Aluminide Diffusion Coatings on IN 718 by Pack Cementation
Abstract
:1. Introduction
- the active component, suppliers of Al in the active state: frequently aluminum powder (about 50 wt.%), or high-content Ferroaluminum powder (about 40%), which is 98% of the powder mixture;
- the neutral component, with the role of preserving the uniform distribution of the active component in the entire volume of the powder mixture and at the same time avoiding the tendency of particles’ mutual sintering of the active component during the aluminizing process. Alumina (Al2O3) is the most frequently used component with the role of dispersing and blocking the sintering tendency. If FeAl40 is used as an active component of the pack aluminizing mixture, it is no longer necessary to use a neutral component alongside the active one;
- the component with the role of activating the metal surface subjected to processing and, at the same time, directly participating, most often, in the reactions generating aluminum in the active state, is ammonium chloride (NH4Cl).
- Checking the behavior of the Al-Ni diffusion couple (solid or powdery, with Al as an active component) in the conditions of holding temperatures below and above the Al melting temperature;
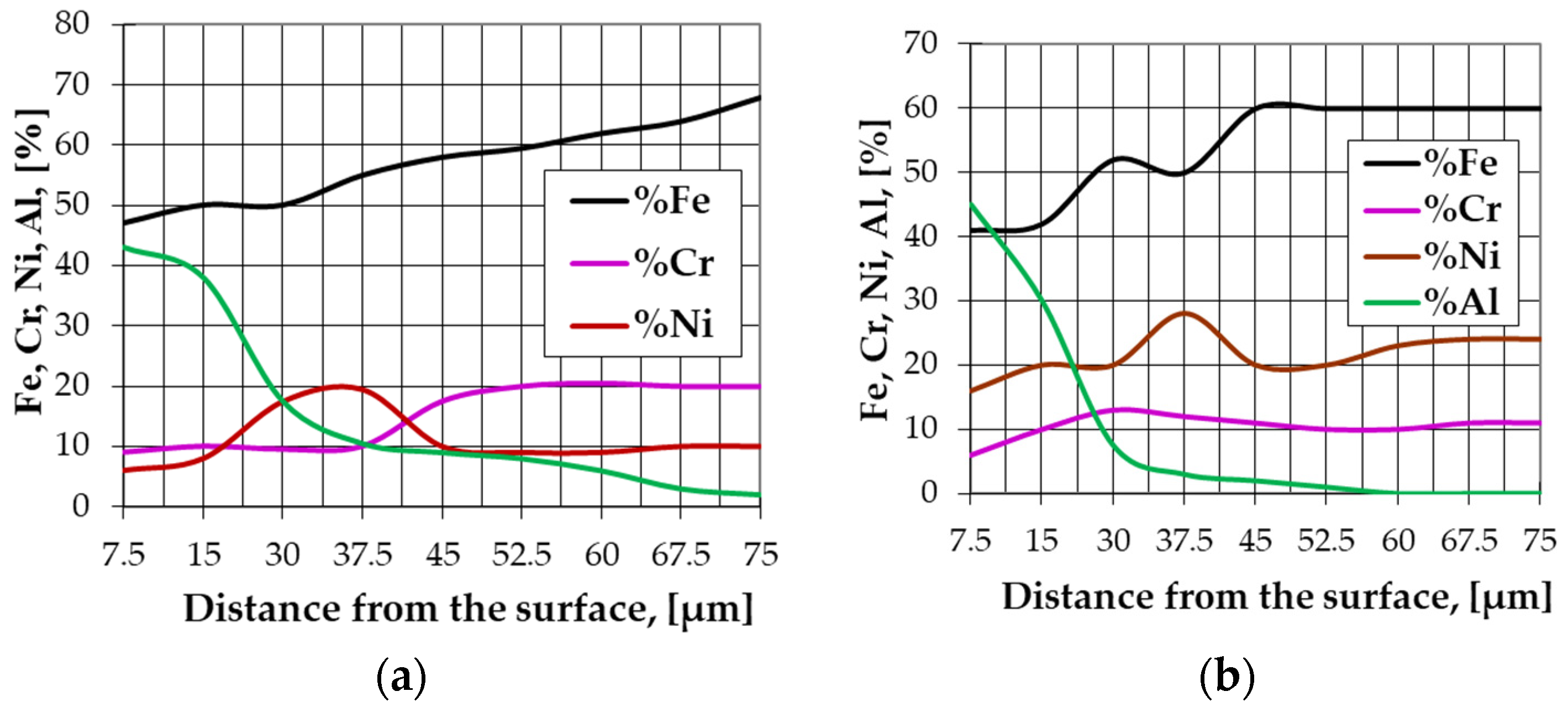
- Explaining the effects of the phase composition variation of the pack cementation mixture used in the IN 718 superalloy aluminizing, on the level of the Al/Ni weight proportion ratio in the top diffusion coating, the maximum %Al value in the diffusion coating and the diffusion coating thickness.
2. Materials and Methods
- On the diffusion twosome:
- (A1)—Massive Al-Ni, under pressure at 100 KPa, at 650 °C, for 50 h—air atmosphere;
- (A2)—Massive Ni—powder mixture (Al-Al2O3 + NH4Cl), at 1000 °C for 50 h—air atmosphere;
- B.
- On the Ni-based superalloy diffusion twosome/INCONEL 718—powdery solid aluminum supplier, at 900 °C for 5 h;
- (B1)—IN 718—powdery solid mixture (Al-Al2O3 + NH4Cl);
- (B2)—IN 718—powdery solid mixture (FeAl40 + NH4Cl);
- (B3)—IN 718—powdery solid mixture (Al-Fe + NH4Cl).
- Note: the samples were packed in powders mixtures, in refractory steel boxes (300 × 180 × 180 mm) sealed with clay, in a chamber electric furnace with 10 KW, equipped with an automatic system of programming and temperature control.
