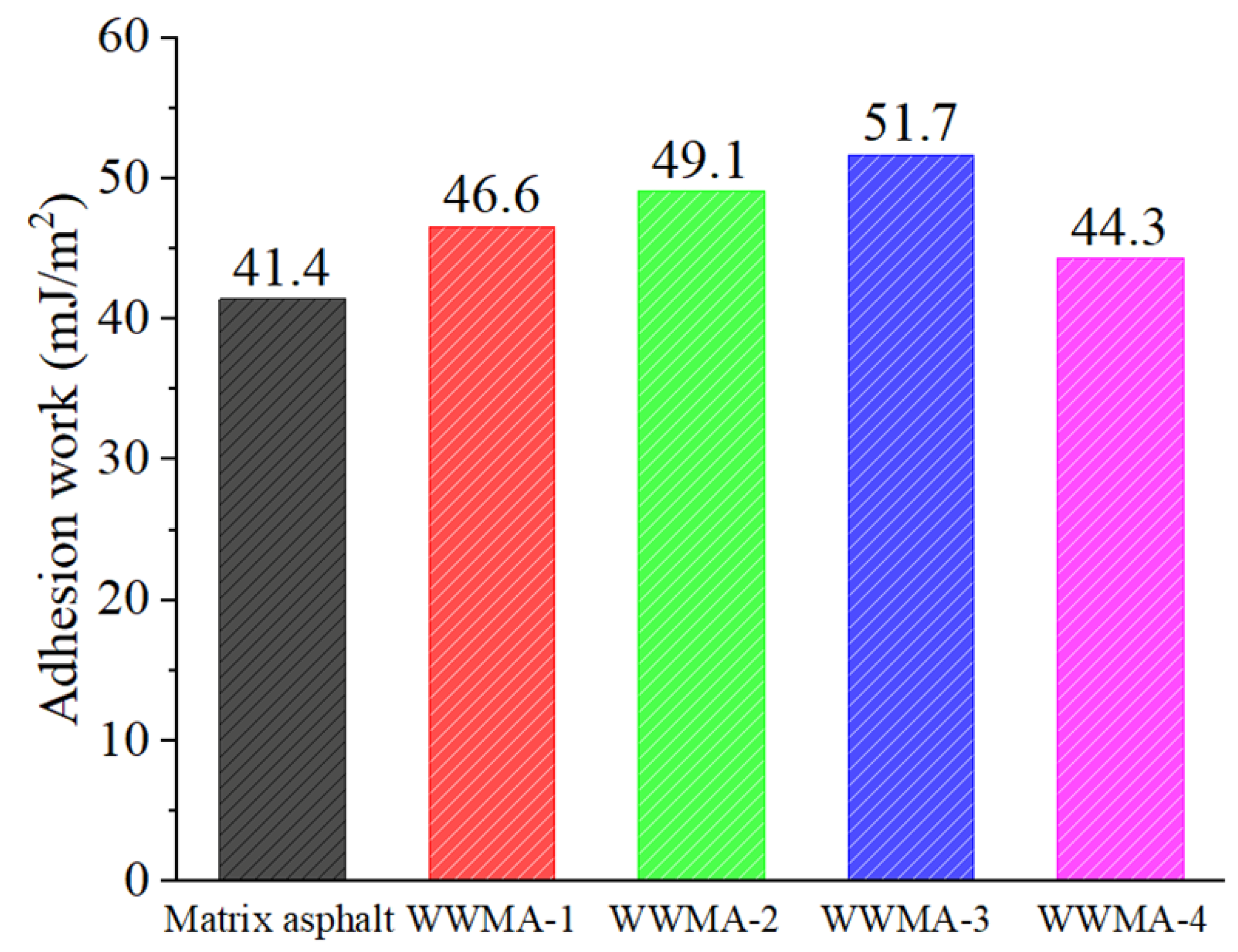
3.5. Contact Angle Experiment
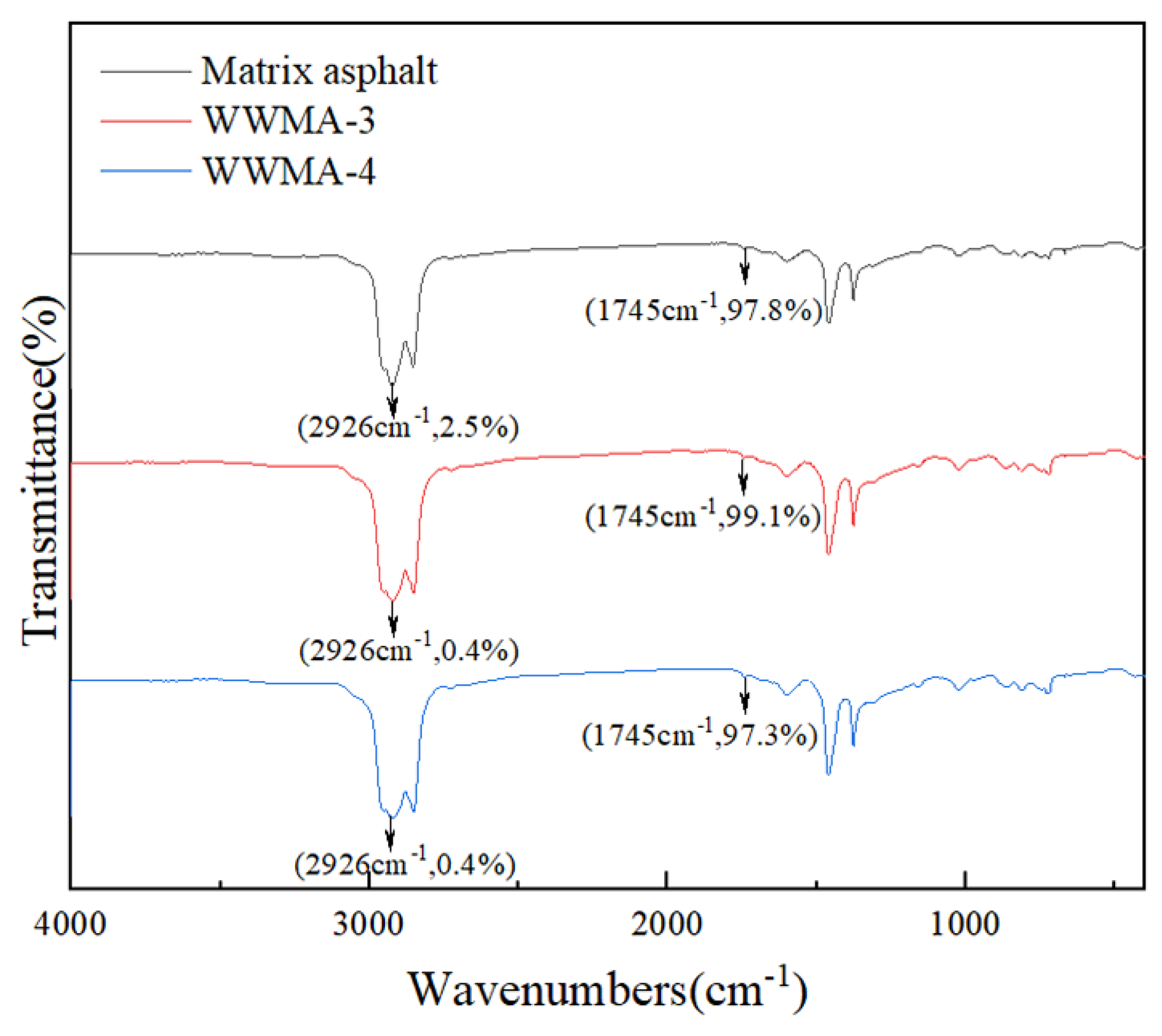
3.6. FTIR
4. Conclusions
- (1)
- In MD simulation, the adhesion work of the WWMA and the two aggregate oxides is larger than that of matrix asphalt and the two aggregate oxides. The adhesion works of the WWMA-3 on SiO2 and CaCO3 increase to the peak values. They increase by 31.2% and 14.0% compared to the matrix asphalt on SiO2 and CaCO3. The pull-off experiments and the contact angle experiments are also in good agreement with that of the MD simulation.
