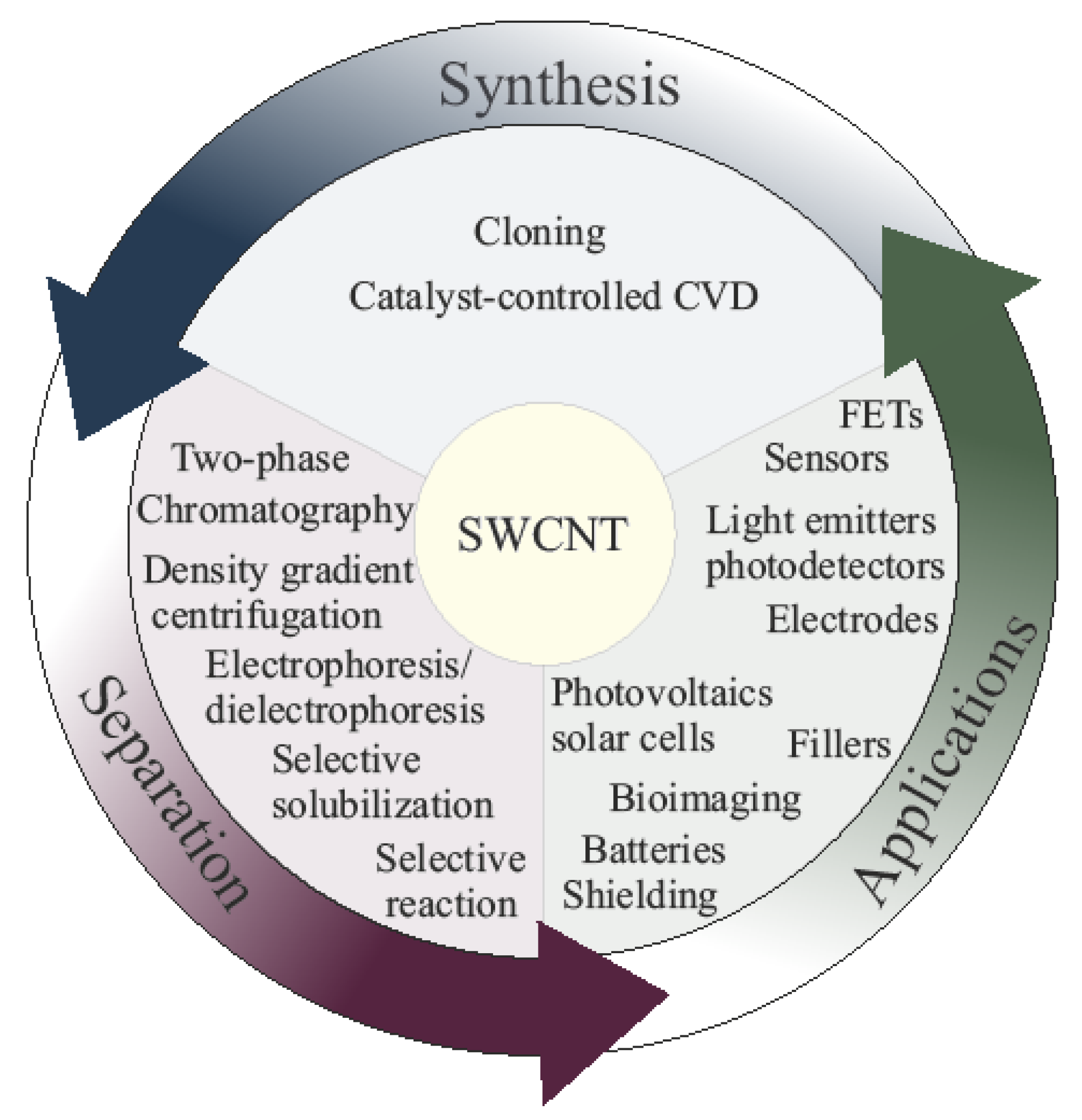
Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Synthesis Methods of Semiconducting, Metallic, and Single-Chirality SWCNTs
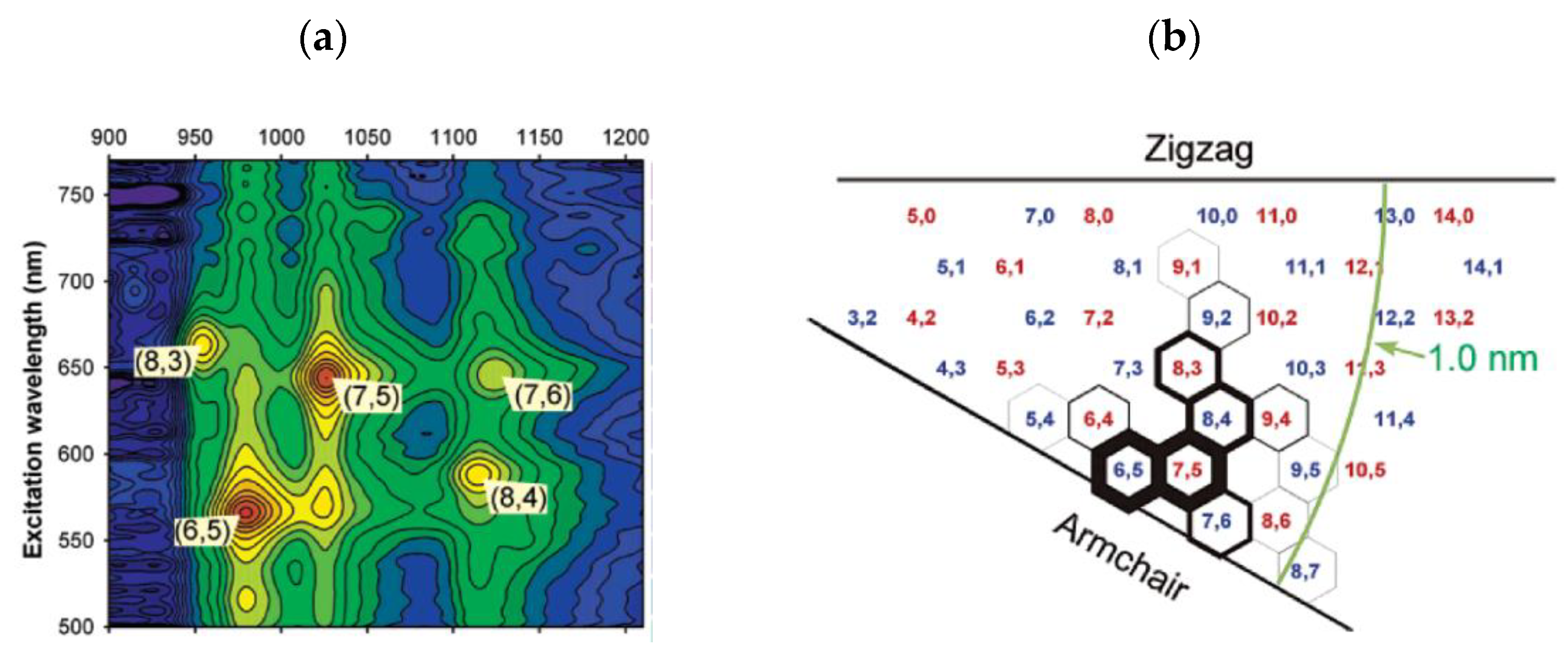
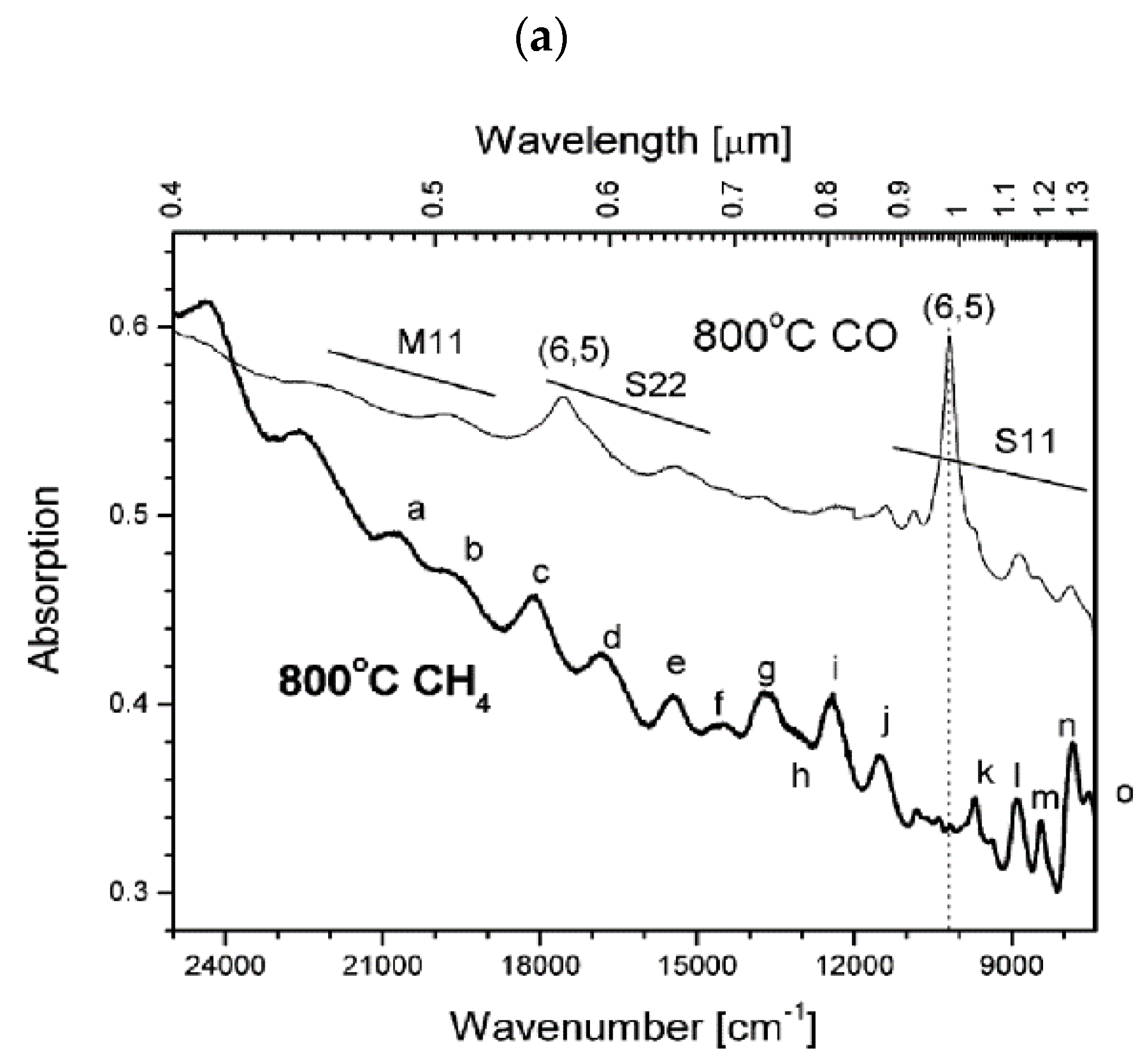
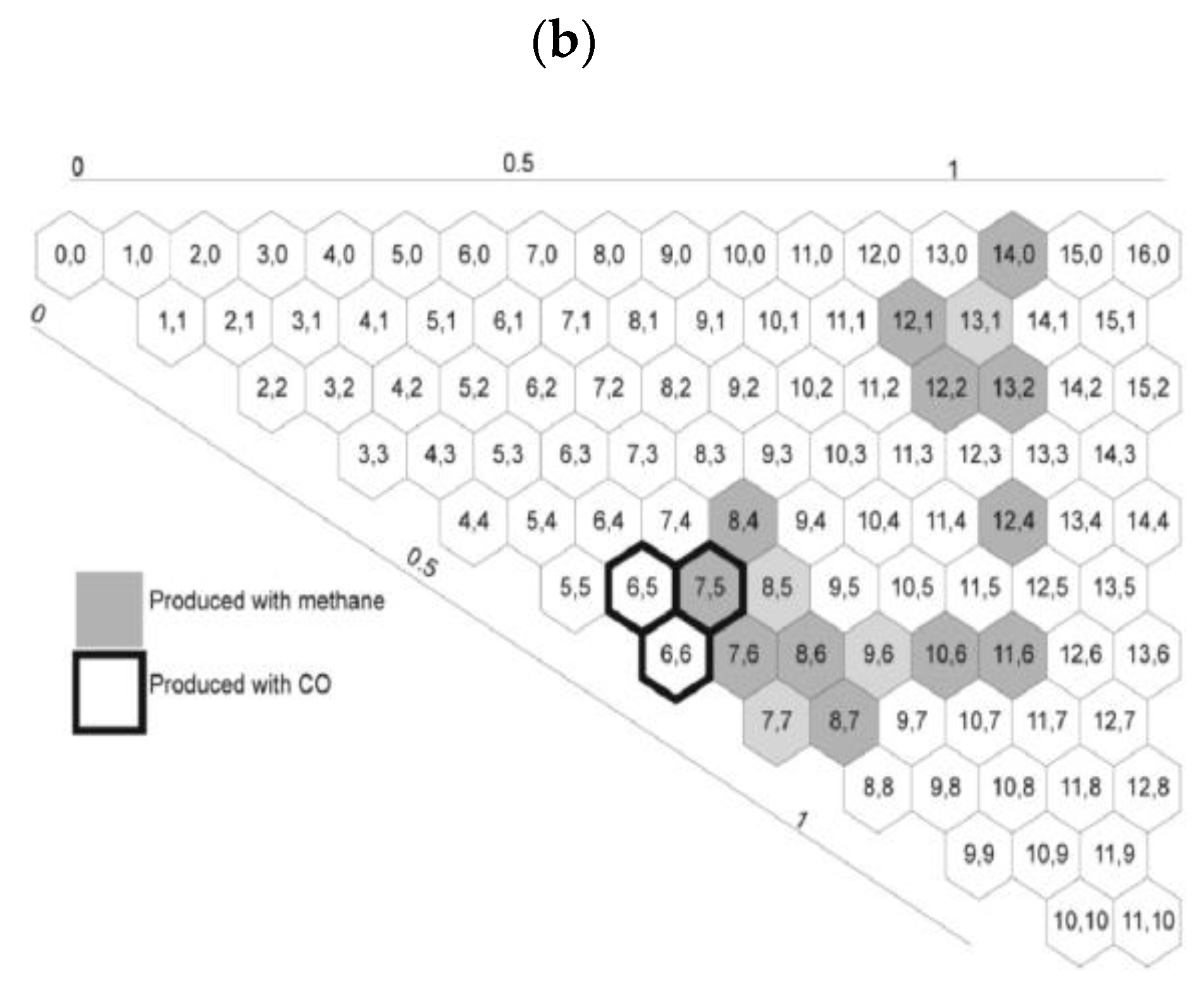
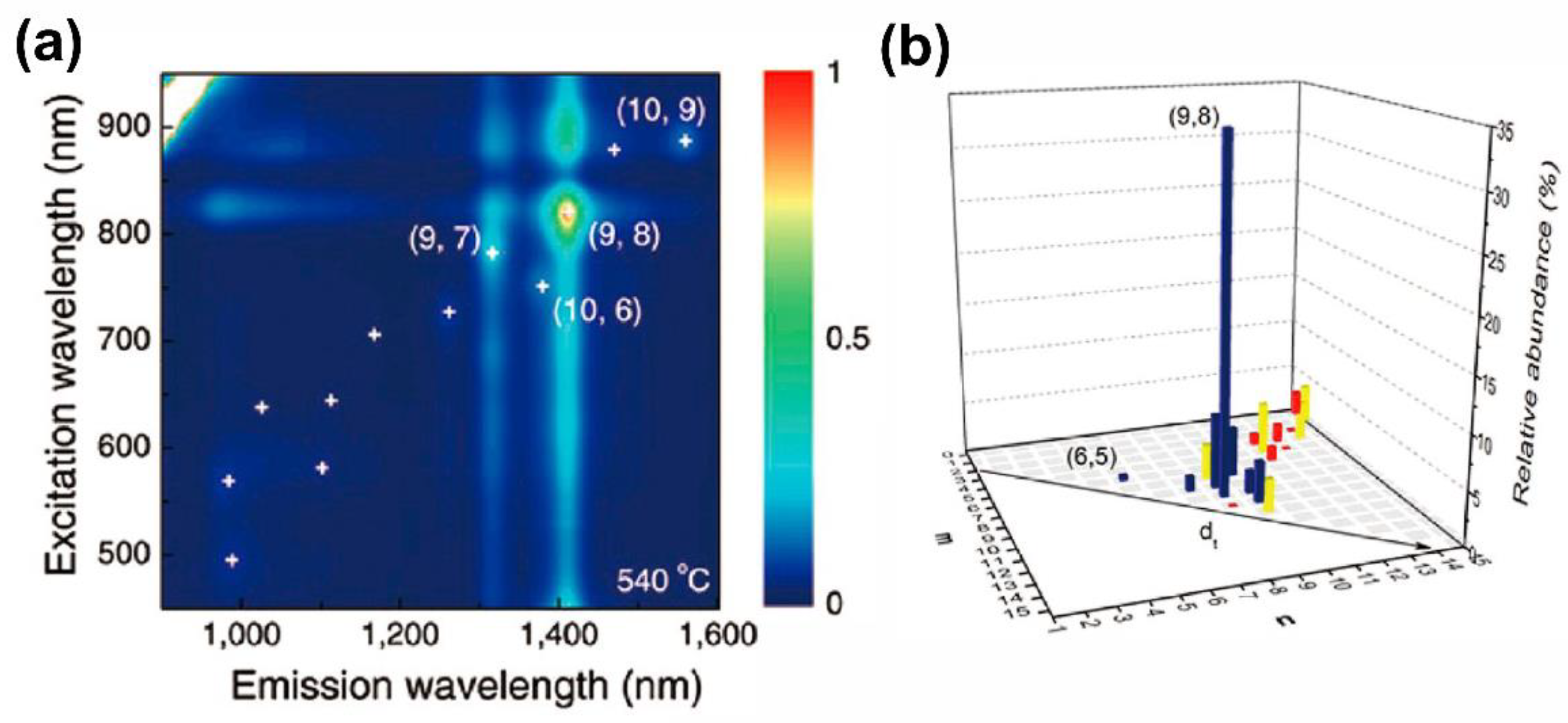
2.1. Catalyst-Controlled CVD Synthesis of Single-Chirality SWCNTs
2.2. The Epitaxial Growth Method of SWCNT Using Nanocarbon Seeds

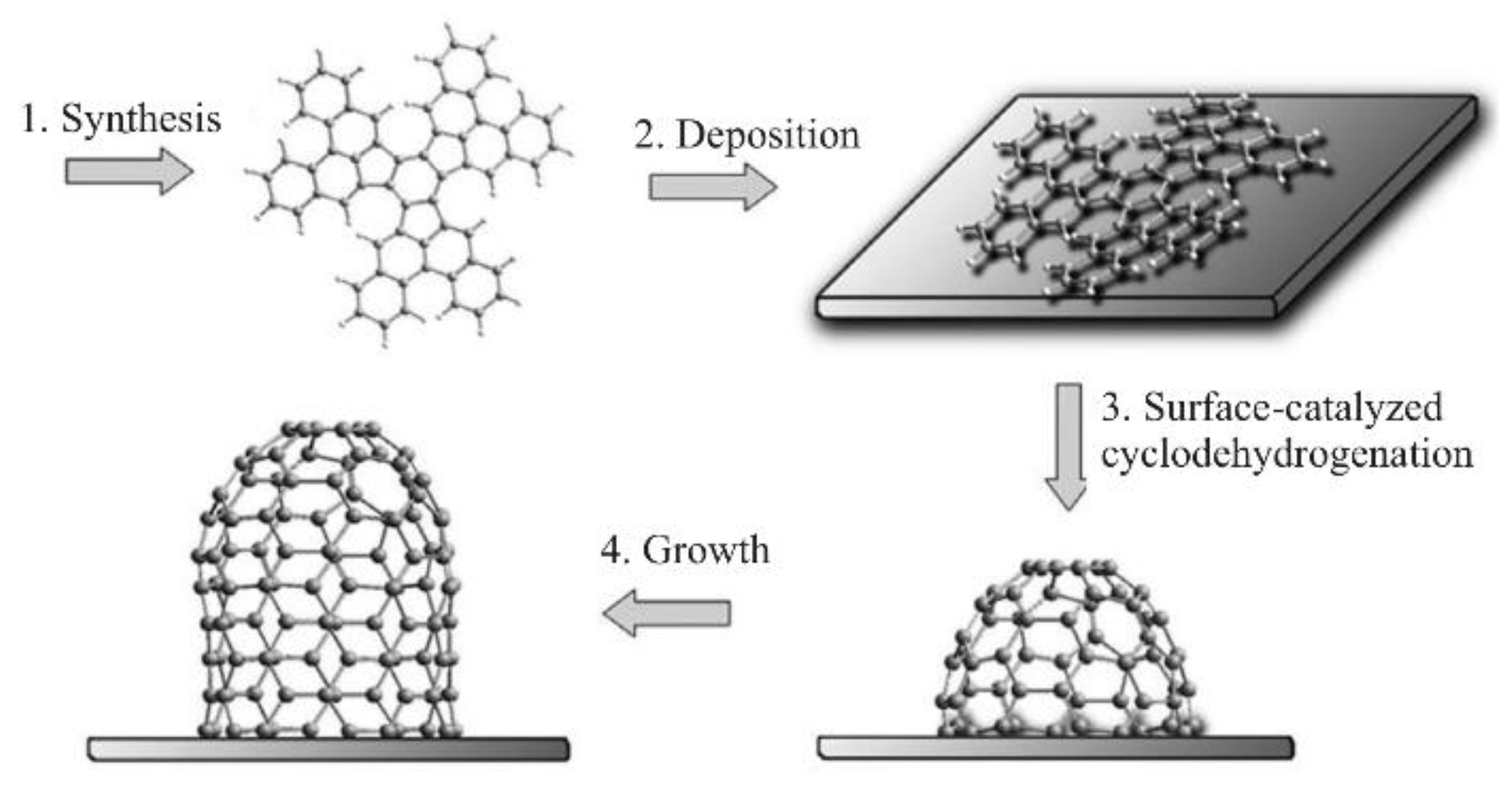
2.3. The Growth Method Using Nanocarbon Segments Obtained by Organic Synthesis
3. Separation of SWCNT
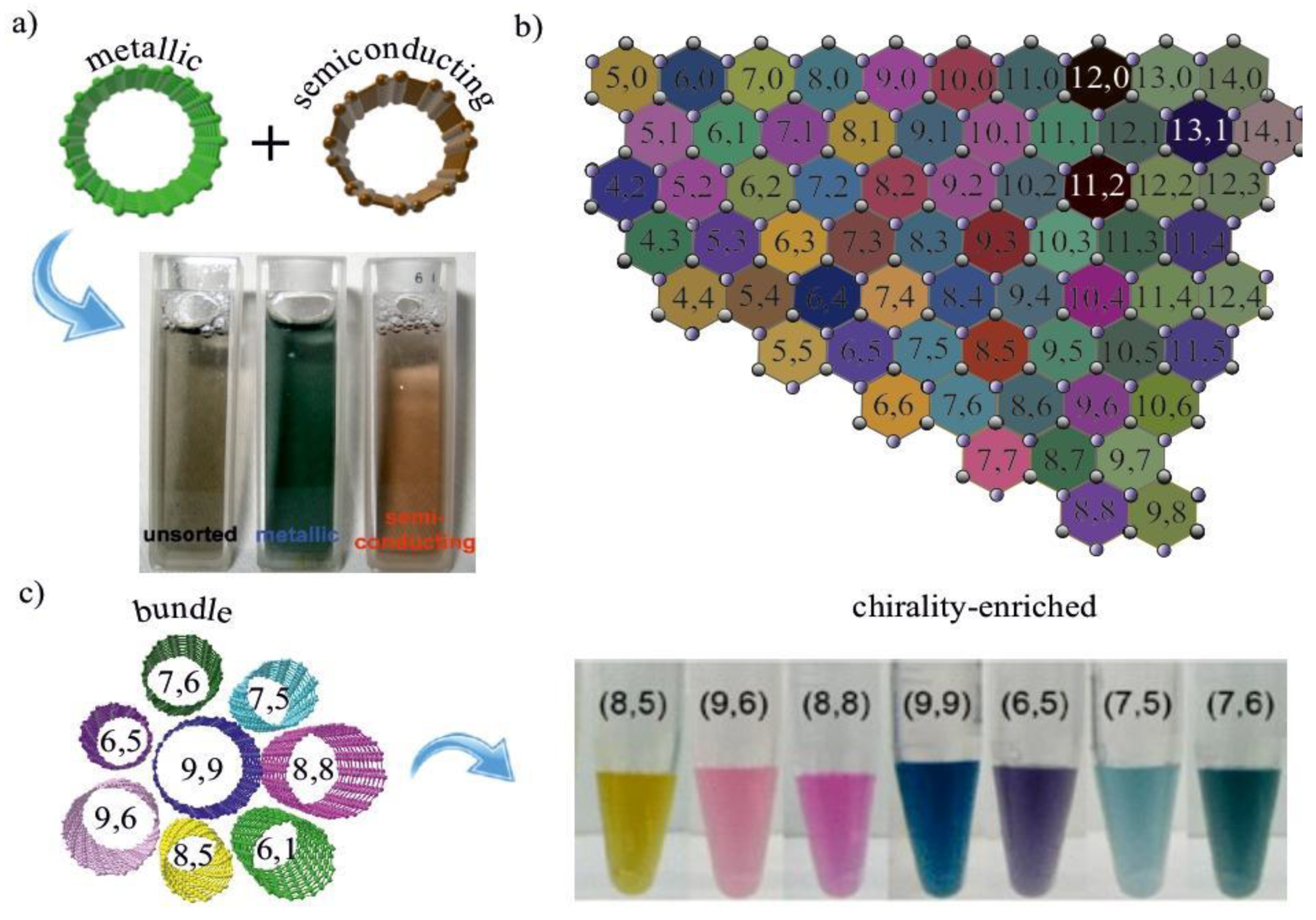
3.1. Separation by Electronic Type
3.1.1. Electrophoresis/Dielectrophoresis
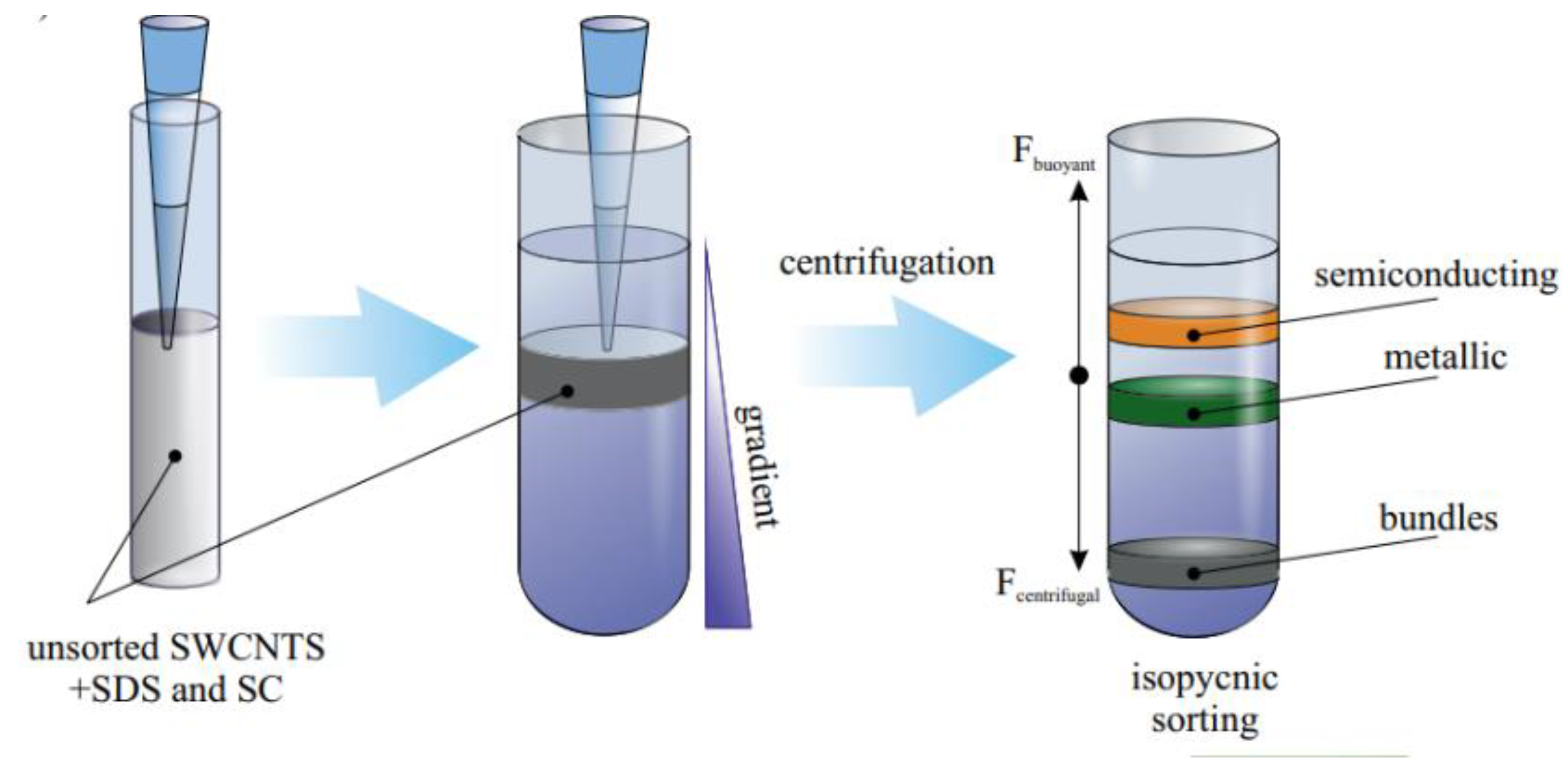
3.1.2. Density Gradient Centrifugation (DGC)
3.1.3. Low-Speed DGC
3.1.4. Ultrahigh DGC
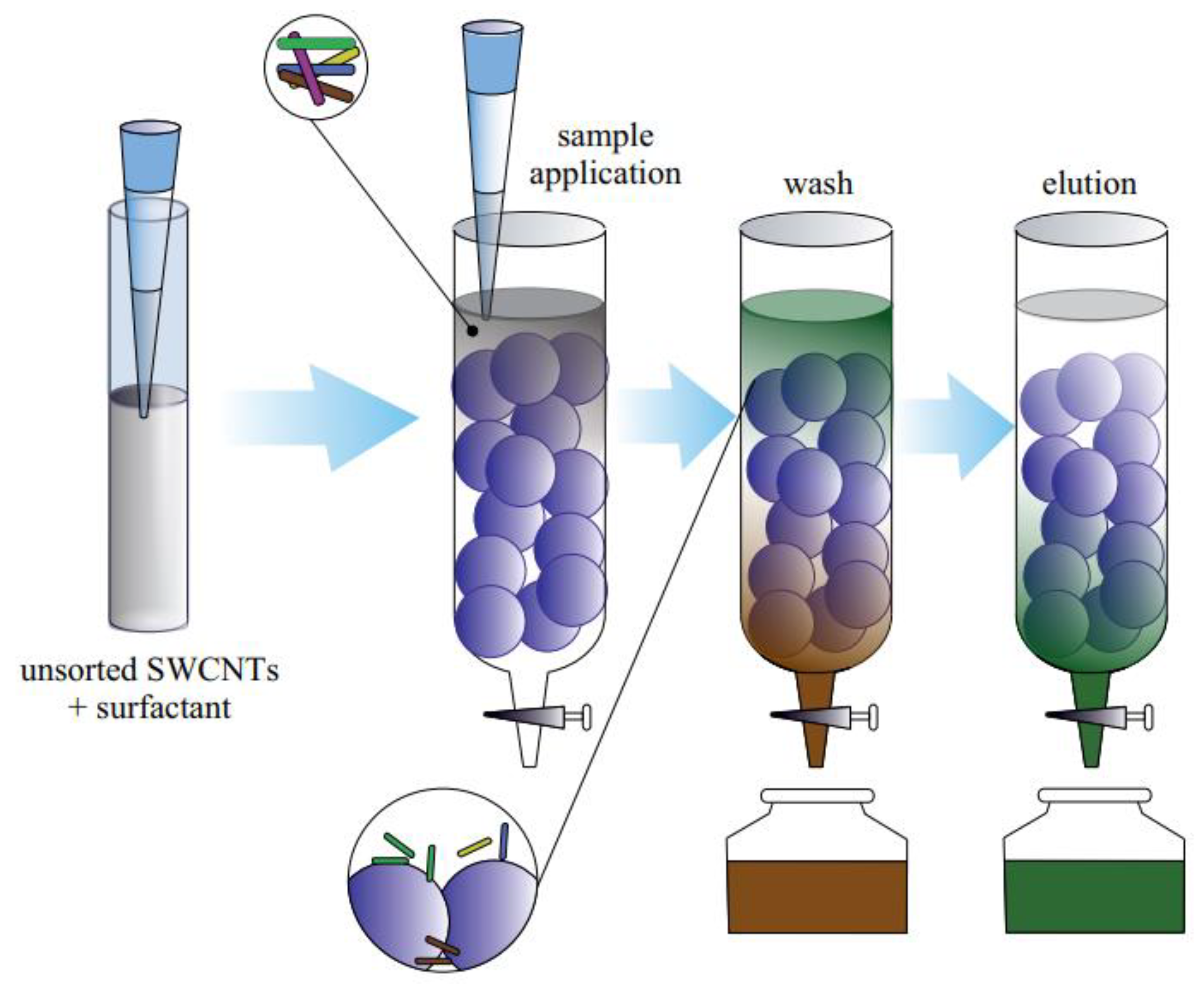
3.1.5. Chromatography
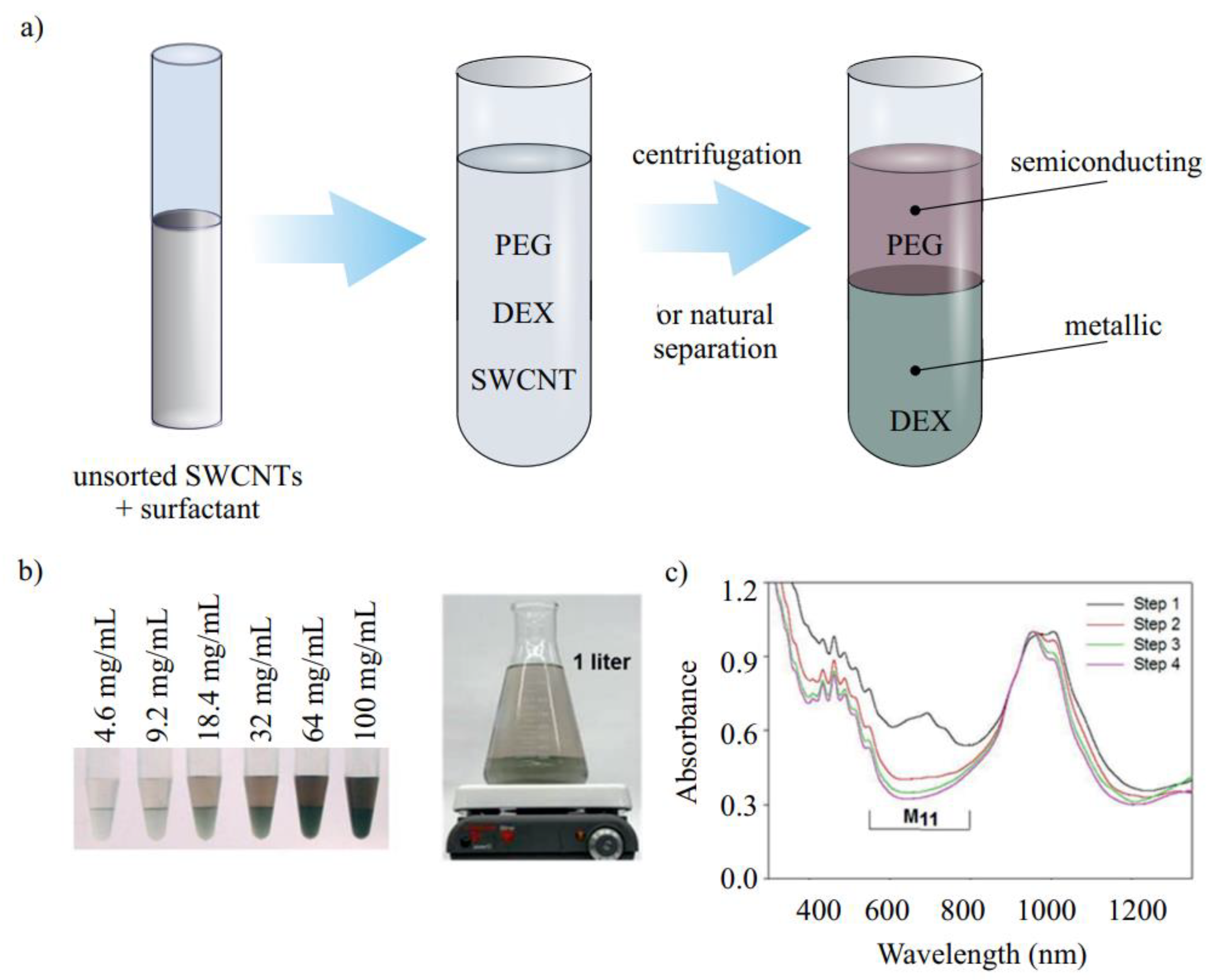
3.1.6. Two-Phase Separation
3.1.7. Selective Solubilization and Selective Reaction
3.2. Separation by Single Chirality
3.2.1. Density Gradient Centrifugation (DGC)
3.2.2. Chromatography
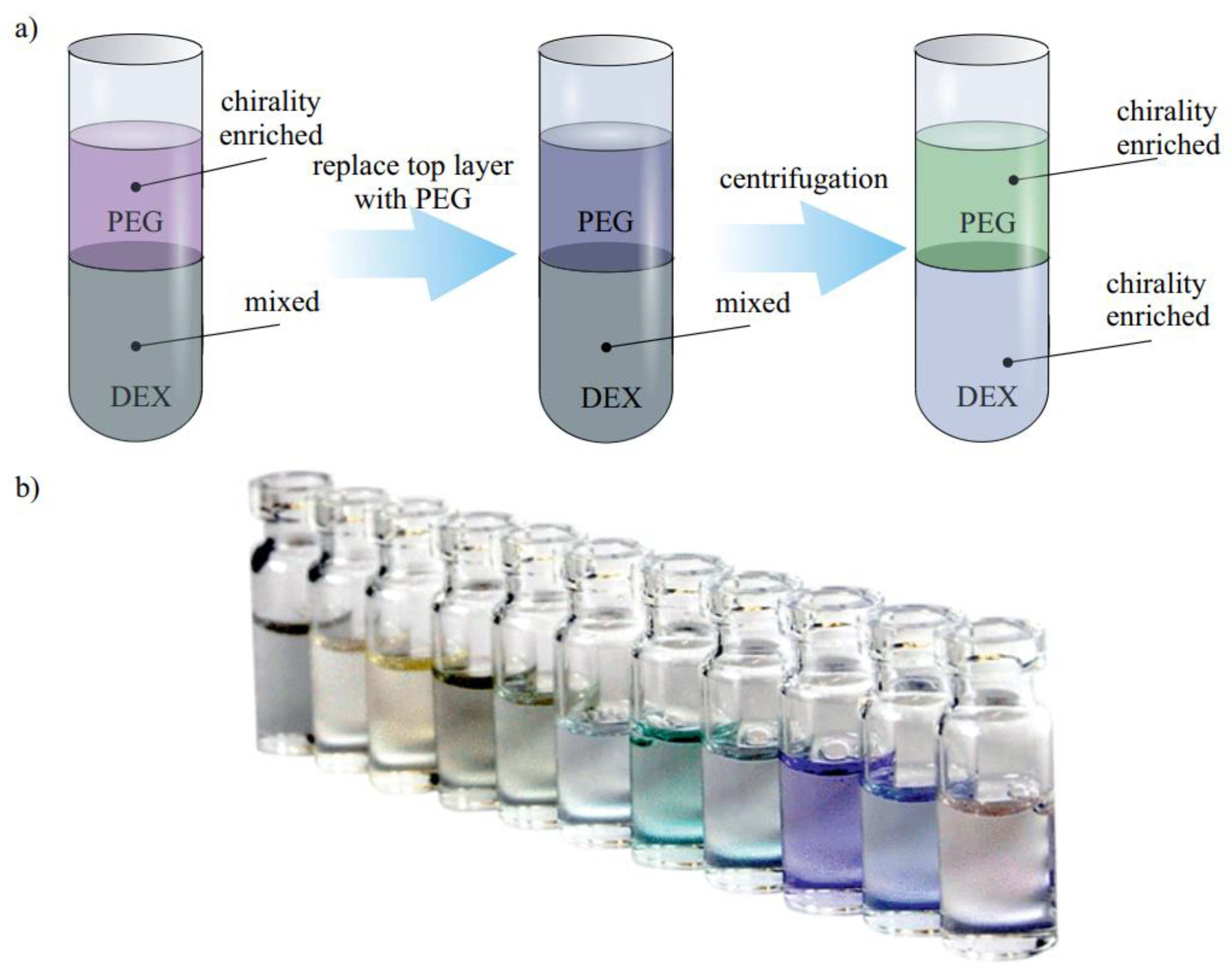
3.2.3. Two-Phase Separation