- -
- Al powder, produced by air spraying in Zlatna (Romania), by the Sherritt hydrometallurgical process, of 99.2% purity (0.15% Fe, 15% O2; 0.5% N2) with an average particle diameter of about 50 μm;
- -
- Fe powder, produced in Höganäs (Sweden), obtained by atomization, with a purity greater than 99.9% and an average diameter of about 45 μm;
- -
- Ferroaluminum (FeAl40) produced by Avon Metals LTD (UK), fragments with an average equivalent diameter of 40 ÷ 50 mm, subsequently ground in ball mills up to equivalent average diameters of 3 ÷ 4 mm;
- -
- Alumina powders, with a purity greater than 98.5%, produced by Alum Tulcea S.A. (Romania), having a maximum 10% for the fraction greater than 150 μm and 12% for the fraction less than 45 μm;
- -
- Ammonium chloride powder of analytical purity, produced by Silver Chemicals (Romania);
- -
- The obtained results involved the following investigations:
- -
- Determination of the oxygen and nitrogen content of the powders used, by ASTM E 1019-11, using the LECO TC-236;
- -
- Hardness testing using a Rockwell Insize ISH-R150 manual hardness tester;
- -
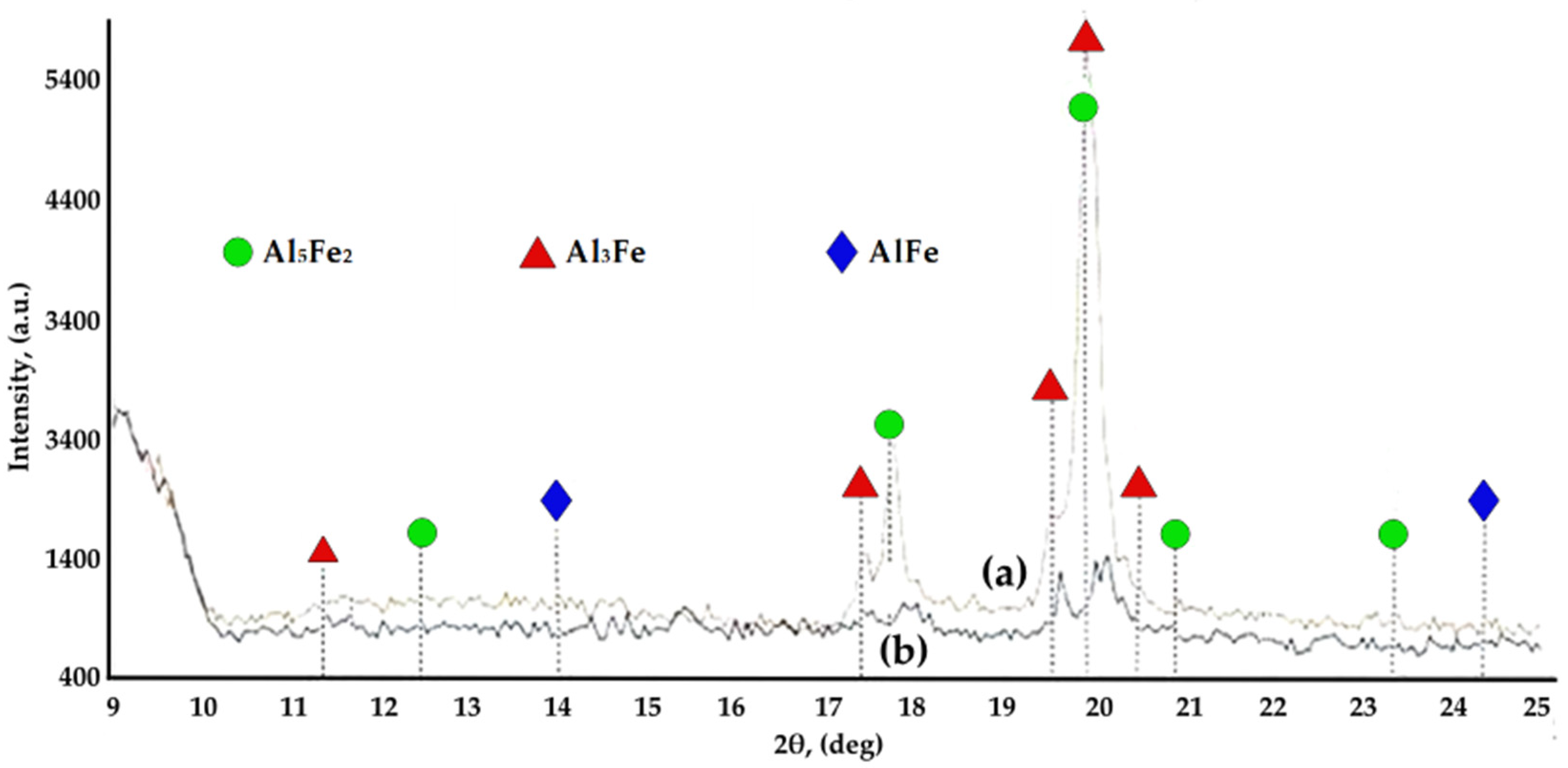
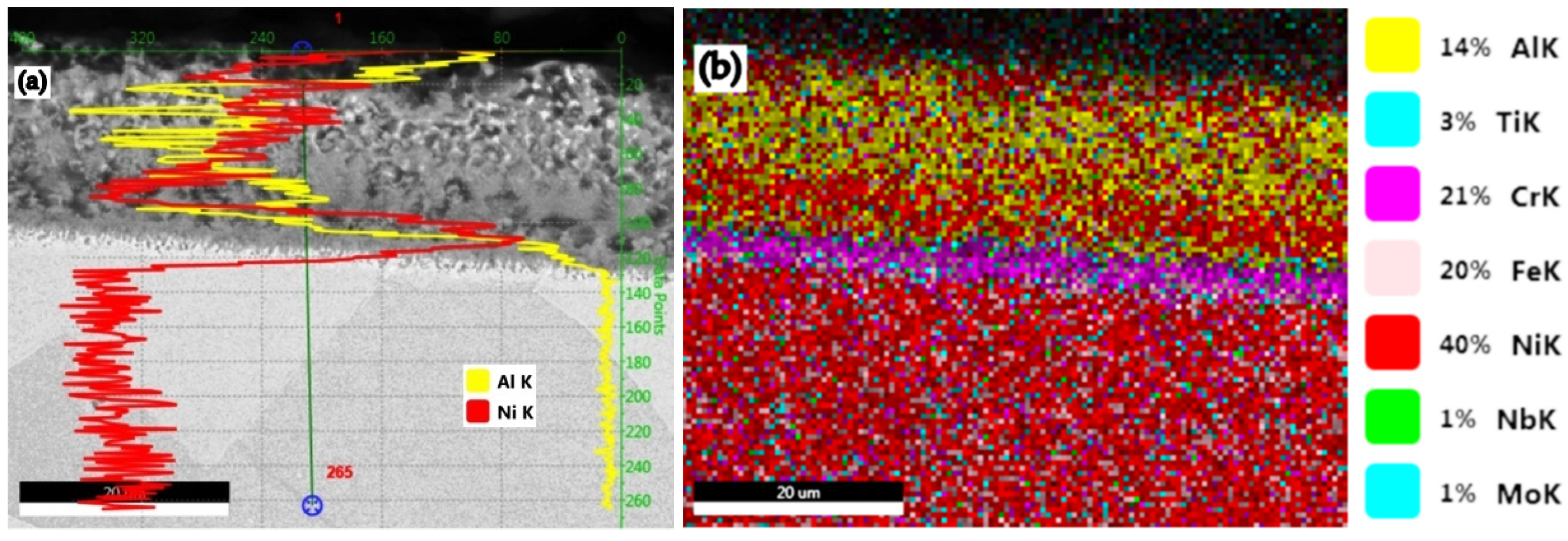
- Highlighting microstructural changes by optical microscopy (Image Analysis System—Buehler Omnimet), scanning electron microscopy (TESCAN VEGA XMU 8 and Philips XL30 ESEM TMP), and EDAX analysis (EDAX Sapphire type dispersive energy spectrometer with the resolution of 128 kV), as well as X-ray diffraction (POWDER X-RAY DIFFRACTOMETER—GNR APD 2000 PRO and D8 Advanced type—BRUKER-AXS).
