Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. SPS of Mechanically Activated Powder Compositions of the Ti-Al-Nb System
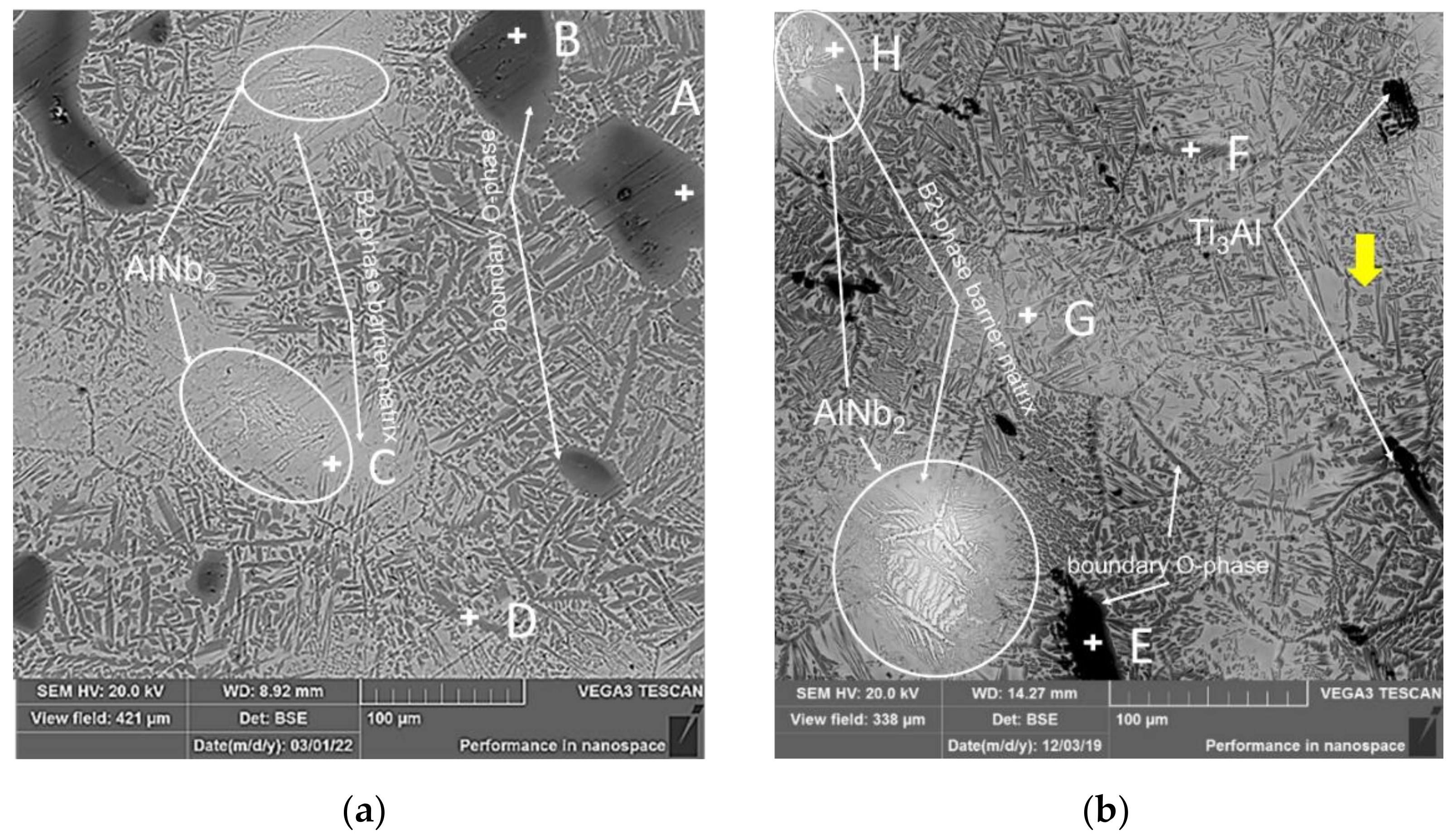
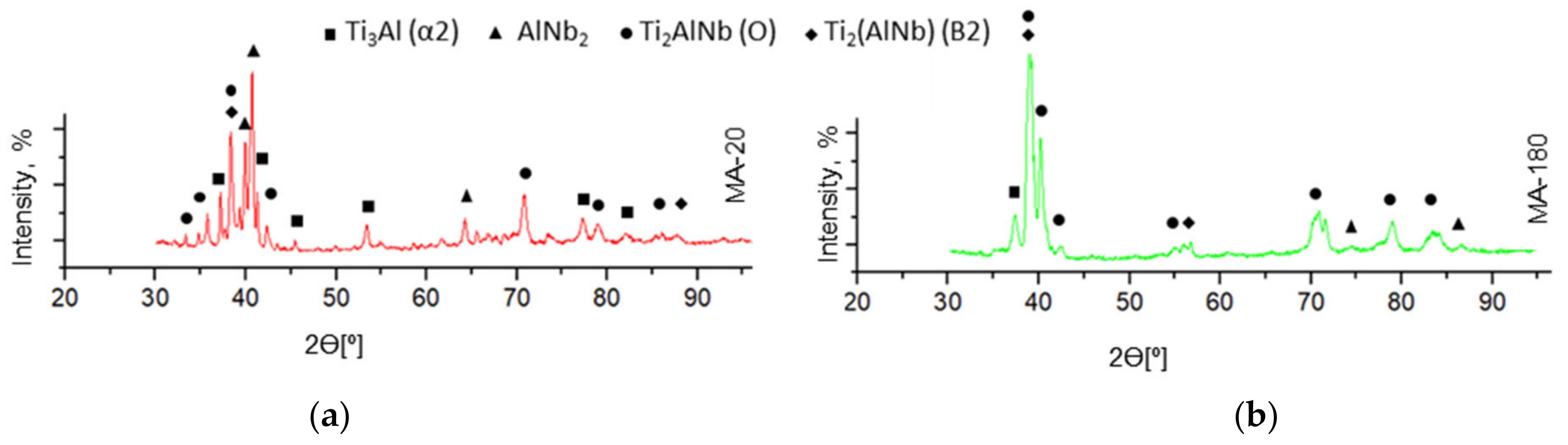
3.2. Structural and Phase State of Samples after Sintering
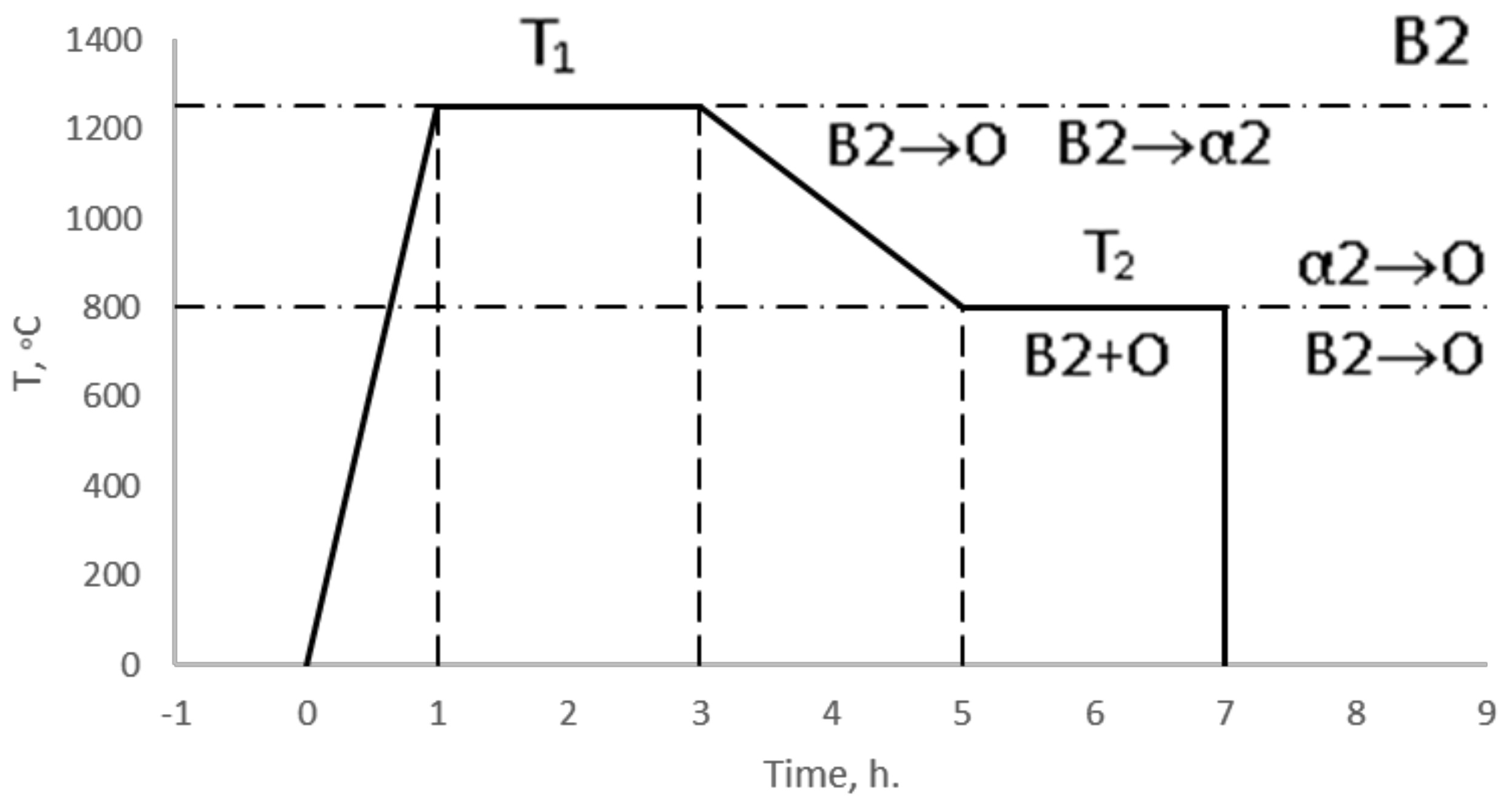
3.3. Structural and Phase State of Samples after Heat Treatment
3.4. Strength Properties of Samples before and after Heat Treatment
4. Conclusions
- SPS of mechanically activated powder mixtures of the Ti-Al-Nb system at a temperature of 1300 °C and a static prepress pressure of 20 MPa with an isothermal holding for 5 min allows obtaining an alloy with a predominantly bimodal (O + B2) structure;
- After SPS, the main structural-phase composition is the matrix B2-phase with a cubic lattice and the volumetric amount of precipitates of the orthorhombic O phase at the boundaries and in the body of grains of the B2-phase. At the same time, traces of a volumetric number of secondary phases are still observed in the structure, such as: AlNb2, α2, and unreacted Nb;
- Mechanical properties of alloys of the Ti-Al-Nb system obtained by combining the MA and SPS processes are characterized by abnormally low values of strength (less than 150 MPa) and ductility. This is due to the formation of a large number of large precipitates of the brittle α2-phase, as well as a strong concentration of deformation at the boundaries of the matrix B2-phase, caused by the nucleation and growth of the acicular O phase;
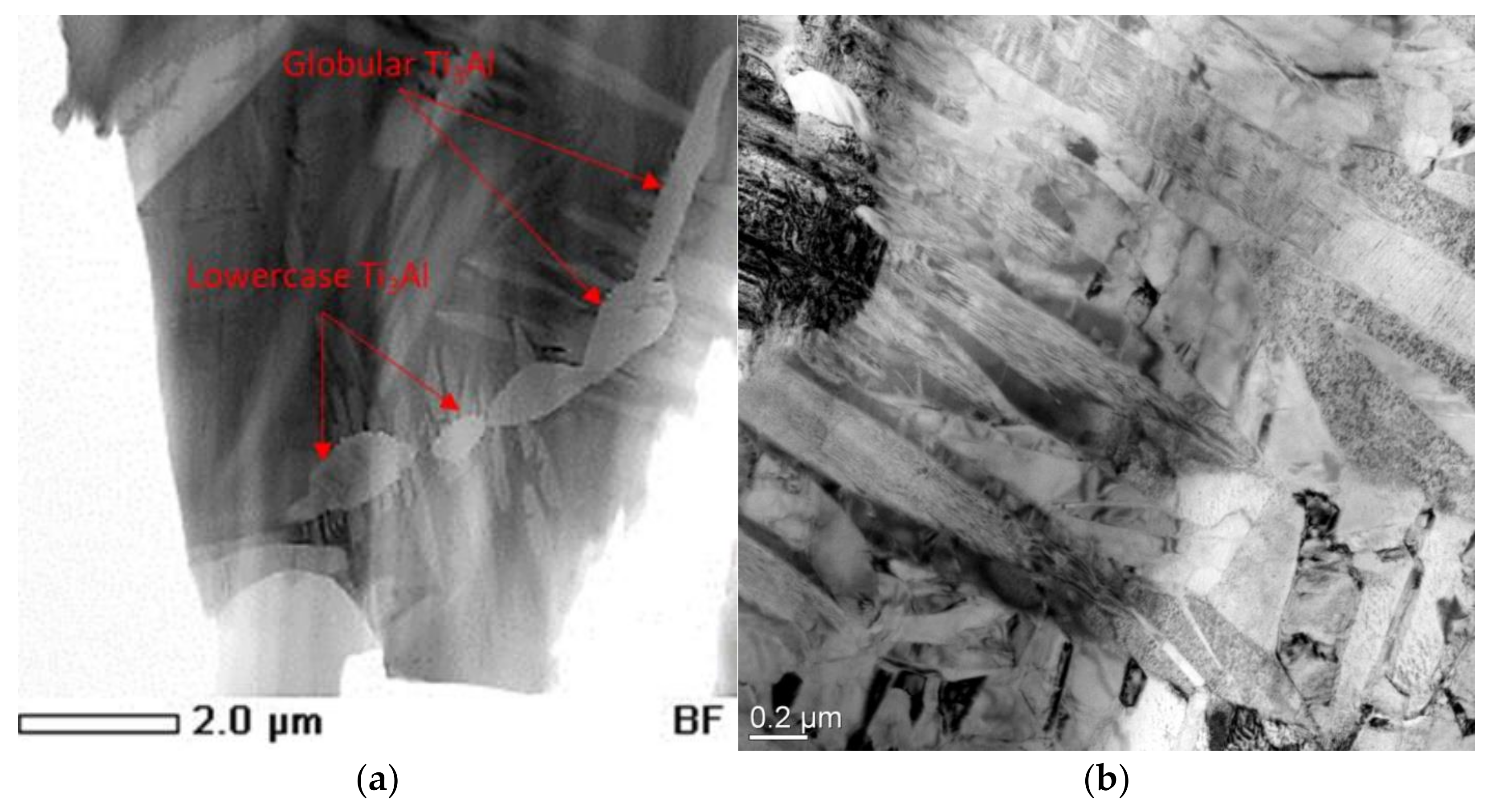
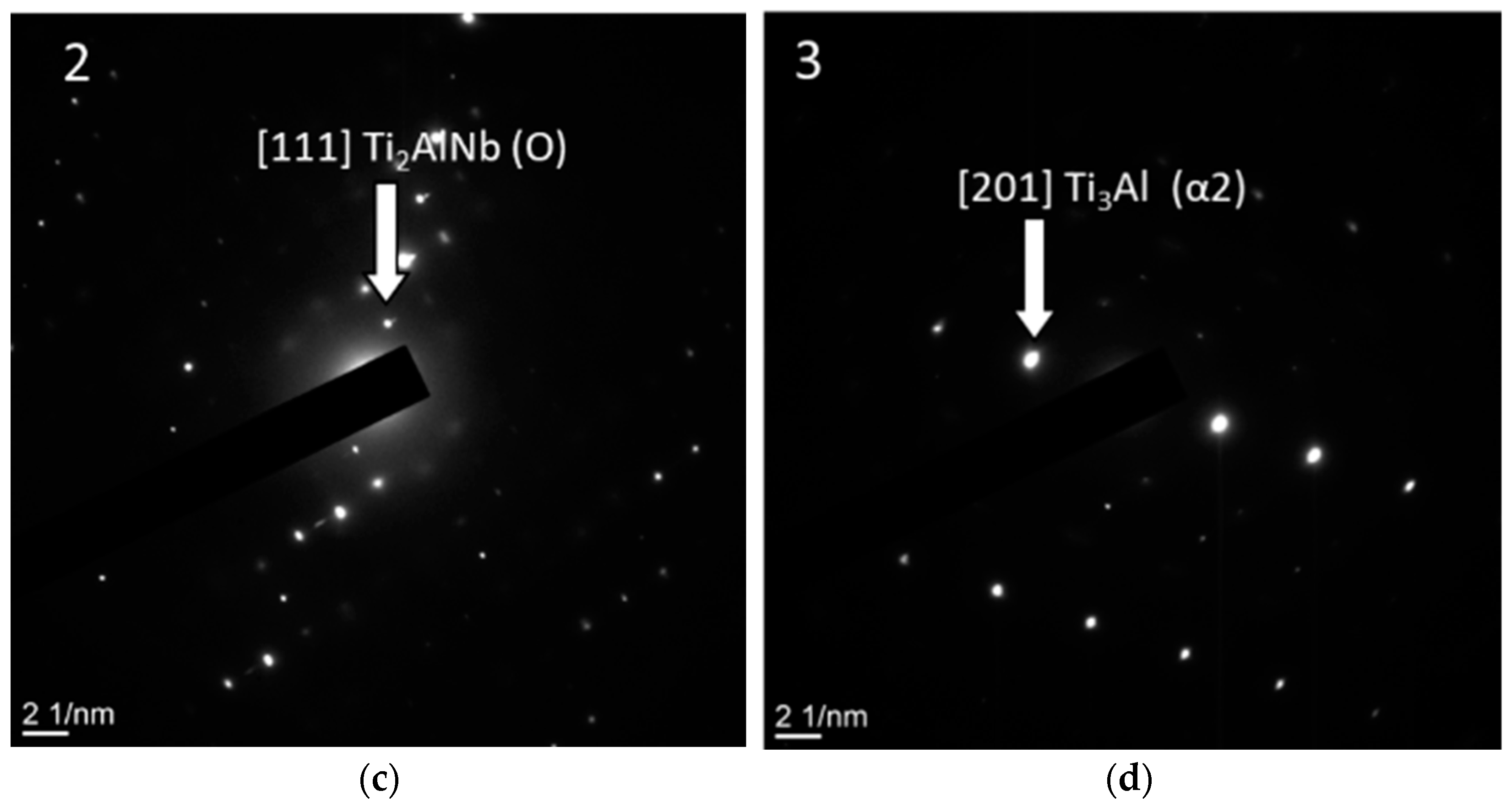