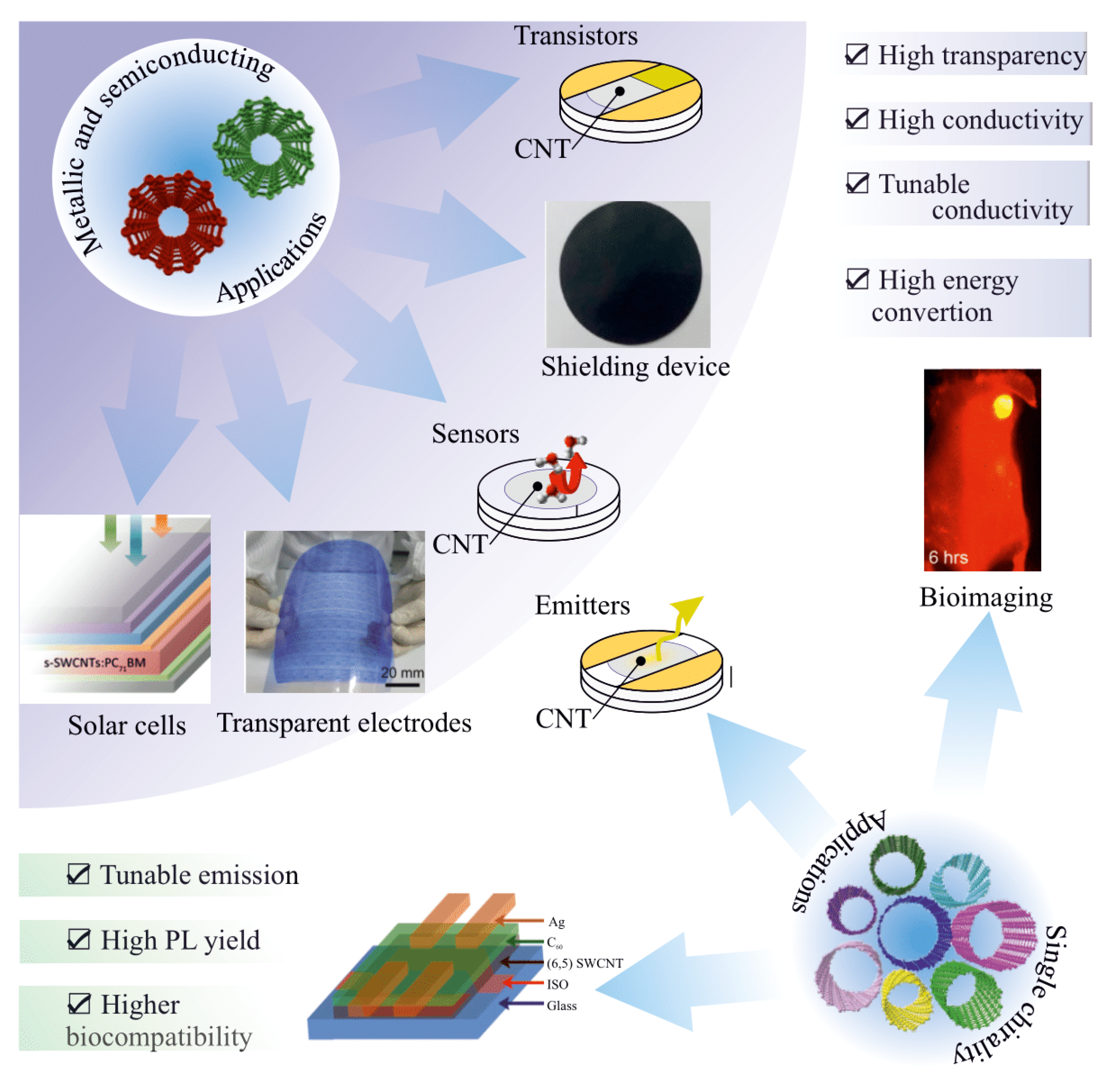
4. Applications of Type and Chirality-Separated SWCNT
4.1. Field Effect Transistors (FETs)
4.2. Sensors
4.3. Light Emitters and Photodetectors
4.4. Transparent Electrodes
4.5. Photovoltaics/Solar Cells
4.6. Batteries
4.7. Neuromorphic Engineering. Quantum Information Processing. Interconnections for Electronic Circuits
4.8. Bioimaging
4.9. Conductive Fillers for Composites. Shielding Devices
5. Remaining Challenges and Future Outlook
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| CNT | carbon nanotube |
| CPP | cyclo(para-phenelene) |
| CVD | chemical vapor deposition |
| DEP | dielectrophoresis |
| DEX | dextran |
| DFT | density functional theory |
| DGC | density gradient centrifugation |
| DGU | density gradient ultracentrifugation |
| DNA | deoxyribonucleic acid |
| DOC | sodium deoxycholate |
| DWCNT | double-walled carbon nanotube |
| EMI SH EF | electromagnetic interference shielding effectiveness |
| F-SWCNT | fluorinated single-walled carbon nanotube |
| FET | field-effect transistor |
| FTIR | Fourier-transform infrared spectra |
| GNR | graphene nanoribbon |
| HRTEM | high-resolution TEM |
| IEC | anion exchange chromatography |
