Two Magnetic Orderings and a Spin–Flop Transition in Mixed Valence Compound Mn3O(SeO3)3
Abstract
:1. Introduction
2. Experimental Section
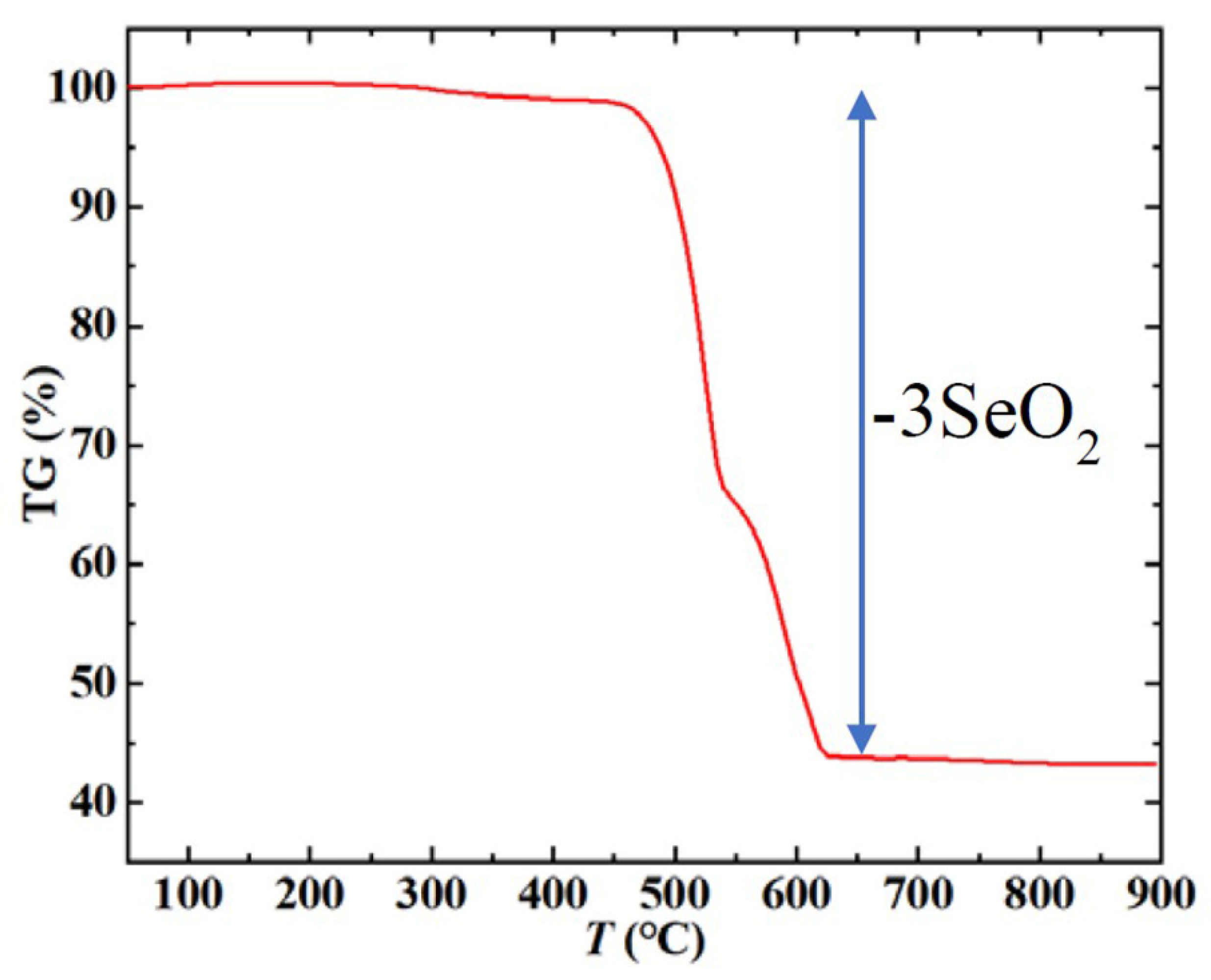
2.1. Synthesis of Mn3O(SeO3)3
2.2. Methods
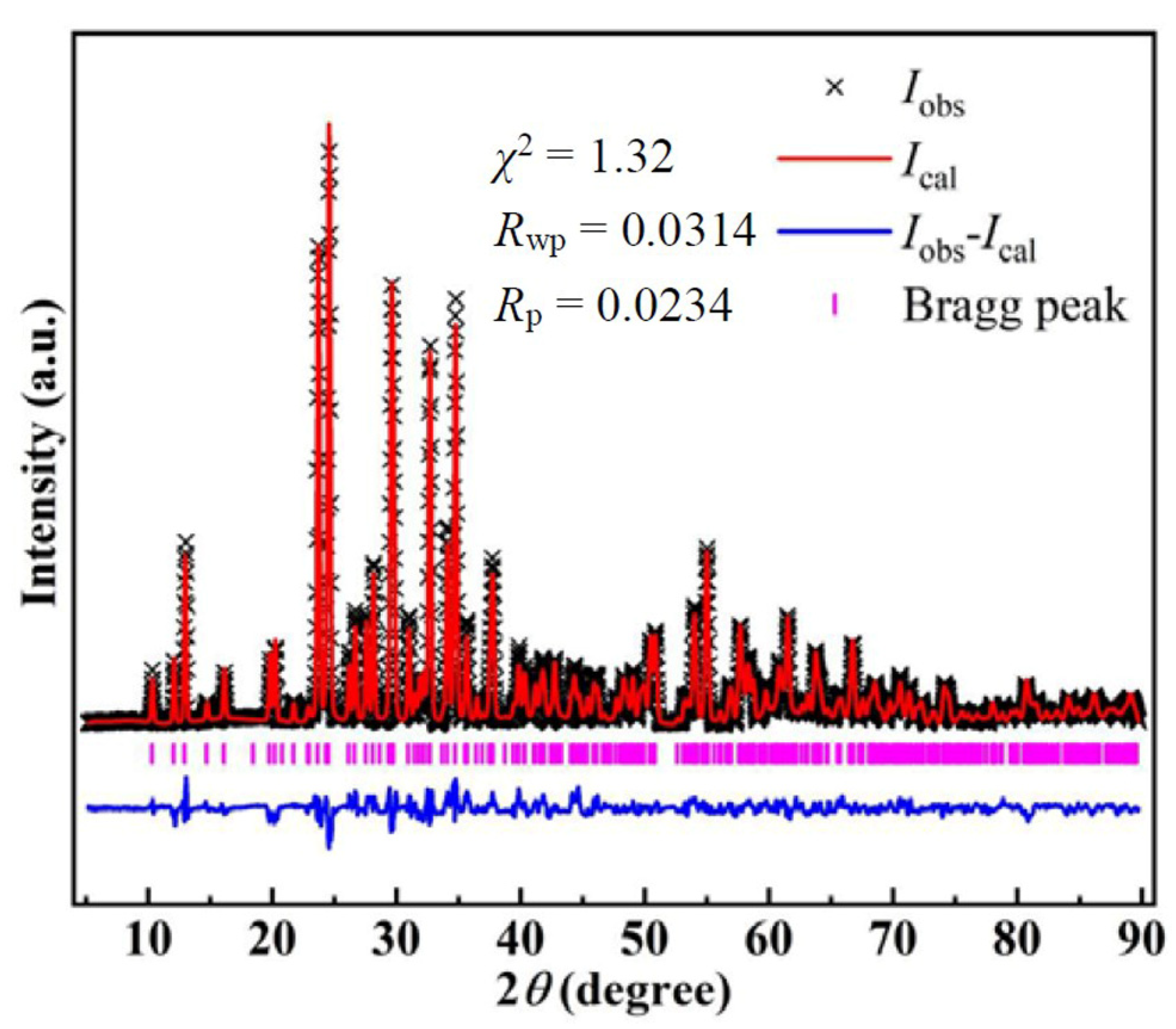
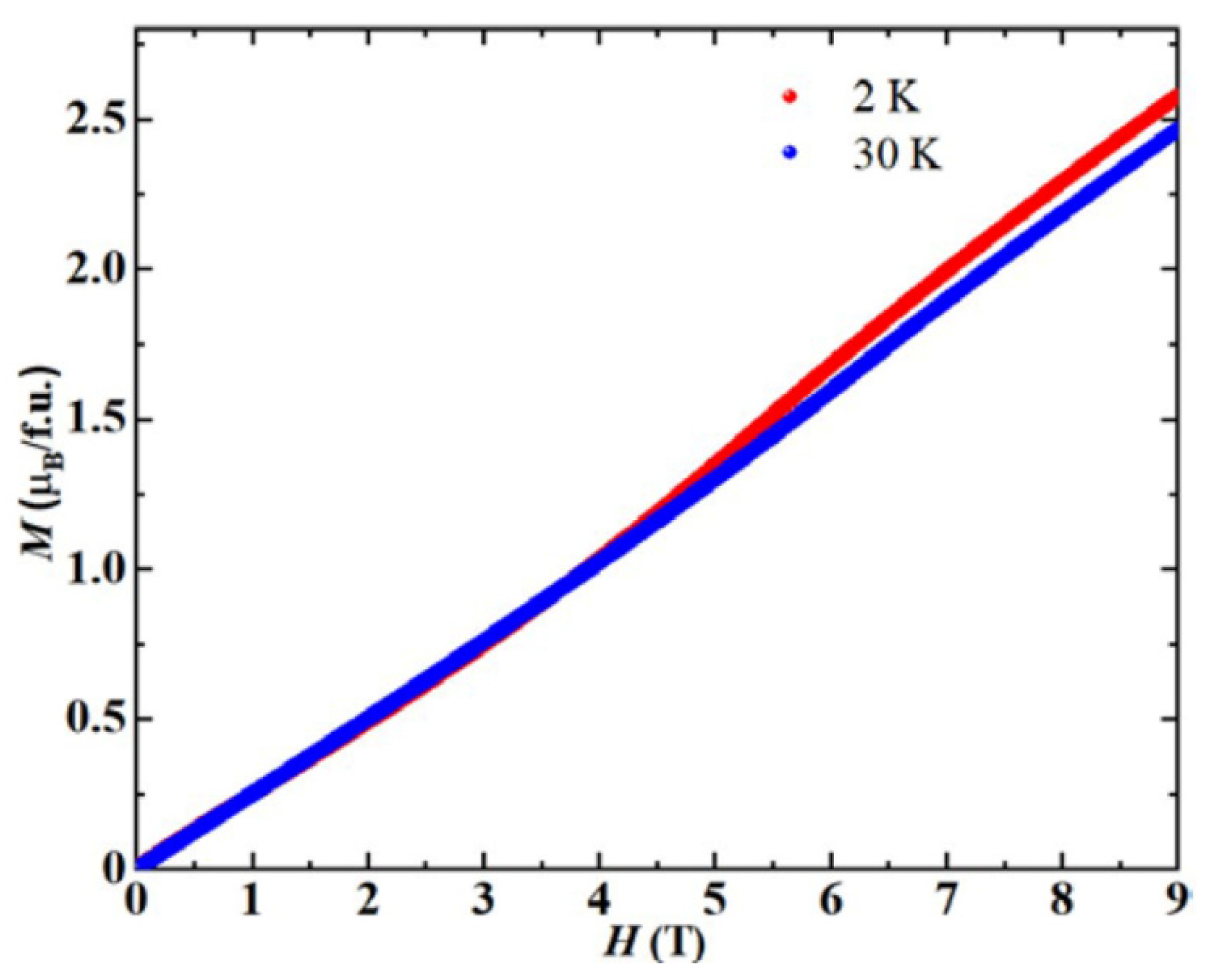
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varma, C.M. Mixed-valence compounds. Rev. Mod. Phys. 1976, 48, 219–238. [Google Scholar] [CrossRef]
- Brown, D.B. Mixed-Valence Compounds: Theory and Applications in Chemistry, Physics, Geology, and Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sleight, A.W. Chemistry of high-temperature superconductors. Science 1988, 242, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Mori, S.; Chen, C.H.; Cheong, S.W. Percolative phase separation underlies colossal magnetoresistance in mixed-valent manganites. Nature 1999, 399, 560–563. [Google Scholar] [CrossRef]
- Tranquada, J.M. John Goodenough and the many lives of transition-metal oxides. J. Electrochem. Soc. 2022, 169, 010535. [Google Scholar] [CrossRef]
