Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analytical and Characterization Methods
3. Results and Discussion
3.1. Effect of Different Factors on Removal Efficiency of SAD
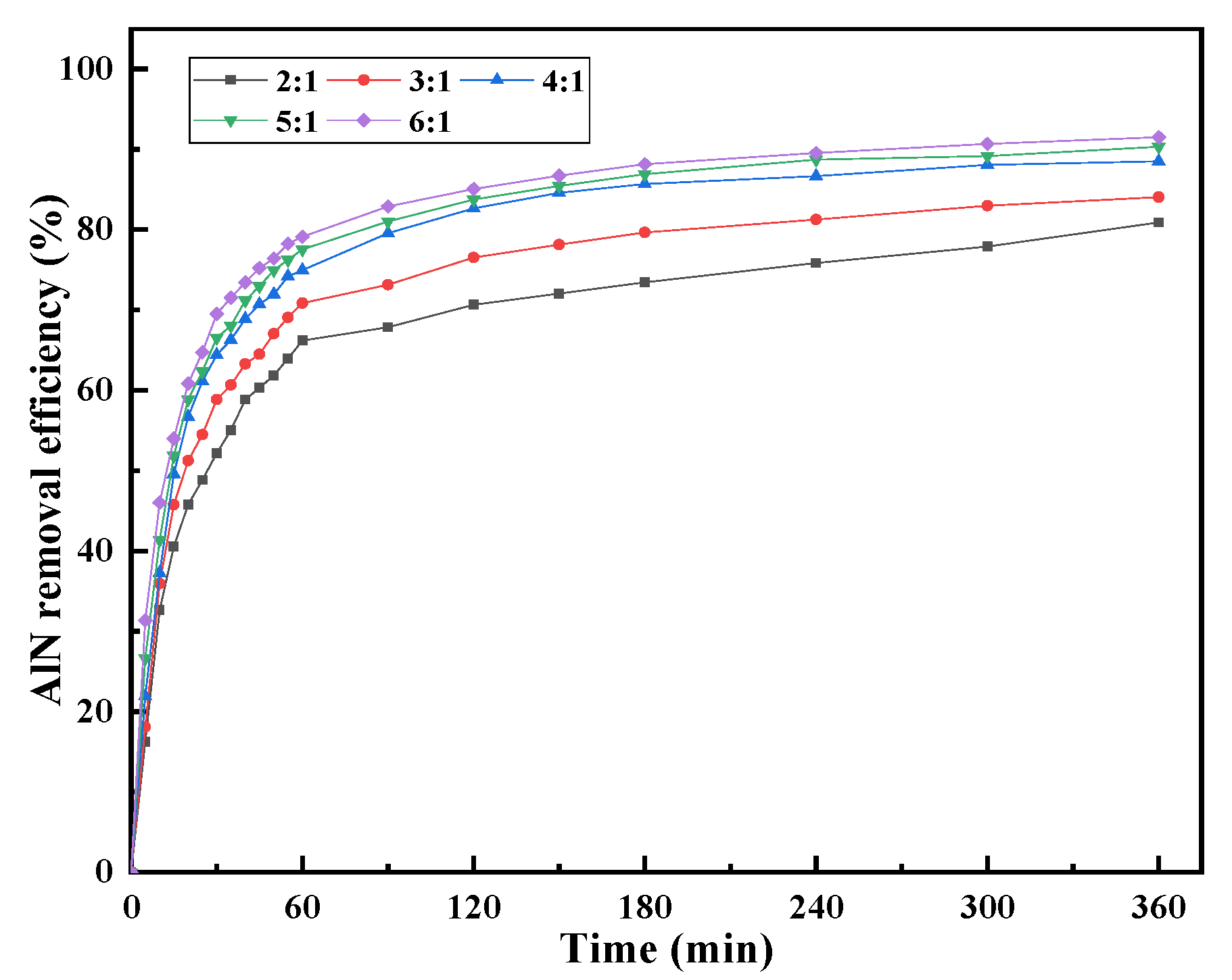
3.1.1. Effect of Liquid–Solid Ratio
3.1.2. Effect of Temperature
3.2. Analysis of Hydrolysis Products
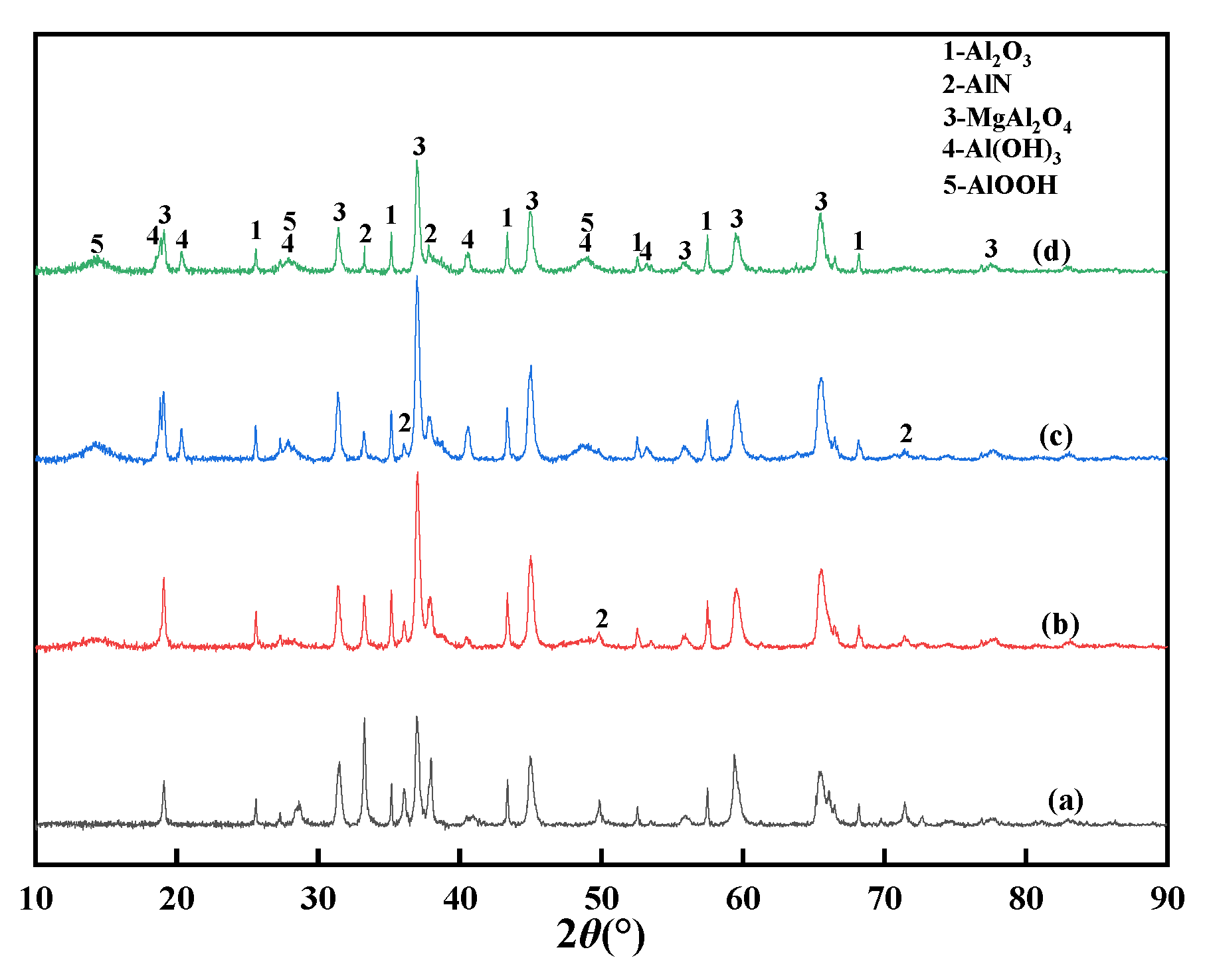
3.2.1. Analysis of XRD
3.2.2. Analysis of SEM and EDS
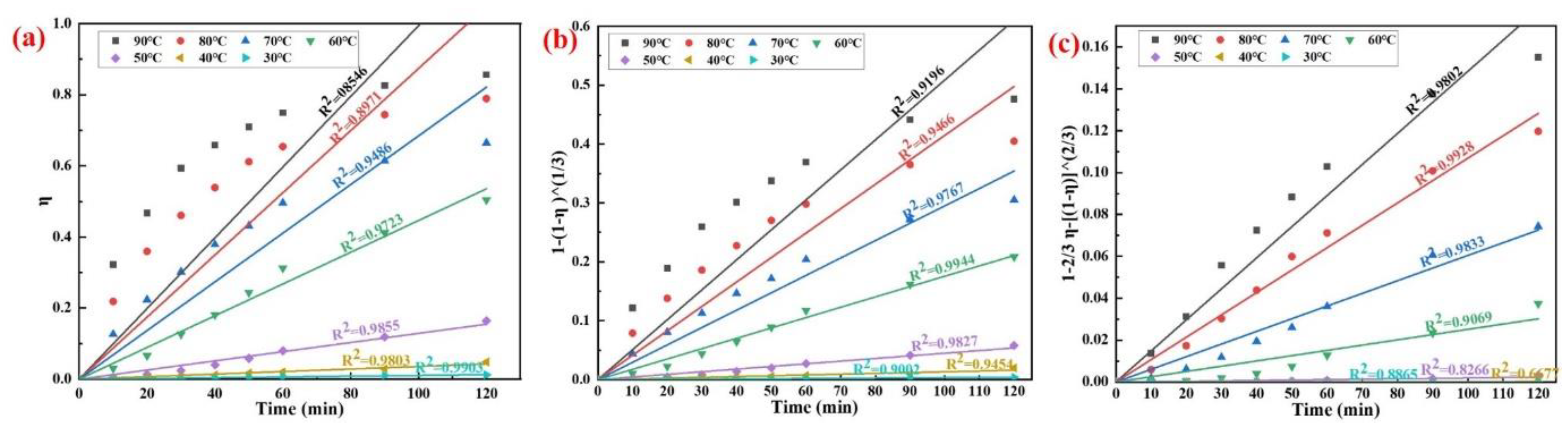
3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, M.C.; Hypolito, R. Solid waste from aluminum recycling process: Characterization and reuse of its economically valuable constituents. Waste Manag. 2005, 25, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, P.E. Aluminium salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 217–218, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, O.; Wuth, W.; Bohlinger, I. Characterizing the physical and chemical properties of aluminum dross. J. Miner. Met. Mater. Soc. 1997, 49, 48–51. [Google Scholar] [CrossRef]