- Two-stage heat treatment of alloys of the Ti-Al-Nb system leads to a decrease in the size of the precipitates of the O phase and to an increase in its content while maintaining the bimodal structure. After heat treatment, the O phase becomes dominant in the structure of the alloys, while the AlNb2 and Nb phases are completely dissolved. Coarse precipitates of the α2-phase are transformed into nuclei of the O phase due to saturation with niobium, fine precipitates of the α2-phase depleted in niobium precipitate in the form of globular and line precipitates at the boundaries of the B2-phase;
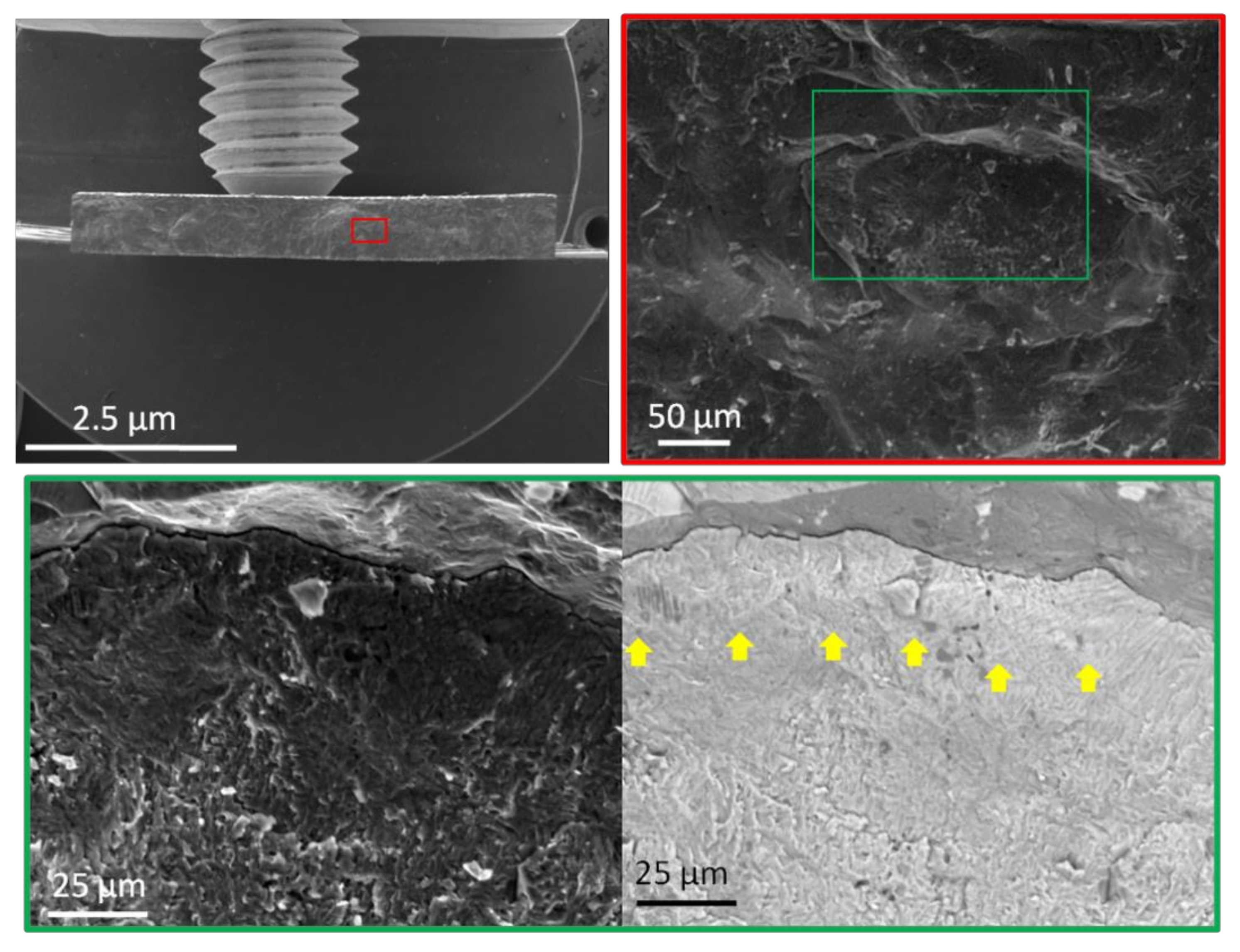
- The strength and ductility of Ti-Al-Nb alloy samples after heat treatment increases by 10 (1310 MPa, MA-180) and 2 (1322 Mpa, MA-20) times, respectively. The surface fracture of the samples obtained after testing is characterized by intergranular (intercrystalline) brittle fracture with “river” or “step” features. In a crystal structure with a bcc lattice, the critical stress of the sliding drive system is greater, the number of sliding systems is less, and therefore, the samples after testing demonstrate the performance characteristics of high strength and relatively low ductility.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.; Yang, J.; Zhang, X.; Zhong, H.; Ma, J.; Li, F.; Fu, L.; Bi, Q.; Li, J.; Liu, W. High temperature tribological behavior of a Ti-46Al-2Cr-2Nb intermetallics. Intermetallic 2012, 31, 120–126. [Google Scholar] [CrossRef]