- Saha, R.A.; Bandyopadhyay, A.; Schiesaro, I.; Bera, A.; Mondal, M.; Meneghini, C.; Ray, S. Colossal electroresistance response accompanied by metal-insulator transition in a mixed-valent vanadate. Phys. Rev. B 2021, 104, 045149. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, H.; Zhu, X.; Xing, Z.; Liu, Q.; Liu, T.; Gao, S.; Lu, S.; Chen, G.; Asiri, A.M.; et al. Identifying the origin of Ti3+ activity toward enhanced electrocatalytic N2 reduction over TiO2 nanoparticles modulated by mixed-valent copper. Adv. Mater. 2020, 32, 2000299. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Cai, T.; Dong, W.; Tang, L.; Xia, X.; Wang, L.; Li, T. Boosting photocatalytic performance in mixed-valence MIL-53(Fe) by changing FeII/FeIII ratio. ACS Appl. Mater. Inter. 2019, 11, 28791–28800. [Google Scholar] [CrossRef]
- Michel, C.; Hervieu, M.; Borel, M.M.; Grandin, A.; Deslandes, F.; Provost, J.; Raveau, B. Superconductivity in the Bi-Sr-Cu-O system. Z. Phys. B 1987, 68, 421–423. [Google Scholar] [CrossRef]
- Raveau, B. Copper mixed valence concept: “Cu(I)–Cu(II)” in thermoelectric copper sulfides—an alternative to “Cu(II)–Cu(III)” in superconducting cuprates. J. Supercond. Nov. Magn. 2019, 33, 259–263. [Google Scholar] [CrossRef]
