Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review
Abstract
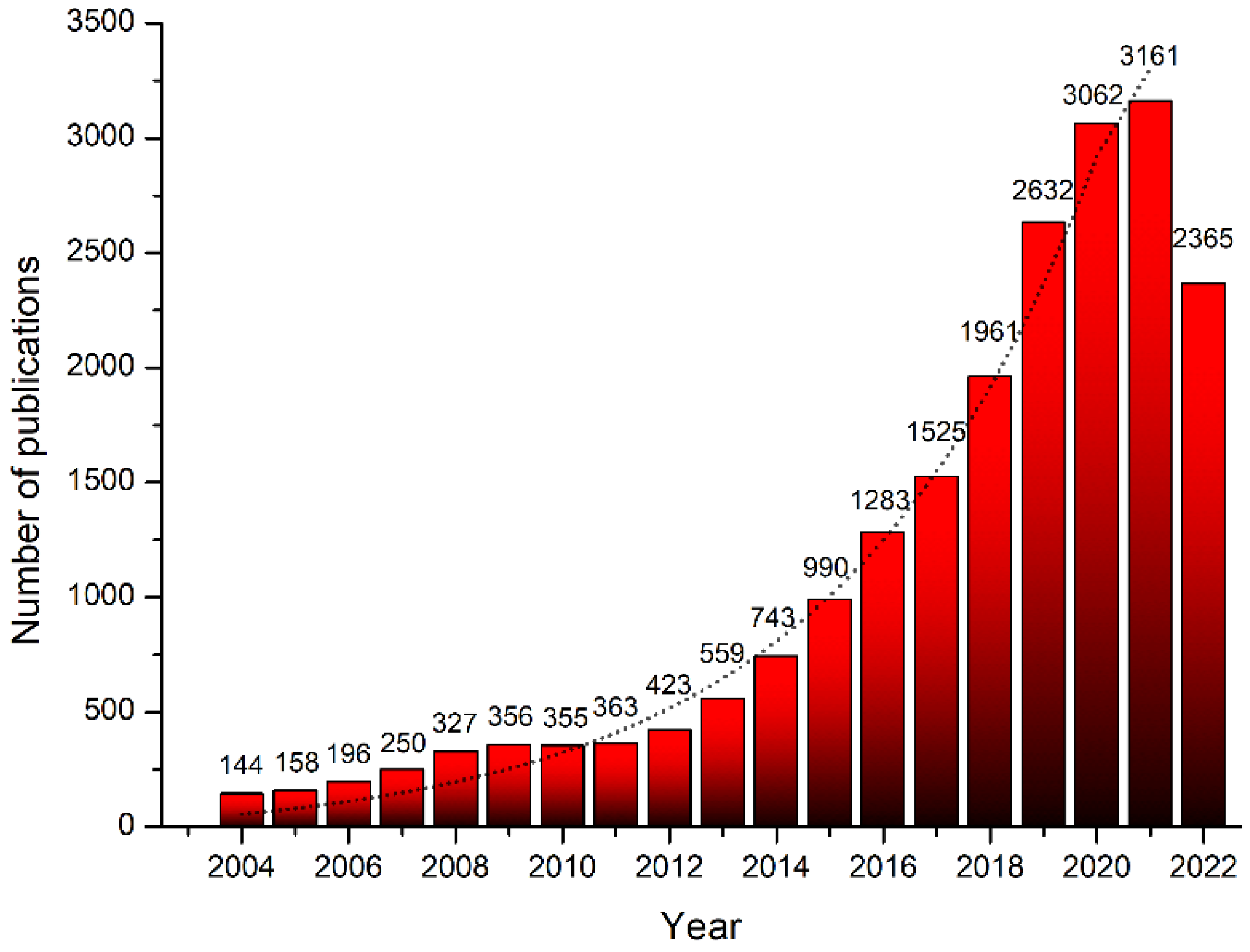
:1. Introduction
2. Materials for Frequency Down-Conversion LEDs