- Cinca, N.; Roberto, C.; Lima, C.; Guilemany, J.M. An overview of intermetallic research and application: Status of heat spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Baklanov, V.; Zhanbolatova, G.; Skakov, M.; Miniyazov, A.; Sokolov, I.; Tulenbergenov, T.; Kozhakhmetov Ye Bukina, O.; Orazgaliev, N. Study of the Temperature Dependence of a Carbidized Layer Formation on the Tungsten Surface Under Plasma Irradiation. Mater. Res. Express 2022, 9, 016403. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Liang, Y.; Xu, X.; Gao, S.; Wang, Y.; He, J.; Lin, J. Phase equilibria of the Ti-Al-Nb system at 1300 °C. J. Alloys Compd. 2017, 724, 339–347. [Google Scholar] [CrossRef]
- Shuleshova, O.; Holland-Moritz, D.; Löser, W.; Voss, A.; Hartmann, H.; Hecht, U.; Witusiewicz, V.T.; Herlach, D.M.; Büchner, B. In situ observations of solidification processes in γ-TiAl alloys by synchrotron radiation. Acta Mater. 2010, 58, 2408–2418. [Google Scholar] [CrossRef]
- Kenel, C.; Leinenbach, C. Influence of cooling rate on microstructure formation during rapid solidification of binary TiAl alloys. J. Alloys Compd. 2015, 637, 242–247. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, L.; Li, X.W.; Yang, D.Z.; Umakoshi, Y. Mechanical properties and oxidation behavior of the Ti–24Al–14Nb–3V–0.5Mo alloy sheet. Mater. Sci. Eng. A 2006, 427, 42–50. [Google Scholar] [CrossRef]
- Jafari, R.; Eghbali, B.; Adhami, M. Influence of annealing on the microstructure and mechanical properties of Ti/Al and Ti/Al/Nb laminated composites. Mater. Chem. Phys. 2018, 213, 313–323. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, J.F.; Chang, J.; Wang, H.P. Microstructure evolution and nano-hardness modulation of rapidly solidified Ti–Al–Nb alloy. J. Alloys Compd. 2020, 836, 155538. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, H.; Yu, H.; Chen, C.