3.1. Effect of Infiltration Time on the Microstructure of SiC Matrix Composites
To investigate the microstructure evolution of the SiC matrix composites, the effect of infiltration holding time were studied. During the infiltration procedure, SiC ceramic samples were immersed in a molten Al base alloy and then the Al base alloy infiltrated the SiC ceramic.
Figure 1 shows the SEM and corresponding element distribution map of the infiltration and no infiltration parts of SiC matrix ceramics. From
Figure 1a,b, it can be seen that the left side of the dashed line is the SiC ceramic sample with melted Al base alloy, and the right side is the part without alloy. There are very obvious differences between the two parts. As shown in
Figure 1c, purple represents the distribution of element Si. The Si distributed relatively uniform at the right side without the Al base alloy, because the SiC matrix ceramic is mainly composed of SiC particles and Si embedding in the gaps of SiC particles. However, the purple Si elements mainly present as irregular and blocky in the infiltrated part, and the gaps of SiC particles present as dark. Combined with
Figure 1d, the green color represents the Al element distribution, and it can be found that the green color mainly distributes in the left part infiltrated the Al base alloy and does not exist in the right part without the Al base alloy. Moreover, from the green color distribution, it can be seen that Al elements are mainly distributed around the irregular mass around. Therefore, it can be inferred that Al elements are mainly distributed in the gaps of SiC particles during the infiltration process.
Figure 1e–g are the distribution maps of C, Ti, and Cu elements, respectively. However, the color of Ti and Cu are rarely collected, indicating that the contents of Ti and Cu in the SiC matrix composites are relatively low through the infiltration process.
Figure 2a shows the image of no infiltration (up) and infiltration (down). It is obvious that Al alloy infiltrated the SiC matrix ceramics exists at the gaps of SiC particles. According to the EDS analysis of Point 1 from
Figure 2b, the ratio of Si and Al is higher than the Al base alloy. It can be inferred that the Al base alloy dissolved the Si existing in the gaps of the SiC particles and then penetrated the SiC ceramic sample. It also can be found that the content of Ti and Cu is low, and corresponds to the element maps in