3. Results and Discussion
4. Conclusions
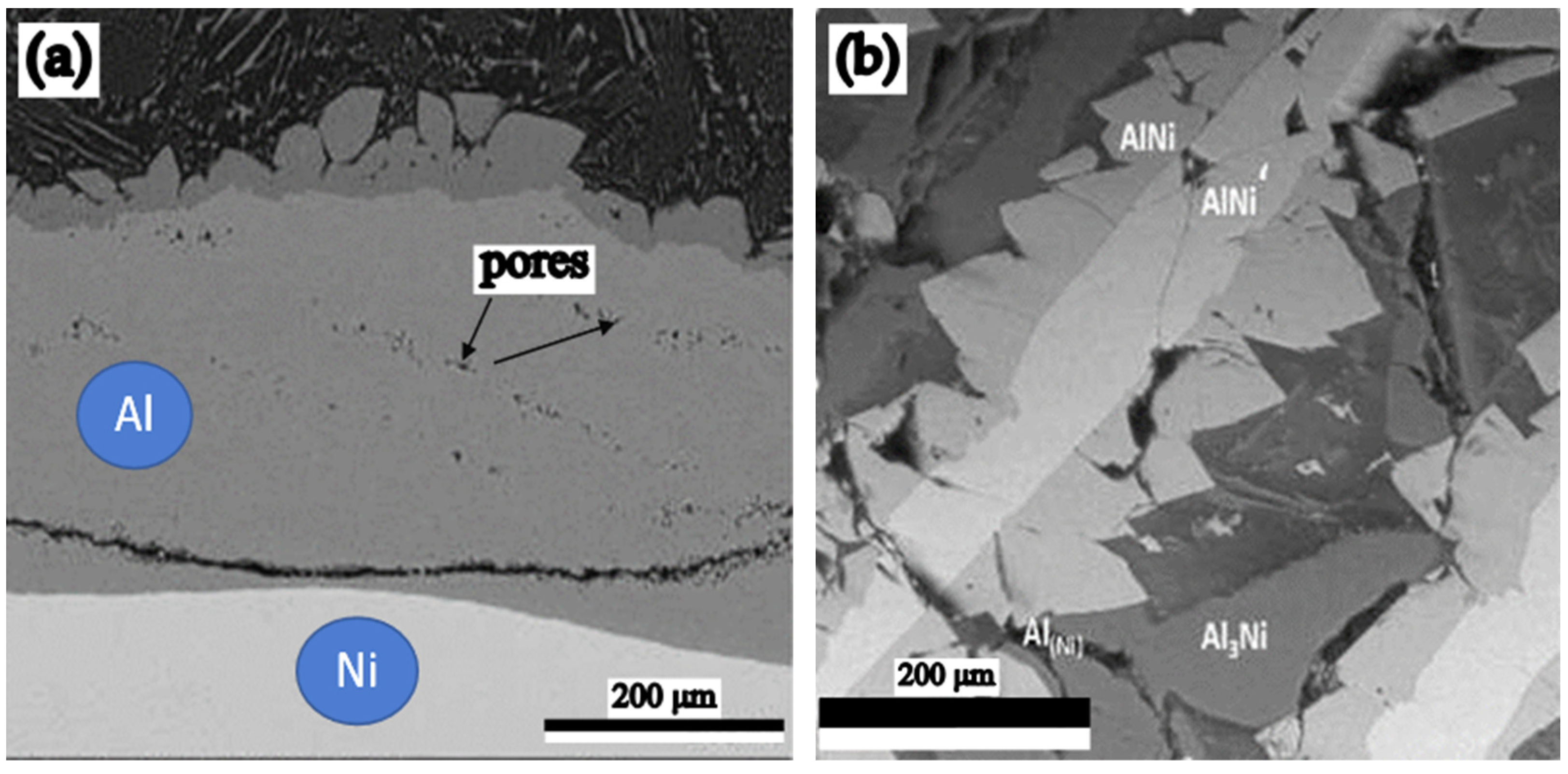
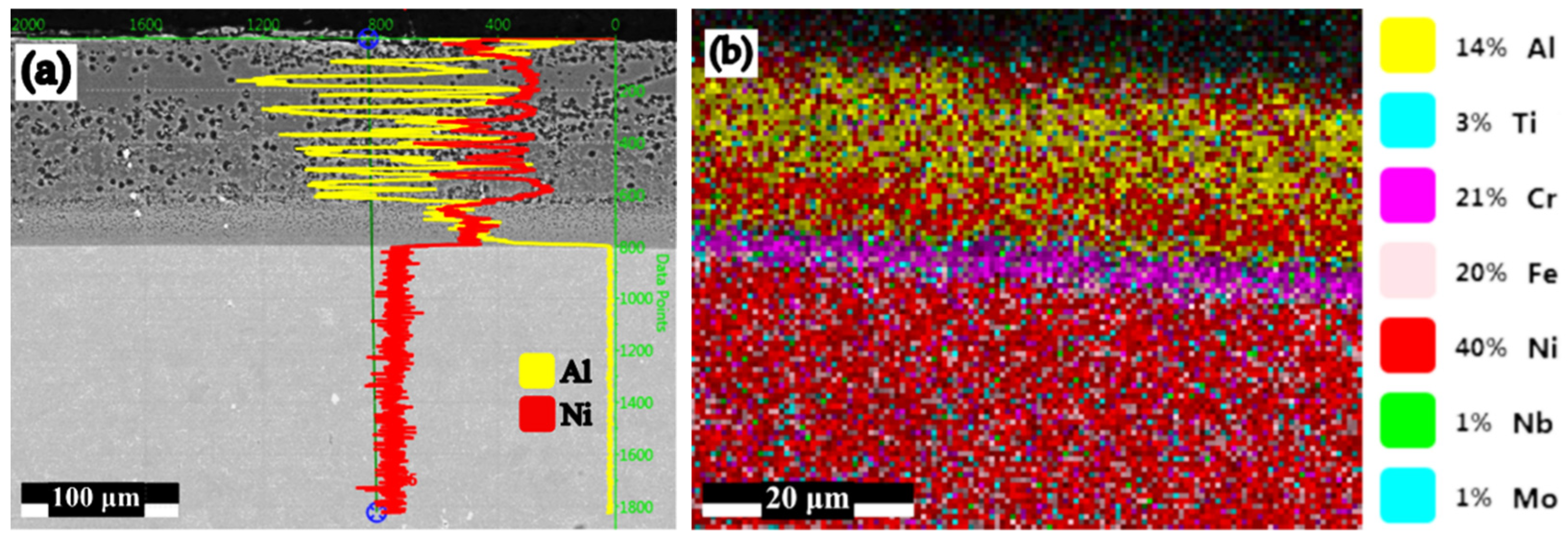
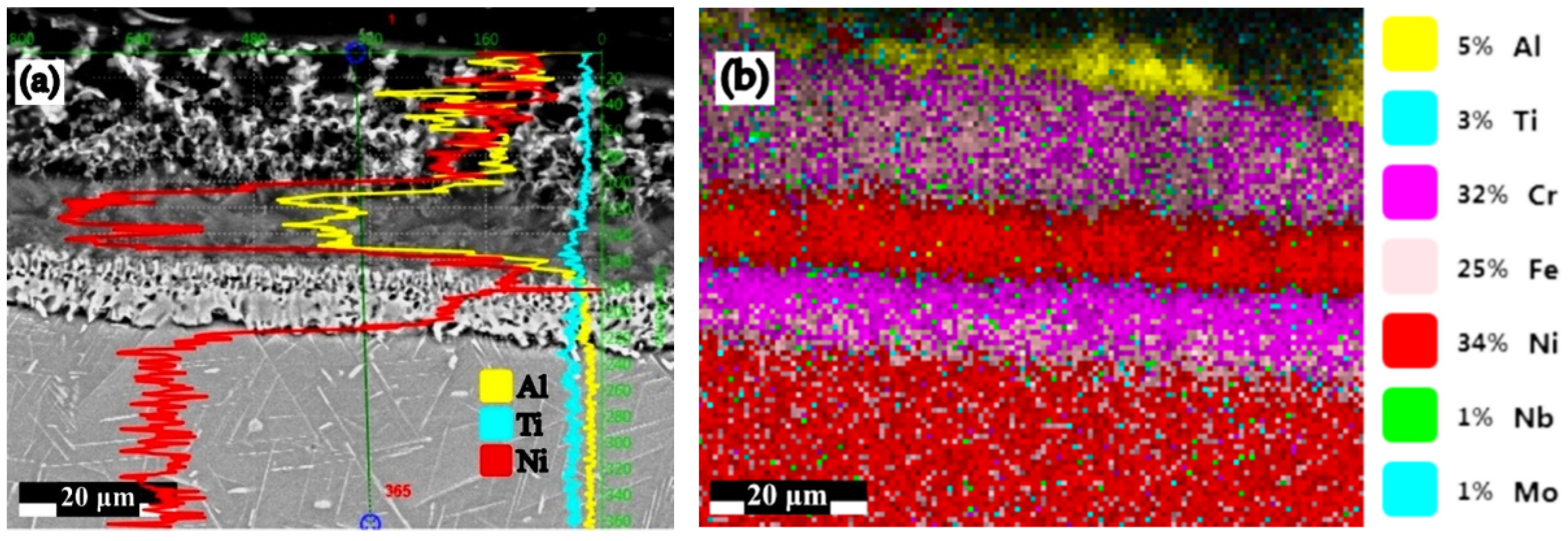
- The differences between the values of the diffusion coefficients of Al in Ni, and vice versa, cause the Kirkendall–Frenkel effect to be manifested in this diffusion couple. Increasing the diffusion couple temperature has the effect of reducing the porosity.