- (2)
- The pull-off and contact angle experiments prove that WWMA can increase the tensile strength and adhesion work between the asphalt and the limestone cube. The tensile strength and the adhesion work between the aggregate and the asphalt containing 3 wt% wax modifier reach the peak values. These values are 140.7% and 124.9% compared with those between the aggregate and the matrix asphalt.
- (3)
- FTIR reveals that the carbonyl content in WWMA is much higher than that in matrix asphalt. It explains well that carbonyl can enhance the adhesion strength of asphalt and aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abed, A.; Thom, N.; Grenfell, J. A novel approach for rational determination of warm mix asphalt production temperatures. Constr. Build. Mater. 2019, 200, 80–93. [Google Scholar]
- Abd, D.M.; Al-Khalid, H.; Akhtar, R. An investigation into the impact of warm mix asphalt additives on asphalt mixture phases through a nano-mechanical approach. Constr. Build. Mater. 2018, 198, 296–306. [Google Scholar]
- Zhang, Y.; Leng, Z.; Zou, F.; Wang, L.; Chen, S.S.; Tsang, D.C.W. Synthesis of zeolite A using sewage sludge ash for application in warm mix asphalt. J. Clean. Prod. 2018, 172, 687–695. [Google Scholar]
- Feitosa, J.P.M.; Alencar, A.E.V.; Filho, N.W.; Souza, J.R.R. Evaluation of sun-oxidized carnauba wax as warm mix asphalt additive. Constr. Build. Mater. 2016, 115, 294–298. [Google Scholar]
- Woszuk, A.; Zofka, A.; Bandura, L.; Franus, W. Effect of zeolite properties on asphalt foaming. Constr. Build. Mater. 2017, 139, 247–255. [Google Scholar]
- Han, X.; Cao, Z.; Wang, R.; He, P.; Zhang, Y.; Yu, J.; Ge, Y. Effect of silane coupling agent modified zeolite warm mix additives on properties of asphalt. Constr. Build. Mater. 2020, 259, 119713. [Google Scholar]
- Wan, Z.; Zheng, B.; Xie, X.; Yang, J.; Zhou, H. Preparation method and performance test of Evotherm pre-Wet treatment aluminum hydroxide type warm-Mixed flame-Retardant asphalt. Constr. Build. Mater. 2020, 262, 120618. [Google Scholar]
- Wu, S.; Zhang, W.; Shen, S.; Li, X.; Muhunthan, B.; Mohammad, L.N. Field-Aged asphalt binder performance evaluation for Evotherm warm mix asphalt: Comparisons with hot mix asphalt. Constr. Build. Mater. 2017, 156, 574–583. [Google Scholar]