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Li, C.; Ma, Z.; Yu, L.; Li, H. Microstructure evolution and phase transformations in Ti-22Al-25Nb alloys tailored by super-transus solution treatment. Vacuum 2019, 161, 209–219. [Google Scholar] [CrossRef]
- Goyal, K.; Sardana, N. Phase stability and microstructural evolution of Ti2AlNb alloys-a review. Mater. Today Proc. 2021, 41, 951–968. [Google Scholar] [CrossRef]
- Dadé, M.; Esin, V.A.; Nazé, L.; Sallot, P. Short- and long-term oxidation behavior of an advanced Ti2AlNb alloy. Corros. Sci. 2019, 148, 379–387. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Kozhakhmetov Ye Skakov, M.; Kurbanbekov, S.H.; Mukhamedov, N. Microstructural stability of a two-phase (O+B2) alloy of the Ti-25Al-25Nb at.% system during heat cycling in a hydrogen atmosphere. AIMS Mater. Sci. 2022, 9, 206–218. [Google Scholar]
- Senkevich, K.S.; Pozhoga, O.Z. Experimental investigation of hydrogen absorption by commercial high alloyed Ti2AlNb-based alloy in cast and rapidly solidified state. Vacuum 2021, 191, 110379. [Google Scholar] [CrossRef]
- Zhang, L.T.; Ito, K.; Vasudevan, V.K.; Yamaguchi, M. Hydrogen absorption and desorption in a B2 single-phase Ti–22Al–27Nb alloy before and after deformation. Acta Mater. 2001, 49, 751–758. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, K.; Zhou, H.; Ni, L.; Chen, Y. A comparative study of microstructure and tensile properties of Ti2AlNb joints prepared by laser welding and laser-additive welding with the addition of filler powder. J. Mater. Process. Technol. 