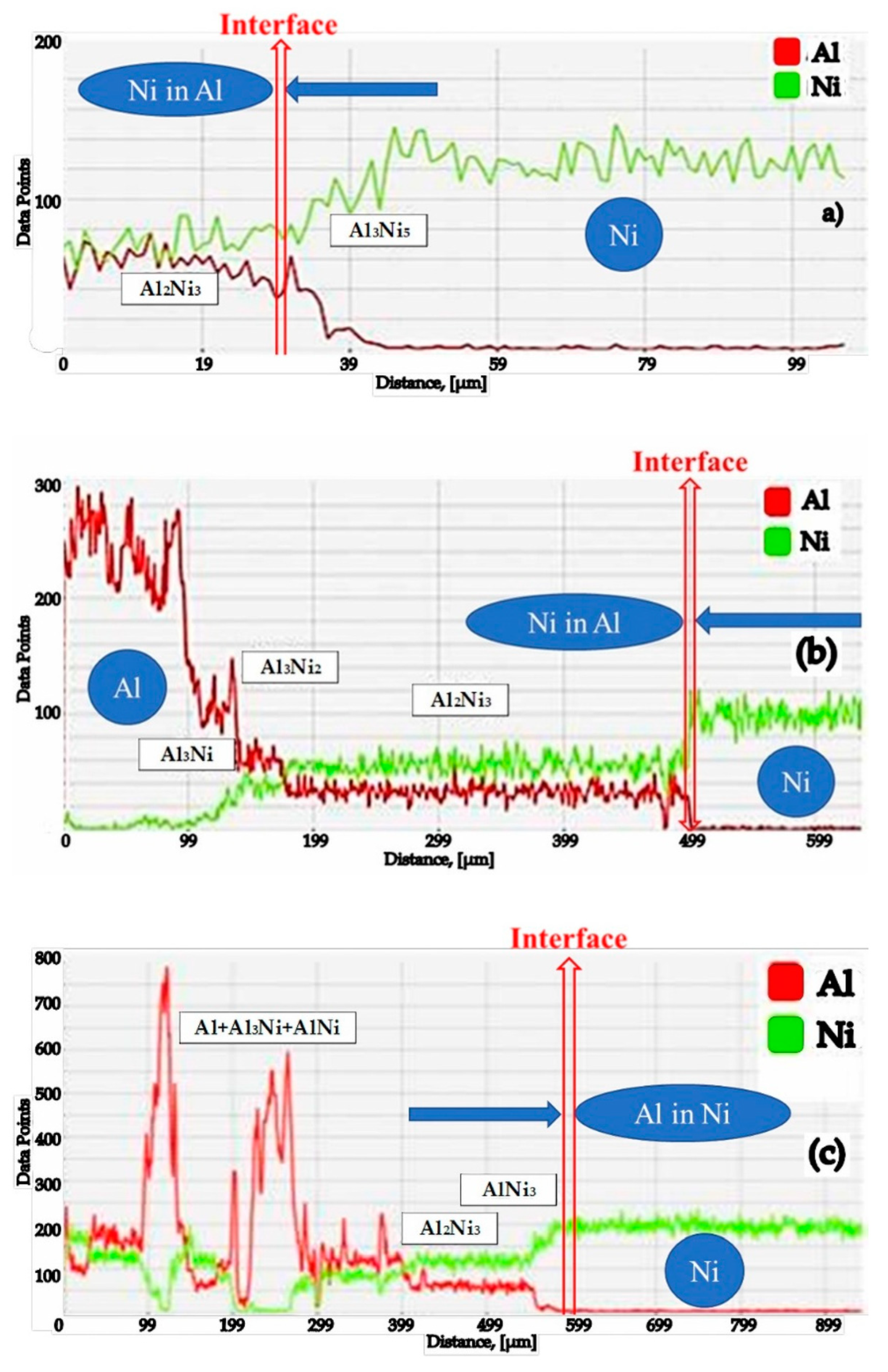
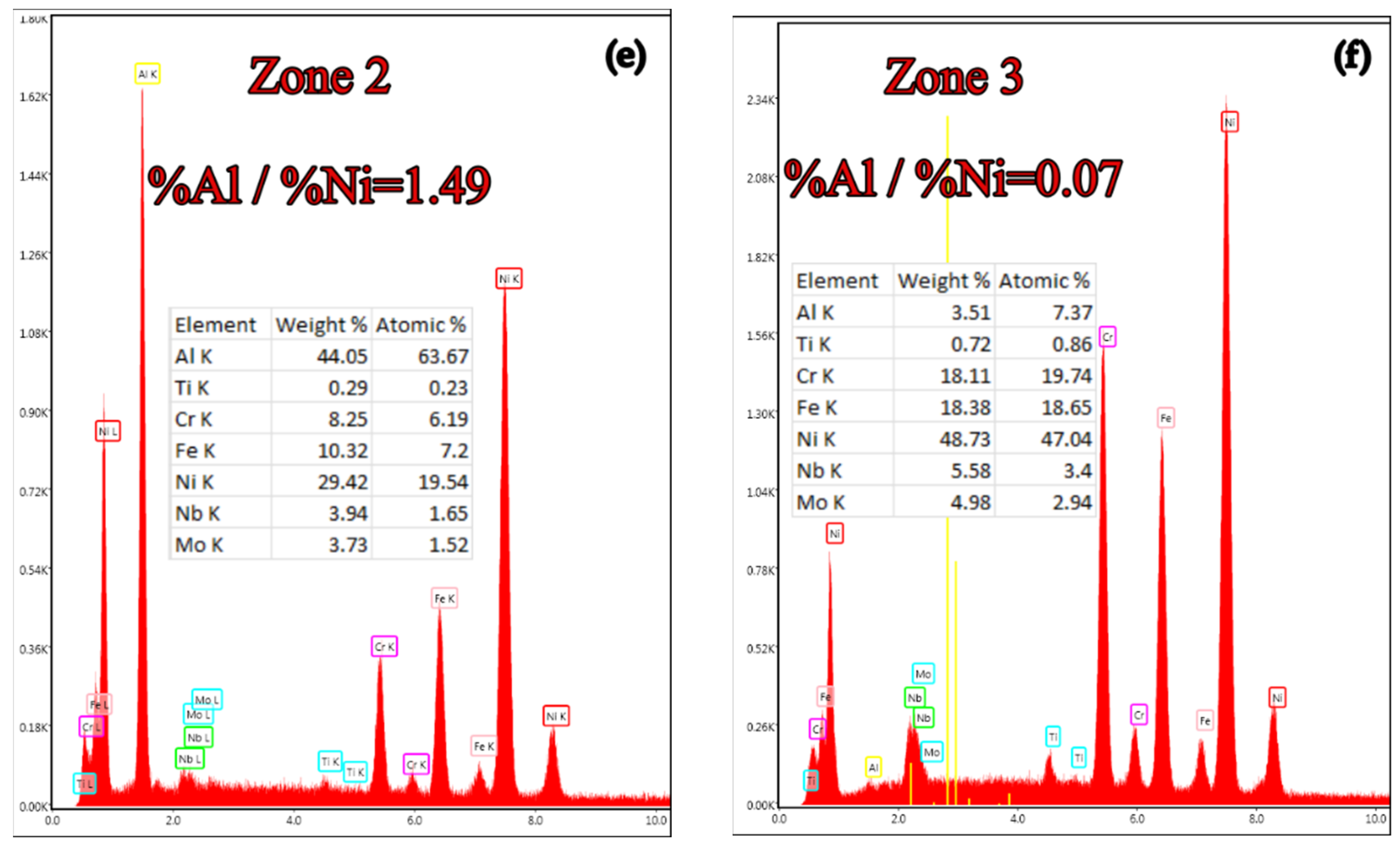
- Between the two elements of the diffusion couple, during the process of aluminide synthesis and regardless of the temperature, a compositional gradient appears, which has as a consequence a continuous variation of the aluminide types.