Figure 1.
Through analysing the
Figure 1 and
Figure 2, it is found that the distribution of Al elements can be used to determine whether the alloy infiltrates SiC ceramics. The content of Al can be detected when it infiltrates the SiC ceramic sample. Accordingly,
Figure 3 shows the EDS analysis of the sample centre infiltrated at 800 °C for a different time.
Figure 3a shows that original SiC ceramic mainly contains 40 at.% C and 60 at.% Si.
Figure 3b–d exhibit the EDS analysis of the sample centre infiltrated at 800 °C for 30 min, 2 h, and 6 h, respectively. The corresponding Al content was 15.72 at.%, 13.69 at.% and 14.81 at.%. It can be inferred that melting Al can infiltrate the SiC ceramic sample with 5 mm thickness completely when the infiltration time is above 30 min at 800 °C.
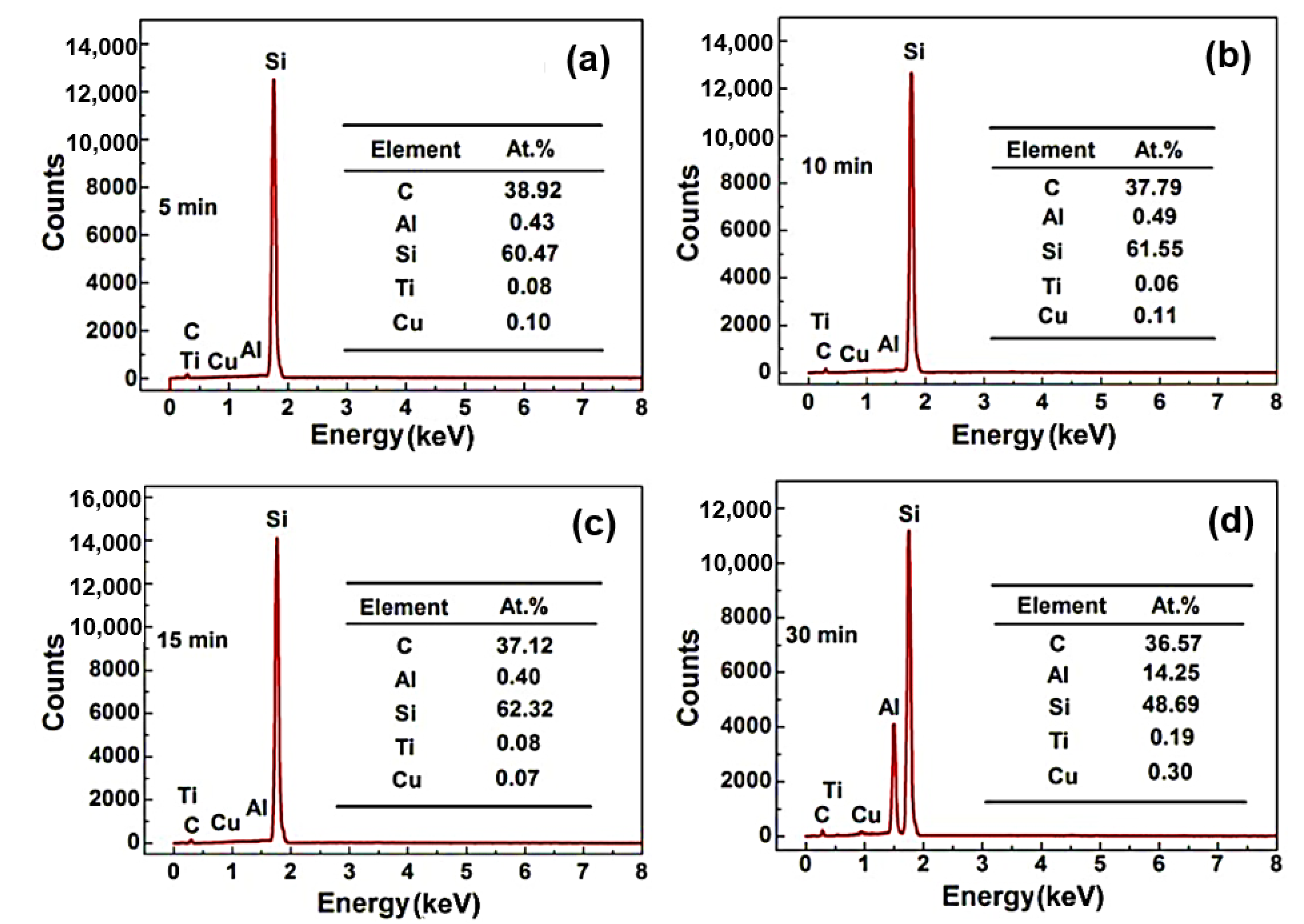
Figure 4 shows the EDS analysis of SiC ceramic sample centre infiltrated by the Al base alloy at 900 °C for different times (5 min, 10 min, 15 min, 30 min). As seen from
Figure 4a–c, the centre of sample mainly contains C, Si elements, and trace amounts of Al, Ti, and Cu.
Figure 4d shows the sample centre after being infiltrated for 30 min and containing 14.25 at.% Al, 36.57 at.% C, and 48.69 at.% Si. It is noted that the centre of the sample does not contain the Al element when the infiltration time is less than 15 min, which indicates that the alloy does not infiltrate the sample centre. From the above analysis, the sample with 5 mm thickness would be completely infiltrated by the alloy after 30 min at 800 °C and 900 °C. Therefore, it can be inferred that the sample with 5 mm thickness could be infiltrated completely under an infiltration condition with a temperature higher than 800 °C and a time longer than 30 min.
The porosity of the original SiC ceramic is 1.48 vol.%, measured by the Archimedes method [
3]. Molten Al base alloy could infiltrate into SiC ceramic samples with 5 mm thickness at 800 °C, 900 °C and 1000 °C for a time longer than 30 min. It is obviously different from traditional impregnation relying on the open porous. Melton Al alloy made contactwith the Si, embedding in the gaps of SiC particles, and then dissolving Si gradually. The channels for Al alloy infiltrating into SiC samples were built, therefore the Al alloy can infiltrate the SiC sample.
