Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZNPs Using Spin Coating Method
2.3. Preparation of Vertically Aligned ZNRAs Using Hydrothermal Method
2.4. Structural, Morphological, and Optical Characterizations of ZNRAs
2.5. Photoelectrochemical Characterizations of ZNRAs
3. Results and Discussion
3.1. Structures and Morphologies of ZNRAs
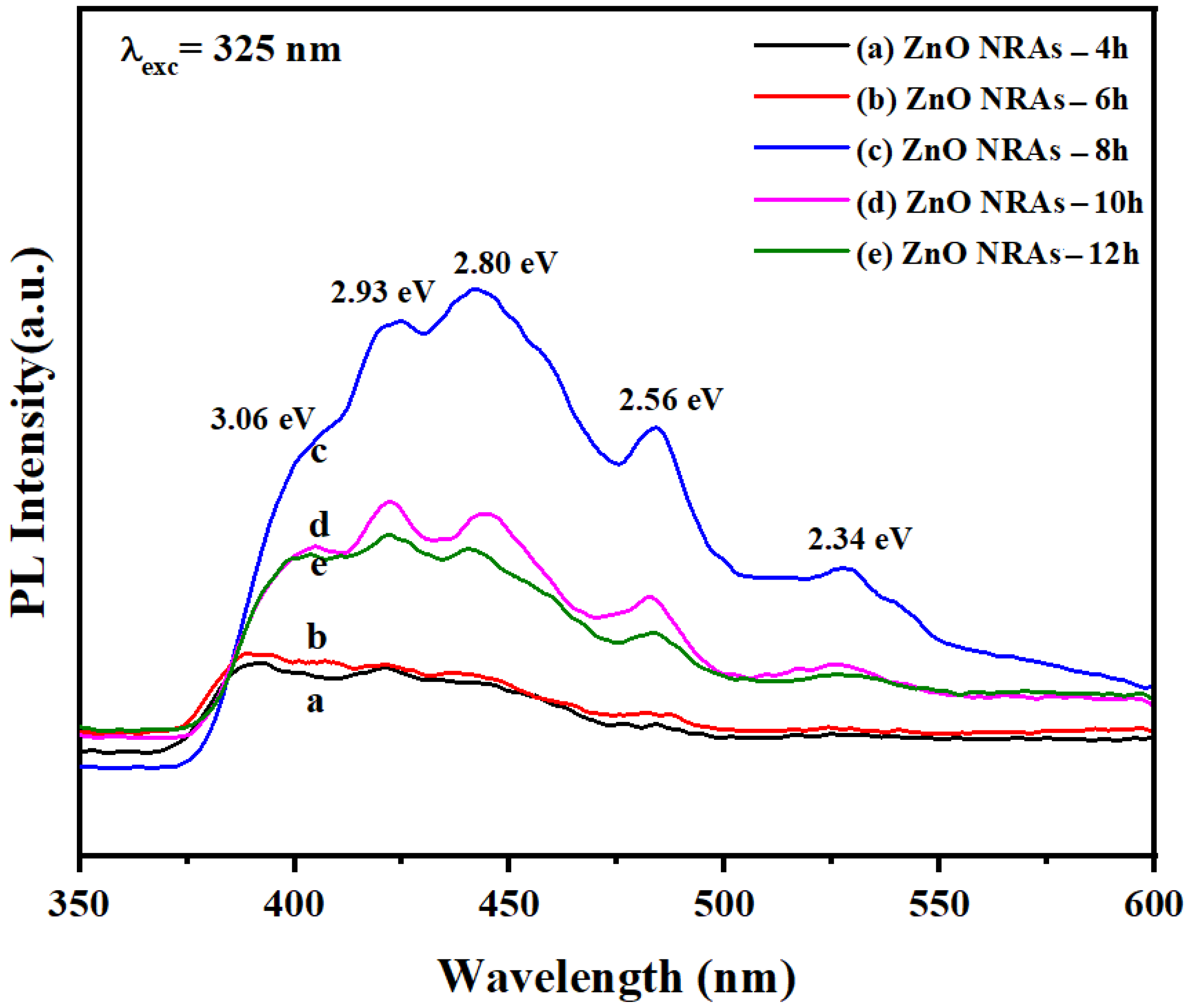
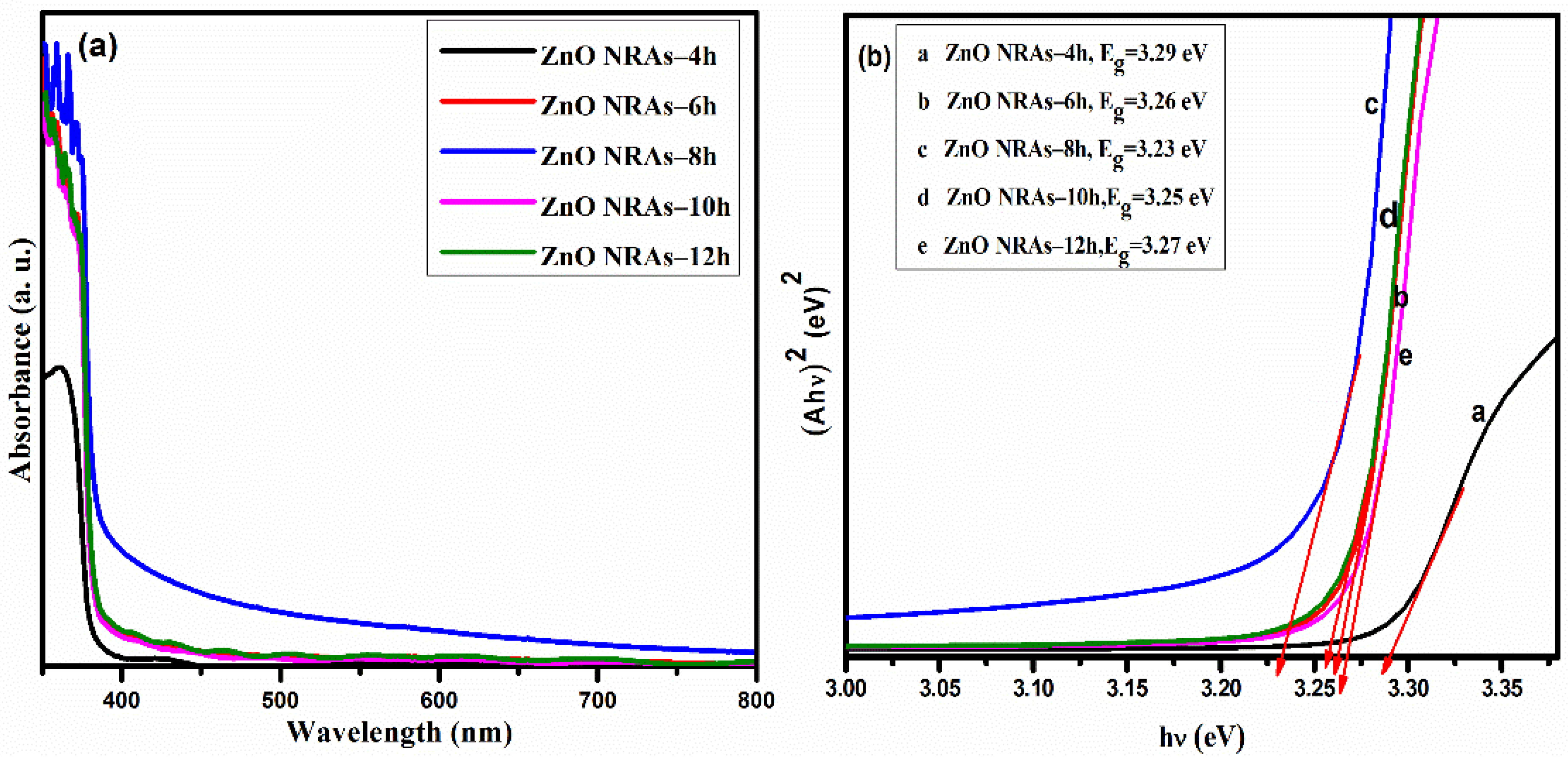
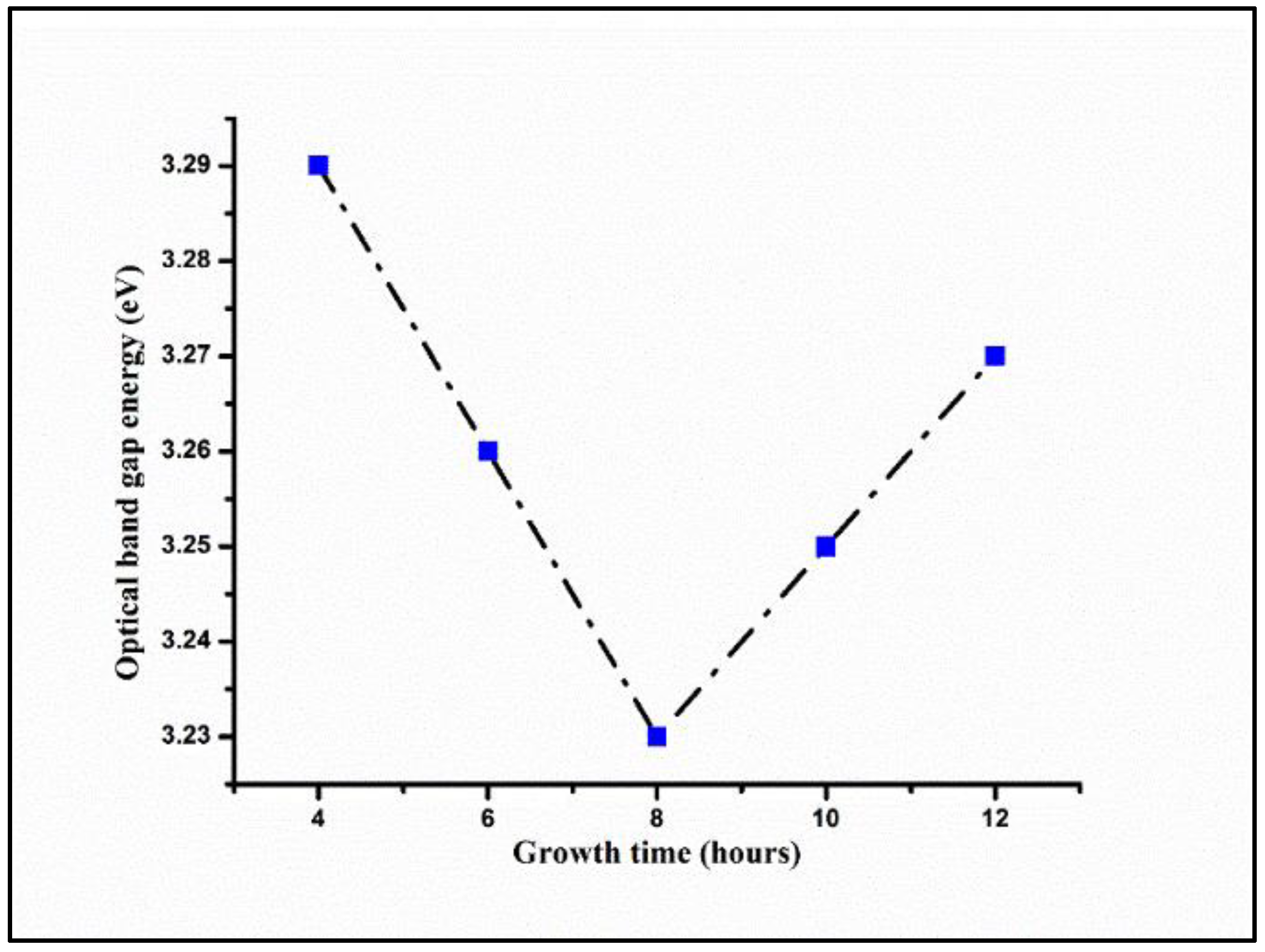
3.2. Effects of Growth Times on Optical Properties of ZNRAs
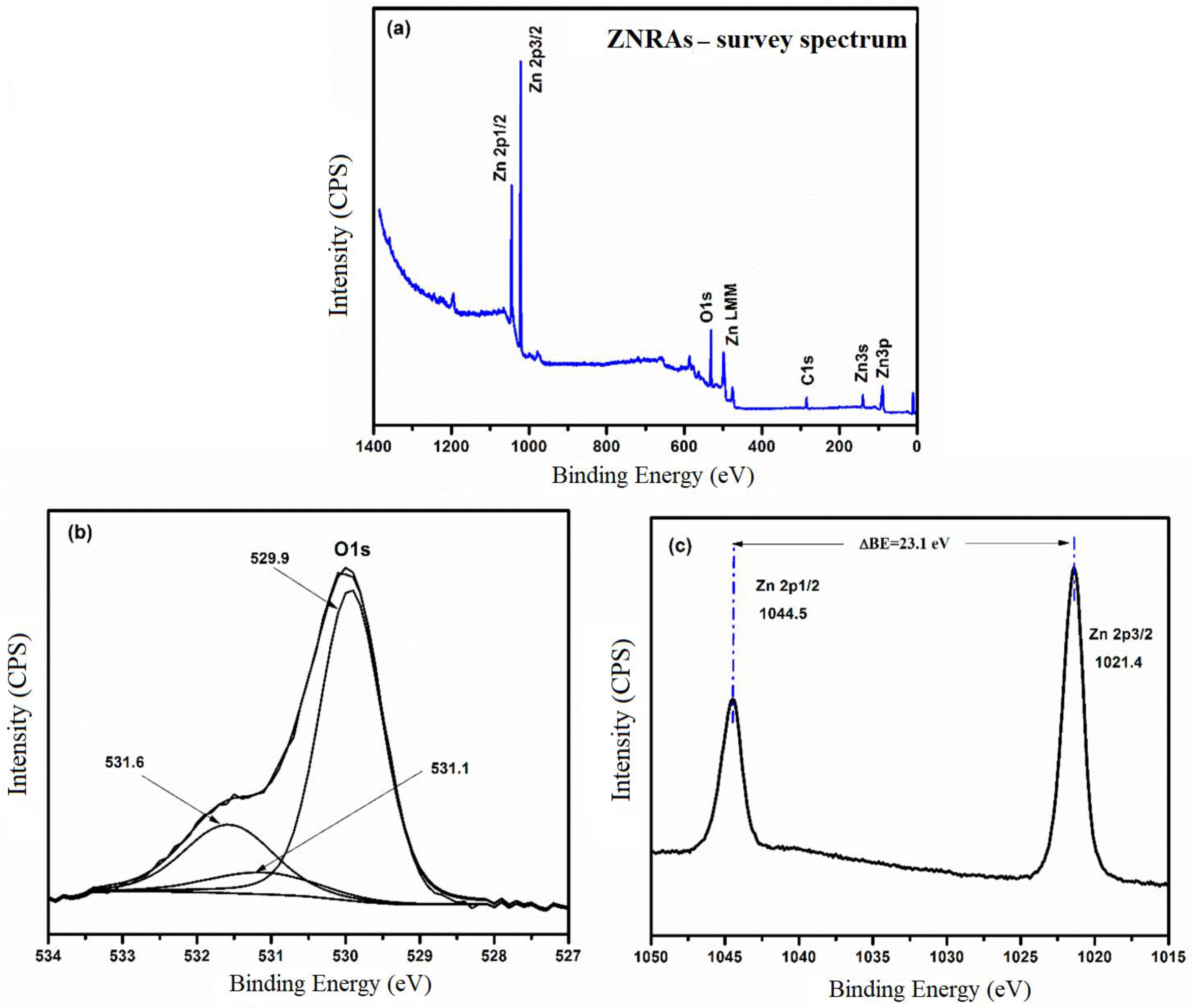
3.3. XPS Spectra of ZNRAs
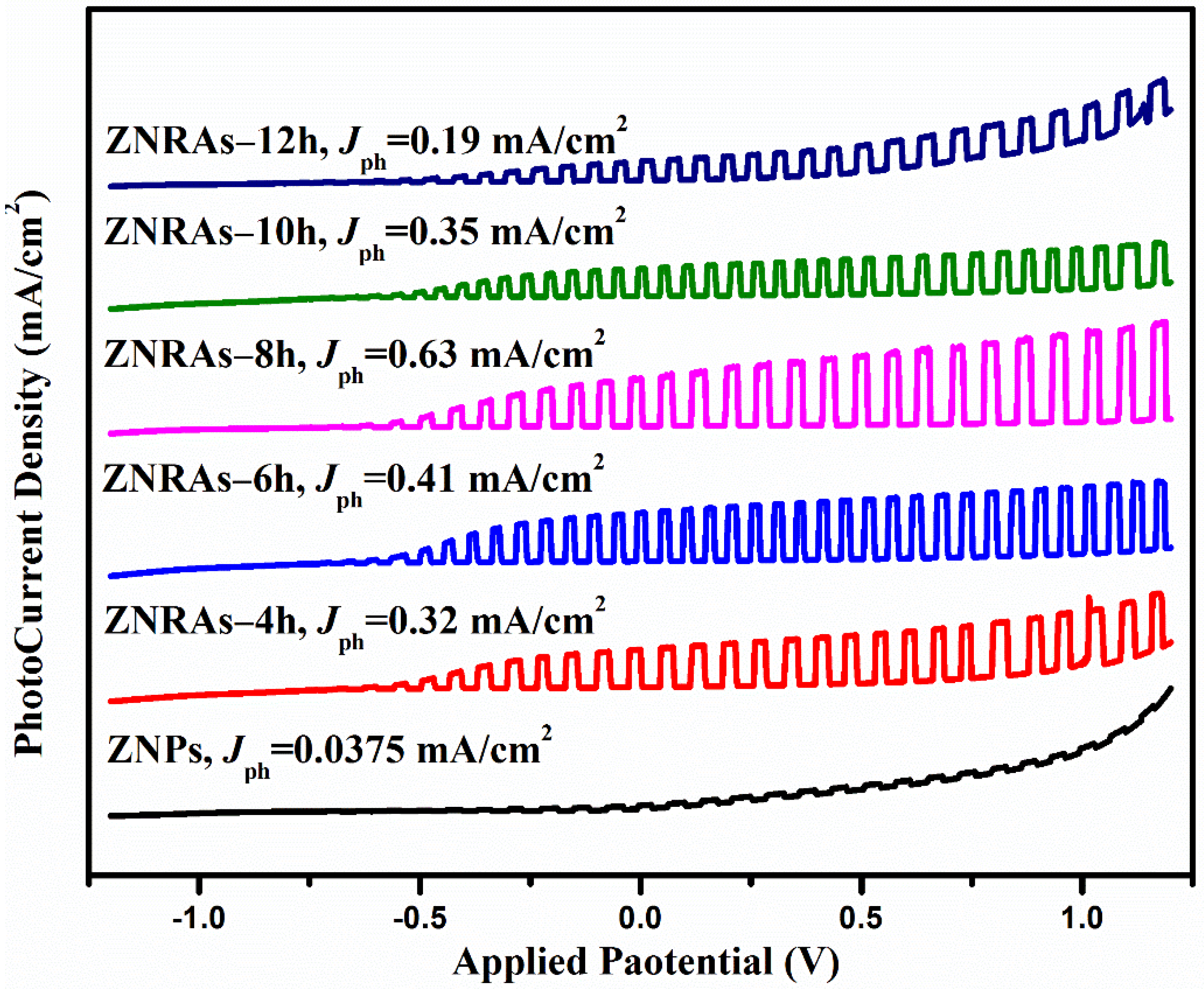
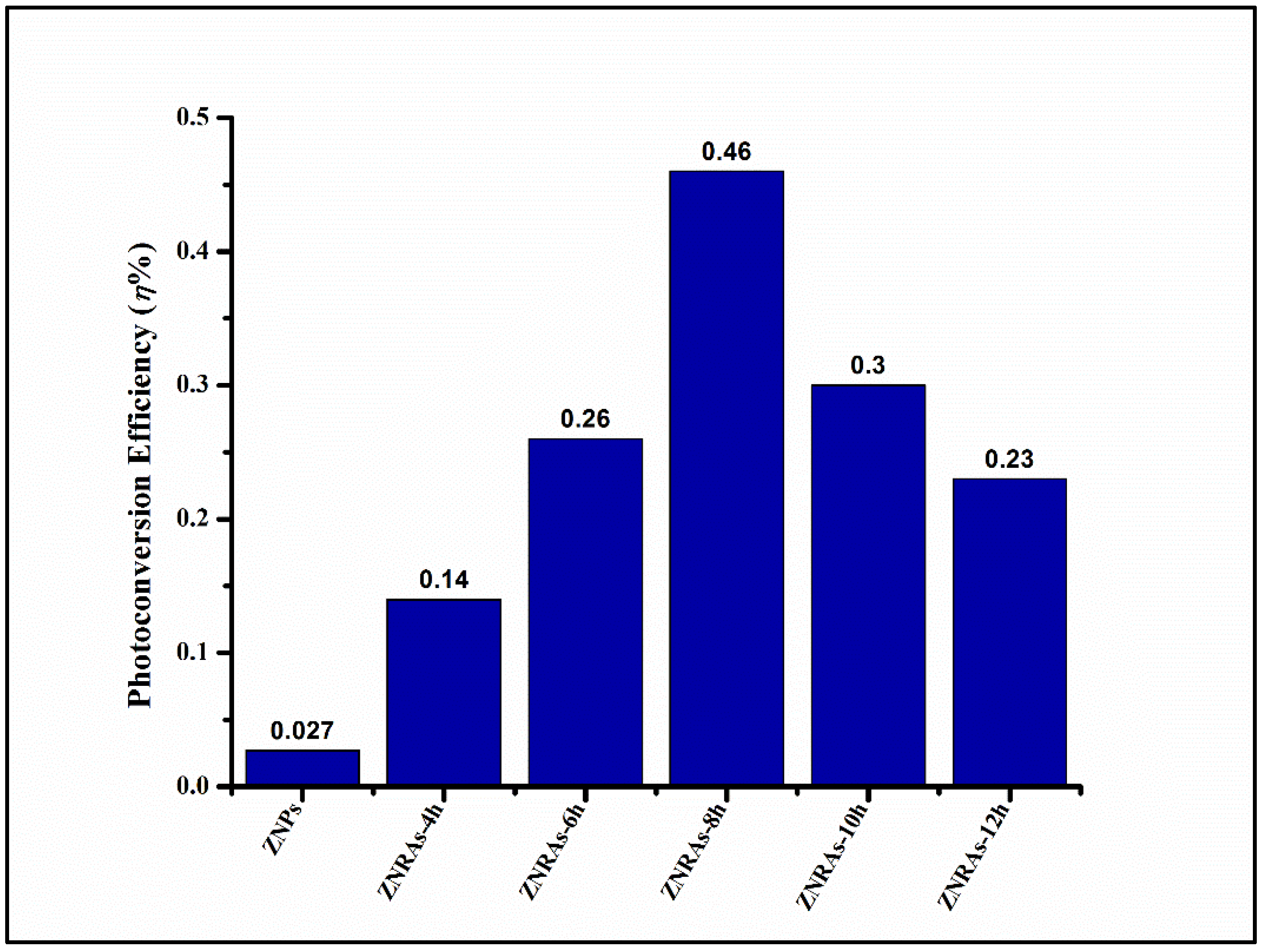
3.4. Photoelectrochemical Performance of the ZNRAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Photoelectrochemical cells. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; Nature Publishing Group: London, UK, 2011; pp. 26–32. [Google Scholar]
- Grimes, C.A.; Varghese, O.K.; Ranjan, S. Hydrogen generation by water splitting. In Light, Water, Hydrogen; Springer: Boston, MA, USA, 2008; pp. 35–113. [Google Scholar]
- Van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2012; Volume 90. [Google Scholar]
- Eswar, K.A.; Husairi, F.S.; Ab Aziz, A.; Rusop, M.; Abdullah, S. Effect of Post Annealing Temperature on Surface Morphology and Photoluminescence Properties of ZnO Thin Film. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014; Volume 832, pp. 654–658. [Google Scholar]
- Kuo, S.-Y.; Chen, W.-C.; Lai, F.-I.; Cheng, C.-P.; Kuo, H.-C.; Wang, S.-C.; Hsieh, W.-F. Effects of doping concentration and annealing temperature on properties of highly-oriented Al-doped ZnO films. J. Cryst. Growth 2006, 287, 78–84. [Google Scholar] [CrossRef]
- Gopal, V.R.V.; Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: A comparative study. Appl. Nanosci. 2017, 7, 75–82. [Google Scholar] [CrossRef]