- Shinzato, M.C.; Hypolito, R. Effect of disposal of aluminum recycling waste in soil and water bodies. Environ. Earth Sci. 2016, 75, 628–638. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Huang, X.; Xu, Z. Recovery of Metals from Aluminum Dross and Saltcake. J. Miner. Mater. Charact. 2006, 5, 47–62. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater. 2012, 209–210, 501–509. [Google Scholar] [CrossRef]
- Dash, B.; Das, B.; Tripathy, B.; Bhattacharya, I.; Das, S. Acid dissolution of alumina from waste aluminium dross. Hydrometallurgy 2008, 92, 48–53. [Google Scholar] [CrossRef]
- Murayama, N.; Maekawa, I.; Ushiro, H.; Miyoshi, T.; Shibata, J.; Valix, M. Synthesis of various layered double hydroxides using aluminum dross generated in aluminum recycling process. Int. J. Miner. Processing 2012, 110–111, 46–52. [Google Scholar] [CrossRef]
- Jones, L.; Nizam, M.; Reynolds, B.; Bareham, S.; Oxley, E. Upwind impacts of ammonia from an intensive poultry unit. Environ. Pollut. 2013, 180, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Li, Q.; Zhang, G.; Shi, Q.; Feng, H. Investigation of leaching kinetics of aluminum extraction from secondary aluminum dross with use of hydrochloric acid. Hydrometallurgy 2019, 187, 158–167. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Song, S.J.; Lee, M.S. Development of a hydrometallurgical process for the recovery of pure alumina from black dross and synthesis of magnesium spinel. J. Mater. Res. Technol. 2020, 9, 2568–2577. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, M.F.; Ding, Y.D.; Nan, S.U.; Quan, Y.A. Hydrolysis behavior of AlN in aluminum dross. Chin. J. Nonferrous Met. 2012, 22, 3555–3561. [Google Scholar] [CrossRef]
- Lu, S.S.; Ni, W.; Ni, H.J. Hydrolysis behavior of AlN in aluminum dross and its multivariate nonlinear regression analysis. Chin. J. Nonferrous Met. 2020, 30, 920–926. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.-H.; Han, Z.-Y.; Xiao, X.-Y. Effects of AlN hydrolysis on fractal geometry characteristics of residue from secondary aluminium dross using response surface methodology. Trans. Nonferrous Met. Soc. China 2018, 28, 2574–2581. [Google Scholar] [CrossRef]