3.2. Effect of Infiltration Temperature on the Microstructure of SiC Matrix Composites
Figure 5 shows XRD patterns of the surface of SiC ceramic that infiltrated the Al base alloy at 800, 900, and 1000 °C for 6 h. After infiltration at 800 °C, there was no obvious change to the SiC structure. The surface of the SiC ceramic contains mainly SiC and Al, with a small amount of oxides and Si. As the infiltration temperature increased to 900 °C, diffraction peaks of Ti
3Si(Al)C
2 at 2
θ = 34.13°, 39.58°, 42.55°, and 60.33° became more intense, with that of SiC simultaneously decreasing. After infiltration at 1000 °C, diffraction peaks of TiC at 2
θ = 35.89°, 41.68° were more intense than those of the SiC and Ti
3Si(Al)C
2 phases, indicating that more TiC was generated.
The XRD analysis implies that the constituent phases in the reaction layer change significantly when the infiltration temperature changes. To explore this in more detail, elemental analyses were carried out using X-ray photoelectron spectroscopy (XPS). As shown in
Figure 6a, the Si 2p spectrogram of samples infiltrated at 800 °C for 6 h mainly consists of three peaks (99.7, 100.6, and 101.9 eV), corresponding to the Si–Si bond of Si, Si–C bond of SiC, and Si–O bond of SiO
x, respectively. The samples infiltrated at 900 °C for 6 h gave mainly four peaks (98.8, 99.8, 100.2, and 101.9 eV), corresponding to the Si–Ti bond of TiSi
2, Si–Si bond of Si, Si–C bond of SiC, and Si–O bond of SiO
x, respectively. The samples infiltrated at 1000 °C had similar peaks to that at 900 °C, but no peak corresponding to the Si–Si bond was present. The Ti 2p spectra in
Figure 6b for the samples infiltrated at 800 °C consist of three peaks (453.3, 457.5, and 458.9 eV). The former peak corresponds to the binding energy of Ti–Al of Al
3Ti, and the latter two peaks correspond to the energy of the Ti–O bond in TiO
2. The samples infiltrated at 900 °C displayed five peaks (453.86, 454.89, 458.37, 459.1, and 464.19 eV), which correspond to the Si–Ti, Ti–C, Ti–O, Si–Ti, and Ti–O bonds. Infiltration at 1000 °C gave four peaks (454.6, 455.3, 458.4, and 460.51 eV). The former peak corresponds to the Ti–C bond in TiC, and the latter three peaks correspond to the Ti–O bond in TiO
2. The C 1 s spectra in
Figure 6c demonstrates that all the samples included the C–C bond. After infiltration at 800 °C, the main peak is of the C–Si bond, whereas those after infiltration at 900 °C were of C–Si and C–Ti bonds. At 1000 °C, the C–Ti bond dominates. The Al 2p spectra in
Figure 6d shows that the main bonds in the samples infiltrated at 800 °C are Al–O and Al–Al, whereas after infiltration at 900 or 1000 °C, the major bond is of Al–O alone. The XPS results demonstrate that there are slight oxides on the surface. The major phases of the composites are SiC and Al after 800 °C infiltration, SiC and Ti
3SiC
2 after 900 °C infiltration, and SiC and TiC after 1000 °C infiltration, which coincide with the results of the XRD phase analysis in
Figure 5.
The effects of infiltration temperature can be clearly seen from the above analysis. After infiltration at 800 °C, the diffraction peaks revealed that there was no formation of Ti3Si(Al)C2, whereas at 900 °C, Ti3Si(Al)C2 formed obviously. As the infiltration temperature increased further to 1000 °C, diffraction peaks of TiC appeared, and the intensity of Ti3Si(Al)C2 decreased.
Previously, El-Raghy [
11] and Harimkar [
25] reported that the intermediate phases in the formation of Ti
3SiC
2 (from Ti/SiC/C powders) were TiC
x and Ti
5Si
3C
x. Using 3Ti/1Si/2C powder, Zhang et al. found that Ti
5Si
3C
x formed at about 820 °C, and TiC
x formed at about 980 °C, by DSC, XRD, and EDS analyses [
26]. Hence, we performed DSC analyses on mixtures of Al base alloy and SiC ceramic (
Figure 7) to determine the formation temperatures of phases in the infiltrated samples. An endothermic peak at 570 °C and exothermic peaks starting at about 900 and 1000 °C can be observed. In the DSC curve of the Al base alloy, only the endothermic peak appears at about 570 °C and hence it can be inferred to be the melting point of the Al base alloy, noting that the composition of Al is 76.5 wt.%. Feng et al. [
27] reported that the reaction to form Ti
3SiC
2 is exothermic. Hence, by combining the results with those of XRD and EDS, the exothermic peak starting at 900 °C was assigned to the formation of Ti
3Si(Al)C
2. The lack of peaks around 820 °C show that the formation process of Ti
3Si(Al)C
2 is different from that in the works of El-Raghy [
11] and Harimkar [
25], and therefore that TiC
x and Ti
5Si
3C
x were not intermediate phases in the formation of Ti
3SiC
2 from infiltrating molten Al base alloy into SiC ceramic.