- Regonini, D.; Teloeken, A.C.; Alves, A.K.; Berutti, F.A.; Gajda-Schrantz, K.; Bergmann, C.P.; Graule, T.; Clemens, F. Electrospun TiO2 fiber composite photoelectrodes for water splitting. ACS Appl. Mater. Interfaces 2013, 5, 11747–11755. [Google Scholar] [CrossRef]
- Ingler, W.B.; Khan, S.U. A Self-Driven p/n-Fe2O3 Tandem Photoelectrochemical Cell for Water Splitting. Electrochem. Solid-State Lett. 2006, 9, G144. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, H.; Fan, W.; Wang, L.; Zhao, H.; Zhao, X.; Yuan, S. Enhanced photoelectrochemical performance of tungsten oxide film by bifunctional Au nanoparticles. RSC Adv. 2017, 7, 15201–15210. [Google Scholar] [CrossRef]
- Miao, J.; Liu, B. II–VI semiconductor nanowires: ZnO. In Semiconductor Nanowires; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–28. [Google Scholar]
- Rattanawarinchai, P.; Khemasiri, N.; Jessadaluk, S.; Chananonnawathorn, C.; Horprathum, M.; Klamchuen, A.; Kayunkid, N.; Rahong, S.; Phromyothin, D.; Nukeaw, J. Growth time dependence on photoelectrochemical property of zinc oxide nanorods prepared by hydrothermal synthesis. Surf. Rev. Lett. 2018, 25 (Suppl. 1), 1840001. [Google Scholar] [CrossRef]
- Kim, D.; Leem, J.Y. Effect of the pH of an aqueous solution on the structural, optical, and photoresponse properties of hydrothermally grown ZnO nanorods and the fabrication of a high performance ultraviolet sensor. J. Korean Phys. Soc. 2018, 72, 400–405. [Google Scholar] [CrossRef]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Film. 1997, 306, 78–85. [Google Scholar] [CrossRef]
- Mah, C.F.; Yam, F.K.; Hassan, Z. Investigation and characterization of ZnO Nanostructures synthesized by electrochemical deposition. Procedia Chem. 2016, 19, 83–90. [Google Scholar] [CrossRef][Green Version]