2018, 255, 477–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yu, L.; Liang, H.; Huang, Y.; Ma, Z. Microstructures and tensile properties of Ti2AlNb and Mo-modified Ti2AlNb alloys fabricated by hot isostatic pressing. Mater. Sci.Eng. A 2020, 776, 139043. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Zhang, Y.; Liang, H. Thermal stability and mechanical properties of Ti–22Al–25Nb alloy with different initial microstructures. J. Alloys Compd. 2020, 842, 155794. [Google Scholar] [CrossRef]
- Germann, L.; Banerjee, D.; Guedou, J.Y.; Strudel, J.-L. Effect of composition on the mechanical properties of newly developed Ti2AlNb-based titanium aluminide. Intermetallics 2005, 13, 920–924. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.A.; Batyrbekov, E.G.; Skakov, M.K.; Kurbanbekov, S.R.; Mukhamedzhanova, R.M.; Mukhamedova, N.M. Method for Producing Hydrogen-Storage Rechargeable Intermetallic Compounds. Innovative patent of RK No. 5809, 29 January 2021. [Google Scholar]
- Kundu, J.; Chakraborty, A.; Kundu, S. Bonding pressure effects on characteristics of microstructure, mechanical properties, and mass diffusivity of Ti-6Al-4V and TiAlNb diffusion-bonded joints. Weld. World 2020, 64, 2129–2143. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, N.; Liang, H.; Liu, Y.J. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2021, 80, 203–216. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.; Skakov, M.; Mukhamedova, N.; Kurbanbekov, S.; Ramankulov, S.; Wieleba, W. Changes in the microstructural state of Ti-Al-Nb-based alloys depending on the temperature cycle during spark plasma sintering. Mater. Test. 2021, 63, 119–123. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.; Skakov, M.; Wieleba, W.; Kurbanbekov, S.; Mukhamedova, N. Evolution of intermetallic compounds in Ti-Al-Nb system by the action of mechanical activation and spark plasma sintering. J. AIMS Mater. Sci. 2020, 7, 182–191. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.A.; Skakov, M.K.; Kurbanbekov, S.R.; Mukhamedov, N.M.; Mukhamedov, N.Y. Powder composition structurization of the Ti-25Al-25Nb at.% system upon mechanical activation and subsequent spark plasma sintering. Eurasian Chem.-Technol. J. 2021, 23, 37–44. [Google Scholar] [CrossRef]
- Polkin, I.S.; Skvortsova, S.V.; Turichin, G.A.; Novikova, M.B. Structure formation in A.M. processes of Titanium and Ni-base alloys. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–98. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Majumbar, B.S.; Seetharaman Miracle, D.B. Part I. The microstructural evolution in Ti-Al-Nb O + BCC orthorhombic alloys. Metall. Mater. Trans. A 1999, 30, 2305–2323. [Google Scholar] [CrossRef]
- Sha, J.; Gao, C.; Lee, S.-K.; Li, Y.; Zhao, N.; Tour, J.M. Preparation of Three-Dimensional Graphene Foams Using Powder Metallurgy Templates. Am. Chem. Soc. 2015, 10, 1411–1416. [Google Scholar] [CrossRef]
- Emura, S.; Araoka, A.; Hagiwara, M. B2 Grain Size Refinement of (O+B2) Ti-22Al-27Nb Alloy. In Proceedings of the 10th World Conference on Titanium, Hamburg, Germany, 13–18 July 2003; WILEY-VCH GmbH& Co. KGaA: Hamburg, Germany, 2003; Volume 4, pp. 2153–2159. [Google Scholar]
- Kazantseva, N.V.; Demakov, S.L.; Popov, A.A. Microstructure and plastic deformation of orthorhombic titanium aluminides Ti2AlNb. III. Formation of transformation twins upon the B2 → O phase transformation. B Phys. Met. Metallogr. 2007, 103, 378–387. [Google Scholar] [CrossRef]