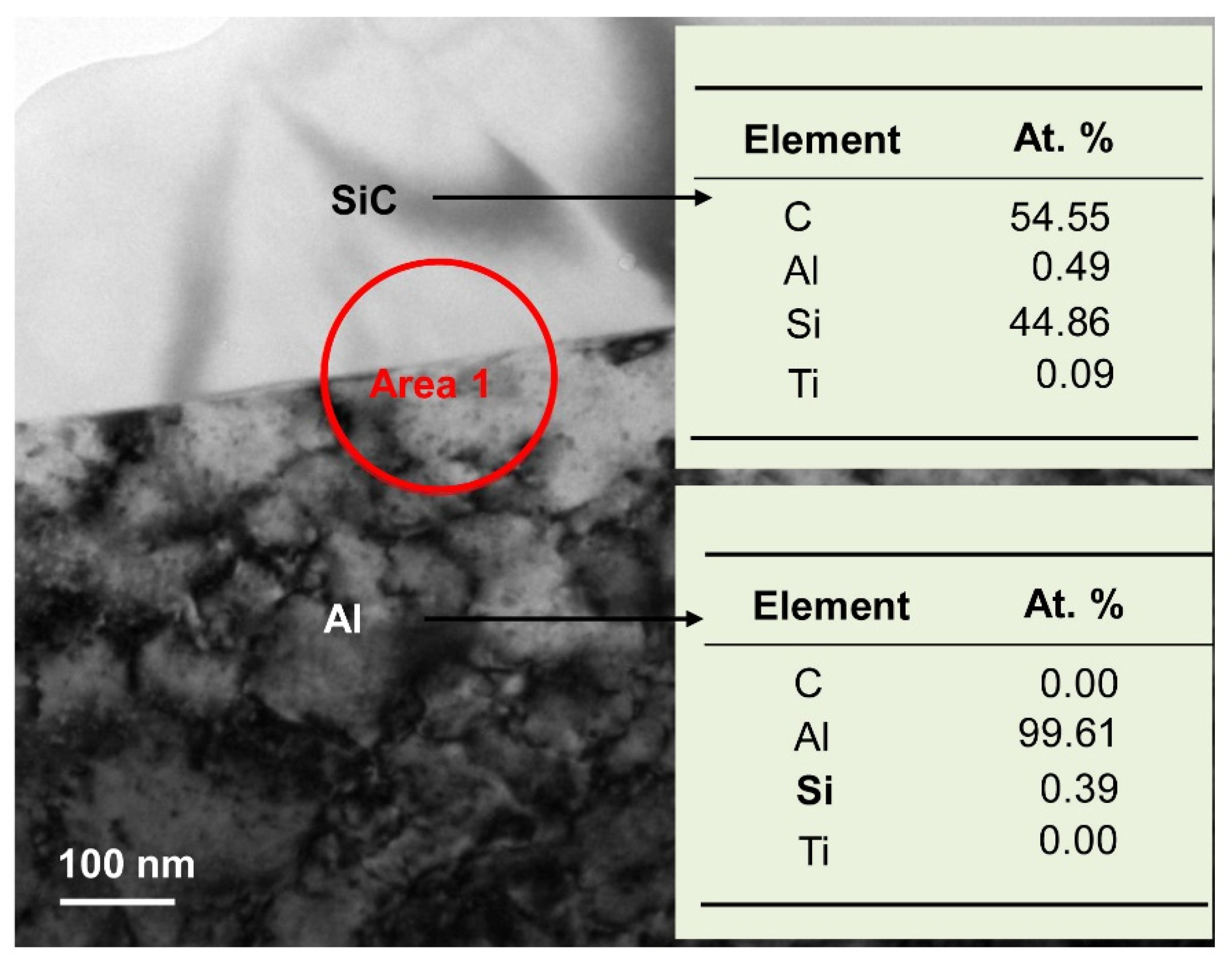
To obtain more information on the microstructures, cross sectional TEM and EDS analyses were performed, as shown in
Figure 8. SiC was seen to coexist with Al, which is consistent with the XRD results (
Figure 5). HRTEM images and SAED patterns of area 1 (from
Figure 8) are shown in
Figure 9. SiC phase and Al phase are evidenced by the HRTEM images in
Figure 9a,b; high magnification micrographs of the interface between SiC (ICDD PDF: 48-0708, hexagonal,
a = 0.3081 nm,
c = 0.503 nm) and Al (ICDD PDF: 04-0787, cubic,
a = 0.4049 nm) are shown in
Figure 9b. Infiltration at 800 °C produced an incoherent interface, which implies that the interfacial energy is large, and the formation of new phases at the interface is difficult. About three atomic layers at the interface occurred dislocation and some vacancies occurred in the interface of Al and SiC phases, but the Al phase is in contact with the SiC phase. The corresponding SAED pattern (shown in
Figure 9c) reveals no other compounds formed at the interface, which is in good agreement with the XRD results. Therefore, Al alloy dissolved Si existing in the SiC particles gap gradually, and the channels for Al alloy infiltrating SiC samples were built. Then, the Al alloy can infiltrate the SiC sample without new phase formation.
SEM, EDS, and TEM analyses were performed on SiC ceramics infiltrated by Al base alloy at 900 °C for 6 h to investigate the in situ synthesis process of Ti
3Si(Al)C
2. From
Figure 10a,b, and
Table 1, it can be seen that Ti
3Si(Al)C
2 is generated in situ on the SiC ceramic during infiltration. The size of the SiC grains gradually decreased as the Ti
3Si(Al)C
2 grains grew. A higher magnification image of the sample is shown in
Figure 10), where a white Ti
3Si(Al)C
2 phase can be observed near a grey SiC phase and a black Al–Si alloy structure. The EDS line scanning analyses in
Figure 10d,e indicate that a transition layer of Al base alloy exists on the SiC phase, from which we can infer that molten Al penetrates into the SiC ceramic samples. According to the distributions of Ti, Si, Al, and C (