2.1. Phosphors
2.2. Quantum Dots (QDs)
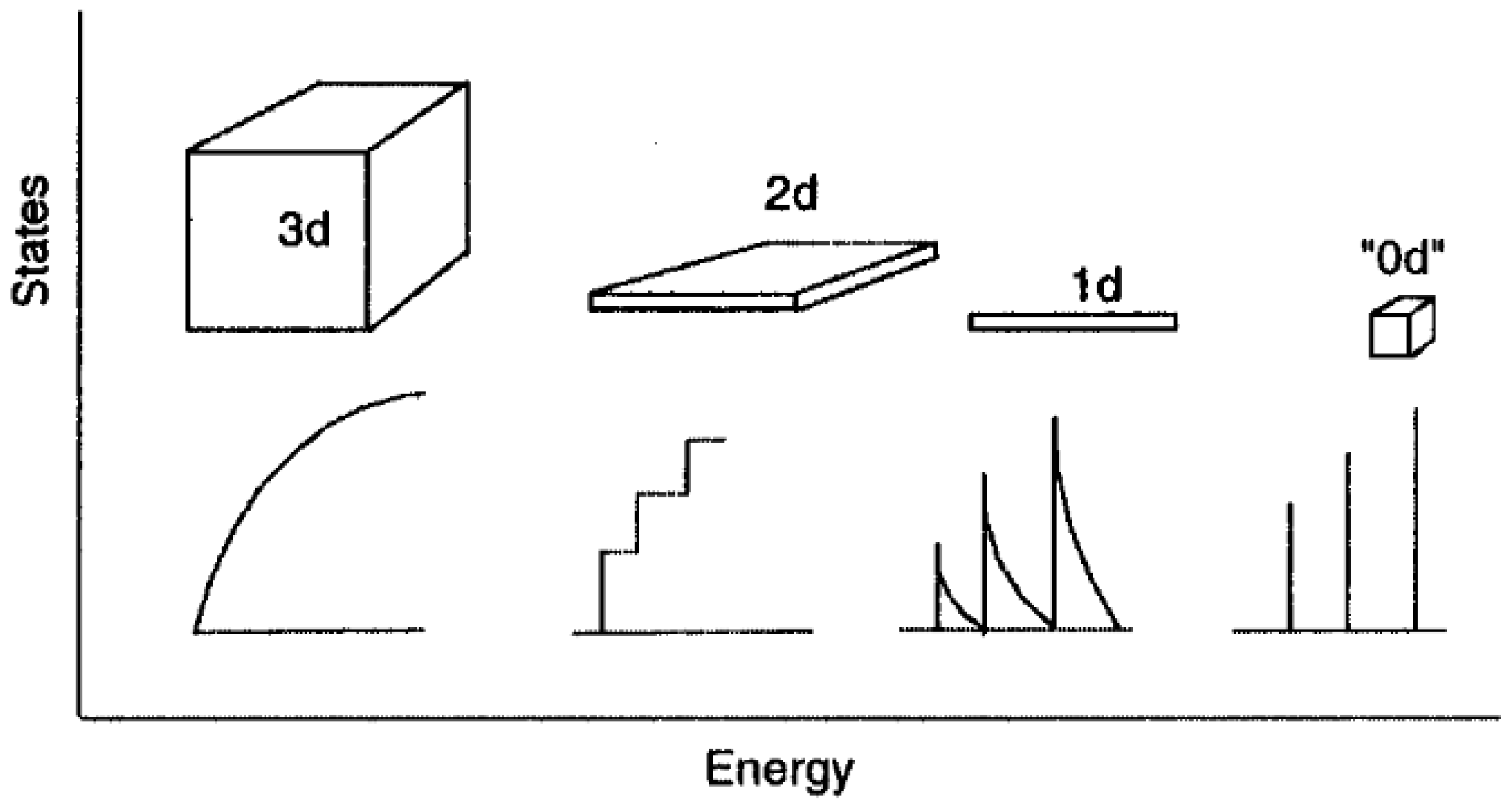