- Thongsuksai, W.; Panomsuwan, G.; Rodchanarowan, A. Fast and convenient growth of vertically aligned ZnO nanorods via microwave plasma-assisted thermal evaporation. Mater. Lett. 2018, 224, 50–53. [Google Scholar] [CrossRef]
- Murkute, P.; Ghadi, H.; Saha, S.; Pandey, S.K.; Chakrabarti, S. Enhancement in optical characteristics of c-axis-oriented radio frequency–sputtered ZnO thin films through growth ambient and annealing temperature optimization. Mater. Sci. Semicond. Process. 2017, 66, 1–8. [Google Scholar] [CrossRef]
- Husna, J.; Aliyu, M.M.; Islam, M.A.; Chelvanathan, P.; Hamzah, N.R.; Hossain, M.S.; Karim, M.R.; Amin, N. Influence of annealing temperature on the properties of ZnO thin films grown by sputtering. Energy Procedia 2012, 25, 55–61. [Google Scholar] [CrossRef]
- Meng, F.; Ge, F.; Chen, Y.; Xu, G.; Huang, F. Local structural changes induced by ion bombardment in magnetron sputtered ZnO: Al films: Raman, XPS, and XAS study. Surf. Coat. Technol. 2019, 365, 2–9. [Google Scholar] [CrossRef]
- Nasirpouri, F. Electrodeposition of Nanostructured Materials; Springer International Publishing: Cham, Switzerland, 2017; Volume 62. [Google Scholar]
- Fudzi, L.M.; Zainal, Z.; Lim, H.N.; Chang, S.K.; Holi, A.M.; Ali, M.S. Effect of temperature and growth time on vertically aligned ZnO nanorods by simplified hydrothermal technique for photoelectrochemical cells. Materials 2018, 11, 704. [Google Scholar] [CrossRef]
- Worasawat, S.; Masuzawa, T.; Hatanaka, Y.; Neo, Y.; Mimura, H.; Pecharapa, W. Synthesis and characterization of ZnO nanorods by hydrothermal method. Mater. Today Proc. 2018, 5, 10964–10969. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wang, D.; Yang, Z.; Ji, W.; Zhang, C.; Zhao, Y. Synthesis of nanostructural ZnO using hydrothermal method for dye-sensitized solar cells. Sci. China Technol. Sci. 2010, 53, 1146–1149. [Google Scholar] [CrossRef]