- Yue, M.; Yue, J.; Wang, R.; Xiong, Y. Evaluating the fatigue characteristics and healing potential of asphalt binder modified with Sasobit and polymers using linear amplitude sweep test. Constr. Build. Mater. 2021, 289, 123054. [Google Scholar]
- Selvadurai, S.K.; Hasan, M.R.M.; Sani, A.; Hiromitsu, N.; Poovaneshvaran, S. Improvements of TPS-Porous Asphalt Using Wax-Based Additives for the Application on Malaysian Expressway. J. Kejuruter. 2021, 33, 205–215. [Google Scholar]
- Vaitkus, A.; Čygas, D.; Laurinavičius, A.; Vorobjovas, V.; Perveneckas, Z. Influence of warm mix asphalt technology on asphalt physical and mechanical properties. Constr. Build. Mater. 2016, 112, 800–806. [Google Scholar] [CrossRef]
- Janshidi, A.; Golchin, B.; Hamzah, M.O.; Turner, P. Selection of type of warm mix asphalt additive based on the rheological properties of asphalt binders. J. Clean. Prod. 2015, 100, 89–106. [Google Scholar] [CrossRef]
- Dong, Z.; Gong, X.; Zhao, L.; Zhang, L. Mesostructural damage simulation of asphalt mixture using microscopic interface contact models. Constr. Build. Mater. 2014, 53, 665–673. [Google Scholar] [CrossRef]
- Abd, D.M.; Al-Khalid, H.; Akhtar, R. Nano-Scale properties of warm-Modified bituminous binders determined with atomic force microscopy. Road Mater. Pavement Des. 2017, 18, 189–202. [Google Scholar] [CrossRef]
- Wen, B. Adhesion of Warm-Mixed Asphalt Aggregate Interface with Organic Wax and Evaluation of Water Stability of Mixtures. Master’s Thesis, Beijing Architecture University, Beijing, China, 2016. [Google Scholar]
- Yang, S.H.; Rachman, F.; Susanto, H.A. Effect of moisture in aggregate on adhesive properties of warm-Mix asphalt. Constr. Build. Mater. 2018, 190, 1295–1307. [Google Scholar] [CrossRef]
- Tarefder, R.; Rafiqul, A. Molecular dynamic simulation of oxidative aging in asphaltene. Pavements Mater. 2010, 1, 16–30. [Google Scholar]
- Qu, X.; Wang, D.; Hou, Y.; Oeser, M.; Wang, L. Influence of Paraffin on the Microproperties of Asphalt Binder Using MD Simulation. J. Mater. Civ. Eng. 2018, 30, 04018191. [Google Scholar]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Gu, F.; Xu, T.; Wang, H. Impact of minerals and water on bitumen-Mineral adhesion and debonding behaviours using molecular dynamics simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar]