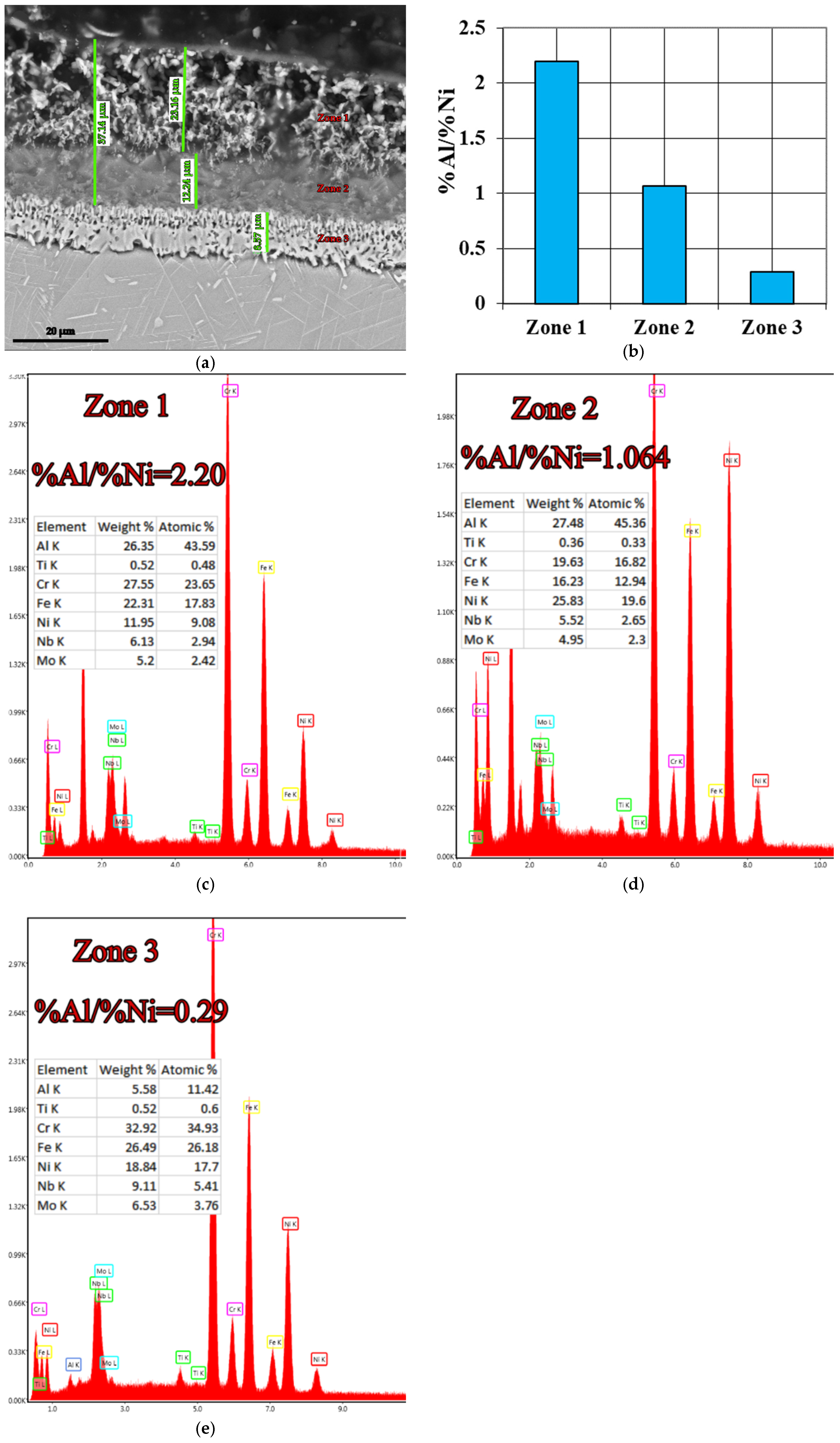
- All the powder mixtures compositions allowed, by aluminizing and by pack cementation, some supraunitary values of the %Al/%Ni weight proportion ratio to the top diffusion coating of the IN 718 superalloy to be achieved.
- (1)
- The activity of the pack aluminizing mixture used is strictly dependent on the state in which the Al is found in the environment (free or bonded).