Figure 10h–k, respectively), it can be seen that white phase mainly contains Ti, Si, C, and a small amount of Al, thus corresponding to the Ti
3Si(Al)C
2 phase. The lower left corner is mainly Al, whereas the lower right corner contains Si and C; therefore, it is inferred that the lower left is Al and the lower right is SiC. Al distribute in the interface between Ti
3Si(Al)C
2 and SiC, which further illustrates that Ti
3Si(Al)C
2 is formed by the reaction of Ti with Al and SiC. At the same time, it can be deduced that Al atoms were substituted into a small number of Si sites in Ti
3SiC
2, thus forming Ti
3Si(Al)C
2.
The above analysis further confirms that neither TiC (TiC
x) nor Ti
5Si
3 (Ti
5Si
3C
x) are present in the interface between Ti
3Si(Al)C
2 and SiC. Hence, to elucidate the synthesis process, SiC ceramics infiltrated with Al base alloy at 900 °C for 0.5 h were examined by TEM and SAED (
Figure 11a). It can be seen that Ti
3Si(Al)C
2 coexists with Al
3Ti and Al phases. Due to the short infiltration time, only a small amount of Ti
3Si(Al)C
2 had formed. Hence, unreacted Al and Ti crystallized to the Al and Al
3Ti phase when cooling after the infiltration process, showing that the molten Al base alloy surrounds and reacts with SiC during melt infiltration. In order to ascertain the growth mechanism, TEM investigations of the interface between SiC and Ti
3Si(Al)C
2 were carried out. The low magnification TEM image in
Figure 11c shows SiC grains surrounded by Ti
3Si(Al)C
2 particles, which were further confirmed by the corresponding SAED pattern in
Figure 11d. The SiC phase belongs to the space group
P6
3/
mmc (ICDD PDF 48-0708), and its lattice parameters are
a = 0.3079 nm and
c = 2.0147 nm. Ti
3Si(Al)C
2 also belongs to the space group
P6
3/
mmc (ICDD PDF 48-1826), and its lattice parameters are
a = 0.3068 nm and
c = 1.767 nm. Hence, SiC and Ti
3Si(Al)C
2 both have a hexagonal crystal unit cell with similar lattice parameters.