- Xu, Z.; Wang, Y.; Cao, J.; Chai, J.; Cao, C.; Si, Z.; Li, Y. Adhesion between asphalt molecules and acid aggregates under extreme temperature: A ReaxFF reactive molecular dynamics study. Constr. Build. Mater. 2021, 285, 122882. [Google Scholar]
- Dong, Z.; Liu, Z.; Wang, P.; Gong, X. Nanostructure characterization of asphalt-Aggregate interface through molecular dynamics simulation and atomic force microscopy. Fuel 2017, 189, 155–163. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-Field optimized for condensed-Phase applications-Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Jones, D.R. SHRP Materials Reference Library, Asphalt Cements: A Concise Data Compilation; In Strategic Highway Research Program National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Samieadel, A.; Oldham, D.; Fini, E.H. Multi-Scale characterization of the effect of wax on intermolecular interactions in asphalt binder. Constr. Build. Mater. 2017, 157, 1163–1172. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Yalghouzaghaj, M.N.; Sarkar, A.; Hamedi, G.H.; Hayati, P. Application of the surface free energy method on the mechanism of low-Temperature cracking of asphalt mixtures. Constr. Build. Mater. 2021, 268, 121194. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Peng, C.; Huang, S.; You, Z.; Xu, F.; You, L. Effect of a lignin-Based polyurethane on adhesion properties of asphalt binder during UV aging process. Constr. Build. Mater. 2020, 247, 118547. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Effects of Polymer Modification on Properties and Microstructure of Model Asphalt Systems. Energy Fuels 2008, 22, 3363–3375. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, H.; Kuang, D.; Song, L.; Wang, L. Effect of chemical composition of aggregate on interfacial adhesion property between aggregate and asphalt. Constr. Build. Mater. 2017, 146, 231–237. [Google Scholar] [CrossRef]
- Guo, M. Study on Mechanism and Multiscale Evaluation Method of Interfacial Interaction between Asphalt Binder and Mineral Aggregate. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Peng, C.; Chen, P.; You, Z.; Lv, S.; Zhang, R.; Xu, F.; Zhang, H.; Chen, H. Effect of silane coupling agent on improving the adhesive properties between asphalt binder and aggregates. Constr. Build. Mater. 2018, 169, 591–600. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Abdelhady, A.; Jia, M.; Xie, N. Investigation on surface free energy and moisture damage of asphalt mortar with fine solid waste. Constr. Build. Mater. 2020, 231, 117140. [Google Scholar] [CrossRef]
