Fast Removal of Methylene Blue via Adsorption-Photodegradation on TiO2/SBA-15 Synthesized by Slow Calcination
Abstract
:1. Introduction
2. Material
2.1. SBA-15 Synthesis
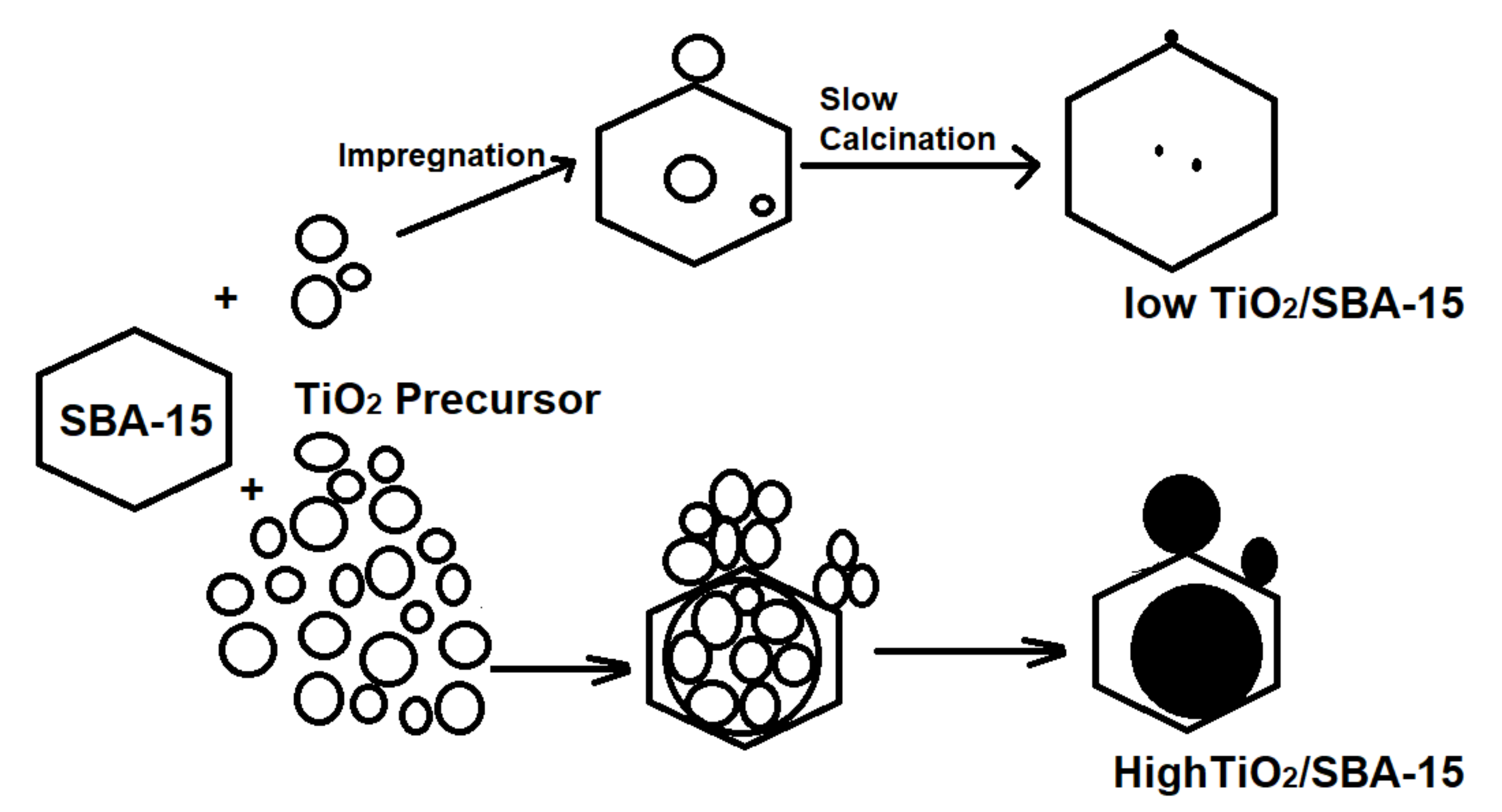
2.2. Synthesis of TiO2/SBA-15
2.3. Characterization
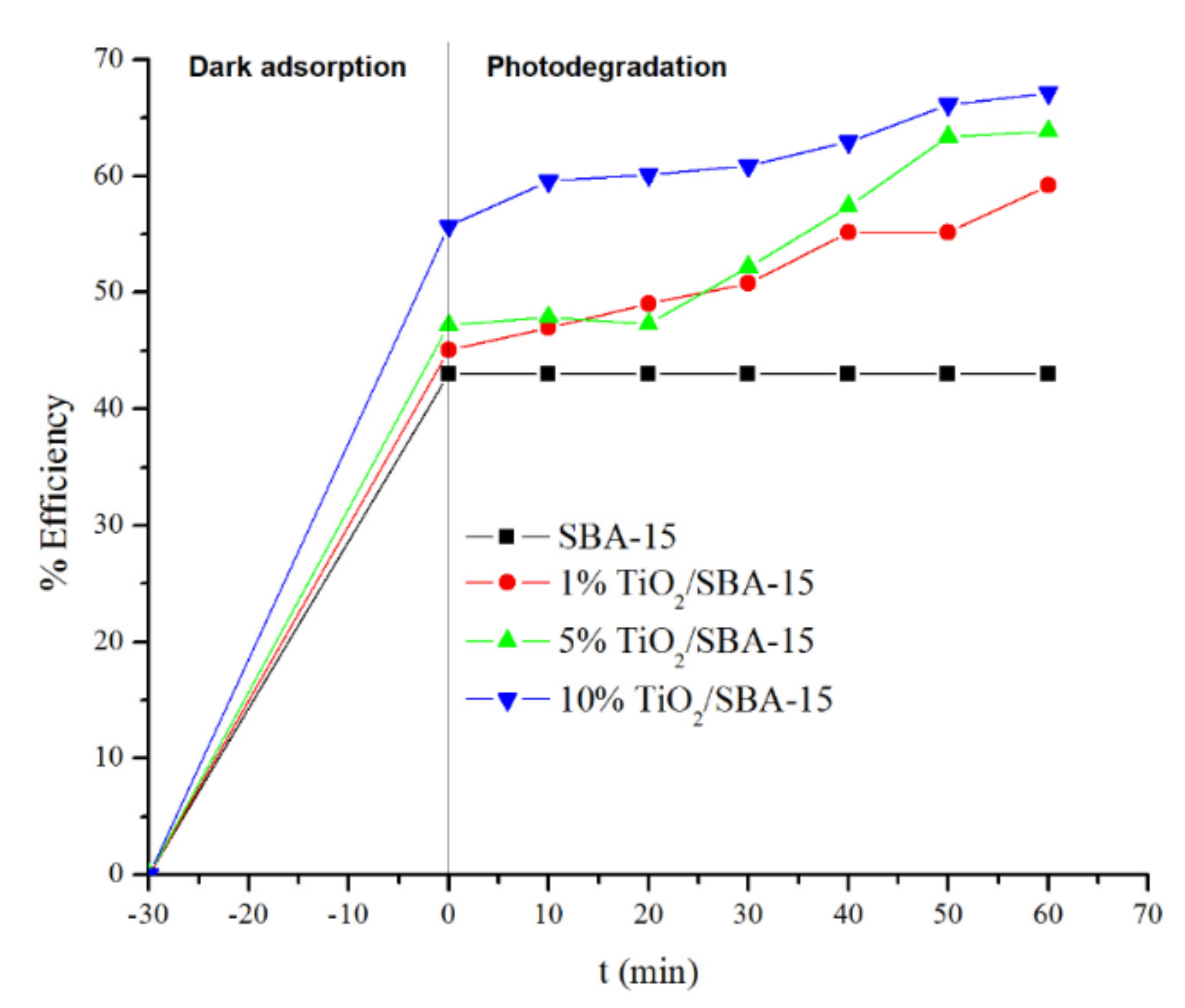
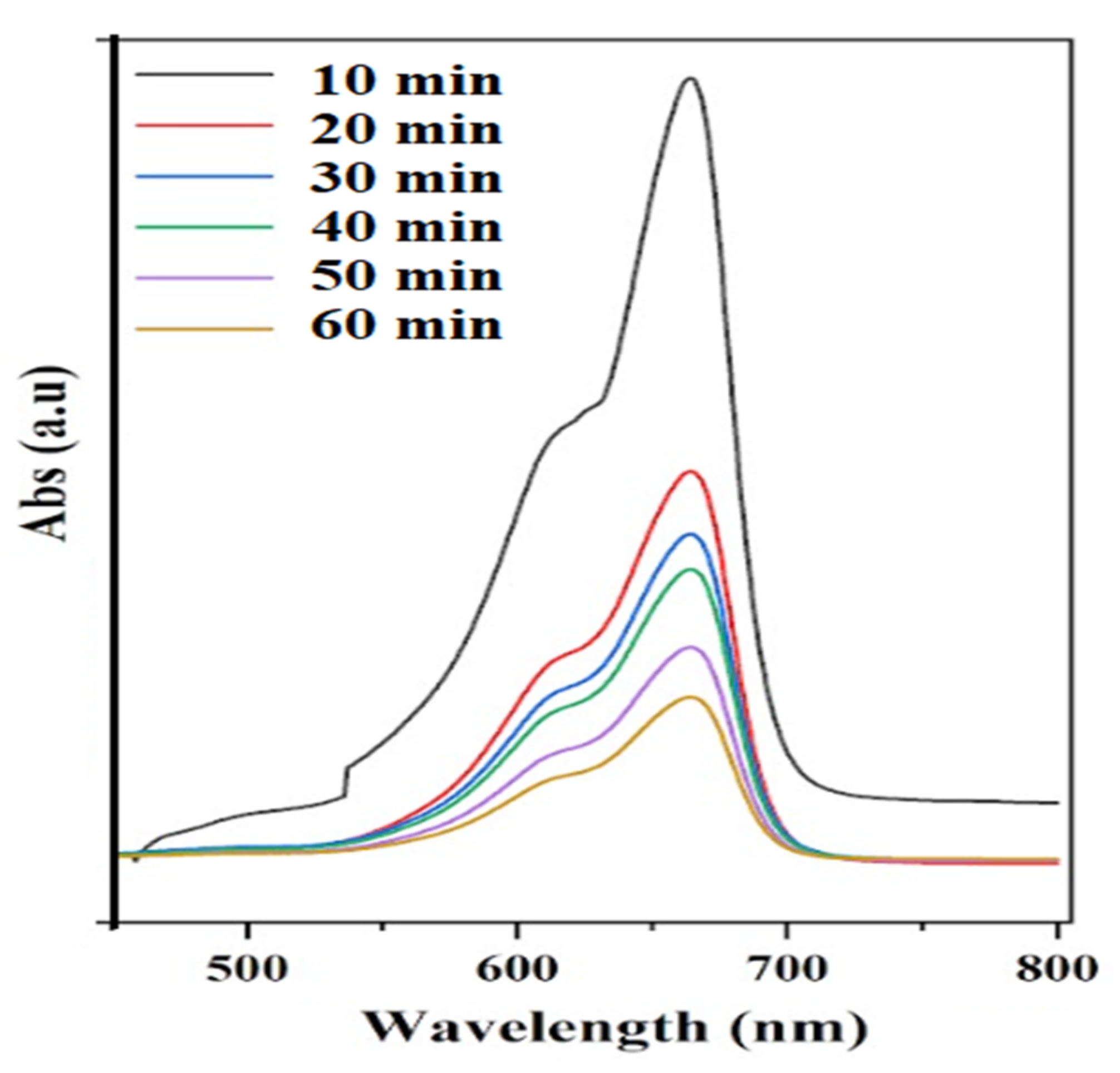
2.4. Methylene Blue Degradation
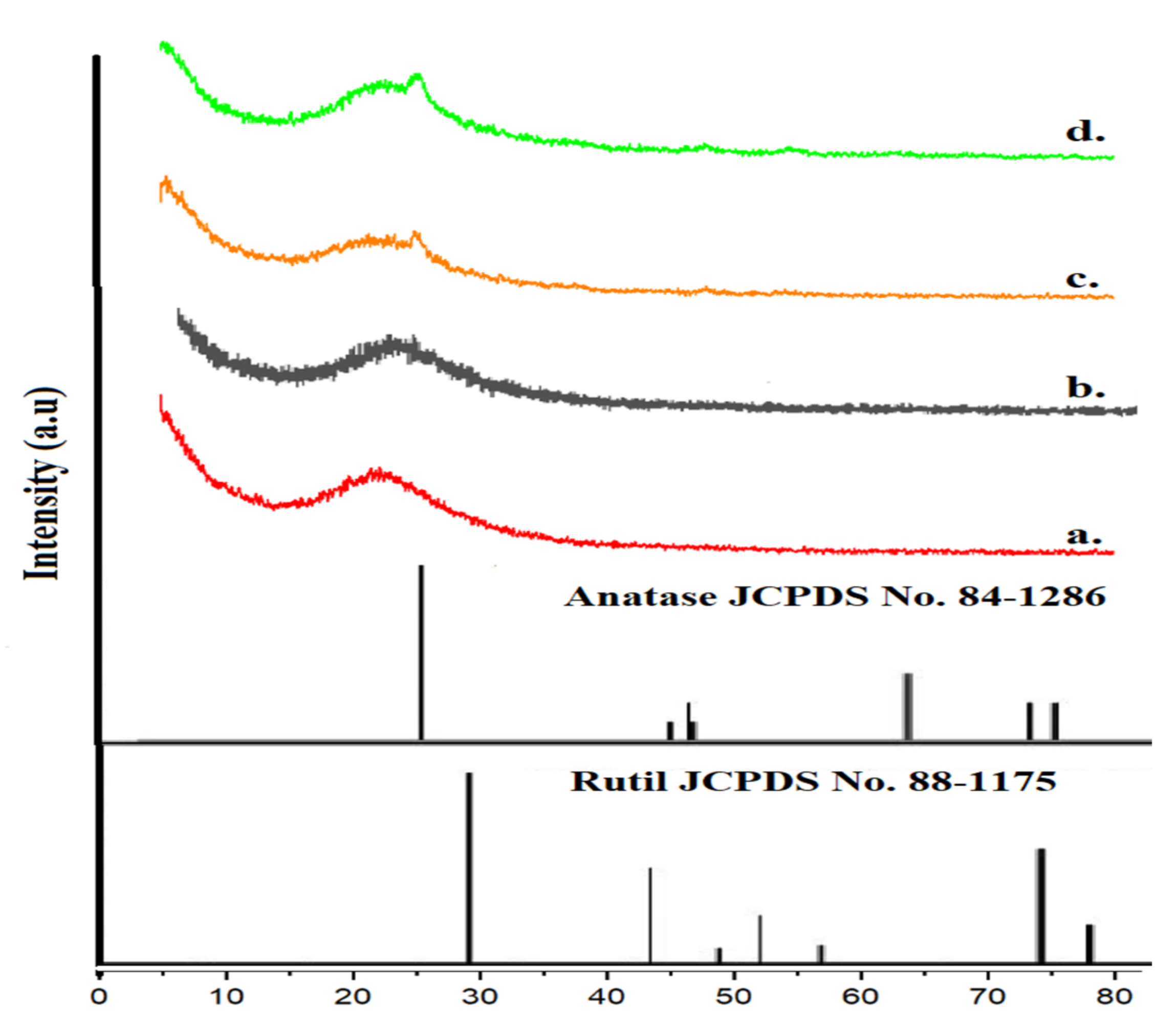
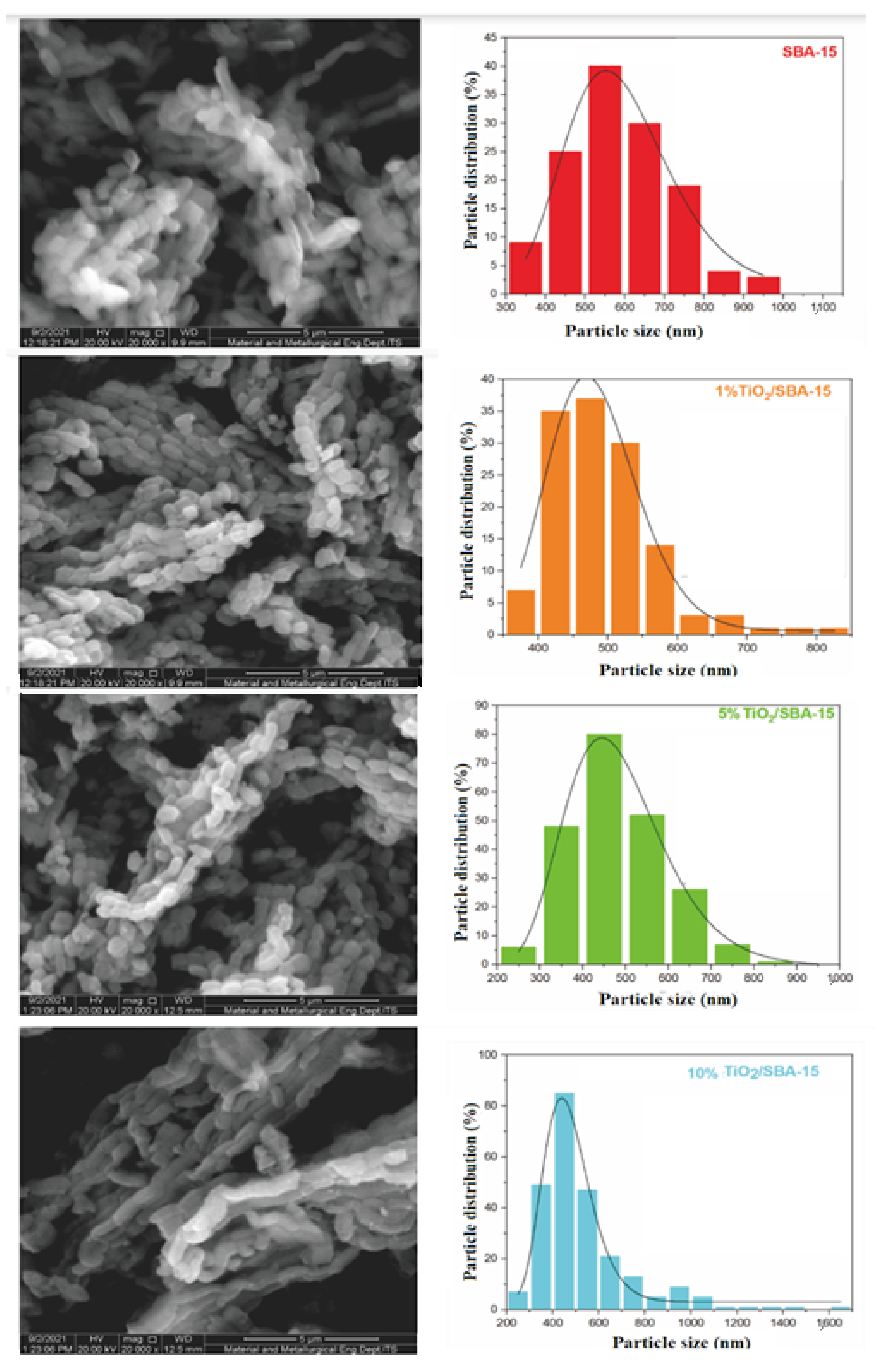
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Rajagopal, S.; Paramasivam, B.; Muniyasamy, K. Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep. Purif. Technol. 2020, 252, 117444. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Shukla, A.K.; Kombaiah, K.; Vijaya, J.J.; Tawfeek, A.M. Synthesis, characterization and optical properties of sulfur and fluorine doped ZnO nanostructures for visible light utilized catalysis. Optik 2017, 148, 325–331. [Google Scholar] [CrossRef]
- Veisi, H.; Tamoradi, T.; Karmakar, B.; Hemmati, S. Green tea extract–modified silica gel decorated with palladium nanoparticles as a heterogeneous and recyclable nanocatalyst for Buchwald-Hartwig C–N cross-coupling reactions. J. Phys. Chem. Solids 2019, 138, 109256. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; et al. Facile ball-milling synthesis of CeO2/g-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and mechanisms. Chem. Eng. J. 2020, 420, 127719. [Google Scholar] [CrossRef]