| m-/s-SWCNT | metallic/semiconducting SWCNT |
| MWCNT | multiwalled carbon nanotube |
| PAM | polyacrylamine |
| PEG | polyethylene glycol |
| PEG-DA | polyethylene glycol diamine |
| PDMS | polydimethylsiloxane |
| PF8-DPP | poly(9,9-dioctylfluorene) |
| PNA | peptide nucleic acid |
| PVP | polyvinylpyrrolidone |
| SDS | sodium dodecyl sulfate |
| SEC | size-exclusion chromatography |
| STEM | scanning transmission electron microscopy |
| STM | scanning tunneling microscopy |
| SWCNT | single-walled carbon nanotube |
| TEM | transmission electron microscopy |
| TUD-1 | mesoporous SiO2 |
| UV–VIS–NIR | ultraviolet–visible–near-infrared |
| XPS | X-ray photoelectron spectra |
References
- Saito, R.; Dresselhaus, G.; Dresselhaus, M. Physical Properties of Carbon Nanotubes—G Dresselhaus, Mildred S Dresselhaus, Riichiro Saito—Google Libros. 1998. Available online: https://books.google.com.ec/books?hl=es&lr=&id=fv63CgAAQBAJ&oi=fnd&pg=PR5&dq=Dresselhaus,+G.,+%26+Riichiro,+S.+(1998).+Physical+properties+of+carbon+nanotubes&ots=sftOPMiL2s&sig=gAvbxmmvaKwdkn1vybGaXOAeOIE#v=onepage&q=Dresselhaus%2C%20G.%2C%20%26%20Riichiro%2C%20S.%20(1998).%20Physical%20properties%20of%20carbon%20nanotubes&f=false (accessed on 22 March 2022).
