Effect of Different Minerals on Water Stability and Wettability of Soil Silt Aggregates
Abstract
:1. Introduction
2. Materials and Methods
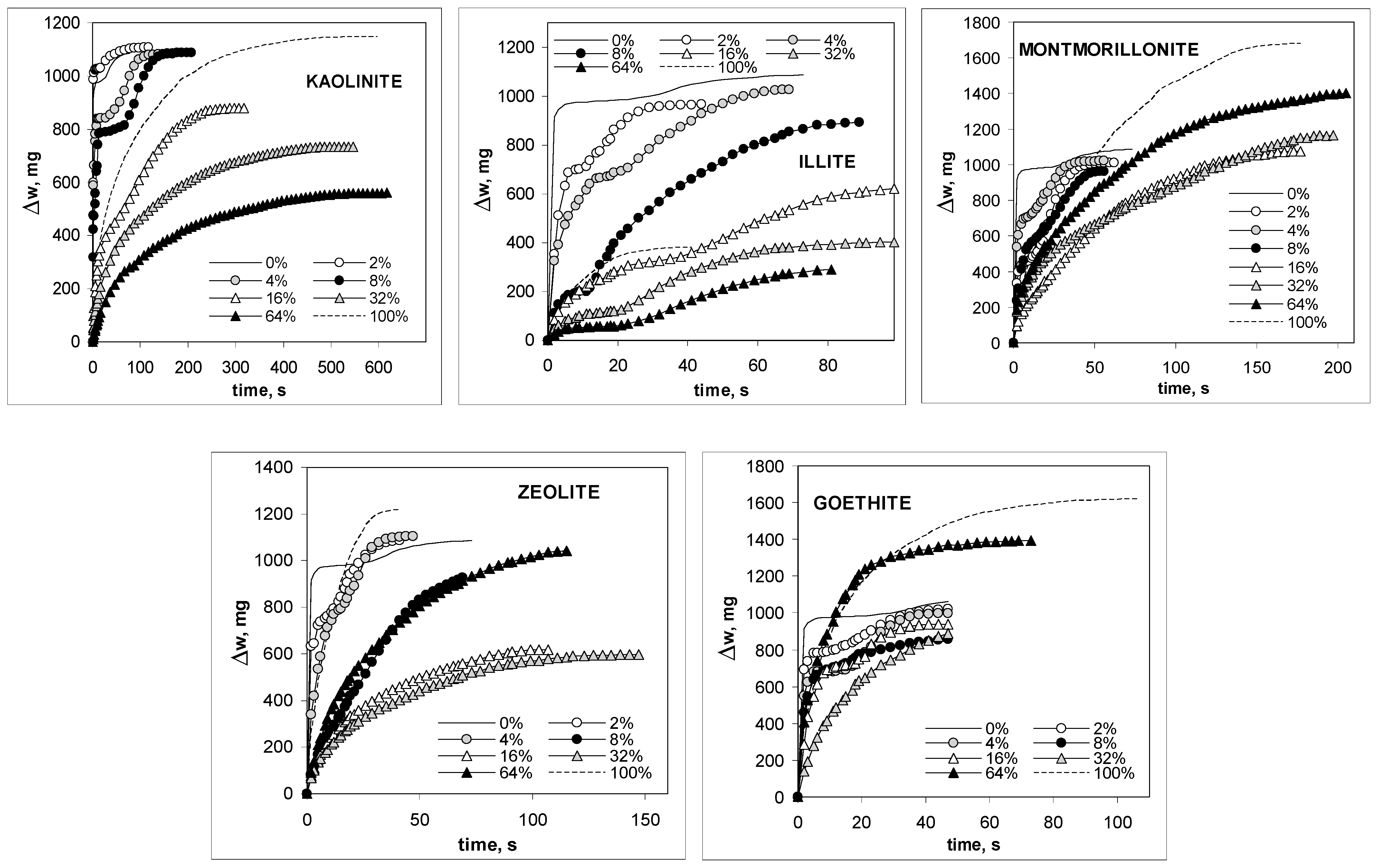
2.1. Materials
- Goethite 71063-100G (Sigma-Aldrich, St Louis, MO, USA),
- Kaolinite containing <5% illite and ~10% quartz,
- Illite containing ~10% kaolinite and ~5% quartz,
- Montmorillonite K10 (Sigma Aldrich Chemie GmbH, Steinheim, Germany),
- Zeolite, coming from a clinoptilolitic tuff deposit in Sokirnitsa, Ukraine.