- (2)
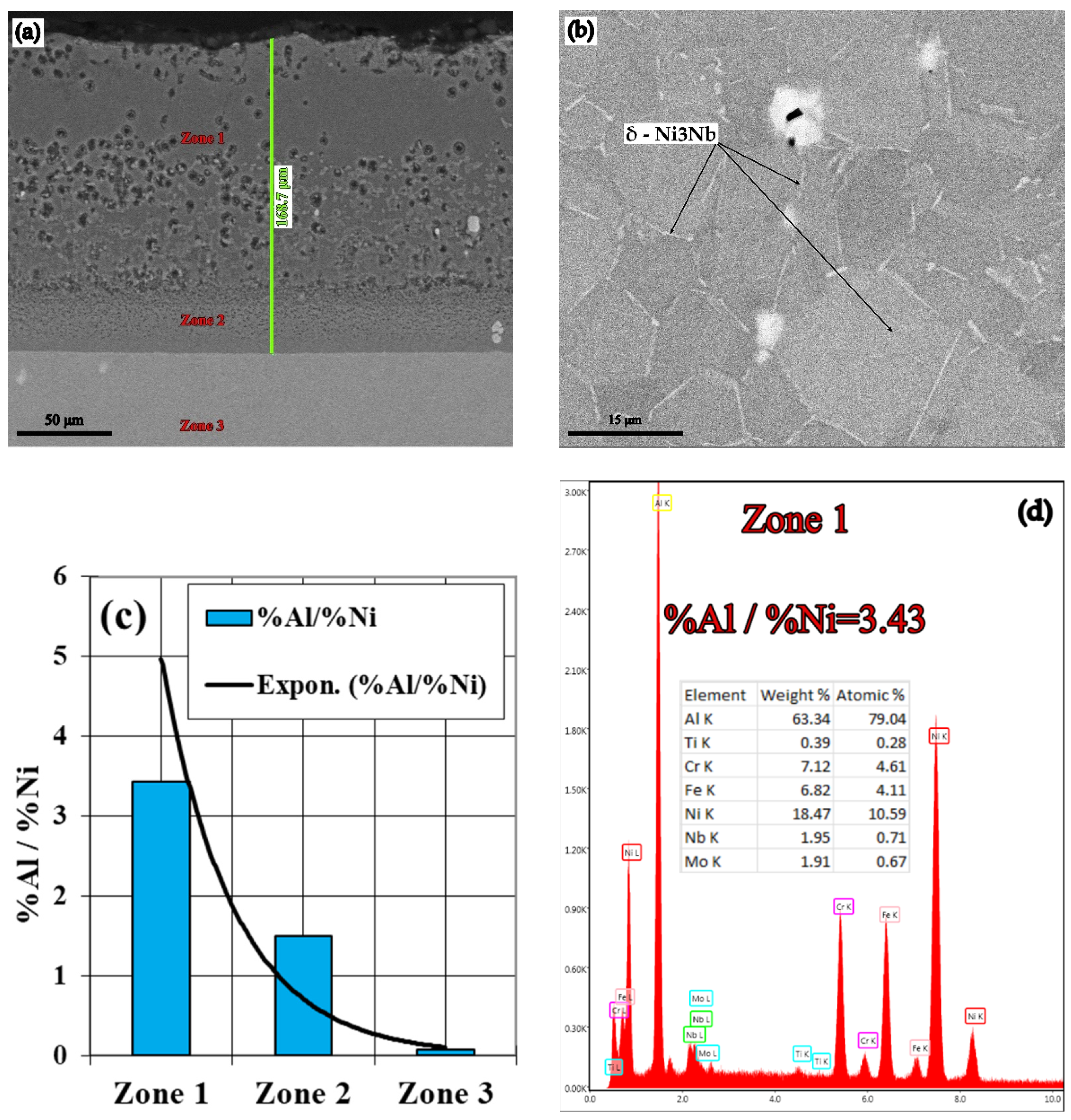
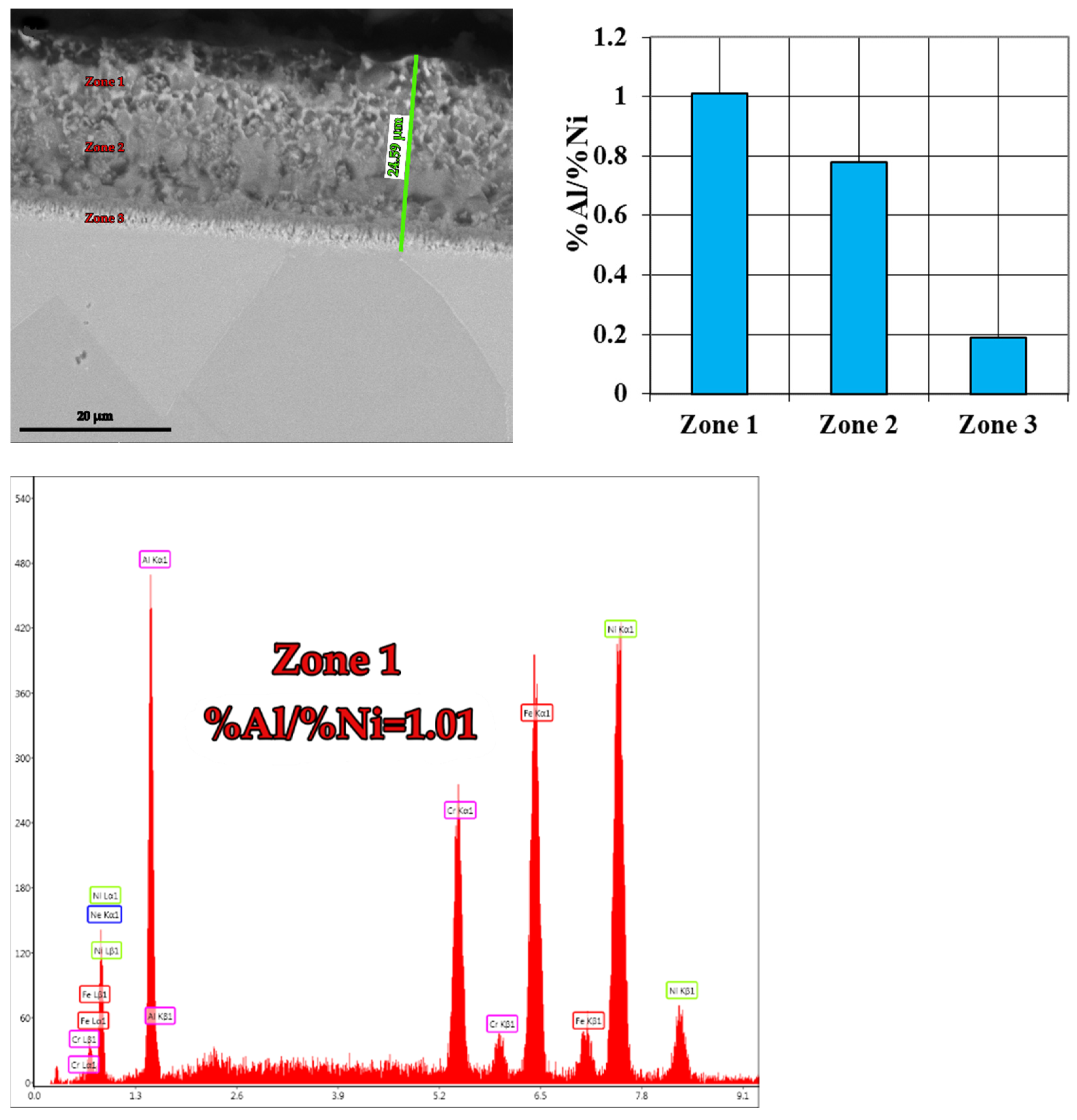
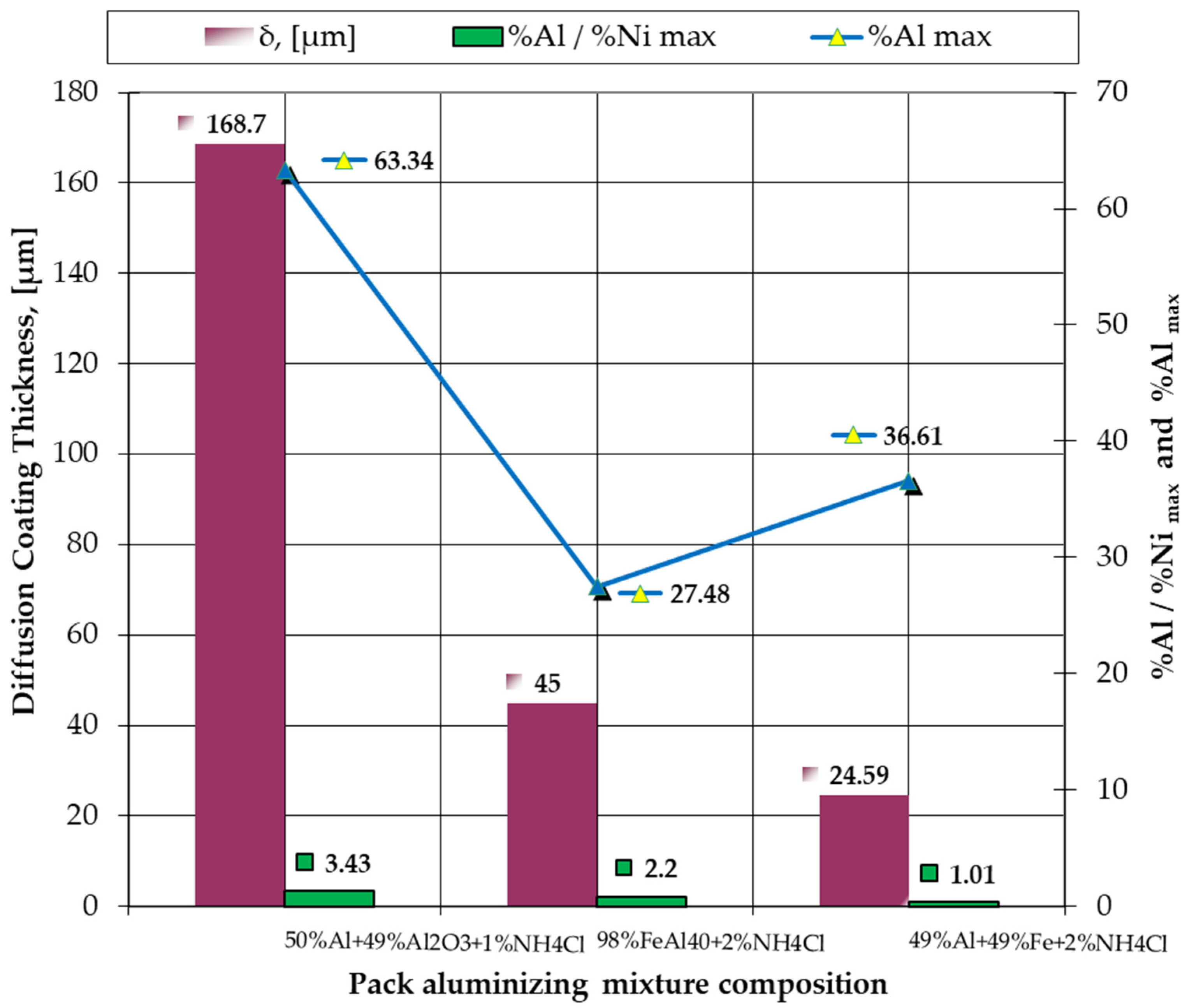
- The most notable effects are obtained when pack cementing with free aluminum, alumina, and ammonium chloride. Thus, at 900 °C, with a 5 h holding time, a higher diffusion coating thickness is generated (168.7 microns), with the concentration of aluminum in the layer also being at the highest level (63.34 %Al). In this case, the %Al/%Ni weight proportion ratio is 3.43.
- (3)
- The layers obtained after pack aluminizing, on an Inconel 718 superalloy matrix, and regardless of the variation in the composition of the pack aluminizing mixture used, are perfectly adherent because they are formed “in situ”.
- (4)
- The Rockwell macro hardness tests revealed an increase of approximately 15 units (from 25 HRC to 40 HRC) in the case of 900 °C holding time heat treatment temperature, above the melting temperature of Al, for 5 h. This increase is due to the massive precipitation of the δ—Ni3Nb hardening phase, in the γ solid solution grains (see Figure 5b).
- (5)
- The increase in the working performances of the parts made of IN 718 superalloy grade can be ensured by their pack cementation. The composition of the powdery mixture consisting of an aluminum powder dispersed in an alumina powder is recommended, as this leads to the formation of the maximum layer thickness, as well as the highest value of the %Al/%Ni weight proportion ratio in the area adjacent to the surface (for the same processing conditions); under these conditions, high resistance and refractoriness of the layer are obtained, for long-term demands. This variant of thermochemical processing does not require specialized equipment or operating personnel with special skills. Heating equipment and refractory steel boxes are required for packing parts into the pack aluminizing mixture.
- The described technological process variants are environmentally friendly, both in terms of the materials used and the resulting reaction products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamoto, H. Al-Ni (Aluminum-Nickel). J. Phase Equilibria Diffus. Sect. III Suppl. Lit. Rev. 2004, 25, 394. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworth & Co. (Publishers) Ltd.: London, UK, 1976; pp. 116–119. ISBN 0-408-70680-5.
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990; Volume 1. [Google Scholar]
- Nash, P.; Singleton, M.F.; Murray, J.L. Al-Ni (Aluminum-Nickel), Phase Diagrams of Binary Nickel Alloys; ASM International: Materials Park, OH, USA, 1991; pp. 3–11. [Google Scholar]
- Ma, Y.; Ardell, A.J. The (γ + γ’)/γ’ phase boundary in the Ni-Al phase diagram from 600 to 1200 °C. Int. J. Mater. Res. 2003, 94, 972–975. [Google Scholar] [CrossRef]
- Dey, G.K. Physical Metallurgy of Nickel Aluminides. Sadhana 2003, 28, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Dey, G.K.; Tewari, R.; Roa, P.; Wadekar, S.L.; Mukhopadhyay, P. Precipitation hardening in nickel copper alloy Monel K500. Metall. Trans. A 1993, 24A, 2709–2719. [Google Scholar] [CrossRef]
- Dey, G.K.; Sekhar, J.A. Micropyretic Synthesis of Tough NiAl Alloys. Metall. Mater. Trans. B 1997, 28B, 905–918. [Google Scholar] [CrossRef]
- Alimadadi, H.; Kjartansdóttir, C.; Burrows, A. Nickel-aluminum diffusion: A study of evolution of microstructure and phase. Mater. Charact. 2017, 130, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Al-Shafaie, S.H.; Nabaa, S.R.; Hussein, M.A. Preparation and Investigation Mechanical Properties of Functionally Graded Materials of Aluminum-Nickel Alloys. J. Mech. Eng. Res. Dev. 2021, 44, 102–109. [Google Scholar]