- Ulfa, M.; Prasetyoko, D.; Bahruji, H.; Nugraha, R.E. Green Synthesis of Hexagonal Hematite (α-Fe2O3) Flakes Using Pluronic F127-Gelatin Template for Adsorption and Photodegradation of Ibuprofen. Materials 2021, 14, 6779. [Google Scholar] [CrossRef]
- Ulfa, M.; Setiarini, I. The Effect of Zinc Oxide Supported on Gelatin Mesoporous Silica (GSBA-15) on Structural Character and Their Methylene Blue Photodegradation Performance. Bull. Chem. React. Eng. Catal. 2022, 17, 363–374. [Google Scholar] [CrossRef]
- Azami, M.; Jalil, A.; Aziz, F.; Hassan, N.; Mamat, C.; Izzudin, N. Influence of the nitrogen pots from graphitic carbon nitride with the presence of wrinkled silica-titania for photodegradation enhancement of 2-chlorophenol. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Barakat, M.; Kumar, R.; Eniola, J.O. Adsorption and photocatalytic scavenging of 2-chlorophenol using carbon nitride-titania nanotubes based nanocomposite: Experimental data, kinetics and mechanism. Data Brief 2020, 34, 106664. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zhu, L.; Chen, S.; Zhang, Y.; Tang, Y.; Zhu, Y.; Li, Y. Synthesis of nano titania particles embedded in mesoporous SBA-15: Characterization and photocatalytic activity. J. Hazard. Mater. 2006, 137, 952–958. [Google Scholar] [CrossRef]