Figure 11e shows that the lattice at the interface is continuous and matches well, and only existing a few of vacancies. According to
Figure 9b, it can be inferred that molten Al lowers the Si–C bonding strength and decompose SiC [
28]. This was similarly found in the work of Fan [
6]. He et al. [
29] also found that when SiC is in contact with molten Ag–Cu–Ti alloy, it can dissolve into Si and C, and then react with active Ti species. The vacancies and special orientation relationship between SiC and Ti
3SiC
2 reduce the interfacial energy, which is beneficial for the formation of Ti
3Si(Al)C
2. We can further infer that Ti
3Si(Al)C
2 is generated on SiC by the infiltration of molten Al base alloy.
During the infiltration procedure, molten Al base alloy penetrates into the SiC ceramic, first by dissolving Si located in the gaps of SiC particles, and then forming Al-Si eutectic with low-melting-point, which constructed the channels for infiltration. Finally, the Al base alloy wets and reacts with the SiC ceramic. The identified Ti
3Si(Al)C
2 formation process is shown in
Figure 11f. Al, Ti, Si, and a small amount of Cu atoms surround the SiC particles. Al atoms decompose the SiC, creating some vacancies, which can be seen from
Figure 9b and
Figure 11e. In addition, the higher affinity of Ti to C than to Al, and the close structural relationships between SiC and Ti
3Si(Al)C
2, enable the in situ growth of Ti
3Si(Al)C
2 on SiC [
30]. Therefore, the formation of Ti
3Si(Al)C
2 can be summarized by Equation (1):
It can be seen that Al not only opens the infiltration channel by dissolving the Si in the gaps of SiC, but also rapidly transports Ti to SiC due to the low melting point. Moreover, the molten Al alloy dissolves the Si released by Reaction (1). When Si is saturated in the Al melt, excess Si will precipitate. At the same time, Reaction (1) will be suppressed. This result corresponds with the results of infiltration for a long time. Indeed, in our previous work [
28], we demonstrated that excessive Si can completely inhibit the formation of Ti
3Si(Al)C
2.
Figure 12a–c shows morphologies of the SiC ceramic infiltrated with Al base alloy at 1000 °C for 4 h. The surface of the sample presented bright white particles, with black SiC phases and grey Ti
3Si(Al)C
2 phases.
Figure 12c,d shows a high magnification image and EDS analysis of the white bright phases. The agglomerate Ti
3Si(Al)C
2 was dissociated into small blocks, and contained 33.89 at.% Ti and 32.99 at.% C. Combined with the XRD patterns in
Figure 2, the small blocks were inferred to be TiC. From
Figure 5 and
Figure 12, it is suggested that Ti
3Si(Al)C
2 was unstable at 1000 °C; prolonged infiltration at 1000 °C resulted in the decomposition of Ti
3Si(Al)C
2.