- Tucho, W.M.; Hansen, V. Studies of Post-Fabrication Heat Treatment of L-PBF-Inconel 718: Effects of Hold Time on Microstructure, Annealing Twins, and Hardness. Metals 2021, 11, 266. [Google Scholar] [CrossRef]
- ASTM F3055-14a; Standard Specification for Additive Manufacturing Nickel Alloy (UNS N07718) with Powder Bed Fusion. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–8. [CrossRef]
- Lahtin, I.M.; Arzamasov, B.N. Himiko-Termiceskaia Obrabotka Metallov (Chemical-Thermal Treatment of Metals), Izd; Metallurghia (Metallurgy Publishing): Moscow, Russia, 1985; pp. 210–221. [Google Scholar]
- Cojocaru, M.O. Capitolul 2, Tehnologii de procesare finala a materialelor metalice. In Tratat de Stiinta si Ingineria Materialelor Metalice, Vol. V (Handbook on Metallic Materials Science and Engineering, Vol. V); Saban, R., Dumitrescu, C., Eds.; Editura AGIR (AGIR Publishing): Bucharest, Romania, 2011; pp. 320–329. [Google Scholar]
- Vilupanur, A.R. Book Chapter: Pack Cementation Coatings. In ASM Handbook Volume 13A, Corrosion: Fundamentals, Testying, and Protection; ASM International: Materials Park, OH, USA, 2003; pp. 763–771. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanism of Formation of Diffusion Aluminide Coatings on Nickel-Base Superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Pichoir, R. Influence of the Mode of Formation on the Oxidation and Corrosion Behavior of NiAl-Type Protective Coatings. In Materials and Coatings to Resist High Temperature Corrosion; Holmes, D.R., Rahmel, A., Eds.; Applied Science Publishers Ltd.: London, UK, 1978; pp. 271–291. [Google Scholar]
- Goward, G.W.; Cannon, L.W. Pack Cementation Coatings for Superalloys: A Review of History, Theory, and Practice. J. Eng. Gas Turbines Power 1988, 110, 150–154. [Google Scholar] [CrossRef]
- Pugacheva, N.B. Current Trends in the Development of Heat-Resistant Coatings Based on Iron, Nickel and Cobalt Aluminides. Diagn. Resour. Mech. Mater. Struct. 2015, 3, 51–82. [Google Scholar] [CrossRef]
- Borzdîka, A.M.; Ţeitlin, V.Z. Termiceskaia Obrabotka Jaroprocinîh Stalei I Splavov (Heat Treatment of Refractory Steels and Alloys); Maşinostroenie (Car Construction Publishing): Moskva, Russia, 1964; pp. 157–169. [Google Scholar]
- Chandler, H. Heat Treater’s Guide: Practices and Procedures for Nonferrous Alloys; ASM International: Materials Park, OH, USA, 1996; ISBN 0-87170-565-6. [Google Scholar]
- Jambor, M.; Bokuvka, O.; Nový, F.; Trško, L.; Belan, J. Phase Transformations in Nickel base Superalloy Inconel 718 during Cyclic Loading at High Temperature. Prod. Eng. Arch. 2017, 15, 15–18. [Google Scholar] [CrossRef]
- Desvalees, Y.; Bouzidi, M.; Bois, F.; Beaude, N. Delta phase in Inconel 718: Mechanical properties and forging process requirements. Superalloys 1994, 718, 281–291. [Google Scholar]
- Donachie, M.J.; Donachie, S.J. Superalloys—A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2002; pp. 213–225. [Google Scholar]
- Kalluri, S.; Rao, K.B.S.; Halford, G.R.; Mcgaw, M. Deformation and damage mechanisms in Inconel 718 superalloy. In Superalloys 718, 625, 706 and Various Derivates; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 1994; pp. 593–606. [Google Scholar]
- Belan, J. High frequency fatigue Test of IN 718 alloy—Microstructure and fractography evaluation. Metalurgija 2015, 54, 59–62. [Google Scholar]