2.3. Carbon Dots (CDs)
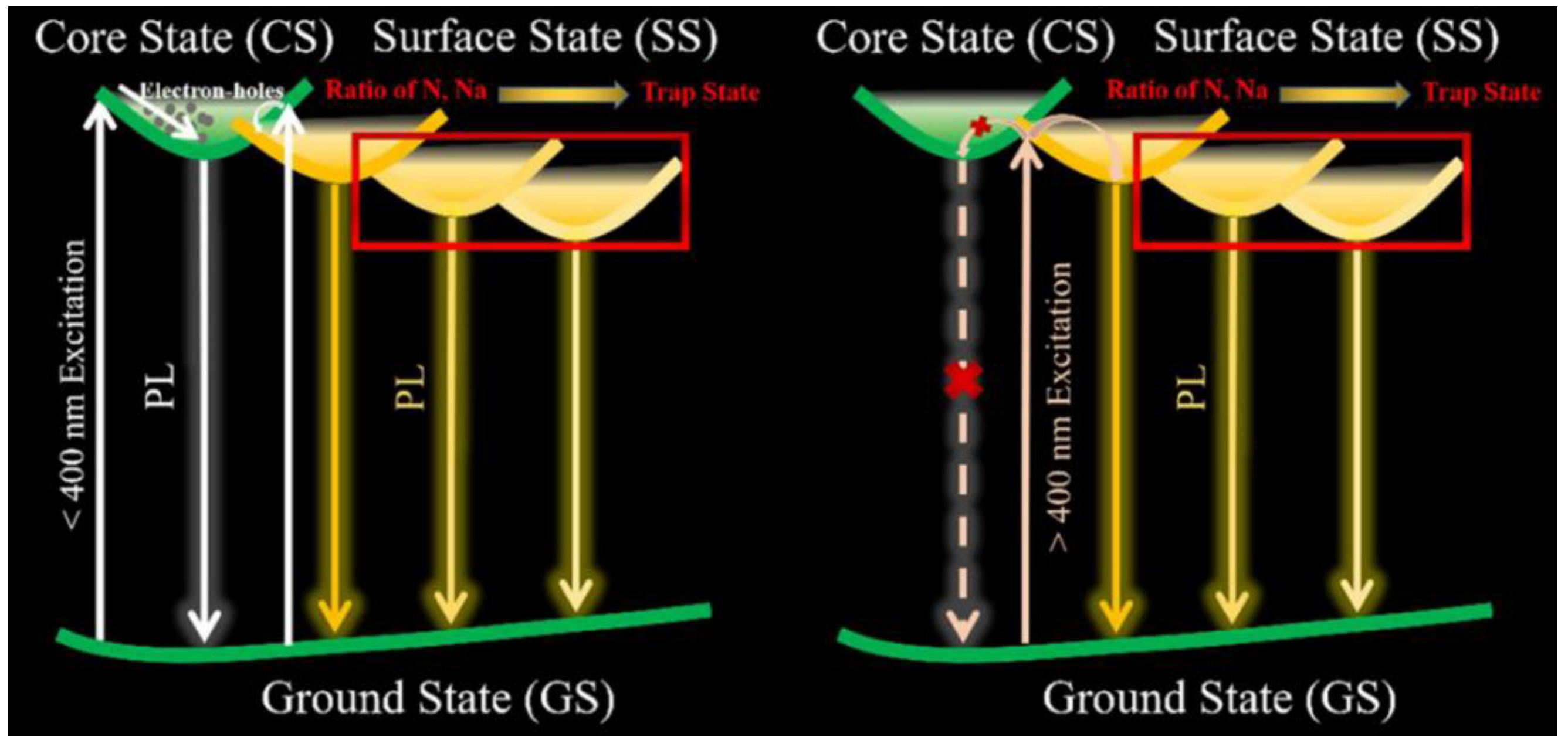
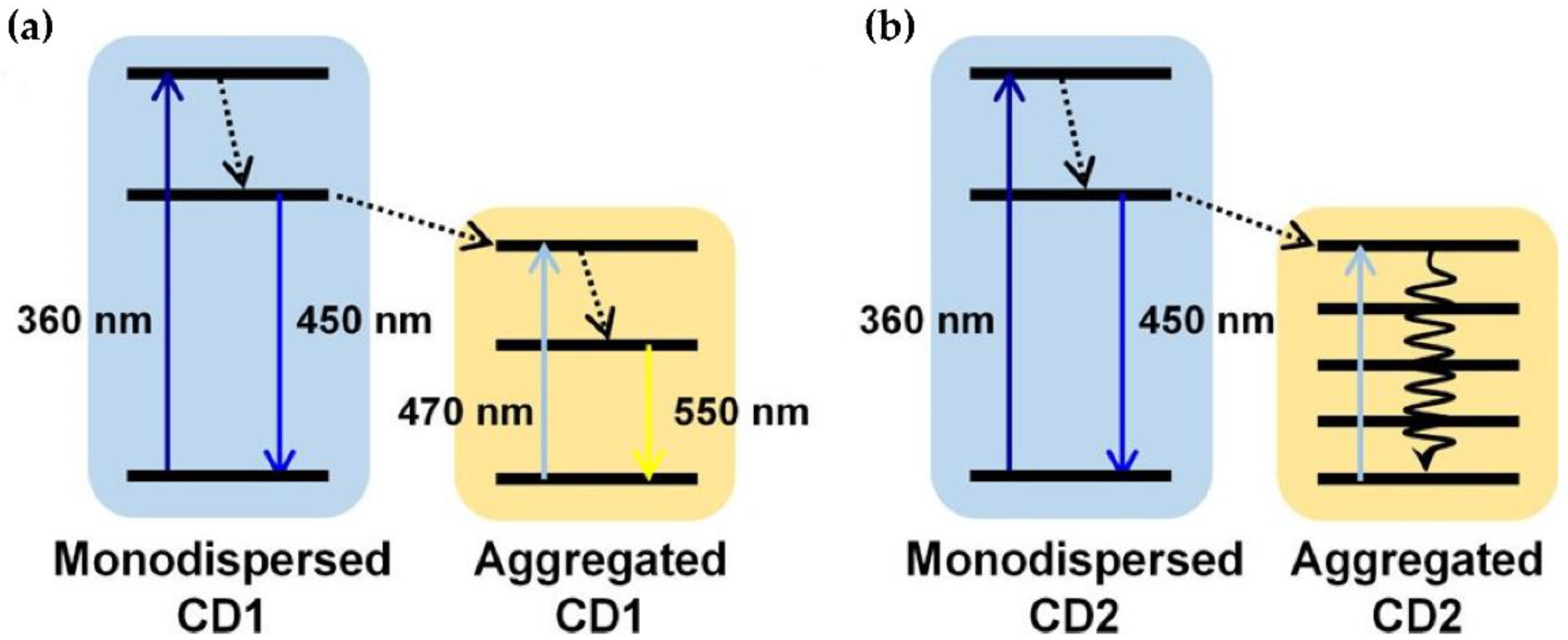