- Zinzuvadiya, S.; Pandya, R.J.; Singh, J.; Joshi, U.S. Low field magnetotransport behavior of barium hexaferrite/ferromagnetic manganite bilayer. J. Appl. Phys. 2021, 130, 024102. [Google Scholar] [CrossRef]
- Hasegawa, K.; Isobe, M.; Yamauchi, T.; Ueda, H.; Yamaura, J.; Gotou, H.; Yagi, T.; Sato, H.; Ueda, Y. Discovery of ferromagnetic-half-metal-to-insulator transition in K2Cr8O16. Phys. Rev. Lett. 2009, 103, 146403. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Bak, S.M.; Shin, Y.; Zhang, R.; Wang, C.; Kisslinger, K.; Ge, M.; Huang, X.; Shadike, Z.; Pattammattel, A.; et al. Hierarchical nickel valence gradient stabilizes high-nickel content layered cathode materials. Nat. commun. 2021, 12, 2350. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Zhao, H.; Hu, Y.; Hao, S.; Liu, J.; Li, H.; Liu, X. Enhancing surface stability of LiNi0.8Co0.1Mn0.1O2 cathode with hybrid core-shell nanostructure induced by high-valent titanium ions for Li-ion batteries at high cut-off voltage. J. Alloy. Compd. 2020, 834, 155099. [Google Scholar] [CrossRef]