- Holi, A.M.; Zainal, Z.; Talib, Z.A.; Lim, H.-N.; Yap, C.-C.; Chang, S.-K.; Ayal, A.K. Effect of hydrothermal growth time on ZnO nanorod arrays photoelectrode performance. Optik 2016, 127, 11111–11118. [Google Scholar] [CrossRef]
- Rana, A.U.H.S.; Chang, S.-B.; Chae, H.U.; Kim, H.-S. Structural, optical, electrical and morphological properties of different concentration sol-gel ZnO seeds and consanguineous ZnO nanostructured growth dependence on seeds. J. Alloys Compd. 2017, 729, 571–582. [Google Scholar] [CrossRef]
- AL-Zahrani, A.A.; Zainal, Z.; Talib, Z.A.; Ngee, J.L.H.; Mohd, L.; Fudzi, A.M.H.; Sarif, M.; Ali, M. Effect of Hydrothermal Growth Temperature and Time on Physical Properties And Photoanode Performance of ZnO Nanorods. Int. J. Nanoelectron. Mater. 2020, 13, 381–400. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prenrice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Shabannia, R. Vertically aligned ZnO nanorods on porous silicon substrates: Effect of growth time. Prog. Nat. Sci. Mater. Int. 2015, 25, 95–100. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ji, Y.; Ma, X.; Xu, J.; Que, D. Low temperature synthesis of flowerlike ZnO nanostructures by cetyltrimethylammonium bromide-assisted hydrothermal process. J. Phys. Chem. B 2004, 108, 3955–3958. [Google Scholar] [CrossRef]
- Krichevsky, O.; Stavans, J. Correlated Ostwald ripening in two dimensions. Phys. Rev. Lett. 1993, 70, 1473. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Study of ZnO sol–gel films: Effect of annealing. Mater. Lett. 2010, 64, 1147–1149. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Kim, S.H.; Al-Hajry, A.; Chauhan, M.S.; Chauhan, S. Growth, properties and dye-sensitized solar cells (DSSCs) applications of ZnO Nanocones and small nanorods. Sci. Adv. Mater. 2011, 3, 695–701. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef]