2.2. Studies of the Aggregates
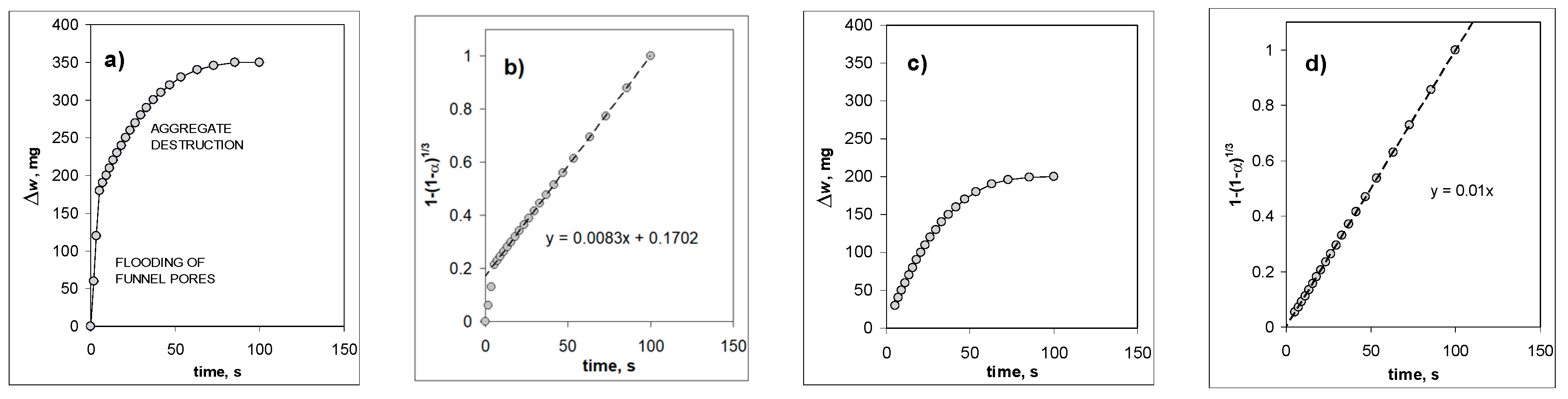
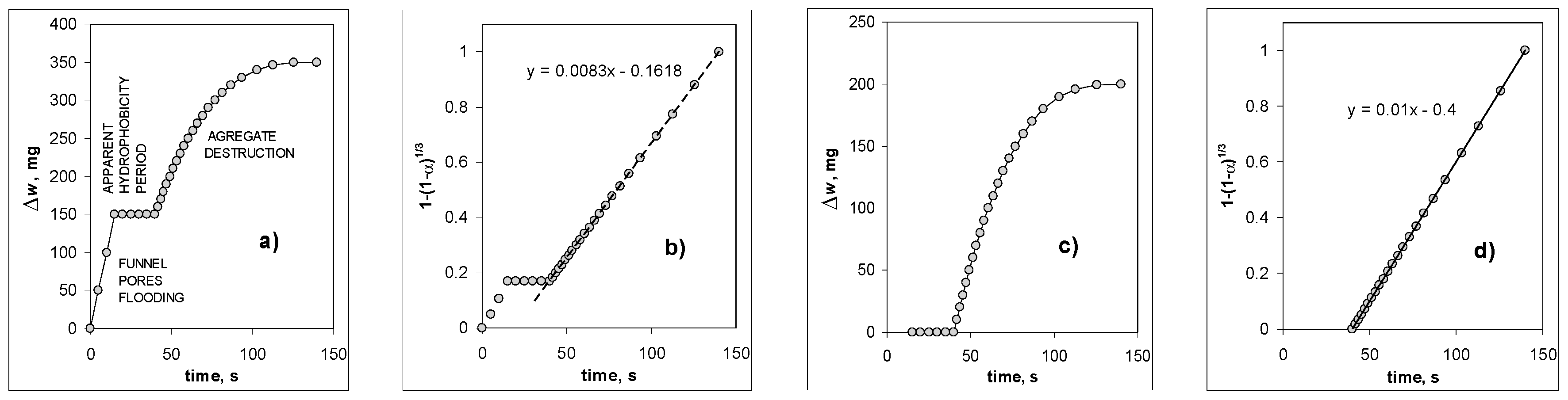
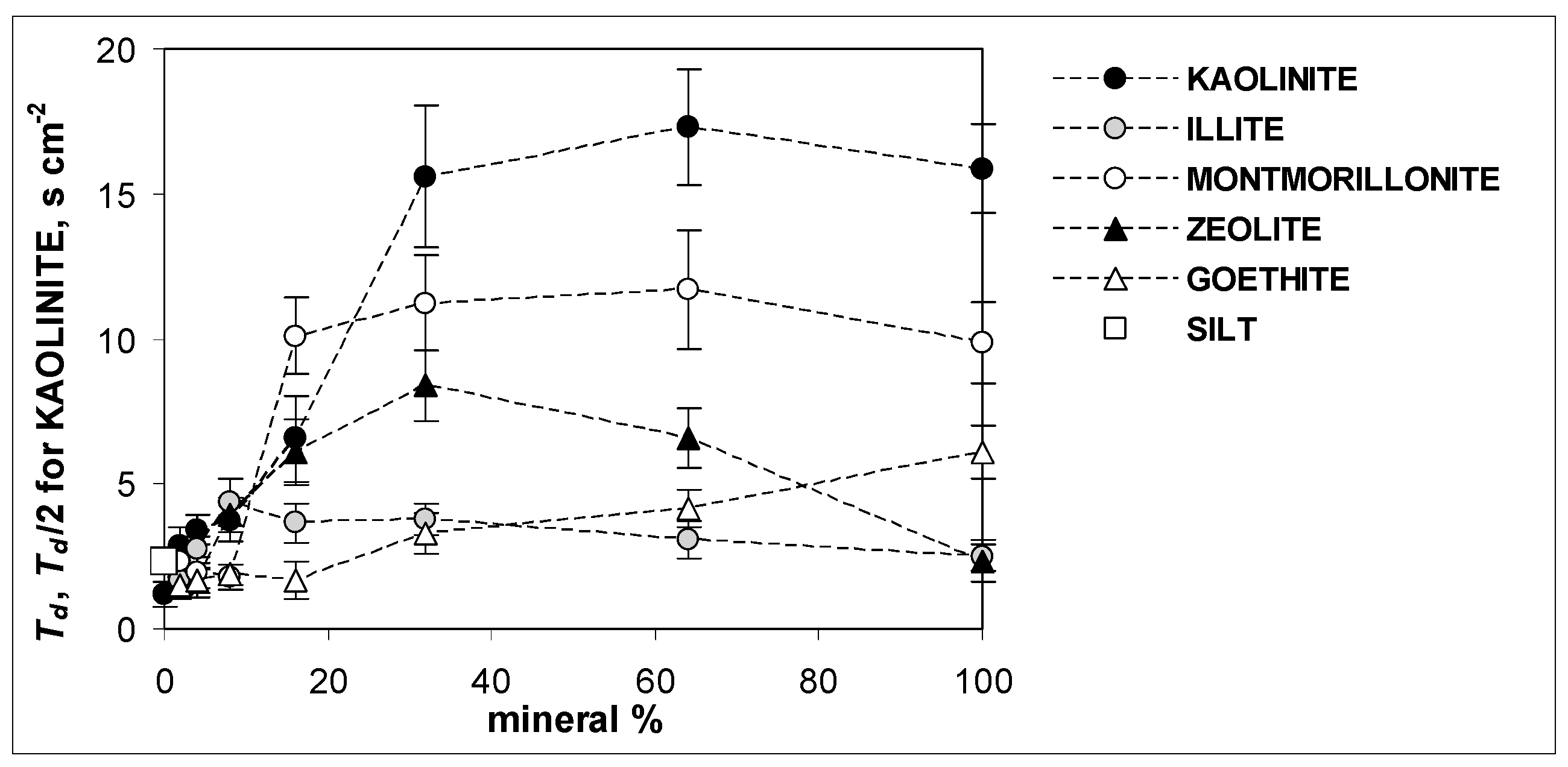
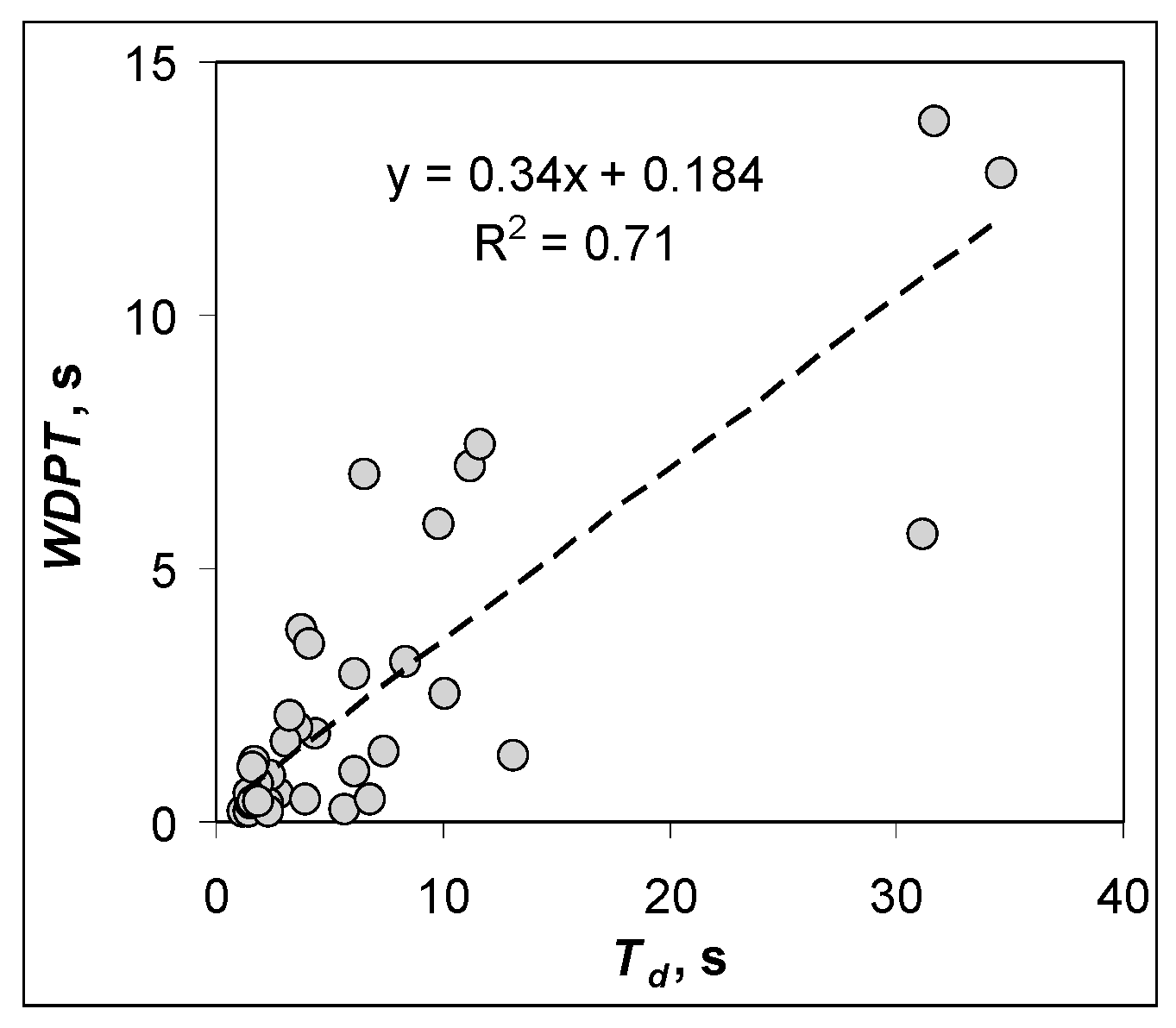
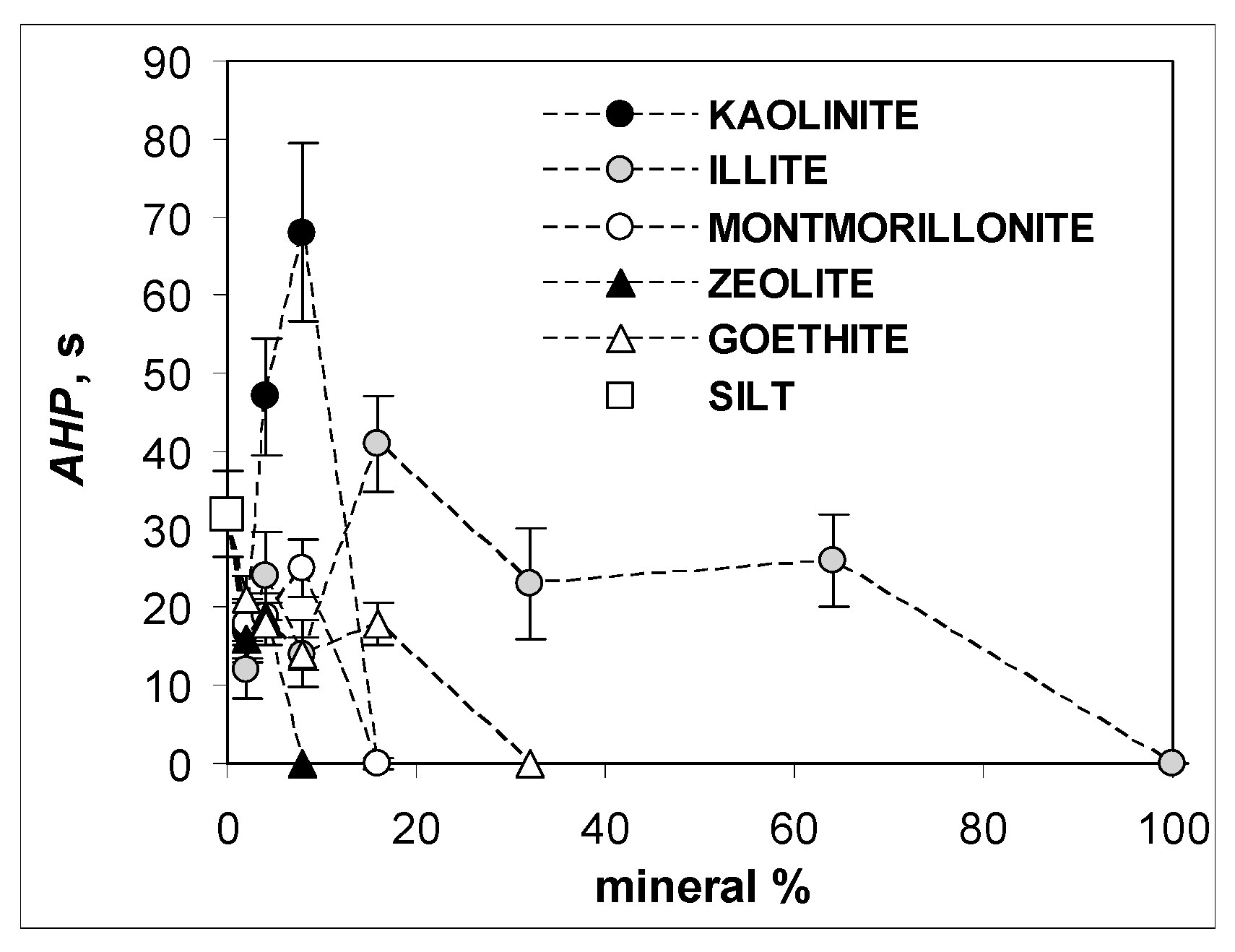
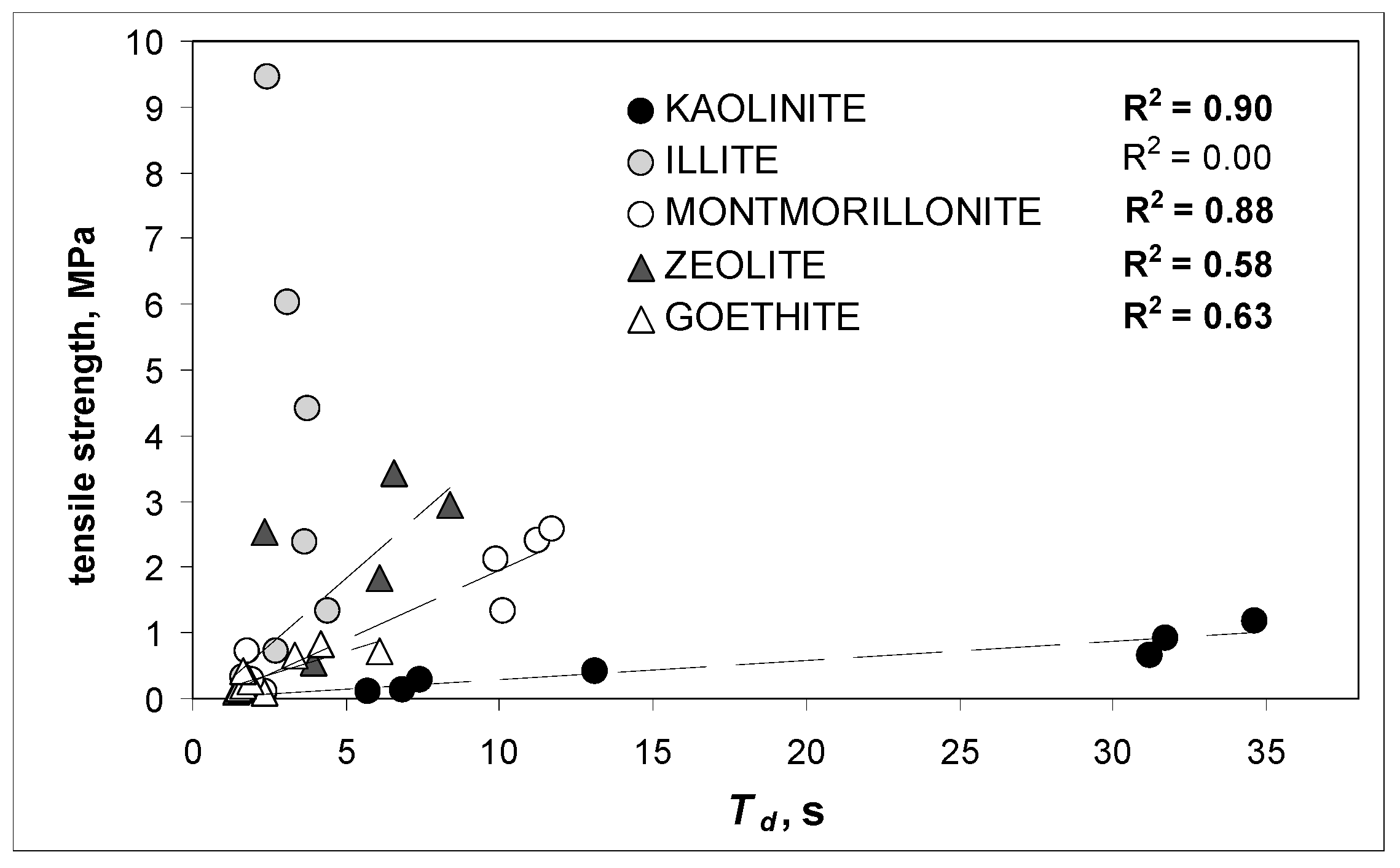
3. Results and Discussion
- (1)
- Some water remains adsorbed on aggregate components’ surfaces at 60% humidity. As it can be read from adsorption isotherms, the thickness of the adsorbed layer at this humidity is around 2–3 molecules of water [45], so an amount of water present on one square meter of the adsorbent is around 3 × 10−4 cm3g−1 per gram. This is a negligible amount even for the studied pure montmorillonite, with a surface area of 200 square meters per gram.
- (2)
- Some bubbles released before the first registration point are not registered. This effect may be important, but only for aggregates exhibiting rapid initial flooding of funnel pores (i.e., for aggregates of low mineral percentages). Since the amount of air registered in the first second of the bubbling may be very high (even up to 0.6 cm3 for 2% kaolinite aggregate, usually less), and assuming the uncertainty of time reading of 0.5 s (one second was a period of time registration during the first few seconds), the nonregistered bubbling may account for up to 0.3 cm3.
- (3)
- Some air remains adhered to the destruction products.
- (4)
- Some air is released directly to the atmosphere due to water replacement (mainly from large pores) during aggregate immersion before its complete flooding.