- Dai, H. Nanotube Growth and Characterization. In Carbon Nanotubes; Springer: Berlin, Germany, 2001; pp. 29–53. [Google Scholar] [CrossRef]
- Joselevich, E.; Dai, H.; Liu, J.; Hata, K.; Windle, A.H. Carbon Nanotube Synthesis and Organization. Top. Appl. Phys. 2007, 111, 101–165. [Google Scholar] [CrossRef]
- Dai, H.; Rinzler, A.G.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Single-wall nanotubes produced by metal-catalyzed disproportionation of carbon monoxide. Chem. Phys. Lett. 1996, 260, 471–475. [Google Scholar] [CrossRef]
- Catalytic Growth of Single-Wall Carbon Nanotubes from Metal Particles|Health & Environmental Research Online (HERO)|US EPA. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/1658370 (accessed on 22 March 2022).
- Lee, Y.H.; Kim, S.G.; Tománek, D. Catalytic Growth of Single-Wall Carbon Nanotubes: An Ab Initio Study. Phys. Rev. Lett. 1997, 78, 2393–2396. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.M.; Dai, H. Chemical vapor deposition of methane for single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Cassell, A.M.; Raymakers, J.A.; Kong, J.; Dai, H. Large Scale CVD Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492. [Google Scholar] [CrossRef]
- Flahaut, E.; Govindaraj, A.; Peigney, A.; Laurent, C.; Rousset, A.; Rao, C. Synthesis of single-walled carbon nanotubes using binary (Fe, Co, Ni) alloy nanoparticles prepared in situ by the reduction of oxide solid solutions. Chem. Phys. Lett. 1999, 300, 236–242. [Google Scholar] [CrossRef]
- Kitiyanan, B.; Alvarez, W.; Harwell, J.; Resasco, D. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co–Mo catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Colomer, J.-F.; Stephan, C.; Lefrant, S.; Van Tendeloo, G.; Willems, I.; Kónya, Z.; Fonseca, A.; Laurent, C.; Nagy, J. Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem. Phys. Lett. 2000, 317, 83–89. [Google Scholar] [CrossRef]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Chen, Y.; Lau, R.; Yang, Y. Preparation of large particle MCM-41 and investigation on its fluidization behavior and application in single-walled carbon nanotube production in a fluidized-bed reactor. Chem. Eng. J. 2008, 142, 331–336. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Y. New technique of synthesizing single-walled carbon nanotubes from ethanol using fluidized-bed over Fe–Mo/MgO catalyst. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Kinloch, I.A.; Shaffer, M.S.; Geng, J.; Johnson, B.; Windle, A.H. Synthesis of single-walled carbon nanotubes by a fluidized-bed method. Chem. Phys. Lett. 2004, 384, 98–102. [Google Scholar] [CrossRef]
- Kim, D.Y.; Sugime, H.; Hasegawa, K.; Osawa, T.; Noda, S. Fluidized-bed synthesis of sub-millimeter-long single walled carbon nanotube arrays. Carbon 2011, 50, 1538–1545. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Zhang, Q.; Huang, J.-Q.; Nie, J.-Q.; Wei, F. Layered double hydroxides as catalysts for the efficient growth of high quality single-walled carbon nanotubes in a fluidized bed reactor. Carbon 2010, 48, 3260–3270. [Google Scholar] [CrossRef]
- Kumar, A.; Parveen, S.; Husain, S.; Ali, J.; Zulfequar, M.; Harsh; Husain, M. A comparative study of nitrogen plasma effect on field emission characteristics of single wall carbon nanotubes synthesized by plasma enhanced chemical vapor deposition. Appl. Surf. Sci. 2014, 322, 236–241. [Google Scholar] [CrossRef]
- Ghorannevis, Z.; Kato, T.; Kaneko, T.; Hatakeyama, R. Growth of Single-Walled Carbon Nanotubes from Nonmagnetic Catalysts by Plasma Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2010, 49, 02BA01. [Google Scholar] [CrossRef]
- Qu, L.; Du, F.; Dai, L. Preferential Syntheses of Semiconducting Vertically Aligned Single-Walled Carbon Nanotubes for Direct Use in FETs. Nano Lett. 2008, 8, 2682–2687. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.; Xu, Z.; Liu, S.; Wang, E. Low temperature growth of single-walled carbon nanotubes: Small diameters with narrow distribution. Chem. Phys. Lett. 2006, 419, 81–85. [Google Scholar] [CrossRef]
- Zhang, G.; Mann, D.; Zhang, L.; Javey, A.; Li, Y.; Yenilmez, E.; Wang, Q.; McVittie, J.P.; Nishi, Y.; Gibbons, J.; et al. Ultra-high-yield growth of vertical single-walled carbon nanotubes: Hidden roles of hydrogen and oxygen. Proc. Natl. Acad. Sci. USA 2005, 102, 16141–16145. [Google Scholar] [CrossRef]
- Bae, E.J.; Min, Y.-S.; Kang, D.; Ko, J.-H.; Park, W. Low-Temperature Growth of Single-Walled Carbon Nanotubes by Plasma Enhanced Chemical Vapor Deposition. Chem. Mater. 2005, 17, 5141–5145. [Google Scholar] [CrossRef]
- Li, Y.; Mann, D.; Rolandi, M.; Kim, W.; Ural, A.; Hung, S.; Javey, A.; Cao, J.; Wang, D.; Yenilmez, E.; et al. Preferential Growth of Semiconducting Single-Walled Carbon Nanotubes by a Plasma Enhanced CVD Method. Nano Lett. 2004, 4, 317–321. [Google Scholar] [CrossRef]
- Kato, T.; Jeong, G.-H.; Hirata, T.; Hatakeyama, R.; Tohji, K.; Motomiya, K. Single-walled carbon nanotubes produced by plasma-enhanced chemical vapor deposition. Chem. Phys. Lett. 2003, 381, 422–426. [Google Scholar] [CrossRef]
- Toni, F.; Xing, H.; Walter, J.; Strauß, V.; Nacken, T.J.; Damm, C.; Wirth, K.-E.; Guldi, D.; Peukert, W. Production of well dispersible single walled carbon nanotubes via a “floating catalyst”-method. Chem. Eng. Sci. 2015, 138, 385–395. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, W.; Chen, Z.-G.; Wang, D.-W.; Liu, B.; Yu, B.; Li, F.; Cong, H.; Cheng, H.-M. Diameter-Selective Growth of Single-Walled Carbon Nanotubes with High Quality by Floating Catalyst Method. ACS Nano 2008, 2, 1722–1728. [Google Scholar] [CrossRef]
- Bhowmick, R.; Clemens, B.M.; Cruden, B.A. Parametric analysis of chirality families and diameter distributions in single-wall carbon nanotube production by the floating catalyst method. Carbon 2008, 46, 907–922. [Google Scholar] [CrossRef]
- Afolabi, A.; Abdulkareem, A.; Iyuke, S.; Pienaar, H.V.Z. Continuous production of carbon nanotubes and diamond films by swirled floating catalyst chemical vapour deposition method. S. Afr. J. Sci. 2010, 105, 278–281. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Xu, X.; Zhang, L.; Huang, S. Controlling the Diameter of Single-Walled Carbon Nanotubes by Improving the Dispersion of the Uniform Catalyst Nanoparticles on Substrate. Nano-Micro Lett. 2015, 7, 353–359. [Google Scholar] [CrossRef][Green Version]
- Song, L.; Ci, L.J.; Sun, L.F.; Jin, C.; Liu, L.; Ma, W.; Liu, D.; Zhao, X.; Luo, S.; Zhang, Z.; et al. Large-Scale Synthesis of Rings of Bundled Single-Walled Carbon Nanotubes by Floating Chemical Vapor Deposition. Adv. Mater. 2006, 18, 1817–1821. [Google Scholar] [CrossRef]
- Huang, S.; Cai, X.; Du, C.; Liu, J. Oriented Long Single Walled Carbon Nanotubes on Substrates from Floating Catalysts. J. Phys. Chem. B 2003, 107, 13251–13254. [Google Scholar] [CrossRef]
- Ci, L.; Xie, S.; Tang, D.; Yan, X.; Li, Y.; Liu, Z.; Zou, X.; Zhou, W.; Wang, G. Controllable growth of single wall carbon nanotubes by pyrolizing acetylene on the floating iron catalysts. Chem. Phys. Lett. 2001, 349, 191–195. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Balzano, L.; Herrera, J.E.; Pompeo, F.; Resasco, D.E.; Weisman, R.B. Narrow (n,m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 2003, 125, 11186–11187. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Y.; Li, L.J.; Chen, Y. Effect of different catalyst supports on the (n,m) selective growth of single-walled carbon nanotube from Co-Mo catalyst. J. Mater. Sci. 2009, 44, 3285–3295. [Google Scholar] [CrossRef]
- Wang, B.; Wei, L.; Yao, L.; Li, L.J.; Yang, Y.; Chen, Y. Pressure-induced single-walled carbon nanotube (n,m) selectivity on Co-Mo catalysts. J. Phys. Chem. C 2007, 111, 14612–14616. [Google Scholar] [CrossRef]
- Wang, B.; Poa, C.H.P.; Wei, L.; Li, L.-J.; Yang, Y.; Chen, Y. (n,m) Selectivity of Single-Walled Carbon Nanotubes by Different Carbon Precursors on Co−Mo Catalysts. J. Am. Chem. Soc. 2007, 129, 9014–9019. [Google Scholar] [CrossRef] [PubMed]
- Lolli, G.; Zhang, L.; Balzano, L.; Sakulchaicharoen, N.; Tan, A.Y.; Resasco, D.E. Tailoring (n,m) Structure of Single-Walled Carbon Nanotubes by Modifying Reaction Conditions and the Nature of the Support of CoMo Catalysts. J. Phys. Chem. B 2006, 110, 2108–2115. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Chiashi, S.; Murakami, Y.; Hayashida, Y.; Maruyama, S. Fluorescence spectroscopy of single-walled carbon nanotubes synthesized from alcohol. Chem. Phys. Lett. 2004, 387, 198–203. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Sankaran, R.M. Linking catalyst composition to chirality distributions of as-grown single-walled carbon nanotubes by tuning NixFe1−x nanoparticles. Nat. Mater. 2009, 8, 882–886. [Google Scholar] [CrossRef]