- Idiawati, R.; Mufti, N.; Taufiq, A.; Wisodo, H.; Laila, I.K.R.; Fuad, A. Effect of growth time on the characteristics of ZnO nanorods. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 202, p. 012050. [Google Scholar]
- Amiruddin, R.; Kumar, M.S. Enhanced visible emission from vertically aligned ZnO nanostructures by aqueous chemical growth process. J. Lumin. 2014, 155, 149–155. [Google Scholar] [CrossRef]
- Ahmed, G.; Hanif, M.; Zhao, L.; Hussain, M.; Khan, J.; Liu, Z. Defect engineering of ZnO nanoparticles by graphene oxide leading to enhanced visible light photocatalysis. J. Mol. Catal. A Chem. 2016, 425, 310–321. [Google Scholar] [CrossRef]
- Sabri, N.S.; Yahya, A.K.; Talari, M.K. Emission properties of Mn doped ZnO nanoparticles prepared by mechanochemical processing. J. Lumin. 2012, 132, 1735–1739. [Google Scholar] [CrossRef]
- Mendil, D.; Challali, F.; Touam, T.; Chelouche, A.; Souici, A.H.; Ouhenia, S.; Djouadi, D. Influence of growth time and substrate type on the microstructure and luminescence properties of ZnO thin films deposited by RF sputtering. J. Lumin. 2019, 215, 116631. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S.G. ZnO nanoparticles: Structural, optical and photoconductivity characteristics. J. Alloys Compd. 2012, 539, 1–6. [Google Scholar] [CrossRef]
- Krishna Reddy, G.; Jagannatha Reddy, A.; Hari Krishna, R.; Nagabhushana, B.M.; Gopal, G.R. Luminescence and spectroscopic investigations on Gd3+ doped ZnO nanophosphor. J. Asian Ceram. Soc. 2017, 5, 350–356. [Google Scholar] [CrossRef]
- Musavi, E.; Khanlary, M.; Khakpour, Z. Red-orange photoluminescence emission of sol-gel dip-coated prepared ZnO and ZnO: Al nano-crystalline films. J. Lumin. 2019, 216, 116696. [Google Scholar] [CrossRef]