4. General Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Saving the face of soil aggregates. Glob. Chang. Biol. 2019, 25, 3574–3577. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.A.; Frankenberger, W.T., Jr. Modification of infiltration rates in an organic-amended irrigated. Agron. J. 1992, 84, 707–717. [Google Scholar] [CrossRef]
- Li, C.; Guo, S. Structural evolution of soil aggregates in a karst rocky desertification area. RSC Adv. 2022, 12, 21004–21013. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, X.; Kang, F.; Han, H. Dynamic characteristics of soil aggregate stability and related carbon and nitrogen pools at different developmental stages of plantations in northern China. J. Environ. Manag. 2022, 316, 1152. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Mawodza, T.; Rabbani, A.; Blaud, A.; Lair, G.J.; Babaei, M.; Kercheva, M.; Rousseva, S.; Banwart, S. Pore system characteristics of soil aggregates and their relevance to aggregate stability. Geoderma 2020, 366, 114259. [Google Scholar] [CrossRef]
- Xia, R.; Shi, D.; Ni, S.; Wang, R.; Zhang, J.; Song, G. Effects of soil erosion and soil amendment on soil aggregate stability in the cultivated-layer of sloping farmland in the Three Gorges Reservoir area. Soil Tillage Res. 2022, 223, 105447. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Yang, W.; Hua, L.; Cai, C. Aggregate stability under long-term fertilization practices: The case of eroded ultisols of south-central China. Sustainability 2019, 11, 1169. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.D.; Alves, P.R.L.; de Paula, A.M.; Nakatani, A.S.; de Moraes Pereira, J.; Nogueira, M.A.; et al. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agri. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Yaroslavtseva, N.V.; Yashin, M.A.; Frid, A.S.; Lazarev, V.I.; Tyugai, Z.N.; Yu Milanovskiy, E. Contact angles of wetting and water stability of soil structure. Eurasian Soil Sci. 2015, 48, 600–607. [Google Scholar] [CrossRef]
- Buchmann, C.; Meyer, M.; Schaumann, G.E. Characterization of wet aggregate stability of soils by H-NMR relaxometry. Magn. Reson. Chem. 2014, 53, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Le Bissonnais, Y. Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Cosentino, D.; Chenu, C.; Le Bissonnais, Y. Aggregate stability and microbial community dynamics under drying–wetting cycles in a silt loam soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Emerson, W.W.; Greenland, D.J. Soil aggregates—Formation and stability. In Soil Colloids and Their Associations in Aggregates, 2nd ed.; De Boodt, M.F., Hayes, M.H.B., Herbillon, A., De Strooper, E.B.A., Tuck, J.J., Eds.; Springer Science + Business Media: New York, NY, USA, 1990; Volume 215, pp. 485–511. [Google Scholar] [CrossRef]
- Emerson, W.W. A classification of soil aggregates based on their coherence in water. Aust. J. Soil Res. 1967, 5, 47–57. [Google Scholar] [CrossRef]
- Kay, B.D.; Anger, D.A. Soil structure. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: New York, NY, USA, 1999; pp. 229–269. [Google Scholar]
- Rawlins, B.G.; Turner, G.; Wragg, J.; McLachlan, P.; Lark, R.M. An improved method for measurement of soil aggregate stability using laser granulometry applied at regional scale. Eur. J. Soil Sci. 2015, 66, 604–614. [Google Scholar] [CrossRef]
- Sullivan, L.A. Soil organic matter, air encapsulation and water-stable aggregation. J. Soil Sci. 1990, 41, 529–534. [Google Scholar] [CrossRef]
- Capriel, P.; Beck, T.; Borchert, H.; Härter, P. Relationship between soil aliphatic fraction extracted with supercritical hexane, soil microbial biomass, and soil aggregate stability. Soil Sci. Soc. Am. J. 1990, 54, 415–420. [Google Scholar] [CrossRef]
- Goldberg, S. Interaction of aluminum and iron oxides and clay minerals and their effect on soil physical properties: A review. Commun. Soil Sci. Plant Anal. 1989, 20, 1181–1207. [Google Scholar] [CrossRef]
- Nagy, N.M.; Kónya, J. Interfacial Chemistry of Rocks and Soils, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–75. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay and Clay Minerals, 1st ed.; Morari Do Nascimento, G., Ed.; IntechOpen: London, UK, 2021; Volume 24, pp. 1–29. [Google Scholar]
- McKissock, I.; Walker, E.L.; Gilkes, R.J.; Carter, D.J. The influence of clay type on reduction of water repellency by applied clays: A review of some West Australian work. J. Hydrol. 2000, 231–232, 323–332. [Google Scholar] [CrossRef]
- McKissock, I.; Gilkes, R.J.; Walker, E.L. The reduction of water repellency by added clay as influenced by clay and soil properties. Appl. Clay Sci. 2002, 20, 225–241. [Google Scholar] [CrossRef]