- Amato, K.N.; Gaytan, S.M.; Murr, L.E.; Martinez, E.; Shindo, P.W.; Hernandez, J.; Collins, S.; Medina, F. Microstructures and mechanical behavior of Inconel 718 fabricated by selective laser melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Maj, P.; Adamczyk-Cieslak, B.; Slesik, M.; Mizera, J.; Pieja, T.; Sirniawski, J.; Gancarczyk, T.; Dudek, S. The Precipitation processes and Mechanical Properties of Aged Inconel 718 Alloy After Annealing. Arch. Metall. Mater. 2017, 62, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Sims, C.; Hagel, W.C. Chapter 12—Pokrâtia i zascita (Coatings and Protection). In Jaroprocinâie Splavâ (The Superalloys); Metallurghia (Merallurgy Publishing): Moskow, Russia, 1976; p. 327. [Google Scholar]
- Wu, Q.; Li, S.S.; Ma, Y.; Gong, S.K. First principles calculations of alloying element diffusion coefficients in Ni using the five-frequency model. Chin. Phys. B 2012, 21, 109102. [Google Scholar] [CrossRef]
- Yuranan Hanlumyuang, Y. Atomistic Simulation of Structural Evolution at Long Time Scales: Diffusion of Aluminum in Nickel at a Low Concentration Limit. J. Sci. Technol. 2017, 5–13. Available online: https://www.thaiscience.info/Journals/Article/JSUU/10990037.pdf (accessed on 16 May 2022).
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Puri, P.; Yang, V. Thermochemical behavior of nano-sized aluminium-coated nickel particles. J. Nanoparticle Res. 2014, 16, 1–16. [Google Scholar] [CrossRef]
- Jimenez-Mena, N.; Simar, A.; Jacques, P.J. On the interplay between intermetallic controlled growth and hot tearing susceptibility in Al-to-steel welding with additional interlayers. Mater. Des. 2019, 180, 107958. [Google Scholar] [CrossRef]
- Tudose, F. Syntesis of Nichel Aluminides by Methodes Specific to Powder Metallurgy. Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Romania, 19 September 2018. [Google Scholar]
- Yan, M.; Fan. Z. Review-Durability of Materials in Molten Aluminum Alloys. J. Mater. Sci. 2001, 36, 285–295. [Google Scholar] [CrossRef]
- HSC Chemistry Software Version 6.12 (Outokumpu); Outotec Research Oy: Pori, Finland, 2007.
- Cserhati, C.; Paul, A.; Kodentsov, A.A.; van Dal, M.J.H.; van Loo, F.J.J. Intrinsic diffusion in Ni3Al system. Intermetallics 2003, 11, 291–297. [Google Scholar] [CrossRef]
- Begunov, A.I.; Kuzmin, M.P. Thermodynamic stability of Intermetallic Compounds in Tehnical Aluminum. J. Sib. Univ. Eng. Technol. 2014, 7, 132–137. [Google Scholar]


| Under Al Meting Temp.—650 °C | Over Al Melting Temp.—1000 °C | ||
|---|---|---|---|
| Ni in Al diffusion [m2/s] | 2.65 × 10−12 [32] | Ni in Al diffusion [m2/s] | 2.86 × 10−9 [33] & 8.18 × 10−9 [34] |
| Al in Ni diffusion [m2/s] | 6.25 × 10−20 [30] | Al in Ni diffusion [m2/s] | 1.37 × 10−15 [31] |
| Pack Aluminizing Mixture Composition | Almax in the Coating, [%] | %Al/%Ni | δmax, [μm] |
|---|---|---|---|
| B1 → 50%Al + 49%Al2O3 + 1%NH4Cl | 63.34 | 3.43 | 168.70 |
| B2 → 8%FeAl40 + 2%NH4Cl | 27.48 | 2.20 | 45.00 |
| B3 → 49%Al + 49%Fe + 2%NH4Cl | 36.61 | 1.01 | 24.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, M.O.; Branzei, M.; Druga, L.N. Aluminide Diffusion Coatings on IN 718 by Pack Cementation. Materials 2022, 15, 5453. https://doi.org/10.3390/ma15155453
Cojocaru MO, Branzei M, Druga LN. Aluminide Diffusion Coatings on IN 718 by Pack Cementation. Materials. 2022; 15(15):5453. https://doi.org/10.3390/ma15155453
Chicago/Turabian StyleCojocaru, Mihai Ovidiu, Mihai Branzei, and Leontin Nicolae Druga. 2022. "Aluminide Diffusion Coatings on IN 718 by Pack Cementation" Materials 15, no. 15: 5453. https://doi.org/10.3390/ma15155453
APA StyleCojocaru, M. O., Branzei, M., & Druga, L. N. (2022). Aluminide Diffusion Coatings on IN 718 by Pack Cementation. Materials, 15(15), 5453. https://doi.org/10.3390/ma15155453