- Zhang, F.; Muhammad, Y.; Liu, Y.; Han, M.; Yin, Y.; Hou, D.; Li, J. Measurement of water resistance of asphalt based on surface free energy analysis using stripping work between asphalt-Aggregate system. Constr. Build. Mater. 2018, 176, 422–431. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.; Liu, K.; Shi, X. Adhesion characteristics of graphene oxide modified asphalt unveiled by surface free energy and AFM-Scanned micro-Morphology. Constr. Build. Mater. 2020, 244, 118404. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Zhang, X.; Guo, Y.; Yu, X. Comparative evaluation of moisture susceptibility of modified/foamed asphalt binders combined with different types of aggregates using surface free energy approach. Constr. Build. Mater. 2020, 256, 119429. [Google Scholar] [CrossRef]
- Peng, C.; Chen, P.; You, Z.; Lv, S.; Xu, F.; Zhang, W.; Yu, J.; Zhang, H. The anti-Icing and mechanical properties of a superhydrophobic coating on asphalt pavement. Constr. Build. Mater. 2018, 190, 83–94. [Google Scholar] [CrossRef]
- Yaylayan, V.A.; Ismail, A.A. Investigation of the enolization and carbonyl group migration in reducing sugars by FTIR spectroscopy. Carbohydr. Res. 1995, 276, 253–265. [Google Scholar] [CrossRef]
- Peng, C.; Jiang, G.; Lu, C.; Xu, F.; Yu, J.; Dai, J. Effect of 4,4’-Stilbenedicarboxylic acid-Intealated layered double hydroxides on UV aging resistance of bitumen. Rsc Adv. 2015, 5, 95504–95511. [Google Scholar] [CrossRef]
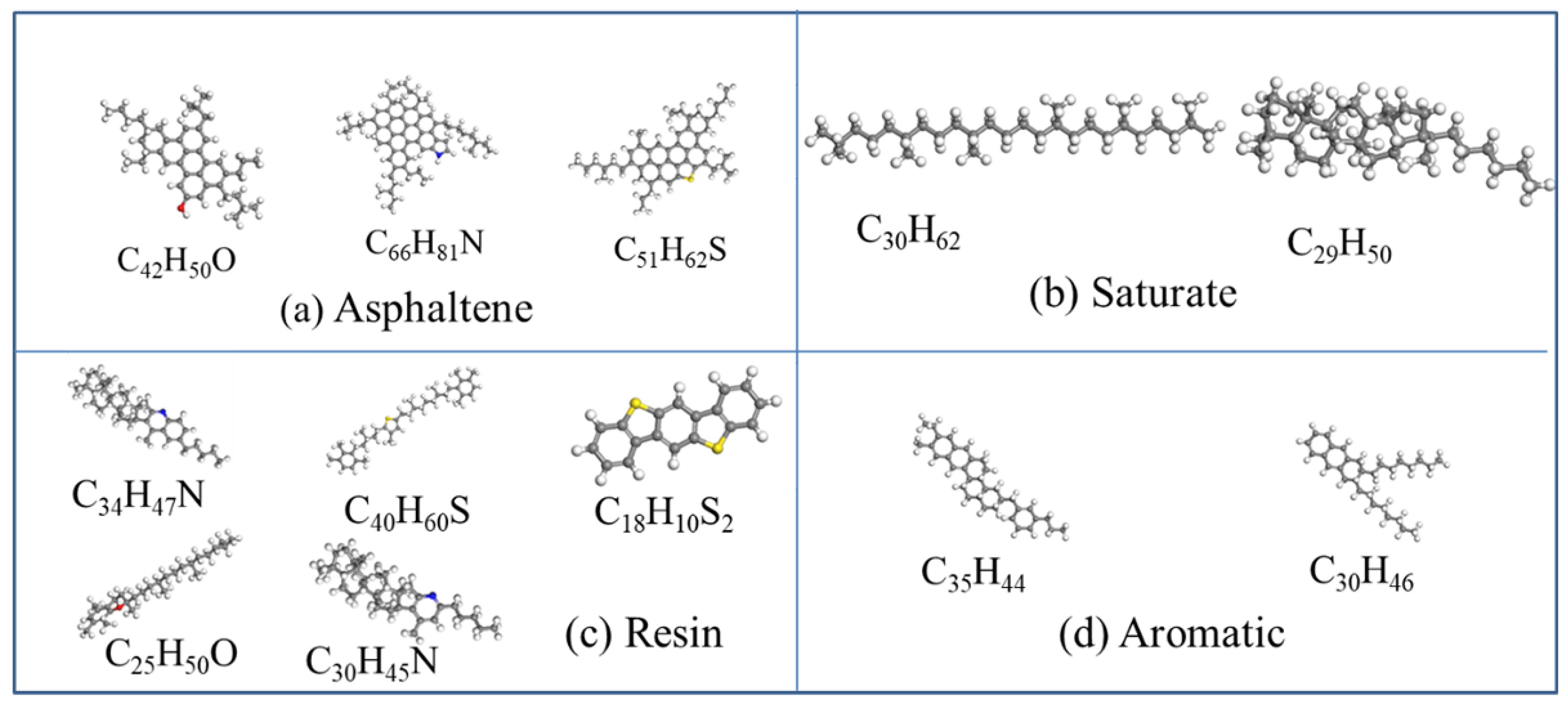
| Molecules in Model | Molar Mass | Molecular Formula | Number of Molecules | |
|---|---|---|---|---|
| Group | Label | |||
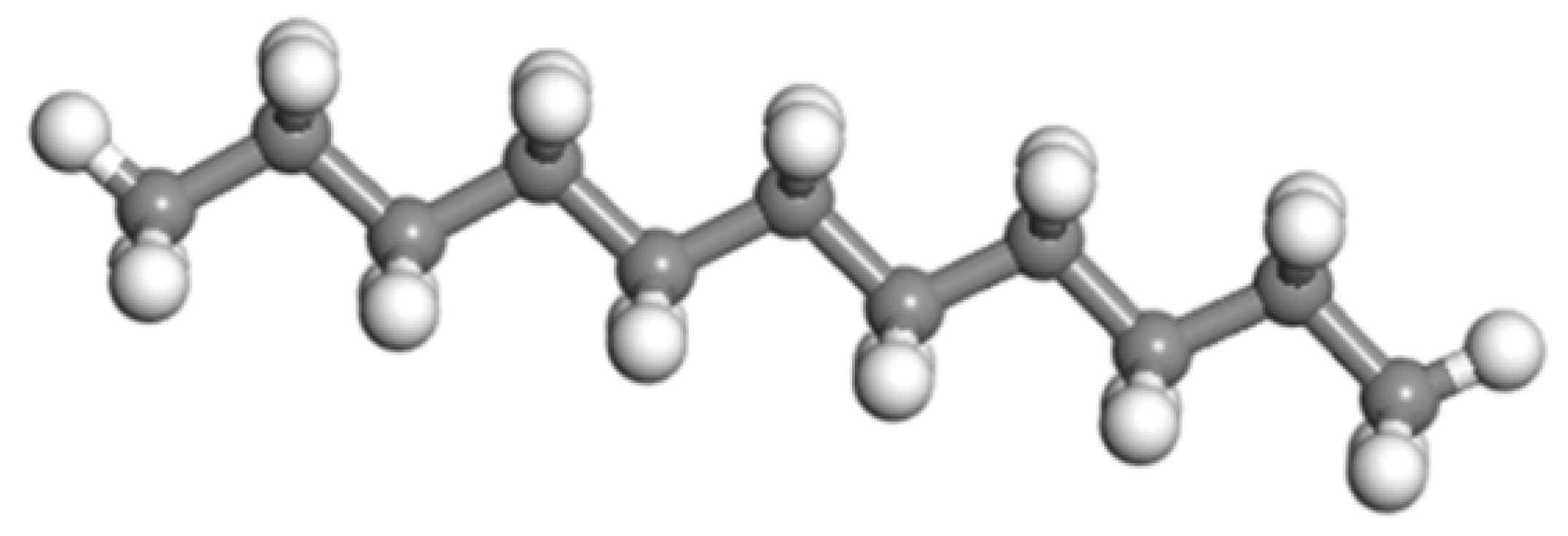
| Saturate | A1 | 422.9 | C30H62 | 4 |
| B1 | 483.0 | C35H62 | 4 | |
| Aromatic | A2 | 464.8 | C35H44 | 11 |
| B2 | 406.8 | C30H46 | 13 | |
| Resin | A3 | 554.0 | C40H59N | 4 |
| B3 | 573.1 | C40H60S | 4 | |
| C3 | 414.8 | C29H50O | 5 | |
| D3 | 530.9 | C36H57N | 4 | |
| E3 | 290.4 | C18H10S2 | 15 | |
| Asphaltene | AS1 | 575.0 | C42H54O | 3 |
| AS2 | 888.5 | C66H81N | 2 | |
| AS3 | 707.2 | C51H63S | 3 | |