- Nochovnaya, N.A.; Shiryaev, A.A.; Alekseev, E.B.; Antashev, V.G. Optimization of Heat Treatment Regimes for Blade Preforms from Experimental Titanium Alloy. Met. Sci. Heat Treat. 2015, 56, 656–660. [Google Scholar] [CrossRef]
- Banejee, D.; Sundarajan, G. lntermetallic Compounds. Ductility and Strength. Ind. J. Technol. 1990, 28, 259–280. [Google Scholar]
- Thomas, M.; Vassel, A.; Veyssiere, P. Dissociation of Super-Dislocations in the Intermetallic Compound Ti3AI. Scr. Metall. 1987, 21, 501–506. [Google Scholar] [CrossRef]
- Ito, K.; Zhang, L.T.; Vasudevan, V.K.; Yamaguchi, M. Multiphase and microstructure effects on the hydrogen absorption/desorption behavior of a Ti–22Al–27Nb alloy. Acta Mater. 2001, 49, 963–972. [Google Scholar] [CrossRef]
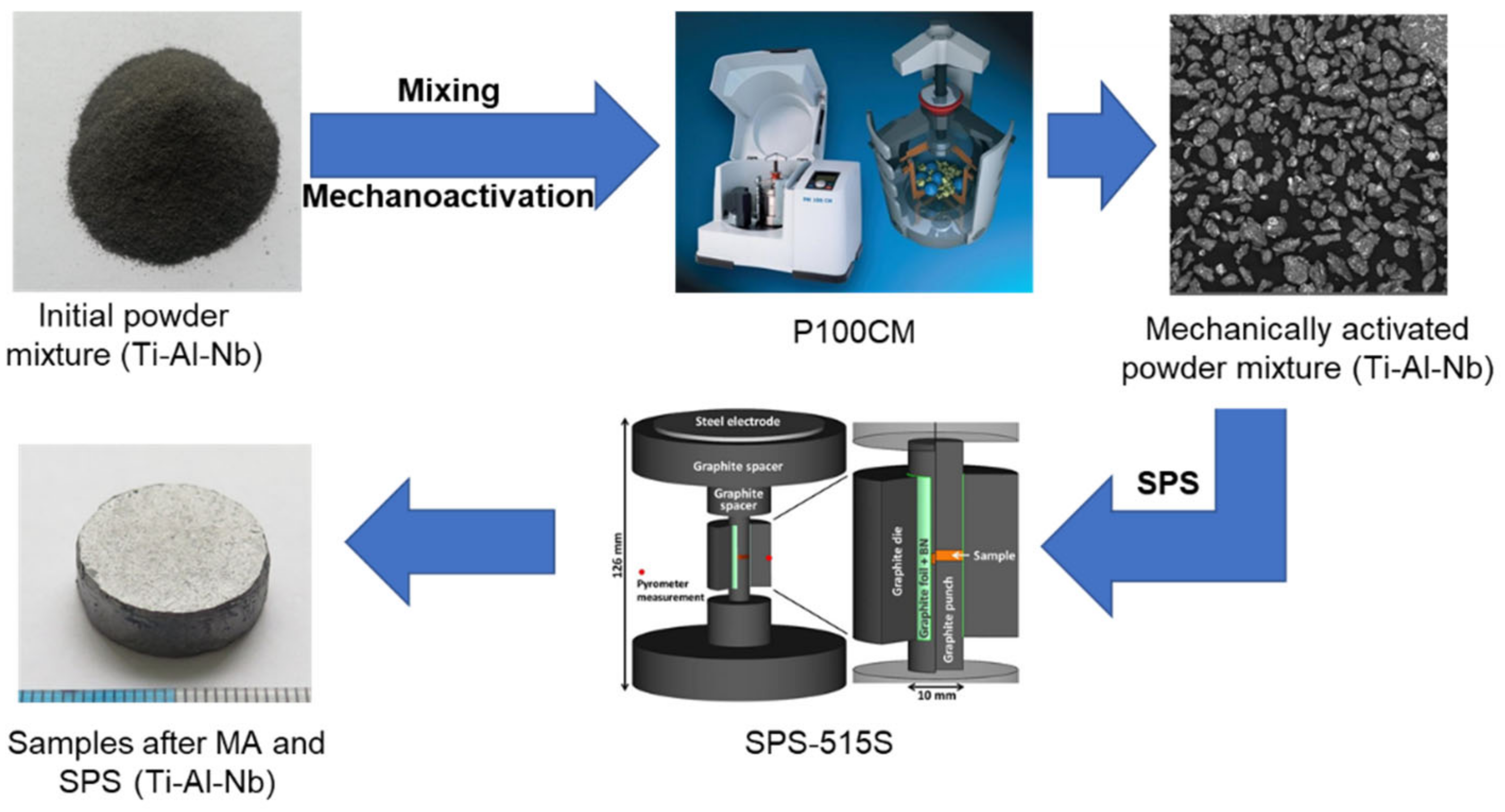

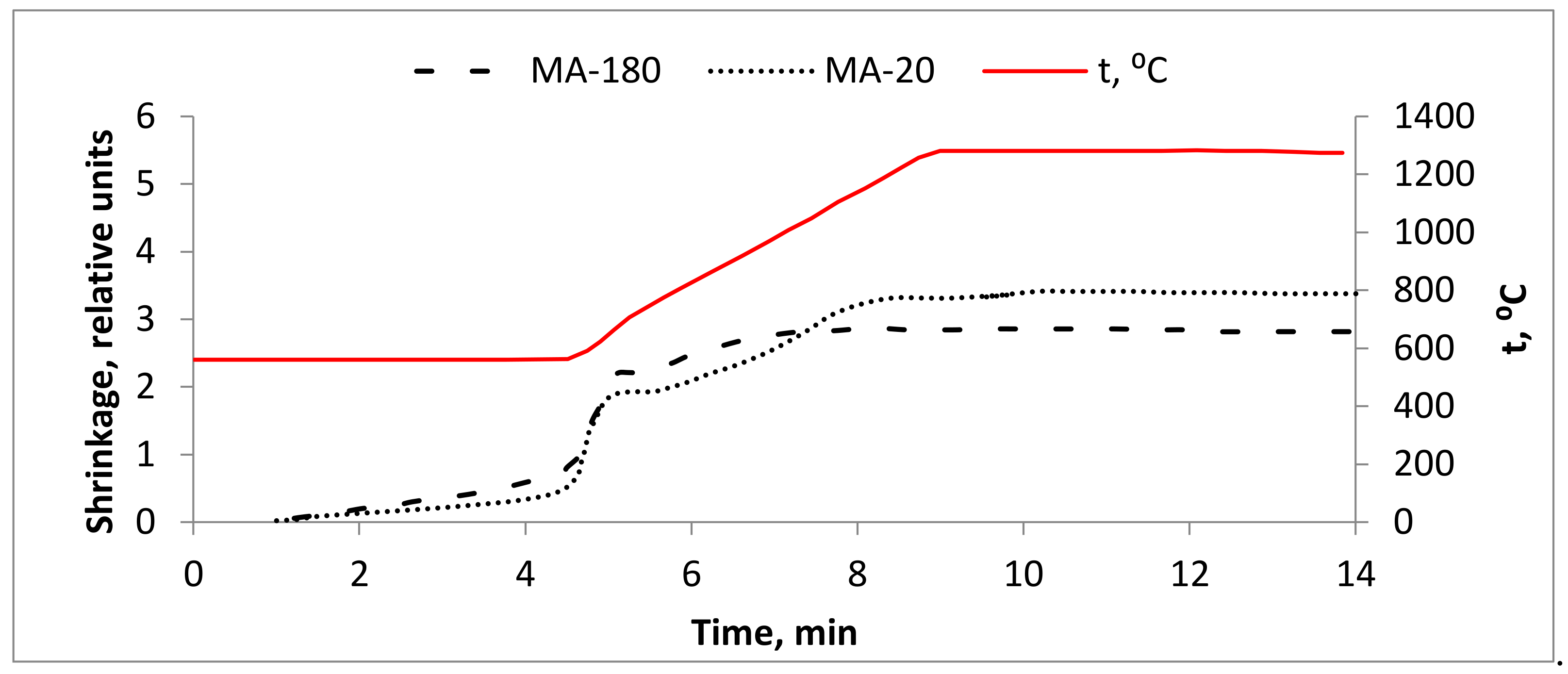





| Chemical Composition, Impurities, wt.%, Maximum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Powder | N | C | H | Fe + Ni | Si | Cu | Ta | W | O |
| Ti | 0.08 | 0.05 | 0.35 | 0.04 | 0.01 | 0.004 | - | - | - |
| Nb | 0.02 | 0.005 | 0.01 | 0.004 | 0.003 | - | 0.06 | 0.003 | 0.08 |
| Al | - | - | ≥0.2 | 0.35 | 0.4 | 0.02 | - | - | - |
| Material | Reference Designation | Duration, Min. | Rotating Speed, rpm | Test Medium |
|---|---|---|---|---|
| Ti-Al-Nb mixture | MA-20 | 20 | 650 | Argon |
| MA-180 | 180 | 350 |
| Name | Al | Ti | Nb | Phase | Name | Al | Ti | Nb | Phase |
|---|---|---|---|---|---|---|---|---|---|
| A | 14.17 | 65.7 | 20.13 | O | E | 27.12 | 67.7 | 5.18 | α2 |