- Ward, P.R.; Oades, J.M. Effect of clay mineralogy and exchangeable cations on water repellency in clay-amended sandy soils. Aust. J. Soil Res. 1993, 31, 351–364. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil structure and soil organic matter: II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000, 64, 1042–1049. [Google Scholar] [CrossRef]
- Aminiyan, M.; Sinegani, A.A.; Sheklabadi, M. Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int. J. Recycl. Org. Waste Agric. 2015, 4, 11–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Q.; Cui, Y.; Zhang, P.; Zhang, X. Use of montmorillonite-enriched siltstone for improving water condition and plant growth in sandy soil. Ecol. Eng. 2020, 145, 105740. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Adamczuk, A.; Skic, K.; Boguta, P. New method for quantifying water stability of soil aggregates from air bubbling after immersion. Measurement 2020, 155, 107569. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Czachor, H. Impact of organic matter, iron oxides, alumina, silica and drying on mechanical and water stability of artificial soil aggregates. Assessment of new method to study water stability. Geoderma 2014, 221–222, 1–10. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Zhu, L. Soil aggregate size distribution and stability of farmland as affected by dry and wet sieving methods. Zemdirbyst 2020, 107, 179–184. [Google Scholar] [CrossRef]
- Loch, R.J. A method for measuring aggregate water stability of dryland soils with relevance to surface seal development. Aust. J. Soil Res. 1994, 32, 687–700. [Google Scholar] [CrossRef]
- Lipiec, J.; Wojciga, A.; Horn, R. Hydraulic properties of soil aggregates as influenced by compaction. Soil Tillage Res. 2009, 103, 170–177. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Skic, K.; Adamczuk, A.; Boguta, P.; Lamorski, K. Structure and strength of artificial soils containing monomineral clay fractions. Materials 2021, 14, 4688. [Google Scholar] [CrossRef] [PubMed]
- Young, T. III An essay on the cohesion of fluids. Philos.Trans.R.Soc. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Tinebra, I.; Alagna, V.; Iovino, M.; Bagarello, V. Comparing different application procedures of the water drop penetration time test to assess soil water repellency in a fire affected Sicilian area. Catena 2019, 177, 41–48. [Google Scholar] [CrossRef]
- Chan, K.Y.; Mullins, C.E. Slaking characteristics of some Australian and British soils. Eur. J. Soil Sci. 1994, 45, 273–283. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Wetting of rough three-dimensional superhydrophobic surfaces. Microsyst. Technol. 2006, 12, 273–281. [Google Scholar] [CrossRef]
- Hajnos, M.; Całka, A.; Józefaciuk, G. Wettability of mineral soils. Geoderma 2013, 206, 63–69. [Google Scholar] [CrossRef]
- Dlapa, P.; Doerr, S.H.; Lichner, L.; Šír, M.; Tesař, M. Effect of kaolinite and Ca-montmorillonite on the alleviation of soil water repellency. Plant Soil Environ. 2004, 50, 358–363. [Google Scholar] [CrossRef]
- Bryant, R.; Doerr, S.H.; Hunt, G.; Conan, S. Effects of compaction on soil surface water repellency. Soil Use Manag. 2007, 23, 238–244. [Google Scholar] [CrossRef]
- Czachor, H. Modelling the effect of pore structure and wetting angles on capillary rise in soils having different wettabilities. J. Hydrol. 2006, 328, 604–613. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Bowanko, G. Effect of acid and alkali treatments on surface areas and adsorption energies of selected minerals. Clays Clay Miner. 2002, 50, 771–783. [Google Scholar] [CrossRef]
- Bartoli, F.; Burtin, G.; Guerif, J. Influence of organic matter on aggregation in Oxisols rich in gibbsite or in goethite. II. Clay dispersion, aggregate strength and water-stability. Geoderma 1992, 54, 259–274. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, A.; Gryta, A.; Skic, K.; Boguta, P.; Jozefaciuk, G. Effect of Different Minerals on Water Stability and Wettability of Soil Silt Aggregates. Materials 2022, 15, 5569. https://doi.org/10.3390/ma15165569
Adamczuk A, Gryta A, Skic K, Boguta P, Jozefaciuk G. Effect of Different Minerals on Water Stability and Wettability of Soil Silt Aggregates. Materials. 2022; 15(16):5569. https://doi.org/10.3390/ma15165569
Chicago/Turabian StyleAdamczuk, Agnieszka, Angelika Gryta, Kamil Skic, Patrycja Boguta, and Grzegorz Jozefaciuk. 2022. "Effect of Different Minerals on Water Stability and Wettability of Soil Silt Aggregates" Materials 15, no. 16: 5569. https://doi.org/10.3390/ma15165569
APA StyleAdamczuk, A., Gryta, A., Skic, K., Boguta, P., & Jozefaciuk, G. (2022). Effect of Different Minerals on Water Stability and Wettability of Soil Silt Aggregates. Materials, 15(16), 5569. https://doi.org/10.3390/ma15165569