- Díaz-Uribe, C.; Vallejo, W.; Campos, K.; Solano, W.; Andrade, J.; Muñoz-Acevedo, A.; Schott, E.; Zarate, X. Improvement of the photocatalytic activity of TiO2 using Colombian Caribbean species (Syzygium cumini) as natural sensitizers: Experimental and theoretical studies. Dye. Pigment. 2018, 150, 370–376. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Papachristou, E.; Korres, D.; Mamma, D.; Kekos, D.; Tarantili, P.A.; Polyzois, G. Titanium Dioxide/Polysiloxane Composites: Preparation, Characterization and Study of Their Color Stability Using Thermochromic Pigments. J. Compos. Sci. 2022, 6, 195. [Google Scholar] [CrossRef]
- Calzada, L.A.; Castellanos, R.; García, L.A.; Klimova, T.E. TiO2, SnO2 and ZnO catalysts supported on mesoporous SBA-15 versus unsupported nanopowders in photocatalytic degradation of methylene blue. Microporous Mesoporous Mater. 2019, 285, 247–258. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, F.-T.; Hao, Y.-J.; Liu, S.-J.; Yang, M.-L. TiO2/SBA-15 composites prepared using H2TiO3 by hydrothermal method and its photocatalytic activity. Mater. Lett. 2013, 99, 38–41. [Google Scholar] [CrossRef]
- Chang, F.; Jiao, M.; Xu, Q.; Deng, B.; Hu, X. Facile fabrication of mesoporous Fe-Ti-SBA15 silica with enhanced visible-light-driven simultaneous photocatalytic degradation and reduction reactions. Appl. Surf. Sci. 2018, 435, 708–717. [Google Scholar] [CrossRef]
- Ali, A.; Shoeb, M.; Li, Y.; Li, B.; Khan, M.A. Enhanced photocatalytic degradation of antibiotic drug and dye pollutants by graphene-ordered mesoporous silica (SBA 15)/TiO2 nanocomposite under visible-light irradiation. J. Mol. Liq. 2020, 324, 114696. [Google Scholar] [CrossRef]