- Yin, J.F.; Zhang, Y.N.; Du, Q.Z.; Chen, J.X.; Yuan, H.B.; Xu, Y.Q. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Mir, M.A.; Bhat, M.A.; Majid, S.A.; Lone, S.H.; Malla, M.A.; Tiwari, K.R.; Pandit, A.H.; Tomar, R.; Bhat, R.A. Hazardous characteristics and transformation mechanism in hydrometallurgical disposing strategy of secondary aluminum dross. J. Environ. Chem. Eng. 2021, 9, 1137–1146. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Q.; Zhang, G.; Shi, Q. Investigations on the hydrolysis behavior of AlN in the leaching process of secondary aluminum dross. Hydrometallurgy 2018, 182, 121–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Sun, J.; Dong, Y. Chemical Composition and Phase Composition of Aluminum Ash with Harmless Treatment. Chin. J. Rare Met. 2022, 46, 340–348. [Google Scholar] [CrossRef]
- Gao, Q. Basic Research on Application of Wet Denitrification and Defluorination Process for Secondary Aluminum Dross. Master’s Thesis, Zhengzhou University, Henan, China, 2021. [Google Scholar]
- Svedberg, L.M.; Arndt, K.C.; Cima, M.J. Corrosion of Aluminum Nitride (AlN) in Aqueous Cleaning Solutions. J. Am. Ceram. Soc. 2000, 83, 41–46. [Google Scholar] [CrossRef]
- Kocjan, A.; Dakskobler, A.; Krnel, K.; Kosmač, T. The course of the hydrolysis and the reaction kinetics of AlN powder in diluted aqueous suspensions. J. Eur. Ceram. Soc. 2011, 31, 815–823. [Google Scholar] [CrossRef]
- Kocjan, A. The Hydrolysis of AlN Powder–A Powerful Tool in Advanced Materials Engineering. Chem. Rec. 2018, 18, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.S.; Ni, W.; Ni, H.J. Study on Hydrolysis of Aluminum Ash Slag Based on Orthogonal Experiment and Nonlinear Regression Analysis. Nonferrous Met. Eng. 2019, 9, 52–56. [Google Scholar] [CrossRef]
- Monroe, D.C.; Blumenfeld, R.S.; Keator, B.D.; Solodkin, A.; Small, S.L. Evolution of aluminum hydroxides at the initial stage of aluminum nitride powder hydrolysis. Ceram. Int. 2016, 42, 11429–11434. [Google Scholar] [CrossRef]