- Hitkari, G.; Singh, S.; Pandey, G. Structural, optical and photocatalytic study of ZnO and ZnO–ZnS synthesized by chemical method. Nano-Struct. Nano-Objects 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Roza, L.; Fauzia, V.; Abd Rahman, M.Y. Tailoring the active surface sites of ZnO nanorods on the glass substrate for photocatalytic activity enhancement. Surf. Interfaces 2019, 15, 117–124. [Google Scholar] [CrossRef]
- Abdulrahman, A.F.; Ahmed, S.M.; Almessiere, M.A. Effect of the growth time on the optical properties of zno nanorods grown by low temperature method. Dig. J. Nanomater. Biostruct. 2017, 12, 1001–1009. [Google Scholar]
- He, H.; Berglund, S.P.; Xiao, P.; Chemelewski, W.D.; Zhang, Y.; Mullins, C.B. Nanostructured Bi₂S₃/WO₃ heterojunction films exhibiting enhanced photoelectrochemical performance. J. Mater. Chem. A 2013, 1, 12826–12834. [Google Scholar] [CrossRef]
- Yang, X.; Hu, L.; Deng, H.; Qiao, K.; Hu, C.; Liu, Z.; Yuan, S.; Khan, J.; Li, D.; Tang, J.; et al. Improving the performance of PbS quantum dot solar cells by optimizing ZnO window layer. Nano-Micro Lett. 2017, 9, 24. [Google Scholar] [CrossRef]
- Hou, T.F.; Boppella, R.; Shanmugasundaram, A.; Kim, D.H.; Lee, D.W. Hierarchically self-assembled ZnO architectures: Establishing light trapping networks for effective photoelectrochemical water splitting. Int. J. Hydrog. Energy 2017, 42, 15126–15139. [Google Scholar] [CrossRef]
- Balachandran, S.; Swaminathan, M. The simple, template free synthesis of a Bi 2 S 3–ZnO heterostructure and its superior photocatalytic activity under UV-A light. Dalton Trans. 2013, 42, 5338–5347. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Basu, R.N. Fabrication of Bi 2 S 3/ZnO heterostructures: An excellent photocatalyst for visible-light-driven hydrogen generation and photoelectrochemical properties. New J. Chem. 2018, 42, 541–554. [Google Scholar] [CrossRef]