- Lachheb, H.; Ahmed, O.; Houas, A.; Nogier, J. Photocatalytic activity of TiO2–SBA-15 under UV and visible light. J. Photochem. Photobiol. A Chem. 2011, 226, 1–8. [Google Scholar] [CrossRef]
- Zhao, S.; Su, D.; Che, J.; Jiang, B.; Orlov, A. Photocatalytic properties of TiO2 supported on SBA-15 mesoporous materials with large pores and short channels. Mater. Lett. 2011, 65, 3354–3357. [Google Scholar] [CrossRef]
- Pertiwi, Y.E.; Ulfa, M.; Saraswati, T.E.; Prasetyoko, D.; Trisunaryanti, W. Understanding Pore Surface Modification of Sucrose-Modified Iron Oxide/Silica Mesoporous Composite for Degradation of Methylene Blue. Bull. Chem. React. Eng. Catal. 2021, 16, 459–471. [Google Scholar] [CrossRef]
- Wei, J.; Chen, X.; Wang, P.; Han, Y.; Xu, J.; Hong, B.; Jin, H.; Jin, D.; Peng, X.; Li, J.; et al. High surface area TiO2/SBA-15 nanocomposites: Synthesis, microstructure and adsorption-enhanced photocatalysis. Chem. Phys. 2018, 510, 47–53. [Google Scholar] [CrossRef]
- Chandrasekar, G.; Son, W.-J.; Ahn, W.-S. Synthesis of mesoporous materials SBA-15 and CMK-3 from fly ash and their application for CO2 adsorption. J. Porous Mater. 2008, 16, 545–551. [Google Scholar] [CrossRef]
- Sahu, D.; Hong, L.; Wang, S.-C.; Huang, J.-L. Synthesis, analysis and characterization of ordered mesoporous TiO2/SBA-15 matrix: Effect of calcination temperature. Microporous Mesoporous Mater. 2009, 117, 640–649. [Google Scholar] [CrossRef]