- Yuan, J.; Luo, L.-K.; Lin, A.-W. Leaching kinetics of aluminum from aluminum dross with hydroloric acid. Nonferrous Met. Extr. Metall. 2022, 6, 37–42. [Google Scholar]
- Mahinroosta, M.; Allahcerdi, A. Enhanced alumina recovery from secondary aluminum dross for high purity nanostructured gamma-alumina powder production: Kinetic study. J. Environ. Manag. 2018, 212, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, A.; Krnel, K.; Kosmac, T. The influence of temperature and time on the AlN powder hydrolysis reaction products. J. Eur. Ceram. Soc. 2008, 28, 1003–1008. [Google Scholar] [CrossRef]
- Li, P.; Guo, M.; Zhang, M.; Teng, L.D.; Seetharaman, S. Leaching process investigation of secondary aluminium dross: Investigation of AlN hydrolysis behaviour in NaCl solution. Miner. Processing Extr. Metall. 2013, 121, 140–146. [Google Scholar] [CrossRef]
- Fukumoto, S.; Hookabe, T.; Tsubakino, H. Hydrolysis behavior of aluminum nitride in various solutions. J. Mater. Sci. 2000, 35, 2743–2748. [Google Scholar] [CrossRef]
- Bowen, P.; Highfield, J.G.; Mocellin, A.; Ring, T.A. Degradation of Aluminum Nitride Powder in an Aqueous Environmet. J. Am. Ceram. Soc. 1990, 73, 724–728. [Google Scholar] [CrossRef]
- Liu, H.-P. Xu, Z. Hydrometallurgy and Leaching Technology. Metall. Ind. Press 2010, 2, 154–157. [Google Scholar]
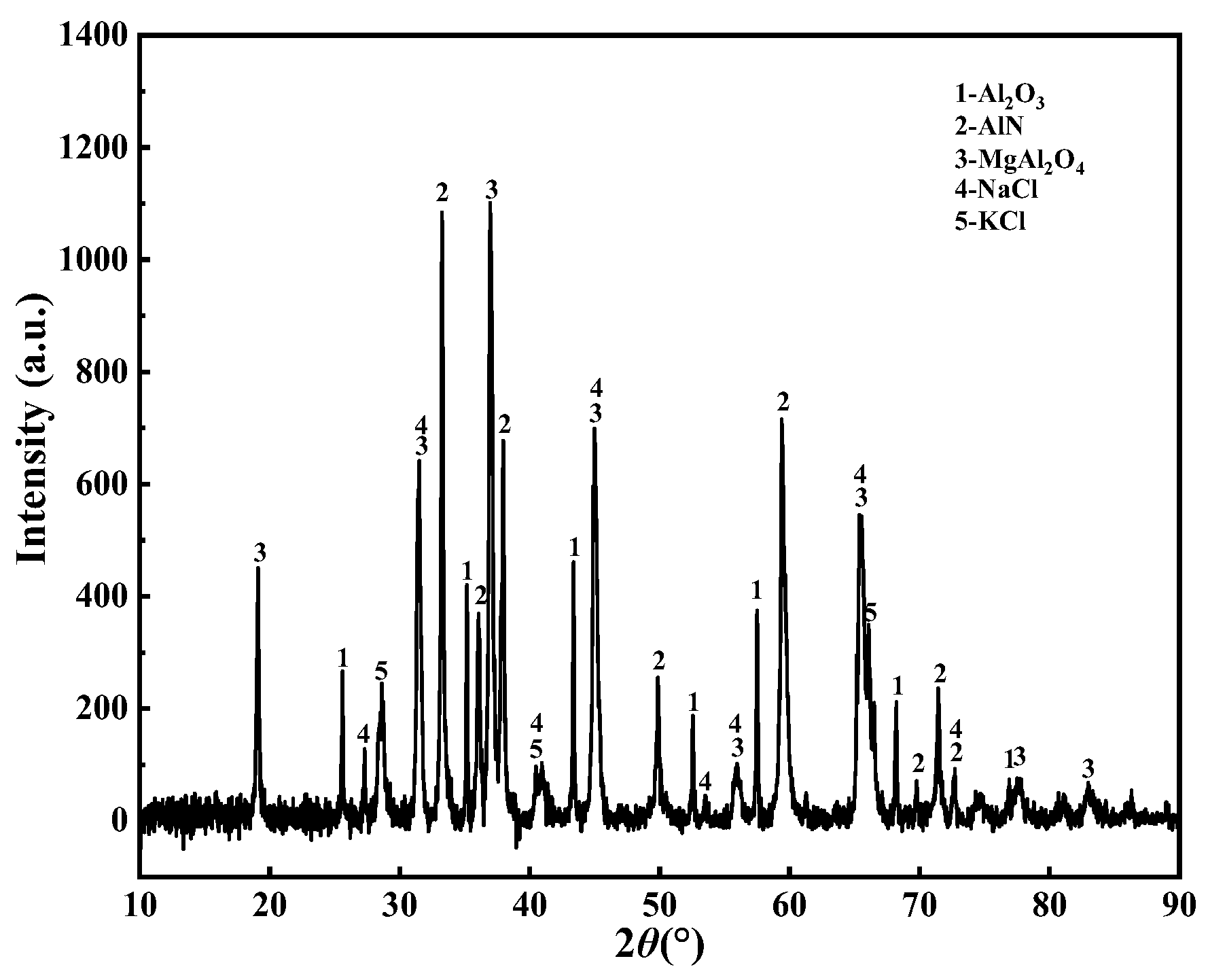
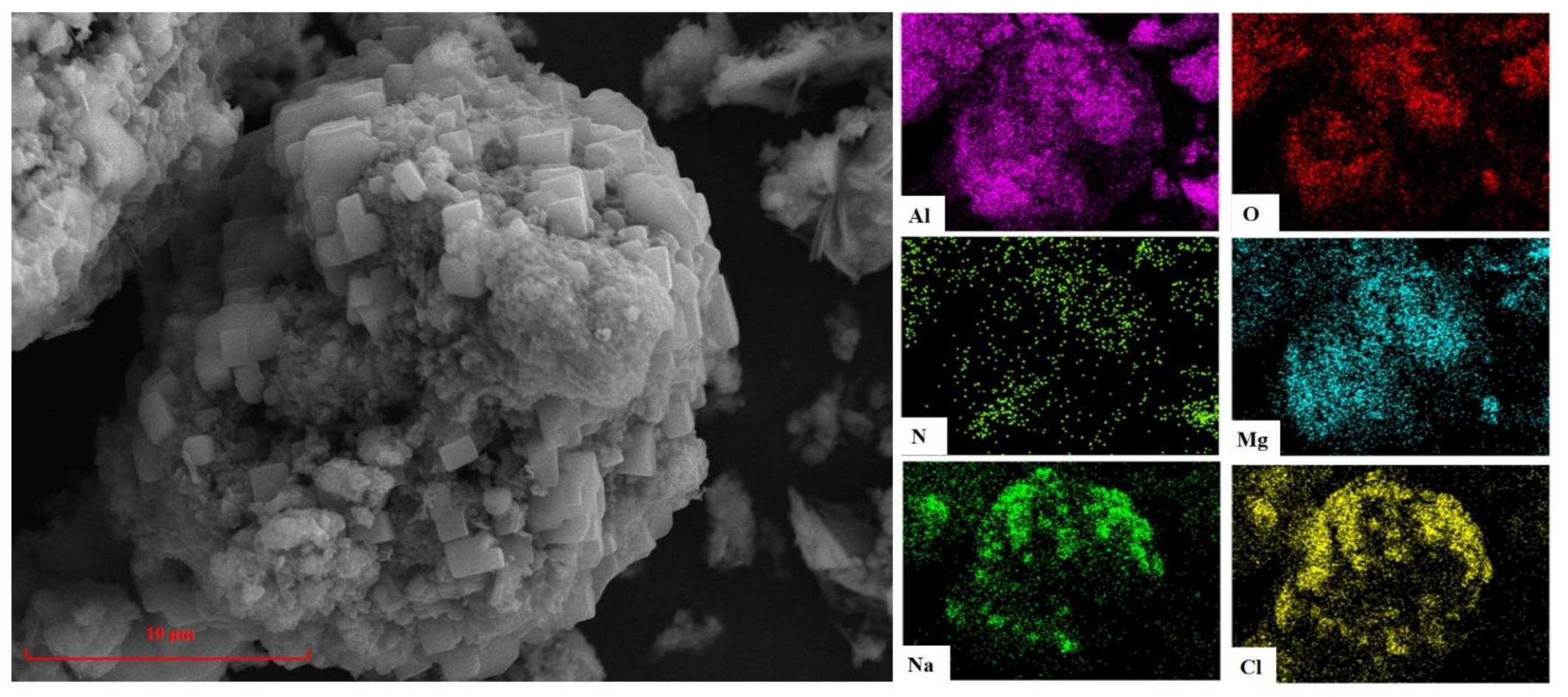

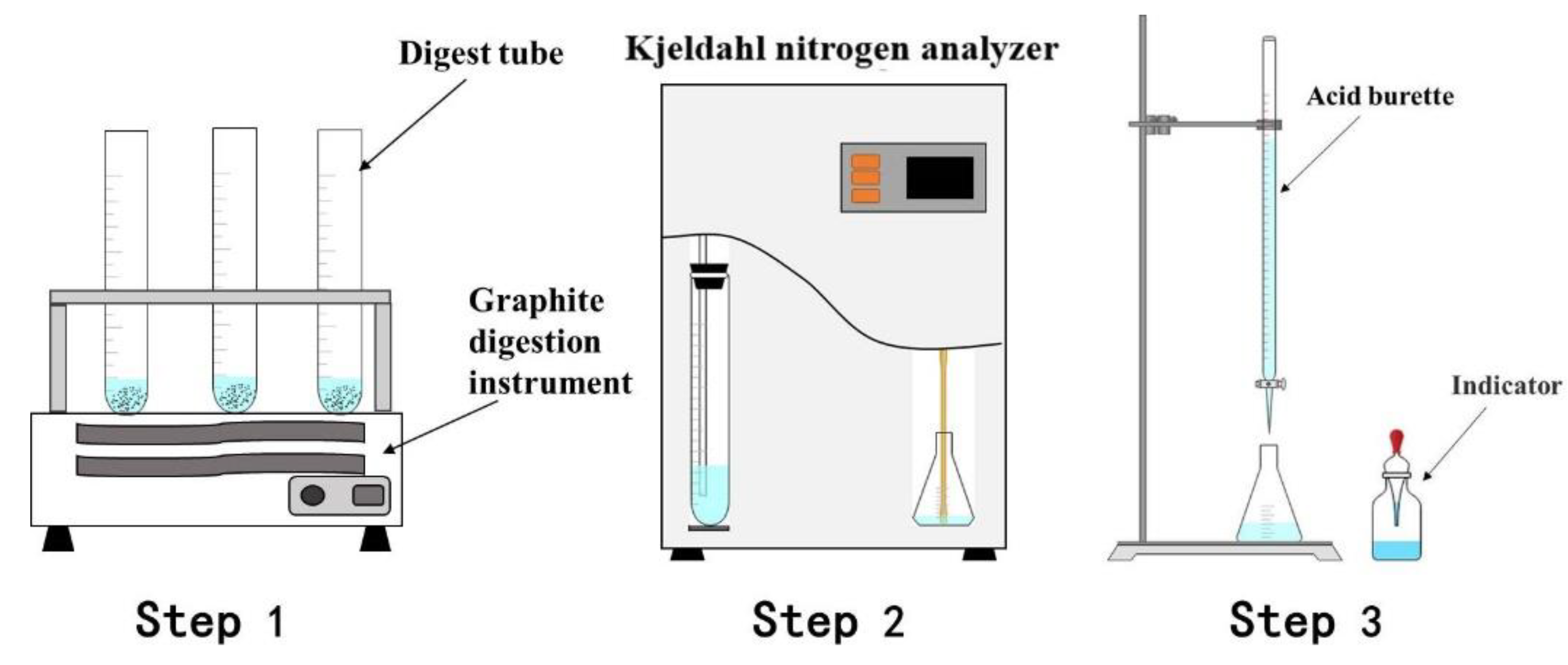



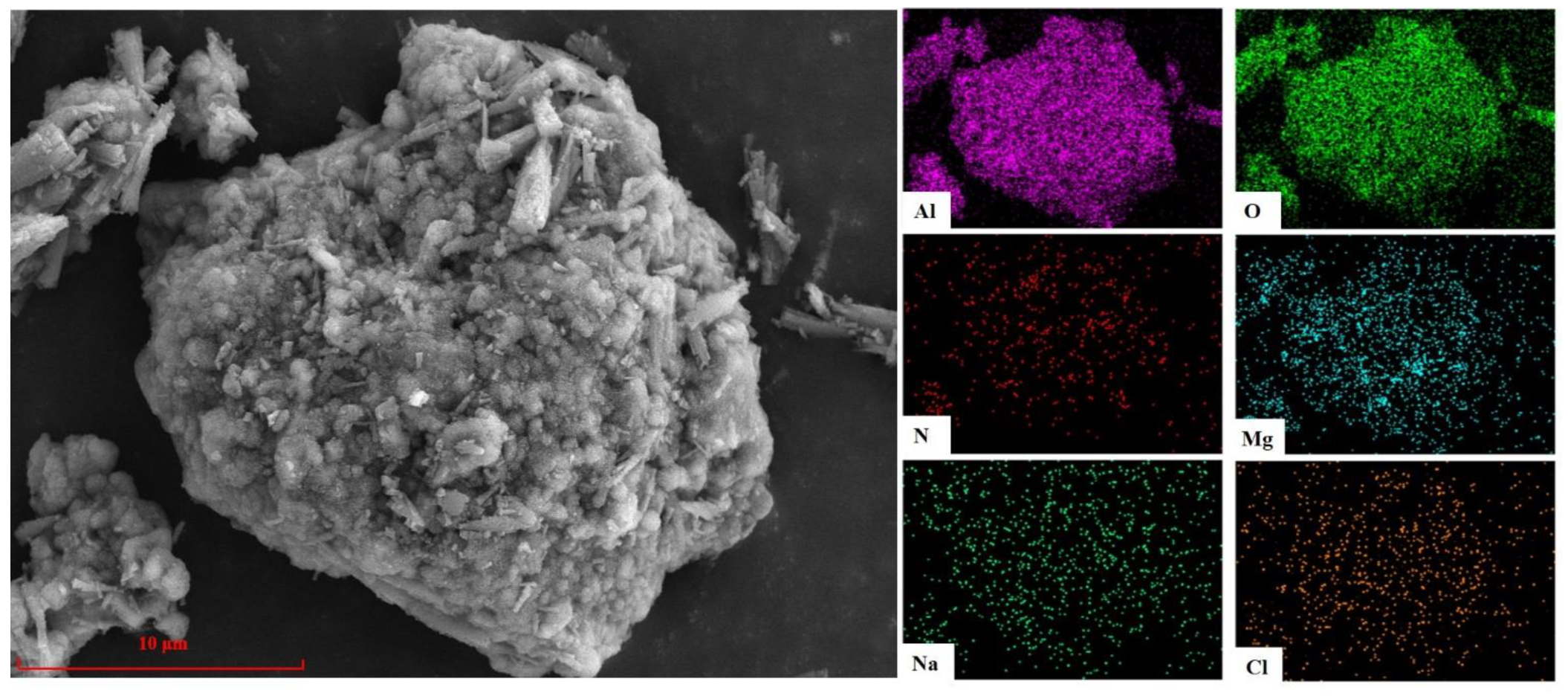
| Element | O | Al | N | Mg | Na | K | Cl | Si | Ca | Ti | Else |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 29.56 | 47.13 | 9.49 | 4.78 | 2.21 | 1.09 | 4.40 | 0.43 | 0.31 | 0.18 | 0.42 |
| Temperature (°C) | Rate-Controlling Step | k | Ea (kJ/mol) |
|---|---|---|---|
| 30 | Boundary layer diffusion | 1.05 × 10−4 | 96.53 |
| 40 | Boundary layer diffusion | 3.57 × 10−4 | |
| 50 | Boundary layer diffusion and chemical reaction | 4.49 × 10−4 | 87.13 |
| 60 | Chemical reaction | 1.76 × 10−3 | |
| 70 | Chemical reaction and product layer diffusion | 2.95 × 10−3 | |
| 80 | Product layer diffusion | 1.02 × 10−3 | 34.59 |
| 90 | Product layer diffusion | 1.42 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-L.; Li, Z.-S.; Ding, Y.-D.; Ge, Q.-Q.; Jiang, L. Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process. Materials 2022, 15, 5499. https://doi.org/10.3390/ma15165499
Yang H-L, Li Z-S, Ding Y-D, Ge Q-Q, Jiang L. Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process. Materials. 2022; 15(16):5499. https://doi.org/10.3390/ma15165499
Chicago/Turabian StyleYang, Hong-Liang, Zi-Shen Li, You-Dong Ding, Qi-Qi Ge, and Lan Jiang. 2022. "Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process" Materials 15, no. 16: 5499. https://doi.org/10.3390/ma15165499
APA StyleYang, H.-L., Li, Z.-S., Ding, Y.-D., Ge, Q.-Q., & Jiang, L. (2022). Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process. Materials, 15(16), 5499. https://doi.org/10.3390/ma15165499