- Al-Saadi, M.J.; Al-Harthi, S.H.; Kyaw, H.H.; Myint, M.T.; Bora, T.; Laxman, K.; Al-Hinai, A.; Dutta, J. Influence of atomic hydrogen, band bending, and defects in the top few nanometers of hydrothermally prepared zinc oxide nanorods. Nanoscale Res. Lett. 2017, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; Lozano, H.; Cases-Utrera, J.; Lee, M.; Esteve, J. Improving morphological quality and uniformity of hydrothermally grown ZnO nanowires by surface activation of catalyst layer. Nanoscale Res. Lett. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.K.; Hashim, M.R.; Allam, N.K. ZnO nano-tetrapod photoanodes for enhanced solar-driven water splitting. Chem. Phys. Lett. 2012, 549, 62–66. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Yan, Y.; Shet, S.; Jones, K.; Deutsch, T.; Turner, J.; Al-Jassim, M. ZnO nanocoral structures for photoelectrochemical cells. Appl. Phys. Lett. 2008, 93, 163117. [Google Scholar] [CrossRef]
- Faid, A.Y.; Allam, N.K. Stable solar-driven water splitting by anodic ZnO nanotubular semiconducting photoanodes. RSC Adv. 2016, 6, 80221–80225. [Google Scholar] [CrossRef]
- Baek, M.; Kim, D.; Yong, K. Simple but effective way to enhance photoelectrochemical solar-water-splitting performance of ZnO nanorod arrays: Charge-trapping Zn(OH)2 annihilation and oxygen vacancy generation by vacuum annealing. ACS Appl. Mater. Interfaces 2017, 9, 2317–2325. [Google Scholar] [CrossRef] [PubMed]



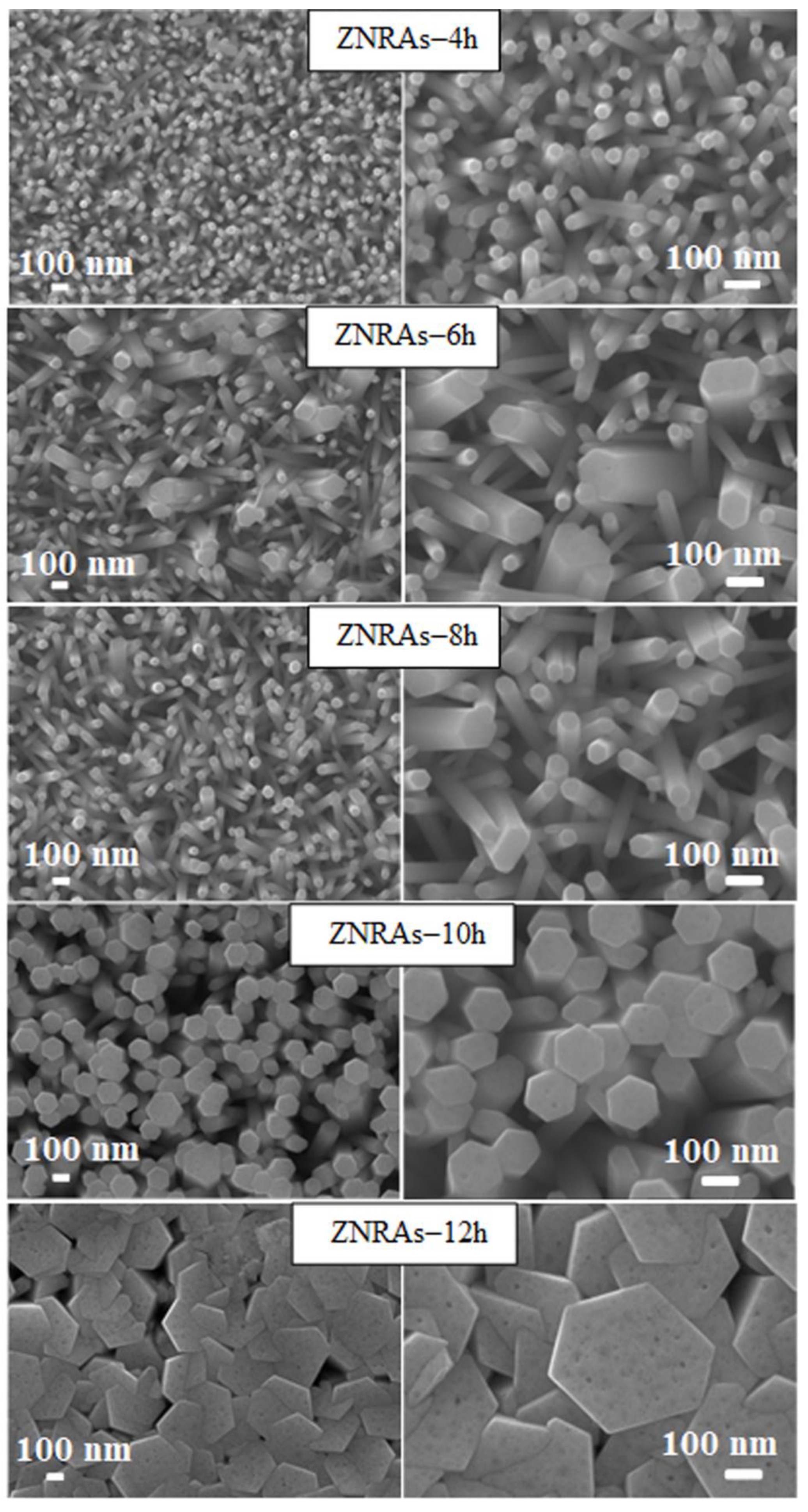
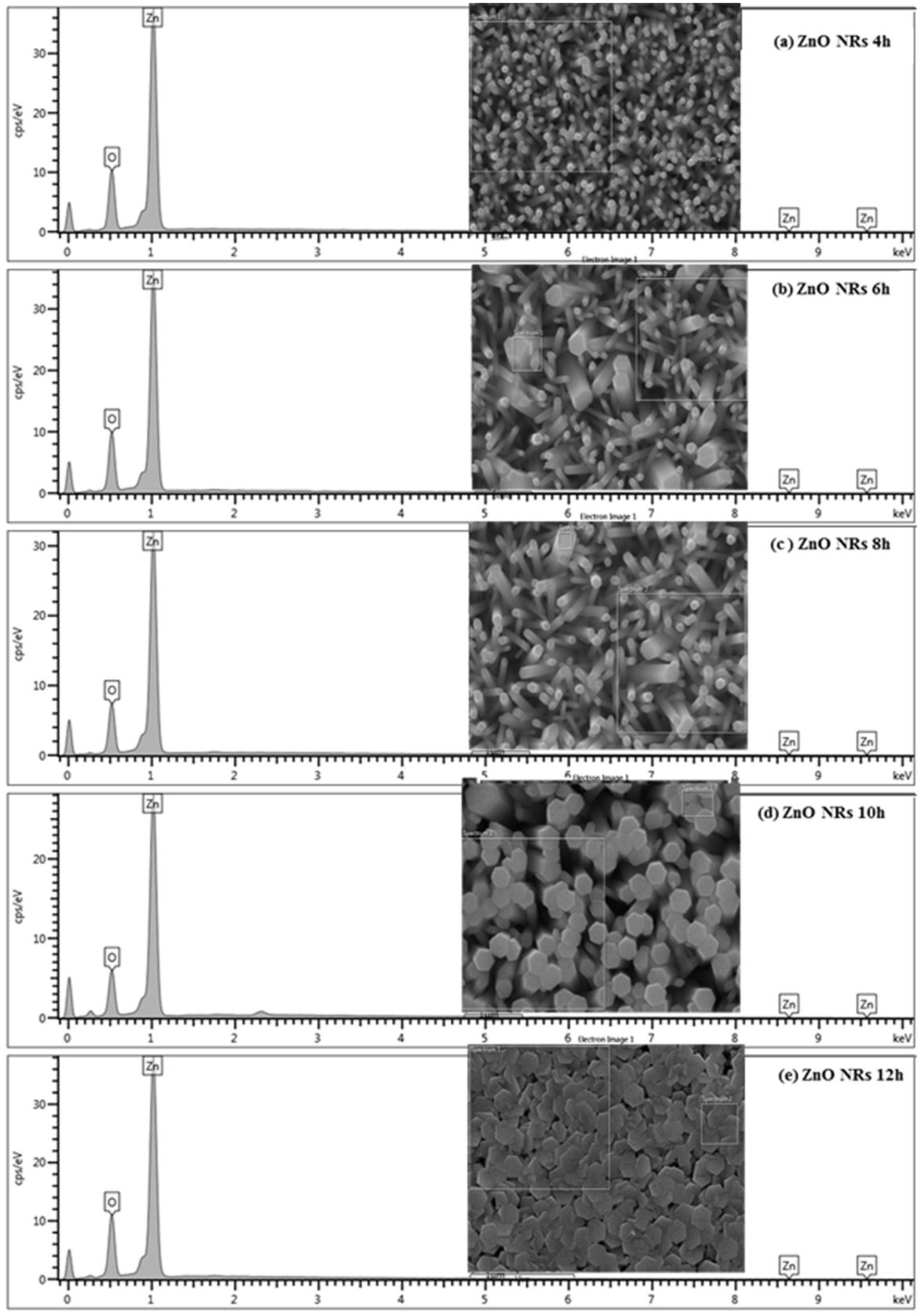
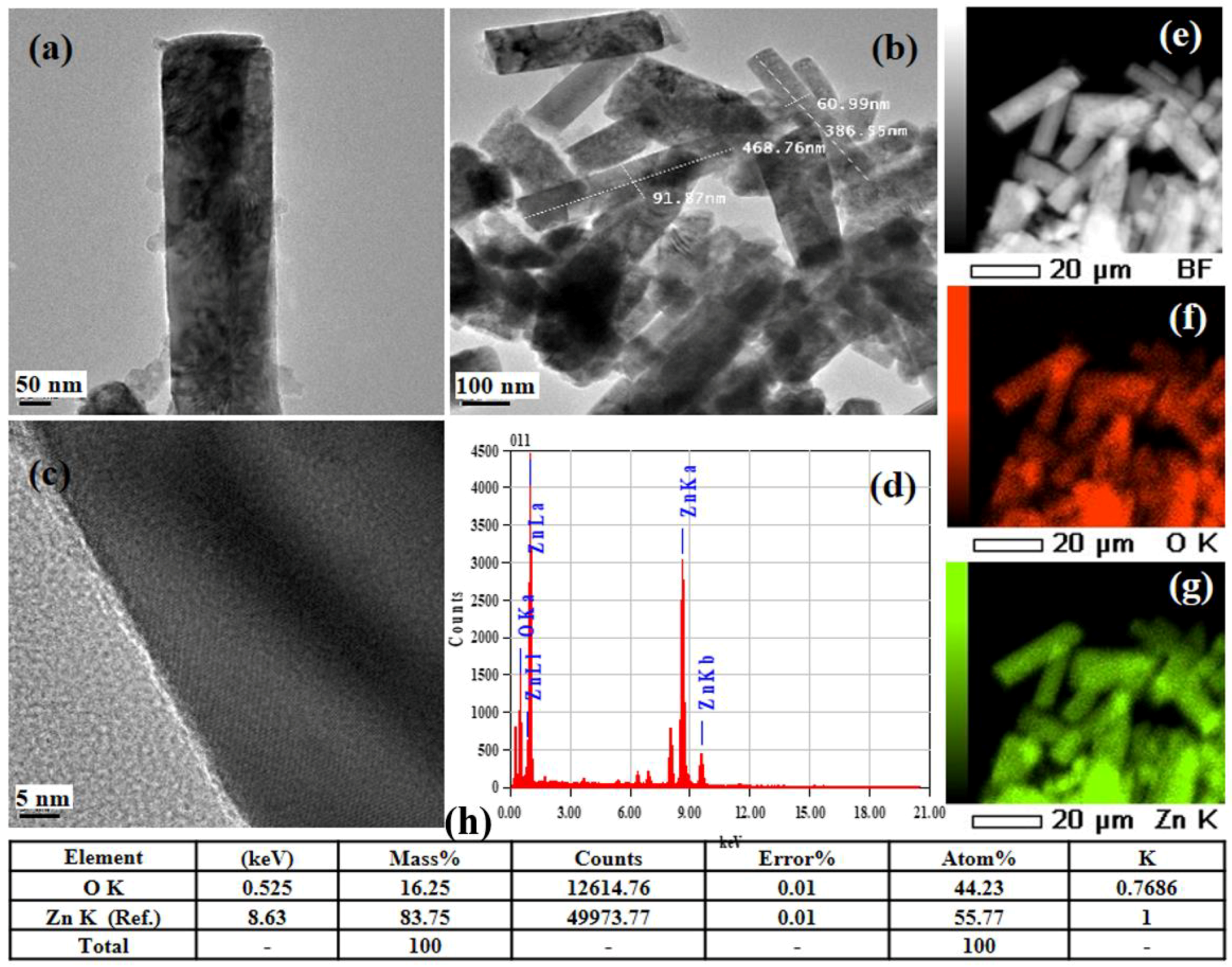
| Element | ZNRAs-4h | ZNRAs-6h | ZNRAs-8h | ZNRAs-10h | ZNRAs-12h |
|---|---|---|---|---|---|
| O | 45.76 | 45.51 | 41.97 | 40.09 | 46.86 |
| Zn | 54.24 | 54.49 | 58.03 | 59.91 | 53.14 |
| Total: | 100 | 100 | 100 | 100 | 100 |
| Photoelectrode | Growth Method | Electrolyte | Illumination Intensity | Jph (mAcm−2) | Ref. |
|---|---|---|---|---|---|