| Oxide Type | Edge Length (Å) | Cross Angle (°) | ||||
|---|---|---|---|---|---|---|
| a | b | c | α | β | γ | |
| SiO2 | 4.98 | 4.98 | 6.95 | 90 | 90 | 90 |
| CaCO3 | 4.99 | 4.99 | 17.06 | 90 | 90 | 120 |
| Physical Properties | Unit | Value | Technical Requirements | Test Method (JTG F40-2004) |
|---|---|---|---|---|
| Softening Point | °C | 49.6 | >42 | T0606 |
| Penetration (25°) | 0.1 mm | 86.1 | 80–100 | T0604 |
| Ductility (15°) | mm | 153 | >100 | T0605 |
| Flashing point | °C | 345 | >245 | T0611 |
| Physical Properties | Unit | Value |
|---|---|---|
| Density | g/cm3 | 0.9 |
| Melting point | °C | 99 |
| Flash point | °C | 285 |
| Surface Free Energy Parameters | Water | Glycerol |
|---|---|---|
| 21.8 | 37.0 | |
| 51.0 | 26.4 | |
| γ | 72.8 | 63.4 |
| Sample | Density (g/cm3) | Reference Value (g/cm3) | Solubility Parameter (J/cm3)0.5 | Reference Value (J/cm3)0.5 |
|---|---|---|---|---|
| Matrix asphalt | 0.987 | 1.00–1.04 | 17.398 | 15.3–23.0 |
| WWMA-1 | 0.986 | 17.586 | ||
| WWMA-2 | 0.983 | 17.544 | ||
| WWMA-3 | 0.981 | 17.514 | ||
| WWMA-4 | 0.983 | 17.697 |
| Sample | limestone | Matrix Asphalt | WWMA-1 | WWMA-2 | WWMA-3 | WWMA-4 |
|---|---|---|---|---|---|---|
| Water | 61.71 | 99.44 | 97.09 | 96.01 | 94.31 | 97.67 |
| Glycerin | 73.02 | 97.33 | 96.95 | 96.85 | 95.79 | 96.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Yang, H.; You, Z.; Ma, H.; Xu, F.; You, L.; Diab, A.; Lu, L.; Hu, Y.; Liu, Y.; et al. Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation. Materials 2022, 15, 5930. https://doi.org/10.3390/ma15175930
Peng C, Yang H, You Z, Ma H, Xu F, You L, Diab A, Lu L, Hu Y, Liu Y, et al. Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation. Materials. 2022; 15(17):5930. https://doi.org/10.3390/ma15175930
Chicago/Turabian StylePeng, Chao, Hanneng Yang, Zhanping You, Hongchao Ma, Fang Xu, Lingyun You, Aboelkasim Diab, Li Lu, Yudong Hu, Yafeng Liu, and et al. 2022. "Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation" Materials 15, no. 17: 5930. https://doi.org/10.3390/ma15175930
APA StylePeng, C., Yang, H., You, Z., Ma, H., Xu, F., You, L., Diab, A., Lu, L., Hu, Y., Liu, Y., Dai, J., & Li, Z. (2022). Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation. Materials, 15(17), 5930. https://doi.org/10.3390/ma15175930