- He, M.; Chernov, A.I.; Fedotov, P.V.; Obraztsova, E.D.; Rikkinen, E.; Zhu, Z.; Sainio, J.; Jiang, H.; Nasibulin, A.G.; Kauppinen, E.I.; et al. Selective growth of SWNTs on partially reduced monometallic cobalt catalyst. Chem. Commun. 2010, 47, 1219–1221. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Quek, X.-Y.; Wei, L.; Zhao, J.; Li, L.-J.; Chan-Park, M.B.; Yang, Y.; Chen, Y. Selective Synthesis of (9,8) Single Walled Carbon Nanotubes on Cobalt Incorporated TUD-1 Catalysts. J. Am. Chem. Soc. 2010, 132, 16747–16749. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Ren, F.; Haller, G.L.; Pfefferle, L.D. Diameter Tuning of Single-Walled Carbon Nanotubes with Reaction Temperature Using a Co Monometallic Catalyst. J. Phys. Chem. C 2009, 113, 10070–10078. [Google Scholar] [CrossRef]
- He, M.; Chernov, A.I.; Fedotov, P.V.; Obraztsova, E.D.; Sainio, J.; Rikkinen, E.; Jiang, H.; Zhu, Z.; Tian, Y.; Kauppinen, E.I.; et al. Predominant (6,5) Single-Walled Carbon Nanotube Growth on a Copper-Promoted Iron Catalyst. J. Am. Chem. Soc. 2010, 132, 13994–13996. [Google Scholar] [CrossRef] [PubMed]
- Ghorannevis, Z.; Kato, T.; Kaneko, T.; Hatakeyama, R. Narrow-Chirality Distributed Single-Walled Carbon Nanotube Growth from Nonmagnetic Catalyst. J. Am. Chem. Soc. 2010, 132, 9570–9572. [Google Scholar] [CrossRef] [PubMed]
- Loebick, C.Z.; Podila, R.; Reppert, J.; Chudow, J.; Ren, F.; Haller, G.L.; Rao, A.M.; Pfefferle, L.D. Selective Synthesis of Subnanometer Diameter Semiconducting Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2010, 132, 11125–11131. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chernov, A.I.; Obraztsova, E.D.; Sainio, J.; Rikkinen, E.; Jiang, H.; Zhu, Z.; Kaskela, A.; Nasibulin, A.G.; Kauppinen, E.I.; et al. Low temperature growth of SWNTs on a nickel catalyst by thermal chemical vapor deposition. Nano Res. 2010, 4, 334–342. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, H.; Susi, T.; Nasibulin, A.G.; Kauppinen, E.I. The use of NH3 to promote the production of large-diameter single-walled carbon nanotubes with a narrow (n,m) distribution. J. Am. Chem. Soc. 2011, 133, 1224–1227. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, Z.; Hu, Y.; Ding, F.; Zhang, J. Chemical vapor deposition synthesis of near-zigzag single-walled carbon nanotubes with stable tube-catalyst interface. Sci. Adv. 2016, 2, e1501729. [Google Scholar] [CrossRef]
- Liu, B.; Ren, W.; Li, S.; Liu, C.; Cheng, H.-M. High temperature selective growth of single-walled carbon nanotubes with a narrow chirality distribution from a CoPt bimetallic catalyst. Chem. Commun. 2012, 48, 2409–2411. [Google Scholar] [CrossRef]
- He, M.; Jiang, H.; Liu, B.; Fedotov, P.V.; Chernov, A.I.; Obraztsova, E.D.; Cavalca, F.; Wagner, J.B.; Hansen, T.W.; Anoshkin, I.V.; et al. Chiral-Selective Growth of Single-Walled Carbon Nanotubes on Lattice-Mismatched Epitaxial Cobalt Nanoparticles. Sci. Rep. 2013, 3, srep01460. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.-Q.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Qi, K.; Yang, J.; Xu, Z.; Li, M.; Zhao, X.; Bai, X.; Li, Y. Growing Zigzag (16,0) Carbon Nanotubes with Structure-Defined Catalysts. J. Am. Chem. Soc. 2015, 137, 8688–8691. [Google Scholar] [CrossRef]
- Yang, F. Water-Assisted Preparation of High-Purity Semiconducting (14,4) Carbon Nanotubes. ACS Nano 2017, 11, 186–193. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, L.; Hu, Y.; Kang, L.; Zhang, J. Diameter-Specific Growth of Semiconducting SWNT Arrays Using Uniform Mo2C Solid Catalyst. J. Am. Chem. Soc. 2015, 137, 8904–8907. [Google Scholar] [CrossRef]
- Sanchez-Valencia, J.R.; Dienel, T.; Gröning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.Y.; Ruffieux, P.; Fasel, R. Controlled synthesis of single-chirality carbon nanotubes. Nature 2014, 512, 61–64. [Google Scholar] [CrossRef]
- Li, X.; Tu, X.; Zaric, S.; Welsher, K.; Seo, W.S.; Zhao, W.; Dai, H. Selective Synthesis Combined with Chemical Separation of Single-Walled Carbon Nanotubes for Chirality Selection. J. Am. Chem. Soc. 2007, 129, 15770–15771. [Google Scholar] [CrossRef]
- Wang, H. Chiral-selective CoSo4/SiO2 catalyst for (9,8) single-walled carbon nanotube growth. ACS Nano 2013, 7, 614–626. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, C.; Zhang, J.; Liu, Z. Cloning of single-walled carbon nanotubes via open-end growth mechanism. Nano Lett. 2009, 9, 1673–1677. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Tu, X.; Liu, B.; Chen, L.; Zheng, M.; Zhou, C. Chirality-controlled synthesis of single-wall carbon nanotubes using vapour-phase epitaxy. Nat. Commun. 2012, 3, 1199. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Tu, X.; Zhang, J.; Zheng, M.; Zhou, C. Chirality-Dependent Vapor-Phase Epitaxial Growth and Termination of Single-Wall Carbon Nanotubes. Nano Lett. 2013, 13, 4416–4421. [Google Scholar] [CrossRef]
- Rao, F.; Li, T.; Wang, Y. Growth of “all-carbon” single-walled carbon nanotubes from diamonds and fullerenes. Carbon 2009, 47, 3580–3584. [Google Scholar] [CrossRef]
- Takagi, D.; Kobayashi, Y.; Homma, Y. Carbon nanotube growth from diamond. J. Am. Chem. Soc. 2009, 131, 6922–6923. [Google Scholar] [CrossRef]
- Terrones, M.; Terrones, G.; Terrones, H. Structure, Chirality, and Formation of Giant Icosahedral Fullerenes and Spherical Graphitic Onions. In Science of Crystal Structures: Highlights in Crystallography; Springer: Berlin, Germany, 2015; pp. 101–112. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Choi, W.; Choi, J.-Y.; Kim, J.M.; Gan, L.; Liu, Z. Cap Formation Engineering: From Opened C60 to Single-Walled Carbon Nanotubes. Nano Lett. 2010, 10, 3343–3349. [Google Scholar] [CrossRef]
- Ibrahim, I.; Bachmatiuk, A.; Grimm, D.; Popov, A.; Makharza, S.; Knupfer, M.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Understanding High-Yield Catalyst-Free Growth of Horizontally Aligned Single-Walled Carbon Nanotubes Nucleated by Activated C60 Species. ACS Nano 2012, 6, 10825–10834. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, S.; Zheng, Z.; Hu, W.; Zhang, J. Microwave-Assisted Regeneration of Single-Walled Carbon Nanotubes from Carbon Fragments. Small 2018, 14, e1800033. [Google Scholar] [CrossRef]
- Mueller, A.; Amsharov, K.Y.; Jansen, M. Synthesis of end-cap precursor molecules for (6,6) armchair and (9,0) zig-zag single-walled carbon nanotubes. Tetrahedron Lett. 2010, 51, 3221–3225. [Google Scholar] [CrossRef]
- Jasti, R.; Bhattacharjee, J.; Neaton, J.B.; Bertozzi, C.R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. [Google Scholar] [CrossRef]
- Merner, B.L.; Dawe, L.N.; Bodwell, G.J. 1,1,8,8-Tetramethyl[8](2,11)teropyrenophane: Half of an Aromatic Belt and a Segment of an (8,8) Single-Walled Carbon Nanotube. Angew. Chem. Int. Ed. 2009, 48, 5487–5491. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.T.; Jackson, E.A.; Zhang, Q.; Steinberg, B.D.; Bancu, M.; Li, B. A Short, Rigid, Structurally Pure Carbon Nanotube by Stepwise Chemical Synthesis. J. Am. Chem. Soc. 2011, 134, 107–110. [Google Scholar] [CrossRef]
- Steinberg, B.D.; Scott, L.T. New strategies for synthesizing short sections of carbon nanotubes. Angew. Chem. Int. Ed. 2009, 48, 5400–5402. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Menning, D.-C.S.; Martín, N. para-Connected Cyclophenylenes and Hemispherical Polyarenes: Building Blocks for Single-Walled Carbon Nanotubes? Angew. Chem. Int. Ed. 2012, 51, 7094–7101. [Google Scholar] [CrossRef]
- Omachi, H.; Segawa, Y.; Itami, K. Synthesis of Cycloparaphenylenes and Related Carbon Nanorings: A Step toward the Controlled Synthesis of Carbon Nanotubes. Accounts Chem. Res. 2012, 45, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Omachi, H.; Nakayama, T.; Takahashi, E.; Segawa, Y.; Itami, K. Initiation of carbon nanotube growth by well-defined carbon nanorings. Nat. Chem. 2013, 5, 572–576. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Li, H.-B.; Bhola, R.; Jackson, E.A.; Scott, L.T.; Page, A.; Irle, S.; Morokuma, K.; Zhou, C. Nearly Exclusive Growth of Small Diameter Semiconducting Single-Wall Carbon Nanotubes from Organic Chemistry Synthetic End-Cap Molecules. Nano Lett. 2014, 15, 586–595. [Google Scholar] [CrossRef]
- Mueller, A.; Amsharov, K.Y. Synthesis of Precursors for Large-Diameter Hemispherical Buckybowls and Precursors for Short Carbon Nanotubes. Eur. J. Org. Chem. 2012, 2012, 6155–6164. [Google Scholar] [CrossRef]
- Segawa, Y.; Yagi, A.; Matsui, K.; Itami, K. Design and Synthesis of Carbon Nanotube Segments. Angew. Chem. Int. Ed. 2016, 55, 5136–5158. [Google Scholar] [CrossRef]
- Jasti, R.; Bertozzi, C.R. Progress and challenges for the bottom-up synthesis of carbon nanotubes with discrete chirality. Chem. Phys. Lett. 2010, 494, 1–7. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, B.; Jiang, L.; Guo, Y.; Huang, L.; Chen, J.; Tan, J.; Geng, D.; Luo, B.; Hu, W.; et al. Low Temperature Growth of Highly Nitrogen-Doped Single Crystal Graphene Arrays by Chemical Vapor Deposition. J. Am. Chem. Soc. 2012, 134, 11060–11063. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Ao, G.; Streit, J.K.; Fagan, J.A.; Zheng, M. Differentiating Left- and Right-Handed Carbon Nanotubes by DNA. J. Am. Chem. Soc. 2016, 138, 16677–16685. [Google Scholar] [CrossRef]