| ZNRs | Hydrothermal | 0.1 M Na2S + 0.1 M Na2SO3 | 100 mW/cm2 | 0.37 at 0.0 V vs. Ag/AgCl | [23] |
| ZNRs | Hydrothermal | 0.1 M Na2S + 0.1 M Na2SO3 | 100 mW/cm2 | 0.337 at 0.5 V vs. Ag/AgCl | [26] |
| ZnO (nanotetrapod) | Thermal evaporation | 0.5 M Na2SO4 | 100 mW/cm2 | 0.40 at 0.8 V vs. Ag/AgCl | [53] |
| ZNRs | Hydrothermal | 0.1 M Na2S + 0.1 M Na2SO3 | 100 mW/cm2 | 0.194 at 0.5 V vs. Ag/AgCl | [20] |
| ZnO (nanocoral) | RF magnetron, thermal oxidation | 0.5 M Na2SO4 | 125 mW/cm2 | 0.25 at 1.2 V vs. Ag/AgCl | [54] |
| ZNRs | Hydrothermal | 0.5 M Na2SO4 | 100 mW/cm2 | 0.62 at 1.2 V vs. RHE | [48] |
| ZnO (nanotubes) | Electrochemical anodization | 0.5 M Na2SO4 | 100 mW/cm2 | 0.52 at 0.25 V vs. SCE | [55] |
| ZNRs | Vacuum annealed | 0.5 M Na2SO4 | 100 mW/cm2 | 0.60 at 1.23 V vs. RHE | [56] |
| ZNRAs-8h | Hydrothermal | 0.1 M Na2S + 0.1 M Na2SO3 | 100 mW/cm2 | 0.63 at 0.5 V vs. Ag/AgCl | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almamari, M.R.; Ahmed, N.M.; Holi, A.M.; Yam, F.K.; Kyaw, H.H.; Almessiere, M.A.; Al-Abri, M.Z. Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time. Materials 2022, 15, 5827. https://doi.org/10.3390/ma15175827
Almamari MR, Ahmed NM, Holi AM, Yam FK, Kyaw HH, Almessiere MA, Al-Abri MZ. Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time. Materials. 2022; 15(17):5827. https://doi.org/10.3390/ma15175827
Chicago/Turabian StyleAlmamari, Mohammed Rashid, Naser M. Ahmed, Araa Mebdir Holi, F. K. Yam, Htet Htet Kyaw, M. A. Almessiere, and Mohammed Z. Al-Abri. 2022. "Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time" Materials 15, no. 17: 5827. https://doi.org/10.3390/ma15175827
APA StyleAlmamari, M. R., Ahmed, N. M., Holi, A. M., Yam, F. K., Kyaw, H. H., Almessiere, M. A., & Al-Abri, M. Z. (2022). Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time. Materials, 15(17), 5827. https://doi.org/10.3390/ma15175827