- Yu, X.; Tokunaga, Y.; Taguchi, Y.; Tokura, Y. Variation of topology in magnetic bubbles in a colossal magnetoresistive manganite. Adv. Mater. 2017, 29, 1603958. [Google Scholar] [CrossRef] [PubMed]
- Wildner, M. Crystal structure of Mn(II)Mn(III)2O(SeO3)3. J. Solid State Chem. 1994, 113, 252–256. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Balents, L. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef]
- Aidoudi, F.H.; Aldous, D.W.; Goff, R.J.; Slawin, A.M.; Attfield, J.P.; Morris, R.E.; Lightfoot, P. An ionothermally prepared S = 1/2 vanadium oxyfluoride kagome lattice. Nat. Chem. 2011, 3, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; McQueen, T.M.; Chisne, R.; Freedman, D.E.; Muller, P.; Lee, Y.S.; Nocera, D.G. A Cu2+ (S = 1/2) kagomé antiferromagnet: MgxCu4−x(OH)6Cl2. J. Am. Chem. Soc. 2010, 132, 5570–5571. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B. Magnetism and the Chemical Bond; John Wiley and Sons: New York, NY, USA, 1966. [Google Scholar]
- Whangbo, M.H.; Koo, H.J.; Dai, D.; Jung, D. Effect of metal-ligand bond lengths on superexchange interactions in Jahn-Teller d4 ion systems: Spin dimer analysis of the magnetic structure of Marokite CaMn2O4. Inorg. Chem. 2002, 41, 5575–5581. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Peng, C.; Ran, S.-J.; Liu, T.; Chen, X.; Su, G. Fermionic algebraic quantum spin liquid in an octa-kagomé frustrated antiferromagnet. Phys. Rev. B 2017, 95, 075140. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Peng, C.; Guo, W.; Wang, J.F.; Su, G.; He, Z. Octa-kagomé lattice compounds showing quantum critical behaviors: Spin gap ground state versus antiferromagnetic ordering. J. Am. Chem. Soc. 2017, 139, 14057–14060. [Google Scholar] [CrossRef] [PubMed]

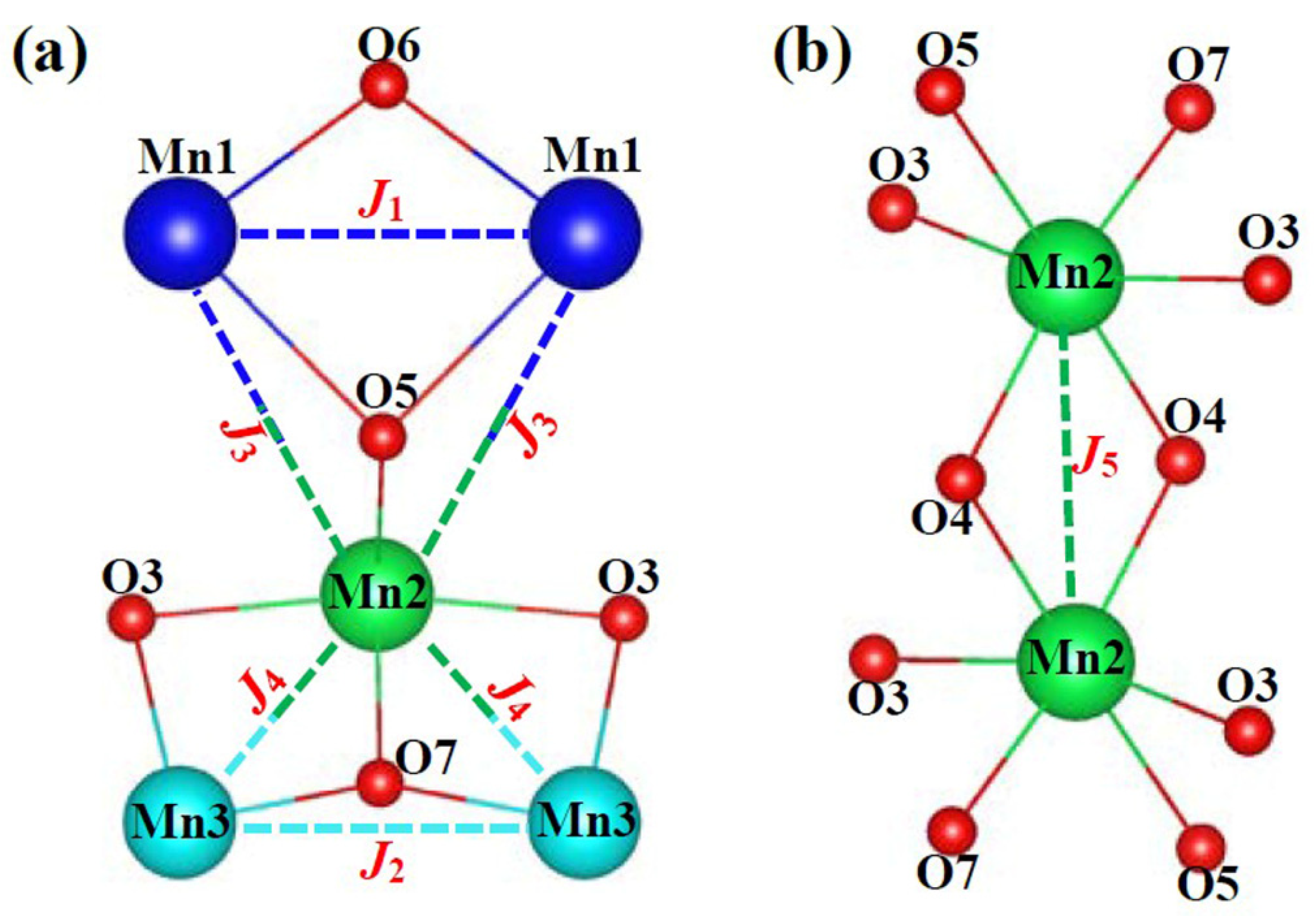

| J1 | d(Mn-O) (Å) | d(Mn-Mn) (Å) | Mn-O-Mn Angle (°) | Magnetism |
|---|---|---|---|---|
| J1 | Mn1-2.130(7)-O6-2.130(7)-Mn1 Mn1-2.361(4)-O5-2.361(4)-Mn1 | 3.332(9) | Mn1-O6-Mn1…102.90(8)AFM-w Mn1-O5-Mn1…89.77(1)FM-S | FM |
| J2 | Mn3-1.857(1)-O7-1.857(1)-Mn3 | 3.332(9) | Mn3-O7-Mn3…127.61(5)AFM-S | AFM |
| J3 | Mn1-2.361(4)-O5-2.278(1)-Mn2 | 3.902(1) | Mn1-O5-Mn2…114.49(4)AFM-S | AFM |