- Mesgari, S.; Poon, Y.F.; Yan, L.Y.; Chen, Y.; Loo, L.S.; Thong, Y.X.; Chan-Park, M.B. High Selectivity cum Yield Gel Electrophoresis Separation of Single-Walled Carbon Nanotubes Using a Chemically Selective Polymer Dispersant. J. Phys. Chem. C 2012, 116, 10266–10273. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F.; Löhneysen, H.V.; Kappes, M.M. Separation of metallic from semiconducting single-walled carbon nanotubes. Science 2013, 301, 344–347. [Google Scholar] [CrossRef]
- Ihly, R.; van Bezouw, S.; Arias, D. High-Yield Separation of Metallic and Semiconducting Single-Wall Carbon Nanotubes by Agarose Gel Electrophoresis. Appl. Phys. Express 2008, 1, 114001. [Google Scholar] [CrossRef]
- Mesgari, S.; Sundramoorthy, A.K.; Loo, L.S.; Chan-Park, M.B. Gel electrophoresis using a selective radical for the separation of single-walled carbon nanotubes. Faraday Discuss. 2014, 173, 351–363. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.-E.; Shim, H.C.; Song, J.-W.; Yoon, J.-H.; Kim, J.; Jeong, S.; Kang, J.; Baik, S.; Han, C.-S. Continuous Extraction of Highly Pure Metallic Single-Walled Carbon Nanotubes in a Microfluidic Channel. Nano Lett. 2008, 8, 4380–4385. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Usrey, M.L.; Strano, M.S. Selective Functionalization and Free Solution Electrophoresis of Single-Walled Carbon Nanotubes: Separate Enrichment of Metallic and Semiconducting SWNT. Chem. Mater. 2007, 19, 1571–1576. [Google Scholar] [CrossRef]
- Ihara, K.; Endoh, H.; Saito, T.; Nihey, F. Separation of Metallic and Semiconducting Single-Wall Carbon Nanotube Solution by Vertical Electric Field. J. Phys. Chem. C 2011, 115, 22827–22832. [Google Scholar] [CrossRef]
- Janas, D. Towards monochiral carbon nanotubes: A review of progress in the sorting of single-walled carbon nanotubes. Mater. Chem. Front. 2018, 2, 36–63. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Paukov, M.; Burdanova, M.G. Nanotube Functionalization: Investigation, Methods and Demonstrated Applications. Materials 2022, 15, 5386. [Google Scholar] [CrossRef]
- Tanaka, T.; Jin, H.; Miyata, Y.; Fujii, S.; Suga, H.; Naitoh, Y.; Minari, T.; Miyadera, T.; Tsukagoshi, K.; Kataura, H. Simple and Scalable Gel-Based Separation of Metallic and Semiconducting Carbon Nanotubes. Nano Lett. 2009, 9, 1497–1500. [Google Scholar] [CrossRef]
- Reis, W.G.; Weitz, R.T.; Kettner, M.; Kraus, A.; Schwab, M.G.; Tomović, Ž.; Krupke, R.; Mikhael, J. Highly Efficient and Scalable Separation of Semiconducting Carbon Nanotubes via Weak Field Centrifugation. Sci. Rep. 2016, 6, 26259. [Google Scholar] [CrossRef]
- Chen, Z.; Du, X.; Du, M.-H.; Rancken, C.D.; Cheng, H.-P.; Rinzler, A.G. Bulk Separative Enrichment in Metallic or Semiconducting Single-Walled Carbon Nanotubes. Nano Lett. 2003, 3, 1245–1249. [Google Scholar] [CrossRef]
- Voggu, R.; Ghosh, S.; Govindaraj, A.; Rao, C.N.R. New Strategies for the Enrichment of Metallic Single-Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2010, 10, 4102–4108. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Galeska, I.; Papadimitrakopoulos, F. A Route for Bulk Separation of Semiconducting from Metallic Single-Wall Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.S.; Green, A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting carbon nanotubes by electronic structure using density differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Hersam, M.C. Colored Semitransparent Conductive Coatings Consisting of Monodisperse Metallic Single-Walled Carbon Nanotubes. Nano Lett. 2008, 8, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Seo, J.-W.T.; Green, A.A.; Hersam, M.C. Sorting Single-Walled Carbon Nanotubes by Electronic Type Using Nonionic, Biocompatible Block Copolymers. ACS Nano 2010, 4, 4725–4732. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Barnes, T.M.; Beard, M.C.; Kim, Y.-H.; Tenent, R.C.; McDonald, T.J.; To, B.; Coutts, T.J.; Heben, M.J. Transparent Conductive Single-Walled Carbon Nanotube Networks with Precisely Tunable Ratios of Semiconducting and Metallic Nanotubes. ACS Nano 2008, 2, 1266–1274. [Google Scholar] [CrossRef]
- Yanagi, K.; Iitsuka, T.; Fujii, S.; Kataura, H. Separations of Metallic and Semiconducting Carbon Nanotubes by Using Sucrose as a Gradient Medium. J. Phys. Chem. C 2008, 112, 18889–18894. [Google Scholar] [CrossRef]
- Niyogi, S.; Densmore, C.G.; Doorn, S.K. Electrolyte Tuning of Surfactant Interfacial Behavior for Enhanced Density-Based Separations of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2008, 131, 1144–1153. [Google Scholar] [CrossRef]
- Liu, H.; Nishide, D.; Tanaka, T.; Kataura, H. Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography. Nat. Commun. 2011, 2, 309. [Google Scholar] [CrossRef]
- Khripin, C.Y.; Fagan, J.A.; Zheng, M. Spontaneous Partition of Carbon Nanotubes in Polymer-Modified Aqueous Phases. J. Am. Chem. Soc. 2013, 135, 6822–6825. [Google Scholar] [CrossRef]
- Subbaiyan, N.K.; Cambré, S.; Parra-Vasquez, A.N.G.; Hároz, E.H.; Doorn, S.K.; Duque, J.G. Role of surfactants and salt in aqueous two-phase separation of carbon nanotubes toward simple chirality isolation. ACS Nano 2014, 8, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Polley, D.; Patra, A.; Barman, A.; Mitra, R.K. Terahertz conductivity engineering in surface decorated carbon nanotube films by gold nanoparticles. Appl. Opt. 2017, 56, 1107. [Google Scholar] [CrossRef] [PubMed]
- Yahya, I.; Bonaccorso, F.; Clowes, S.K.; Ferrari, A.C.; Silva, S.R.P. Temperature dependent separation of metallic and semiconducting carbon nanotubes using gel agarose chromatography. Carbon 2015, 93, 574–594. [Google Scholar] [CrossRef]
- Maeda, Y.; Kimura, S.-I.; Kanda, M.; Hirashima, Y.; Hasegawa, T.; Wakahara, T.; Lian, Y.; Nakahodo, T.; Tsuchiya, T.; Akasaka, T.; et al. Large-Scale Separation of Metallic and Semiconducting Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 10287–10290. [Google Scholar] [CrossRef]
- Lu, J.; Lai, L.; Luo, G.; Zhou, J.; Qin, R.; Wang, D.; Wang, L.; Mei, W.N.; Li, G.; Gao, Z.; et al. Why Semiconducting Single-Walled Carbon Nanotubes are Separated from their Metallic Counterparts. Small 2007, 3, 1566–1576. [Google Scholar] [CrossRef]
- Li, H.; Zhou, B.; Lin, Y.; Gu, L.; Wang, W.; Fernando, K.A.S.; Kumar, S.; Allard, A.L.F.; Sun, Y.-P. Selective Interactions of Porphyrins with Semiconducting Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 1014–1015. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef]
- Hassanien, A.; Tokumoto, M.; Umek, P.; Vrbanic, D.; Mozetič, M.; Mihailović, D.; Venturini, P. Selective etching of metallic single-wall carbon nanotubes with hydrogen plasma. Nanotechnology 2005, 16, 278–281. [Google Scholar] [CrossRef]
- Song, J.W.; Seo, H.W.; Park, J.K.; Kim, J.E.; Choi, D.G.; Han, C.S. Selective removal of metallic SWNTs using microwave radiation. Curr. Appl. Phys. 2008, 8, 725–728. [Google Scholar] [CrossRef]
- Nessim, G.D. Properties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor deposition. Nanoscale 2010, 2, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.S.; Stupp, S.I.; Hersam, M.C. Enrichment of Single-Walled Carbon Nanotubes by Diameter in Density Gradients. Nano Lett. 2005, 5, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bachilo, S.M.; Weisman, R.B. Advanced sorting of single-walled carbon nanotubes by nonlinear density-gradient ultracentrifugation. Nat. Nanotechnol. 2010, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cambré, S.; Wenseleers, W. Separation and Diameter-Sorting of Empty (End-Capped) and Water-Filled (Open) Carbon Nanotubes by Density Gradient Ultracentrifugation. Angew. Chem. Int. Ed. 2011, 50, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Eliseev, A.; Yashina, L.; Kharlamova, M.; Kiselev, N. One-Dimensional Crystals inside Single-Walled Carbon Nanotubes: Growth, Structure and Electronic Properties. In Electronic Properties of Carbon Nanotubes; InTech: Singapore, 2011. [Google Scholar] [CrossRef][Green Version]
- Kashtiban, R.J.; Burdanova, M.G.; Vasylenko, A.; Wynn, J.; Medeiros, P.V.C.; Ramasse, Q.; Morris, A.J.; Quigley, D.; Lloyd-Hughes, J.; Sloan, J. Linear and Helical Cesium Iodide Atomic Chains in Ultranarrow Single-Walled Carbon Nanotubes: Impact on Optical Properties. ACS Nano 2021, 15, 13389–13398. [Google Scholar] [CrossRef]
- Li, H.; Gordeev, G.; Toroz, D.; Di Tommaso, D.; Reich, S.; Flavel, B.S. Endohedral Filling Effects in Sorted and Polymer-Wrapped Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2021, 125, 7476–7487. [Google Scholar] [CrossRef]
- Liu, H.; Tanaka, T.; Kataura, H. One-step separation of high-purity (6,5) carbon nanotubes by multicolumn gel chromatography. Phys. Status Solidi 2011, 248, 2524–2527. [Google Scholar] [CrossRef]