- Fatimah, I.; Prakoso, N.I.; Sahroni, I.; Musawwa, M.M.; Sim, Y.-L.; Kooli, F.; Muraza, O. Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon 2019, 5, e02766. [Google Scholar] [CrossRef]
- Acosta-Silva, Y.; Nava, R.; Hernández-Morales, V.; Macías-Sánchez, S.; Pawelec, B. TiO2/DMS-1 disordered mesoporous silica system: Structural characteristics and methylene blue photodegradation activity. Microporous Mesoporous Mater. 2012, 170, 181–188. [Google Scholar] [CrossRef]
- Li, X.; Wan, T.; Qiu, J.; Wei, H.; Qin, F.; Wang, Y.; Liao, Y.; Huang, Z.; Tan, X. In-situ photocalorimetry-fluorescence spectroscopy studies of RhB photocatalysis over Z-scheme g-C3N4@Ag@Ag3PO4 nanocomposites: A pseudo-zero-order rather than a first-order process. Appl. Catal. B Environ. 2017, 217, 591–602. [Google Scholar] [CrossRef]
- Chang, F.; Wang, G.; Xie, Y.; Zhang, M.; Zhang, J.; Yang, H.-J.; Hu, X. Synthesis of TiO2 nanoparticles on mesoporous aluminosilicate Al-SBA-15 in supercritical CO2 for photocatalytic decolorization of methylene blue. Ceram. Int. 2013, 39, 3823–3829. [Google Scholar] [CrossRef]
- Scala, F.; Chirone, R.; Meloni, P.; Carcangiu, G.; Manca, M.; Mulas, G.; Mulas, A. Fluidized bed desulfurization using lime obtained after slow calcination of limestone particles. Fuel 2013, 114, 99–105. [Google Scholar] [CrossRef]