| B | 21.11 | 69.96 | 8.93 | α2 | F | 24.73 | 53.17 | 22.1 | O |
| C | 14.27 | 56.97 | 28.76 | B2 | G | 28.4 | 58.57 | 1.04 | B2 |
| D | 25.83 | 51.03 | 23.14 | O | H | 14.81 | 63.93 | 21.26 | B2 |
| Sample | Main Phases, Lattice Parameters, nm | Other Phases, Lattice Parameters, nm |
|---|---|---|
| MA-2S | o-AlNbTi2(CmCm; a = 0.689 b = 0.956 Ti2AlNb (B2) (Pm-3m; a = 0.323); | (Ti,Nb,Al)C (Im3m, a = 0.427); Nb(Im3m; a = 0.330); α2 (a= 0,586, c = 0.470); |
| MA-1P | o-AlNbTi2(CmCm; a = 0.610 b = 0.960 c = 0.468); Ti2AlNb (B2) (Pm-3m; a = 0.323); |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skakov, M.; Kozhakhmetov, Y.; Mukhamedova, N.; Miniyazov, A.; Sokolov, I.; Urkunbay, A.; Zhanbolatova, G.; Tulenbergenov, T. Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System. Materials 2022, 15, 5560. https://doi.org/10.3390/ma15165560
Skakov M, Kozhakhmetov Y, Mukhamedova N, Miniyazov A, Sokolov I, Urkunbay A, Zhanbolatova G, Tulenbergenov T. Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System. Materials. 2022; 15(16):5560. https://doi.org/10.3390/ma15165560
Chicago/Turabian StyleSkakov, Mazhyn, Yernat Kozhakhmetov, Nurya Mukhamedova, Arman Miniyazov, Igor Sokolov, Azamat Urkunbay, Gainiya Zhanbolatova, and Timur Tulenbergenov. 2022. "Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System" Materials 15, no. 16: 5560. https://doi.org/10.3390/ma15165560
APA StyleSkakov, M., Kozhakhmetov, Y., Mukhamedova, N., Miniyazov, A., Sokolov, I., Urkunbay, A., Zhanbolatova, G., & Tulenbergenov, T. (2022). Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System. Materials, 15(16), 5560. https://doi.org/10.3390/ma15165560