- Liu, H.; Tanaka, T.; Urabe, Y.; Kataura, H. High-Efficiency Single-Chirality Separation of Carbon Nanotubes Using Temperature-Controlled Gel Chromatography. Nano Lett. 2013, 13, 1996–2003. [Google Scholar] [CrossRef]
- Flavel, B.S.; Kappes, M.M.; Krupke, R.; Hennrich, F. Separation of Single-Walled Carbon Nanotubes by 1-Dodecanol-Mediated Size-Exclusion Chromatography. ACS Nano 2013, 7, 3557–3564. [Google Scholar] [CrossRef]
- Flavel, B.S.; Moore, K.E.; Pfohl, M.; Kappes, M.M.; Hennrich, F. Separation of single-walled carbon nanotubes with a gel permeation chromatography system. ACS Nano 2014, 8, 1817–1826. [Google Scholar] [CrossRef]
- Yomogida, Y.; Tanaka, T.; Zhang, M.; Yudasaka, M.; Wei, X.; Kataura, Y.Y.T.T.M.Y.X.W.H. Industrial-scale separation of high-purity single-chirality single-wall carbon nanotubes for biological imaging. Nat. Commun. 2016, 7, 12056. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.A.; Khripin, C.Y.; Silvera Batista, C.A.; Simpson, J.R.; Hároz, E.H.; Hight Walker, A.R.; Zheng, M.; Fagan, J.A.; Khripin, C.Y.; Silvera Batista, C.A.; et al. Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction. Adv. Mater. 2014, 6, 2800–2804. [Google Scholar] [CrossRef]
- Li, H.; Gordeev, G.; Garrity, O.; Reich, S.; Flavel, B.S. Separation of Small-Diameter Single-Walled Carbon Nanotubes in One to Three Steps with Aqueous Two-Phase Extraction. ACS Nano 2019, 13, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.M.; Ben-Naim, M.; Landry, M.P.; Strano, M.S. Competitive Binding in Mixed Surfactant Systems for Single-Walled Carbon Nanotube Separation. J. Phys. Chem. C 2015, 119, 22737–22745. [Google Scholar] [CrossRef]
- Fagan, J.A. Aqueous two-polymer phase extraction of single-wall carbon nanotubes using surfactants. Nanoscale Adv. 2019, 1, 3307–3324. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, F.; Li, W.; Fischer, R.; Lebedkin, S.; Krupke, R.; Kappes, M.M. Length-Sorted, Large-Diameter, Polyfluorene-Wrapped Semiconducting Single-Walled Carbon Nanotubes for High-Density, Short-Channel Transistors. ACS Nano 2016, 10, 1888–1895. [Google Scholar] [CrossRef]
- Snow, E.S.; Campbell, P.M.; Ancona, M.G.; Novak, J.P. High-mobility carbon-nanotube thin-film transistors on a polymeric substrate. Appl. Phys. Lett. 2005, 86, 033105. [Google Scholar] [CrossRef]
- Snow, E.S.; Novak, J.P.; Campbell, P.M.; Park, D. Random networks of carbon nanotubes as an electronic material. Appl. Phys. Lett. 2003, 82, 2145–2147. [Google Scholar] [CrossRef]
- Cao, Q.; Kim, H.-S.; Pimparkar, N.; Kulkarni, J.P.; Wang, C.; Shim, M.; Roy, K.; Alam, M.A.; Rogers, J.A. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature 2008, 454, 495–500. [Google Scholar] [CrossRef]
- Asada, Y.; Miyata, Y.; Ohno, Y.; Kitaura, R.; Sugai, T.; Mizutani, T.; Shinohara, H. High-Performance Thin-Film Transistors with DNA-Assisted Solution Processing of Isolated Single-Walled Carbon Nanotubes. Adv. Mater. 2010, 22, 2698–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-G.; Liu, X.-W.; Chen, W.-P.; Li, T. Carbon nanotubes humidity sensor based on high testing frequencies. Sensors Actuators A: Phys. 2011, 168, 10–13. [Google Scholar] [CrossRef]
- Shah, M.; Ahmad, Z.; Sulaiman, K.; Karimov, K.; Sayyad, M. Carbon nanotubes’ nanocomposite in humidity sensors. Solid State Electron. 2012, 69, 18–21. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Tange, M.; Xu, W.; Xu, W.; Zhang, K.; Guo, W.; Okazaki, T.; Cui, Z. Sorting semiconducting single walled carbon nanotubes by poly(9,9-dioctylfluorene) derivatives and application for ammonia gas sensing. Carbon 2015, 94, 903–910. [Google Scholar] [CrossRef]
- Karimov, K.S.; Chani, M.T.S.; Khalid, F.A. Carbon nanotubes film based temperature sensors. Phys. E: Low Dimens. Syst. Nanostructures 2011, 43, 1701–1703. [Google Scholar] [CrossRef]
- Antaris, A.L.; Robinson, J.T.; Yaghi, O.K.; Hong, G.; Diao, S.; Luong, R.; Dai, H. Ultra-Low Doses of Chirality Sorted (6,5) Carbon Nanotubes for Simultaneous Tumor Imaging and Photothermal Therapy. ACS Nano 2013, 7, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Ganzhorn, M.; Vijayaraghavan, A.; Dehm, S.; Hennrich, F.; Green, A.A.; Fichtner, M.; Voigt, A.; Rapp, M.; Von Löhneysen, H.; Hersam, M.C.; et al. Hydrogen Sensing with Diameter- and Chirality-Sorted Carbon Nanotubes. ACS Nano 2011, 5, 1670–1676. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Ye, J.; Tange, M.; Zhang, X.; Xu, W.; Zhang, K.; Okazaki, T.; Cui, Z. Printed thin-film transistors and NO2 gas sensors based on sorted semiconducting carbon nanotubes by isoindigo-based copolymer. Carbon 2016, 108, 372–380. [Google Scholar] [CrossRef]
- Battie, Y.; Ducloux, O.; Thobois, P.; Dorval, N.; Lauret, J.S.; Attal-Trétout, B.; Loiseau, A. Gas sensors based on thick films of semi-conducting single walled carbon nanotubes. Carbon 2011, 49, 3544–3552. [Google Scholar] [CrossRef]
- Ruiz-Soria, G.; Paz, A.P.; Sauer, M.; Mowbray, D.J.; Lacovig, P.; Dalmiglio, M.; Lizzit, S.; Yanagi, K.; Rubio, A.; Goldoni, A.; et al. Revealing the adsorption mechanisms of nitroxides on ultrapure, metallicity-sorted carbon nanotubes. ACS Nano 2014, 8, 1375–1383. [Google Scholar] [CrossRef]
- Battie, Y.; Gorintin, L.; Ducloux, O.; Thobois, P.; Bondavalli, P.; Feugnet, G.; Loiseau, A. Thickness dependent sensing mechanism in sorted semi-conducting single walled nanotube based sensors. Anal. 2012, 137, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
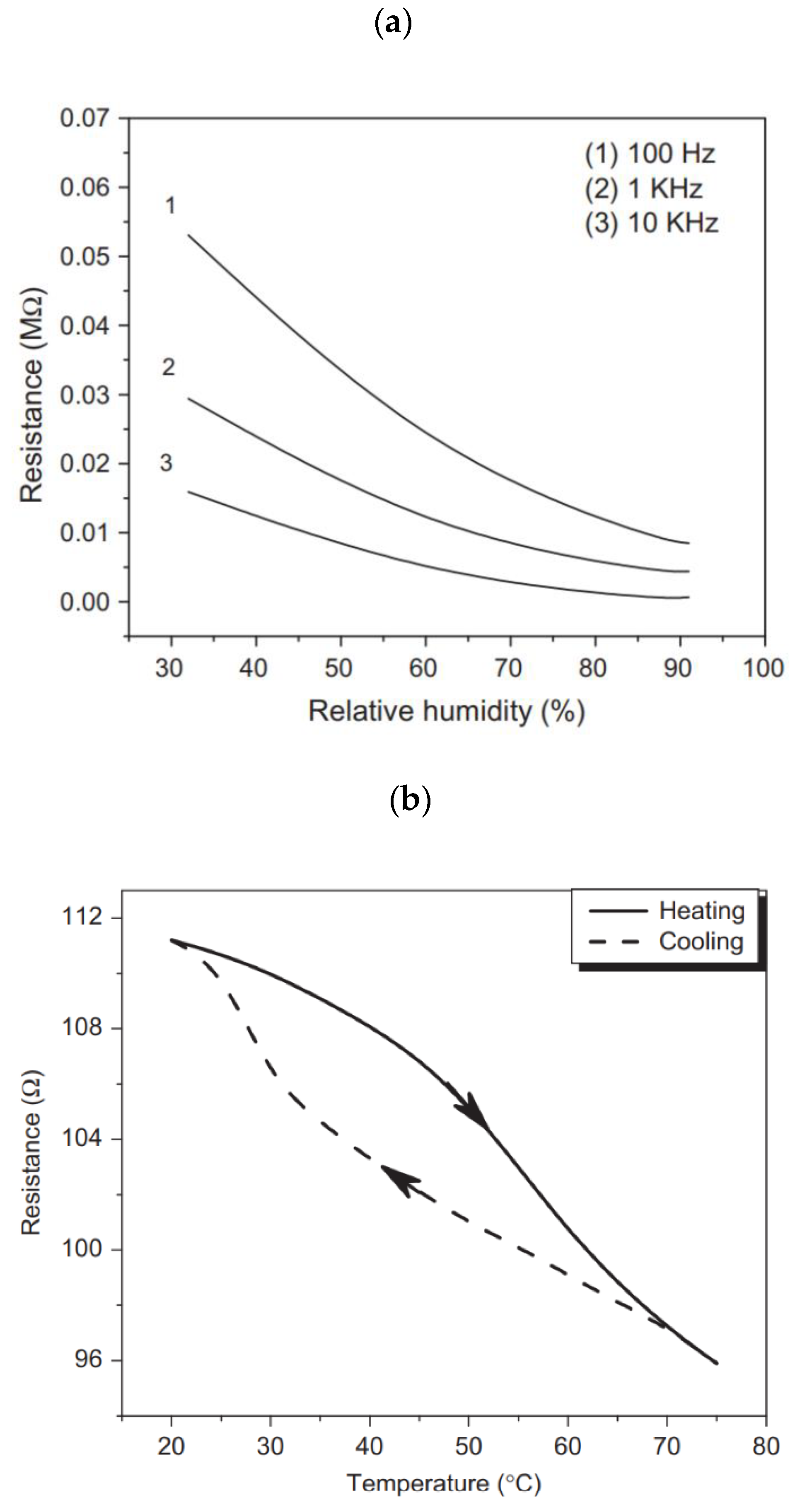
- Besteman, K.; Lee, J.-O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C. Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar] [CrossRef]
- Muguruma, H.; Hoshino, T.; Nowaki, K. Electronically Type-Sorted Carbon Nanotube-Based Electrochemical Biosensors with Glucose Oxidase and Dehydrogenase. ACS Appl. Mater. Interfaces 2014, 7, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Yamauchi, Y.; Honda, S.; Maki, H. An Electrically Driven, Ultrahigh-Speed, on-Chip Light Emitter Based on Carbon Nanotubes. Nano Lett. 2014, 14, 3277–3283. [Google Scholar] [CrossRef] [PubMed]
- Sarti, F.; Biccari, F.; Fioravanti, F.; Torrini, U.; Vinattieri, A.; Derycke, V.; Gurioli, M.; Filoramo, A. Highly selective sorting of semiconducting single wall carbon nanotubes exhibiting light emission at telecom wavelengths. Nano Res. 2016, 9, 2478–2486. [Google Scholar] [CrossRef]
- Balestrieri, M.; Keita, A.-S.; Duran-Valdeiglesias, E.; Alonso-Ramos, C.; Zhang, W.; Le Roux, X.; Cassan, E.; Vivien, L.; Bezugly, V.; Fediai, A.; et al. Polarization-Sensitive Single-Wall Carbon Nanotubes All-in-One Photodetecting and Emitting Device Working at 1.55 µm. Adv. Funct. Mater. 2017, 27, 201702341. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Liu, M.; Staniforth, M.; Zheng, Y.; Xiang, R.; Chiashi, S.; Anisimov, A.; Kauppinen, E.I.; Maruyama, S.; Lloyd-Hughes, J. Intertube Excitonic Coupling in Nanotube Van der Waals Heterostructures. Adv. Funct. Mater. 2021, 32, 2104969. [Google Scholar] [CrossRef]
- Higashide, N.; Yoshida, M.; Uda, T.; Ishii, A.; Kato, Y.K. Cold exciton electroluminescence from air-suspended carbon nanotube split-gate devices. Appl. Phys. Lett. 2017, 110, 191101. [Google Scholar] [CrossRef]
- Jakubka, F.; Grimm, S.B.; Zakharko, Y.; Gannott, F.; Zaumseil, J. Trion Electroluminescence from Semiconducting Carbon Nanotubes. ACS Nano 2014, 8, 8477–8486. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, N.; Zeng, Q.; Han, J.; Huang, H.; Zhong, D.; Wang, F.; Ding, L.; Xia, J.; Xu, H.; et al. Room Temperature Broadband Infrared Carbon Nanotube Photodetector with High Detectivity and Stability. Adv. Opt. Mater. 2015, 4, 238–245. [Google Scholar] [CrossRef]
- Liang, S.; Ma, Z.; Wu, G.; Wei, N.; Huang, L.; Huang, H.; Liu, H.; Wang, S.; Peng, L.-M. Microcavity-Integrated Carbon Nanotube Photodetectors. ACS Nano 2016, 10, 6963–6971. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, F.; Liu, Y.; Wang, S.; Peng, L.-M. Plasmonic Enhanced Performance of an Infrared Detector Based on Carbon Nanotube Films. ACS Appl. Mater. Interfaces 2017, 9, 12743–12749. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Tsapenko, A.P.; Kharlamova, M.V.; Kauppinen, E.I.; Gorshunov, B.P.; Kono, J.; Lloyd-Hughes, J. A Review of the Terahertz Conductivity and Photoconductivity of Carbon Nanotubes and Heteronanotubes. Adv. Opt. Mater. 2021, 9, 2101042. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Katyba, G.M.; Kashtiban, R.; Komandin, G.A.; Butler-Caddle, E.; Staniforth, M.; Mkrtchyan, A.A.; Krasnikov, D.V.; Gladush, Y.G.; Sloan, J.; et al. Ultrafast, high modulation depth terahertz modulators based on carbon nanotube thin films. Carbon 2020, 173, 245–252. [Google Scholar] [CrossRef]
- Rahy, A.; Bajaj, P.; Musselman, I.H.; Hong, S.H.; Sun, Y.-P.; Yang, D.J. Coating of carbon nanotubes on flexible substrate and its adhesion study. Appl. Surf. Sci. 2009, 255, 7084–7089. [Google Scholar] [CrossRef]