- Kim, J.; Jang, E.; Hong, S.; Kim, D.; Kim, E.; Ricther, H.; Simon, A.; Choi, N.; Korelskiy, D.; Fouladvand, S.; et al. Microstructural control of a SSZ-13 zeolite film via rapid thermal processing. J. Membr. Sci. 2019, 591, 117342. [Google Scholar] [CrossRef]
- Yilmaz, M.; Mengelizadeh, N.; Saloot, M.K.; Shahbaksh, S.; Balarak, D. Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater. Sci. Semicond. Process. 2022, 144. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Sahroni, I.; Kamari, A.; Sagadevan, S.; Doong, R.-A. Nanoflower-like composites of ZnO/SiO2 synthesized using bamboo leaves ash as reusable photocatalyst. Arab. J. Chem. 2020, 14, 102973. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Liang, F.; Fang, Y.; Wang, H.; Chen, A. Precise regulation of Ultra-thin platinum decorated Gold/Graphite carbon nitride photocatalysts by atomic layer deposition for efficient degradation of Rhodamine B under simulated sunlight. Arab. J. Chem. 2022, 15, 103951. [Google Scholar] [CrossRef]
- Zuoli, H.; Wenxiu, Q.; Haixia, X.; Jing, C.; Yuan, Y.; Peng, S. Facile Synthesis of Self-Sensitized TiO2Photocatalysts and Their Higher Photocatalytic Activity. J. Am. Ceram. Soc. 2012, 95, 3941–3946. [Google Scholar] [CrossRef]





| Wave Number (cm−1) | Vibration Type [Ref] |
|---|---|
| SBA-15 | |
| 1052–1100 | Symmetric stretching vibration Si-O-Si [18] |
| 950–966 | Stretching vibration of silanol Si-OH [18] |
| TiO2 | |
| 790–800 | stretching band Ti-O-Ti [24] |