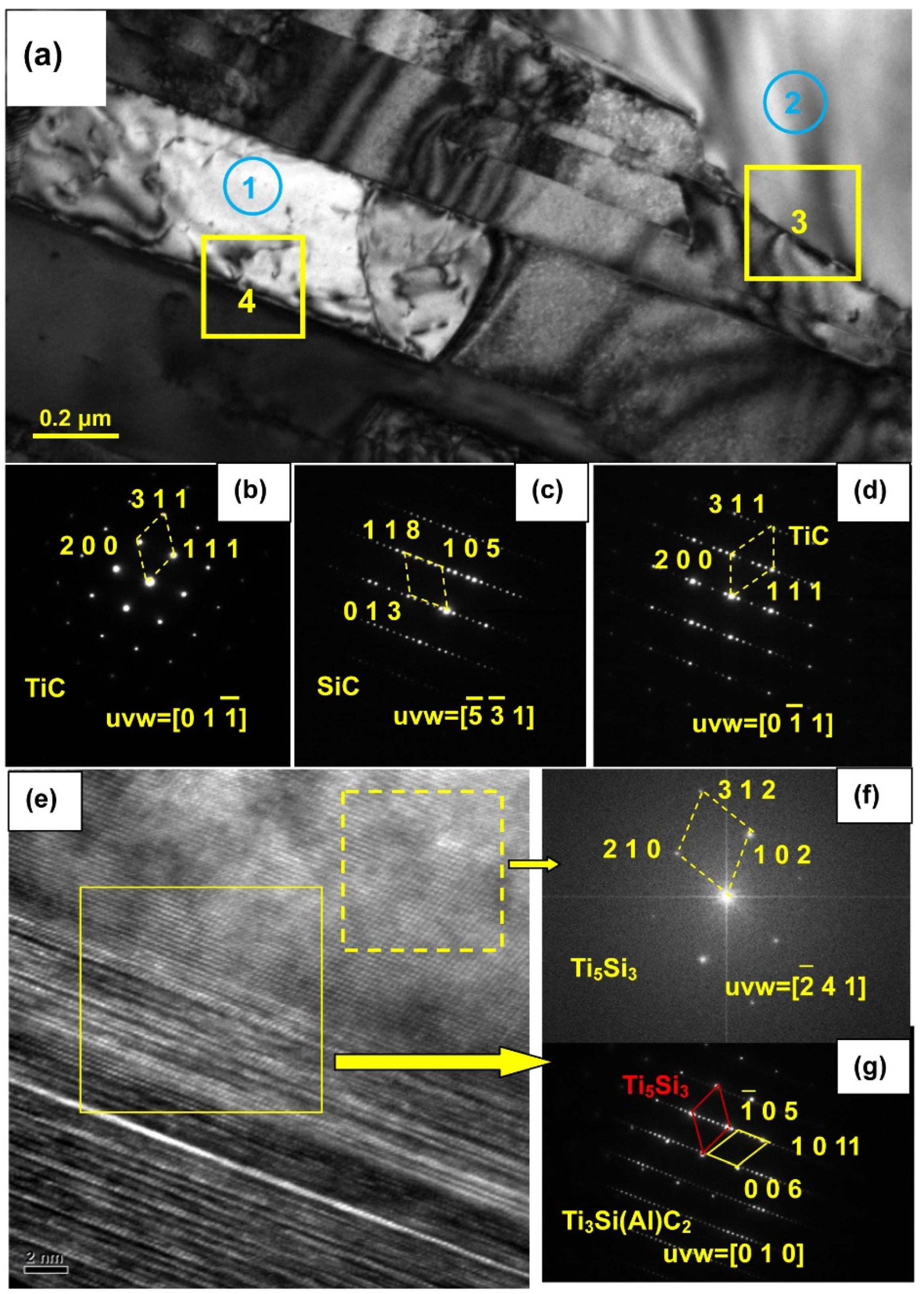
Figure 13 shows HRTEM images and the SAED pattern of the sample infiltrated at 1000 °C for 6 h, which allows identification of the phases in areas 1–4. The general view shows that the banded structure is TiC.
Figure 13b proves that the electron beam is parallel to the [01
] axis. Area 2 is SiC, as seen from the SAED micrograph in
Figure 13c. Area 3 contains SiC (from area 2) and TiC. Moreover, some Ti
3Si(Al)C
2 and Ti
5Si
3 can be identified, from
Figure 13e–g. Therefore, it can be inferred that Reactions (2) and (3) occur at 1000 °C. In the 1000 °C melt infiltration experiment, SiC ceramic surface oxidation film will be removed by deoxidization. Al base alloy infiltrated SiC materials because the Al base alloy wetted with SiC ceramic dissolved Si existing in the gaps of SiC particles. Ti atoms absorbed around the SiC particles, under the condition of high temperatures of 1000 °C are more active. Ti atoms directly reacted with SiC and generated a lot of TiC. A small amount of Ti
3Si(Al)C
2 is also obtained. Ti
3SiC
2 and Ti
3Si(Al)C
2 are not thermodynamically stable at high temperatures [
31]. With the extension of the infiltration time, the TiC and a small amount of Ti
5Si
3 were produced by the reaction between the active Ti atoms and the small amount of Ti
3Si(Al)C
2. Due to the small amount of Ti
5Si
3, it was rarely detected in the XRD phase analysis.