- Hwang, B.-Y.; Choi, S.-H.; Lee, K.-W.; Kim, J.-Y. Highly stretchable and transparent electrode film based on SWCNT/Silver nanowire hybrid nanocomposite. Compos. Part B Eng. 2018, 151, 1–7. [Google Scholar] [CrossRef]
- Zhu, Z.-R.; Geng, W.; Zhu, Q.; Ethiraj, A.S.; Wang, T.; Jing, L.-C.; Ning, Y.-J.; Tian, Y.; Geng, W.-H.; Wu, L.; et al. Highly transparent, low sheet resistance and stable Tannic acid modified-SWCNT/AgNW double-layer conductive network for organic light emitting diodes. Nanotechnology 2020, 32, 015708. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, X.; Gao, J.; Gong, M.; Lin, X.; Zhang, L.; Guo, M.; Wang, D. Customizable Stretchable Transparent Electrodes Based on AgNW/CNT Hybrids via Tailoring Sizes of Building Blocks. ACS Appl. Electron. Mater. 2022, 4, 1186–1195. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Hang, C.-Z.; Wu, X.-Y.; Zhu, L.-Y.; Wen, X.-H.; Wang, Y.; Zhao, X.-F.; Lu, H.-L. Investigation of stretchable strain sensor based on CNT/AgNW applied in smart wearable devices. Nanotechnology 2022, 33, 255501. [Google Scholar] [CrossRef]
- Cai, Y.; Piao, X.; Yao, X.; Gao, W.; Nie, E.; Zhang, Z.; Sun, Z. Transparent conductive film based on silver nanowires and single-wall carbon nanotubes for transparent heating films. Nanotechnology 2019, 30, 225201. [Google Scholar] [CrossRef]
- Ho, M.D.; Ling, Y.; Yap, L.W.; Wang, Y.; Dong, D.; Zhao, Y.; Cheng, W. Percolating Network of Ultrathin Gold Nanowires and Silver Nanowires toward “Invisible” Wearable Sensors for Detecting Emotional Expression and Apexcardiogram. Adv. Funct. Mater. 2017, 27, 20170084. [Google Scholar] [CrossRef]
- Sharma, N.; Nair, N.M.; Nagasarvari, G.; Ray, D.; Swaminathan, P. A review of silver nanowire-based composites for flexible electronic applications. Flex. Print. Electron. 2022, 7, 014009. [Google Scholar] [CrossRef]
- Lee, P.; Ham, J.; Lee, J.; Hong, S.; Han, S.; Suh, Y.D.; Lee, S.E.; Yeo, J.; Lee, S.S.; Lee, D.; et al. Highly Stretchable or Transparent Conductor Fabrication by a Hierarchical Multiscale Hybrid Nanocomposite. Adv. Funct. Mater. 2014, 24, 5671–5678. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.-W.; Park, J.H.; Porte, Y.; Kim, J.-H.; Park, J.-W.; Kim, S.; Myoung, J.-M. SWCNT–Ag nanowire composite for transparent stretchable film heater with enhanced electrical stability. J. Mater. Sci. 2018, 53, 12284–12294. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, Y.; Wang, Z.; Chen, X.; Luis, P.; Sun, L.; Zhang, D.; Zhao, J. Preparation of large-area, high-performance single-walled carbon nanotube (SWCNT)-based heater films by roll-to-roll gravure printing. Flex. Print. Electron. 2022, 7, ac509b. [Google Scholar] [CrossRef]
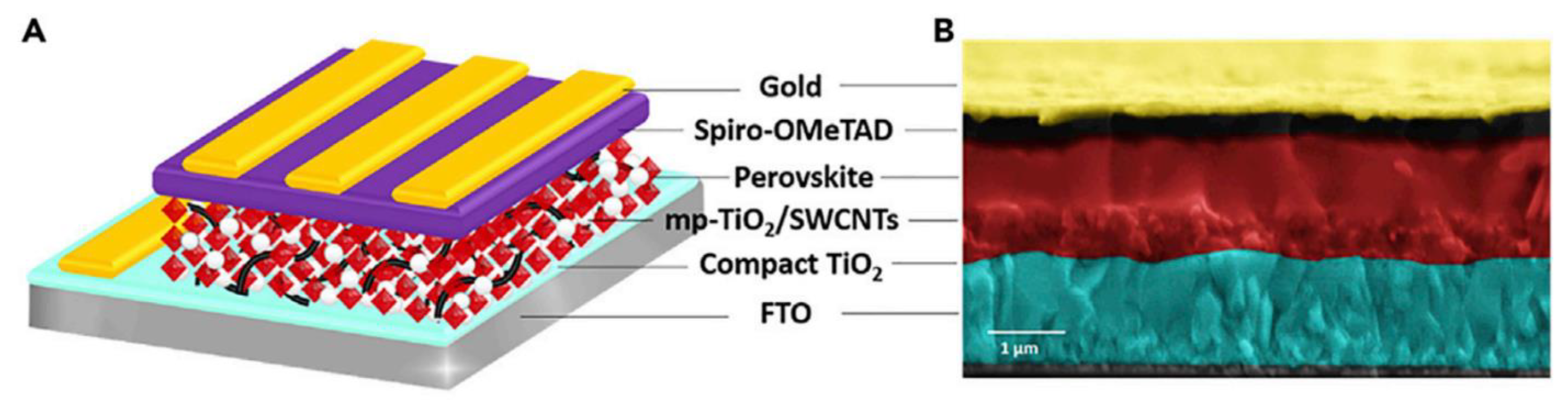
- Bati, A.S.; Yu, L.; Tawfik, S.A.; Spencer, M.J.; Shaw, P.E.; Batmunkh, M.; Shapter, J.G. Electrically Sorted Single-Walled Carbon Nanotubes-Based Electron Transporting Layers for Perovskite Solar Cells. iScience 2019, 14, 100–112. [Google Scholar] [CrossRef]
- Bindl, D.J.; Arnold, M.S. Efficient exciton relaxation and charge generation in nearly monochiral (7,5) carbon nanotube/C60 thin-film photovoltaics. J. Phys. Chem. C 2013, 117, 2390–2395. [Google Scholar] [CrossRef]
- Sandanayaka, A.S.D.; Subbaiyan, N.K.; Das, S.K.; Chitta, R.; Maligaspe, E.; Hasobe, T.; Ito, O.; D’Souza, F. Diameter-Sorted SWCNT-Porphyrin and SWCNT-Phthalocyanine Conjugates for Light-Energy Harvesting. ChemPhysChem 2011, 12, 2266–2273. [Google Scholar] [CrossRef]
- Ramuz, M.P.; Vosgueritchian, M.; Wei, P.; Wang, C.; Gao, Y.; Wu, Y.; Chen, Y.; Bao, Z. Evaluation of Solution-Processable Carbon-Based Electrodes for All-Carbon Solar Cells. ACS Nano 2012, 6, 10384–10395. [Google Scholar] [CrossRef]
- Dang, X.; Yi, H.; Ham, M.-H.; Qi, J.; Yun, D.S.; Ladewski, R.; Strano, M.S.; Hammond, P.T.; Belcher, A.M. Virus-templated self-assembled single-walled carbon nanotubes for highly efficient electron collection in photovoltaic devices. Nat. Nanotechnol. 2011, 6, 377–384. [Google Scholar] [CrossRef]
- Kawasaki, S.; Hara, T.; Iwai, Y.; Suzuki, Y. Metallic and semiconducting single-walled carbon nanotubes as the anode material of Li ion secondary battery. Mater. Lett. 2008, 62, 2917–2920. [Google Scholar] [CrossRef]
- Jaber-Ansari, L.; Iddir, H.; Curtiss, L.A.; Hersam, M.C. Influence of Electronic Type Purity on the Lithiation of Single-Walled Carbon Nanotubes. ACS Nano 2014, 8, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Ju, Y.-W.; Jang, Y.-R.; Gwon, O.; Park, S.; Kim, J.-M.; Lee, C.K.; Lee, S.-Y.; Yeon, S.-H.; Kim, G.; et al. One-pot surface engineering of battery electrode materials with metallic SWCNT-enriched, ivy-like conductive nanonets. J. Mater. Chem. A 2017, 5, 12103–12112. [Google Scholar] [CrossRef]
- Kim, S.; Choi, B.; Lim, M.; Yoon, J.; Lee, J.; Kim, H.-D.; Choi, S.-J. Pattern Recognition Using Carbon Nanotube Synaptic Transistors with an Adjustable Weight Update Protocol. ACS Nano 2017, 11, 2814–2822. [Google Scholar] [CrossRef]
- Esqueda, I.S.; Yan, X.; Rutherglen, C.; Kane, A.; Cain, T.; Marsh, P.; Liu, Q.; Galatsis, K.; Wang, H.; Zhou, C. Aligned Carbon Nanotube Synaptic Transistors for Large-Scale Neuromorphic Computing. ACS Nano 2018, 12, 7352–7361. [Google Scholar] [CrossRef]
- Ishii, A.; He, X.; Hartmann, N.F.; Machiya, H.; Htoon, H.; Doorn, S.K.; Kato, Y.K. Enhanced Single-Photon Emission from Carbon-Nanotube Dopant States Coupled to Silicon Microcavities. Nano Lett. 2018, 18, 3873–3878. [Google Scholar] [CrossRef]
- He, X.; Hartmann, N.F.; Ma, X.; Kim, Y.; Ihly, R.; Blackburn, J.L.; Gao, W.; Kono, J.; Yomogida, Y.; Hirano, A.; et al. Tunable room-temperature single-photon emission at telecom wavelengths from sp3 defects in carbon nanotubes. Nat. Photon. 2017, 11, 577–582. [Google Scholar] [CrossRef]
- Ma, X.; Hartmann, N.F.; Baldwin, J.K.S.; Doorn, S.K.; Htoon, H. Room-temperature single-photon generation from solitary dopants of carbon nanotubes. Nat. Nanotechnol. 2015, 10, 671–675. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef]
- Antaris, A.L.; Yaghi, O.K.; Hong, G.; Diao, S.; Zhang, B.; Yang, J.; Chew, L.; Dai, H. Single Chirality (6,4) Single-Walled Carbon Nanotubes for Fluorescence Imaging with Silicon Detectors. Small 2015, 11, 6325–6330. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mansukhani, N.D.; Guiney, L.M.; Lee, J.-H.; Li, R.; Sun, B.; Liao, Y.-P.; Chang, C.H.; Ji, Z.; Xia, T.; et al. Toxicological Profiling of Highly Purified Metallic and Semiconducting Single-Walled Carbon Nanotubes in the Rodent Lung and E. coli. ACS Nano 2016, 10, 6008–6019. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fernando, K.A.S.; Lin, Y.; Meziani, M.J.; Veca, L.M.; Cao, L.; Zhang, P.; Kimani, A.M.M.; Sun, Y.-P. Metallic Single-Walled Carbon Nanotubes for Conductive Nanocomposites. J. Am. Chem. Soc. 2008, 130, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Yotprayoonsak, P.; Saunajoki, V.; Ahlskog, M.; Virtanen, J.; Kangas, V.; Volodin, A.; Van Haesendonck, C.; Burdanova, M.; Mosley, C.D.W.; et al. Conduction properties of thin films from a water soluble carbon nanotube/hemicellulose complex. Nanotechnology 2018, 29, 145203. [Google Scholar] [CrossRef]
- Yu, I.; Ko, J.; Kim, T.-W.; Lee, D.S.; Kim, N.D.; Bae, S.; Lee, S.-K.; Choi, J.; Lee, S.S.; Joo, Y. Effect of sorted, homogeneous electronic grade single-walled carbon nanotube on the electromagnetic shielding effectiveness. Carbon 2020, 167, 523–529. [Google Scholar] [CrossRef]

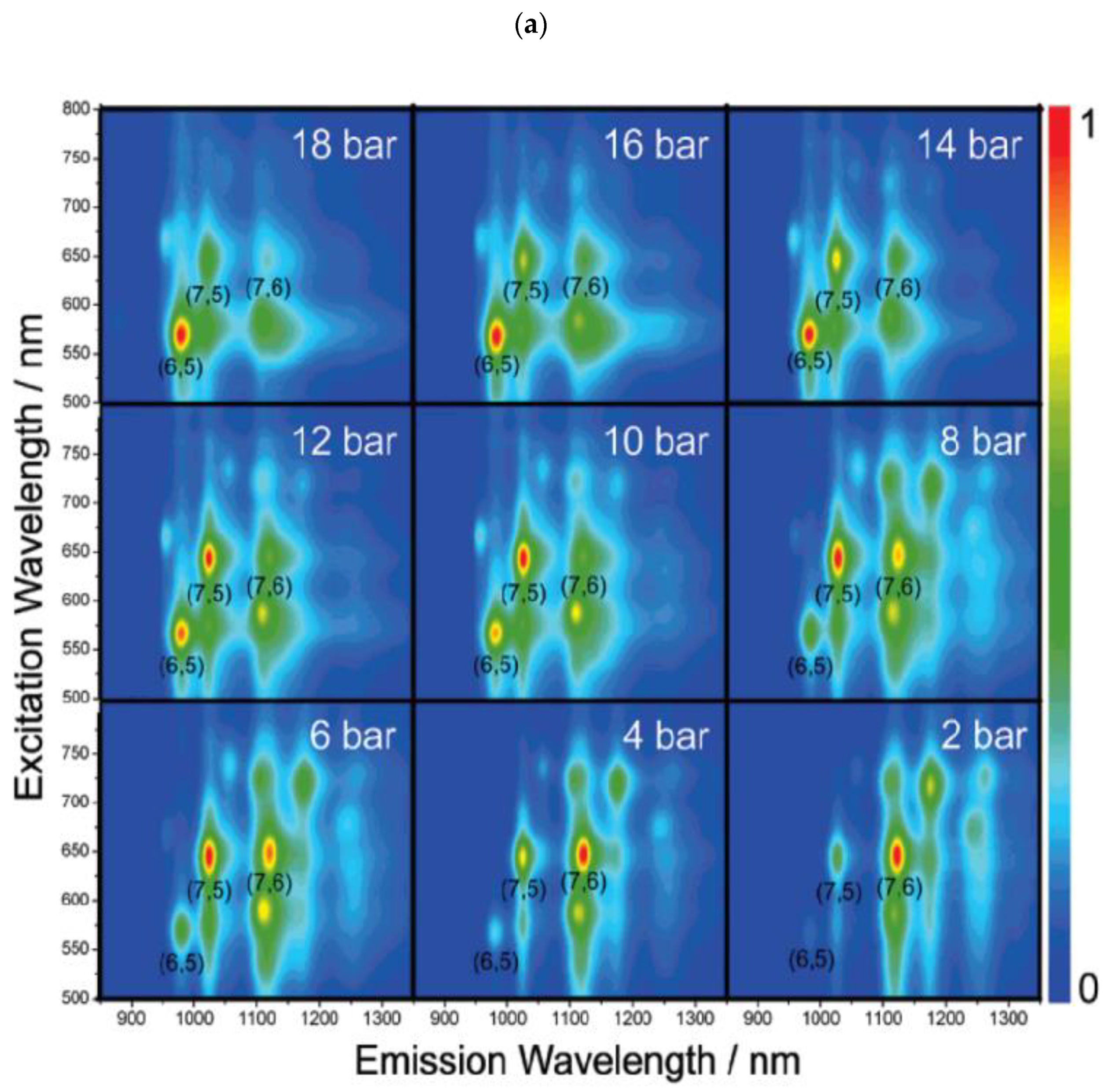
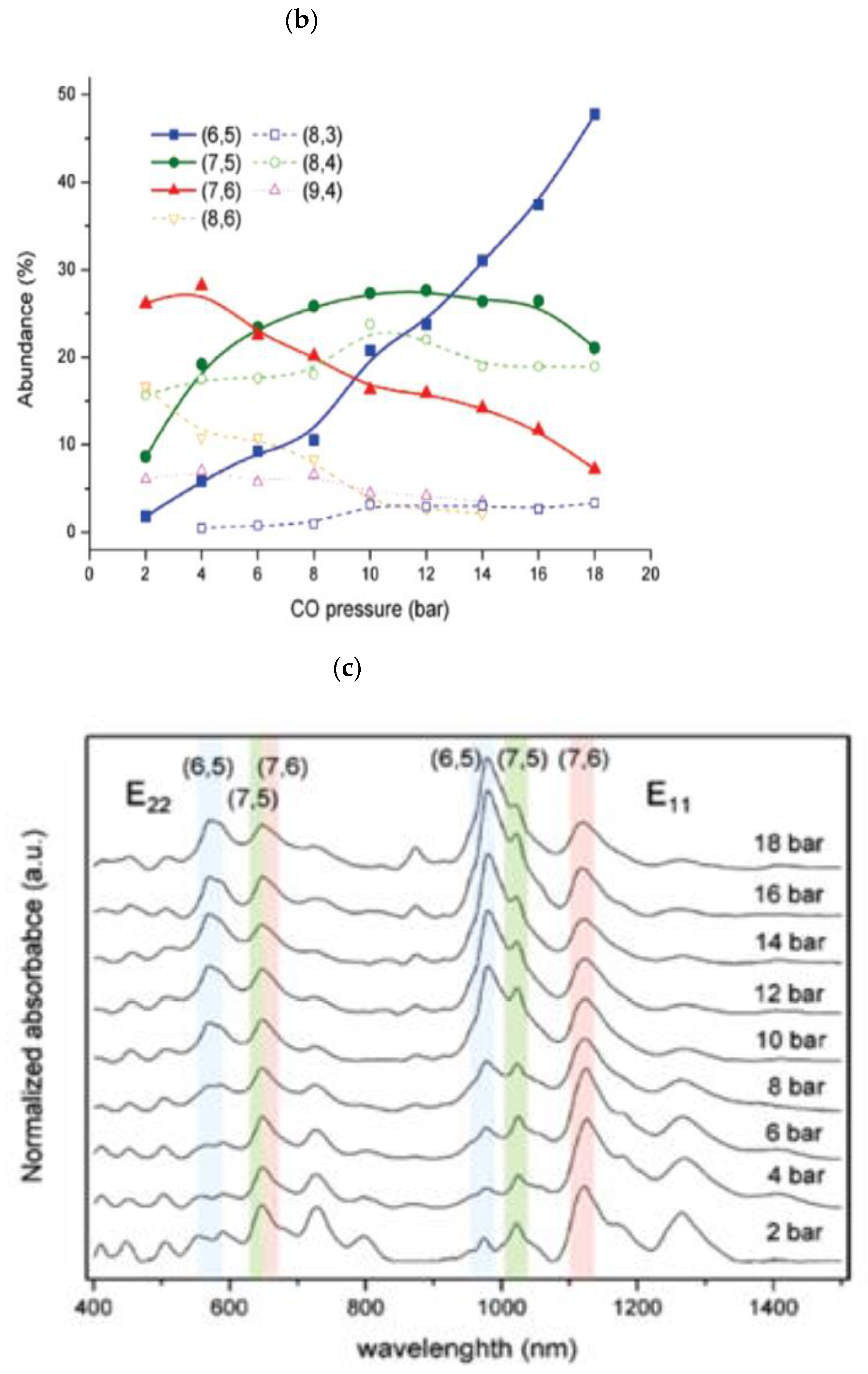
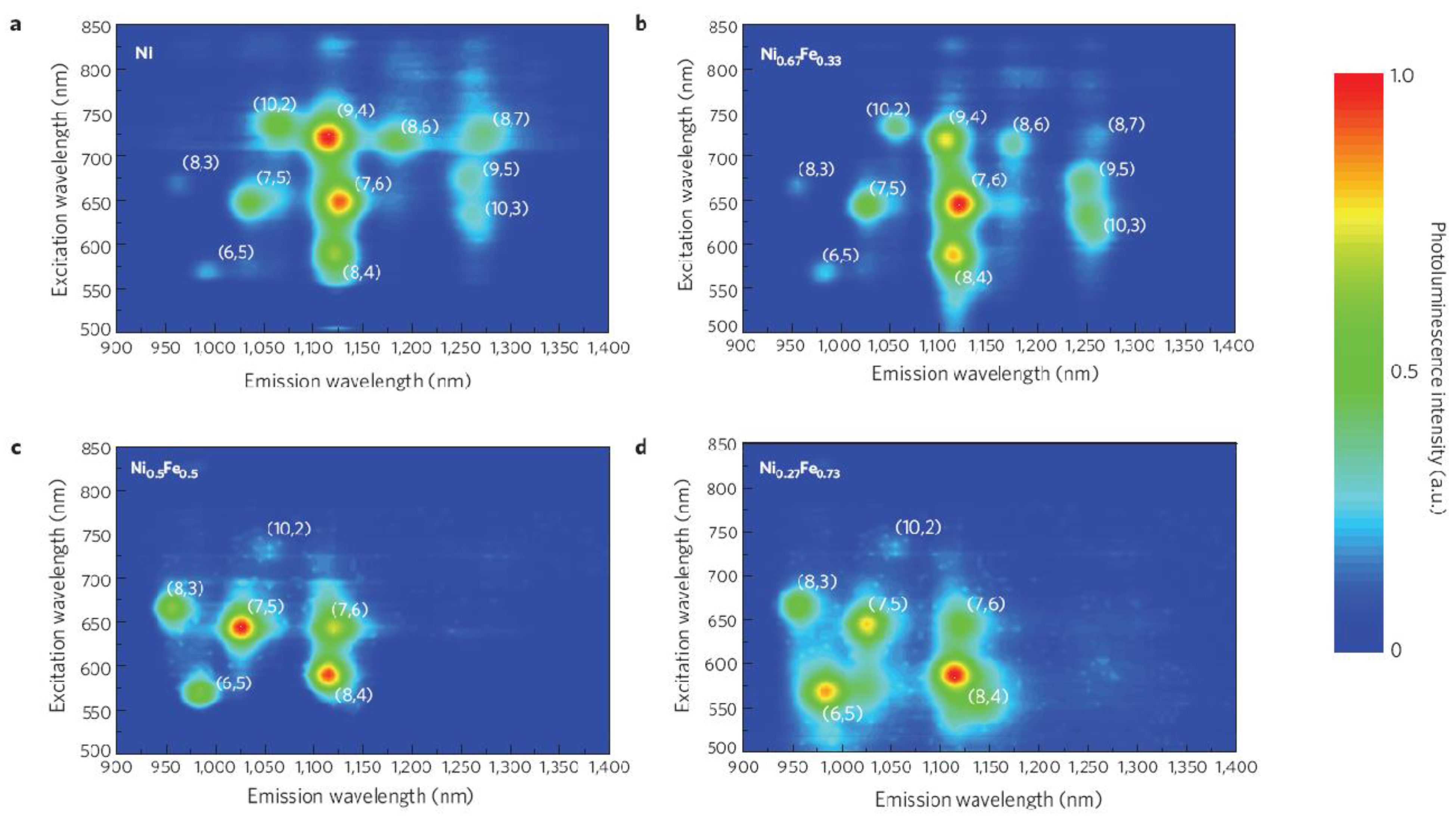
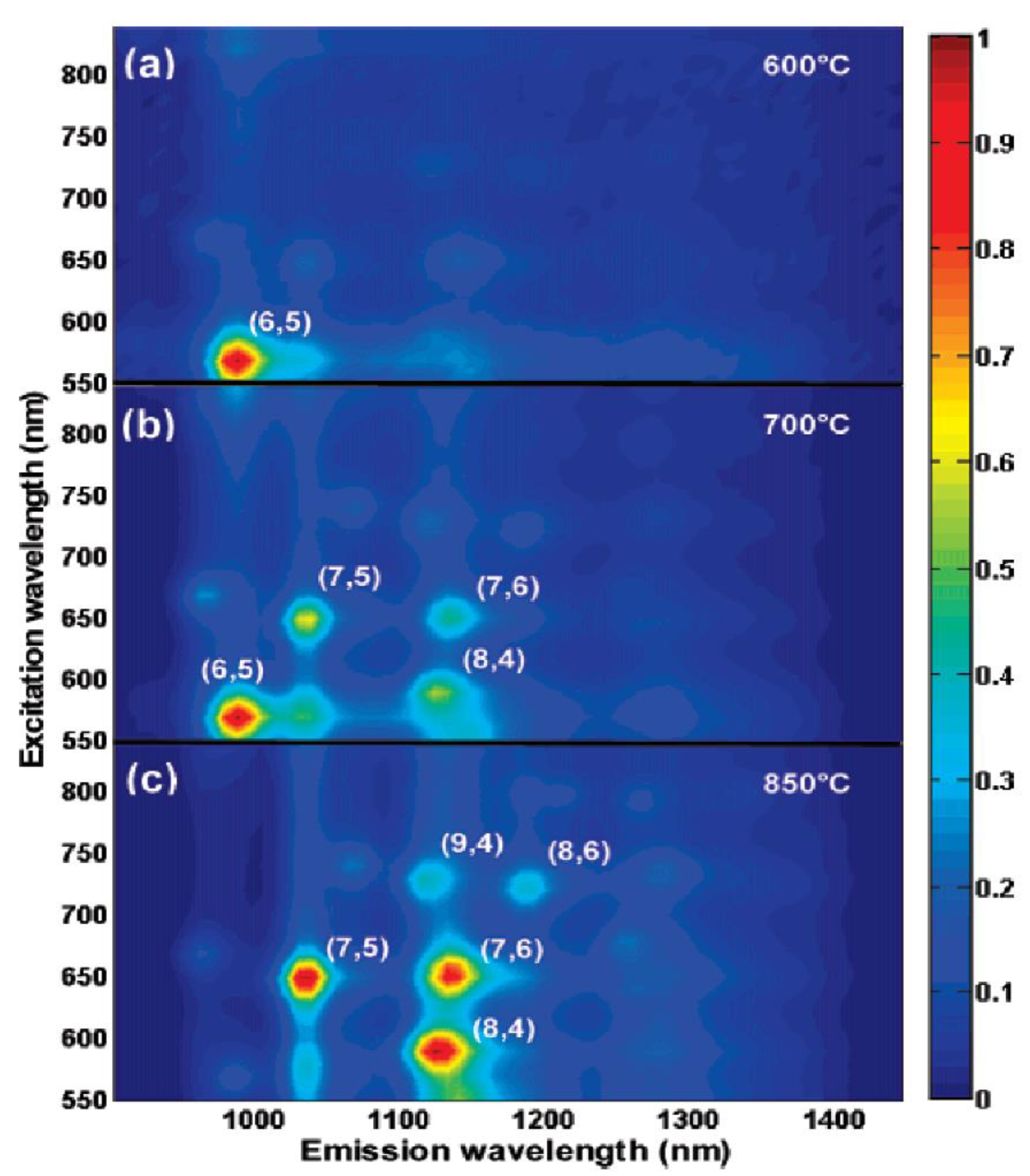




| Synthesis | Electrophoresis | DGC | Chromatography | ATPE | |
|---|---|---|---|---|---|
| Enrichment | Chirality | M, S | M, S, chirality | M, S, chirality | M, S, chirality |
| Scalability | Small scale | Small scale | Moderate scale | Large scale | Large scale |
| Purity | Low | High | High | Low | High |
| Cost | High | Low | High | Low | High |
| Speed | Slow | Fast | Slow | Slow | Fast |
| Simplicity | Complicated | Simple | Complicated | Simple | Simple |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, M.V.; Burdanova, M.G.; Paukov, M.I.; Kramberger, C. Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes. Materials 2022, 15, 5898. https://doi.org/10.3390/ma15175898
Kharlamova MV, Burdanova MG, Paukov MI, Kramberger C. Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes. Materials. 2022; 15(17):5898. https://doi.org/10.3390/ma15175898
Chicago/Turabian StyleKharlamova, Marianna V., Maria G. Burdanova, Maksim I. Paukov, and Christian Kramberger. 2022. "Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes" Materials 15, no. 17: 5898. https://doi.org/10.3390/ma15175898
APA StyleKharlamova, M. V., Burdanova, M. G., Paukov, M. I., & Kramberger, C. (2022). Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes. Materials, 15(17), 5898. https://doi.org/10.3390/ma15175898