| J4 | Mn2-2.049(5)-O3-2.310(4)-Mn3 Mn2-1.854(2)-O7-1.857(1)-Mn3 | 3.069(6) | Mn2-O3-Mn3…89.29(7)FM-S Mn2-O7-Mn3…111.59(8)AFM-S | ? |
| J5 | Mn2-1.896(5)-O4-2.169(5)-Mn2 Mn2-2.169(5)-O4-1.896(5)-Mn2 | 3.150(0) | Mn2-O4-Mn2…101.34(9)AFM-w × 2 | AFM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Cui, M.; Tian, J.; Jiang, P.; Qian, G.; Lu, X. Two Magnetic Orderings and a Spin–Flop Transition in Mixed Valence Compound Mn3O(SeO3)3. Materials 2022, 15, 5773. https://doi.org/10.3390/ma15165773
Zhang W, Cui M, Tian J, Jiang P, Qian G, Lu X. Two Magnetic Orderings and a Spin–Flop Transition in Mixed Valence Compound Mn3O(SeO3)3. Materials. 2022; 15(16):5773. https://doi.org/10.3390/ma15165773
Chicago/Turabian StyleZhang, Wanwan, Meiyan Cui, Jindou Tian, Pengfeng Jiang, Guoyu Qian, and Xia Lu. 2022. "Two Magnetic Orderings and a Spin–Flop Transition in Mixed Valence Compound Mn3O(SeO3)3" Materials 15, no. 16: 5773. https://doi.org/10.3390/ma15165773
APA StyleZhang, W., Cui, M., Tian, J., Jiang, P., Qian, G., & Lu, X. (2022). Two Magnetic Orderings and a Spin–Flop Transition in Mixed Valence Compound Mn3O(SeO3)3. Materials, 15(16), 5773. https://doi.org/10.3390/ma15165773