| 625–550 | Ti-O-Ti stretching vibration [12,18] |
| 453 | Ti-O-Ti stretching vibration for anatase [12,18] |
| 1%, 5%, 10% TiO/SBA-15 | |
| 1052–1100 | Symmetric stretching vibration Si-O-Si [18] |
| 940–960 | The Si–O–Ti linkage stretching band [24] |
| 790–800 | stretching band Ti-O-Ti [24] |
| 440–493 | Ti-O-Ti stretching vibration for anatase [12,18] |
| Photocatalyst | Sa (m2/g) | Vb (cc/g) | Dc (Å) | Vme/Vmid | DAe % | Pf % | DA-Pg (min) | Total Removal (%) |
|---|---|---|---|---|---|---|---|---|
| SBA-15 | 498 | 0.737 | 53.8 | 2.22 | 43 | 0 | 30–60 | 43 |
| 1-TiO2/SBA-15 | 467 | 0.648 | 48.6 | 2.04 | 45 | 14.2 | 30–60 | 59.2 |
| 5-TiO2/SBA-15 | 399 | 0.578 | 48.1 | 1.99 | 47 | 16.8 | 30–60 | 63.8 |
| 10-TiO2/SBA-15 | 384 | 0.550 | 37.4 | 1.97 | 55 | 12.1 | 30–60 | 67.1 |
| SBA-15 [22] | 753.80 | 1.430 | 85.0 | - | 20 | 1.5 | 60–150 | 21.5 |
| 21-TiO2/SBA-15 [22] | 596 | 0.88 | 71.0 | - | 60 | 6 | 60–150 | 66 |
| 80-TiO2/SBA-15 [10] | 142 | 0.240 | 66.5 | - | 37 | 3 | 15–60 | 40 |
| 30-TiO2/SBA-15 [10] | 499 | 0.520 | 41.0 | - | 65 | 34 | 15–60 | 99 |
| 46-TiO2/SBA-15 [22] | 466 | 0.52 | 52.8 | - | 30 | 50 | 60–150 | 80 |
| SBA-15 [24] | - | - | 76 | - | 2 | 54 | 60–420 | 7 |
| 30-TiO2/SBA-15 [24] | - | - | 65 | - | 70 | 28 | 60–420 | 98 |
| 60-TiO2/SBA-15 [24] | - | - | 61 | - | 45 | 44 | 60–420 | 99 |
| SBA-15 [15] | 730 | 1.07 | 80 | - | 21 | 5 | 120–180 | 26 |
| 29-TiO2/SBA-15 [15] | 587 | 0.73 | 65 | - | 22 | 59 | 120–180 | 79 |
| Sample | Pseudo First Order | Pseudo Second Order | % Efficiency | ||
|---|---|---|---|---|---|
| R2 | k1 (min−1) | R2 | k2 (g mg−1min−1) | ||
| SBA–15 | 0.3828 | 2.2 × 10−3 | 0.950 | 1.96 × 10−3 | 43.0 |
| 1% TiO2/SBA-15 | 0.2346 | 3.5 × 10−3 | 0.924 | 1.95 × 10−3 | 59.2 |
| 5% TiO2/SBA-15 | 0.6979 | 4.0 × 10−3 | 0.9558 | 1.36 × 10−3 | 63.8 |
| 10% TiO2/SBA-15 | 0.5344 | 5.2 × 10−3 | 0.9545 | 1.29 × 10−3 | 67.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulfa, M.; Al Afif, H.; Saraswati, T.E.; Bahruji, H. Fast Removal of Methylene Blue via Adsorption-Photodegradation on TiO2/SBA-15 Synthesized by Slow Calcination. Materials 2022, 15, 5471. https://doi.org/10.3390/ma15165471
Ulfa M, Al Afif H, Saraswati TE, Bahruji H. Fast Removal of Methylene Blue via Adsorption-Photodegradation on TiO2/SBA-15 Synthesized by Slow Calcination. Materials. 2022; 15(16):5471. https://doi.org/10.3390/ma15165471
Chicago/Turabian StyleUlfa, Maria, Hafid Al Afif, Teguh Endah Saraswati, and Hasliza Bahruji. 2022. "Fast Removal of Methylene Blue via Adsorption-Photodegradation on TiO2/SBA-15 Synthesized by Slow Calcination" Materials 15, no. 16: 5471. https://doi.org/10.3390/ma15165471
APA StyleUlfa, M., Al Afif, H., Saraswati, T. E., & Bahruji, H. (2022). Fast Removal of Methylene Blue via Adsorption-Photodegradation on TiO2/SBA-15 Synthesized by Slow Calcination. Materials, 15(16), 5471. https://doi.org/10.3390/ma15165471