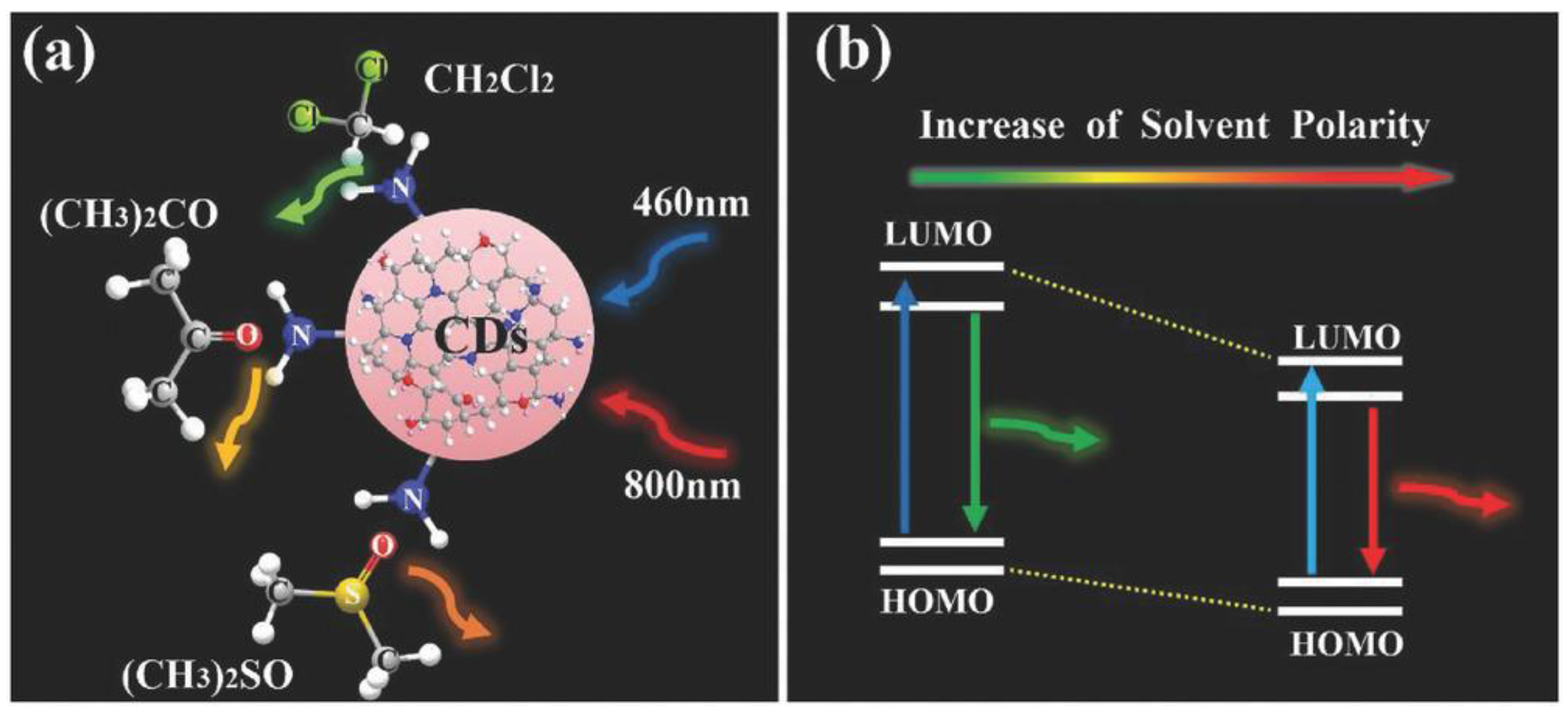
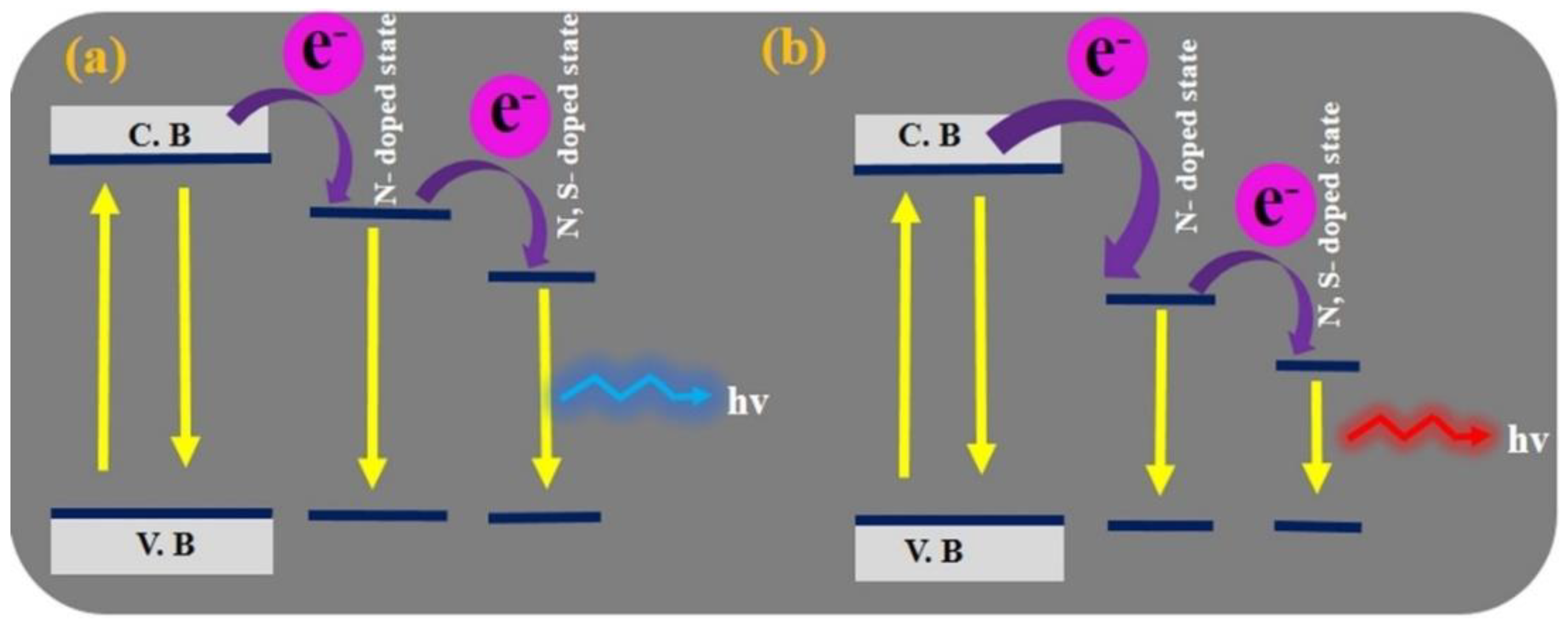
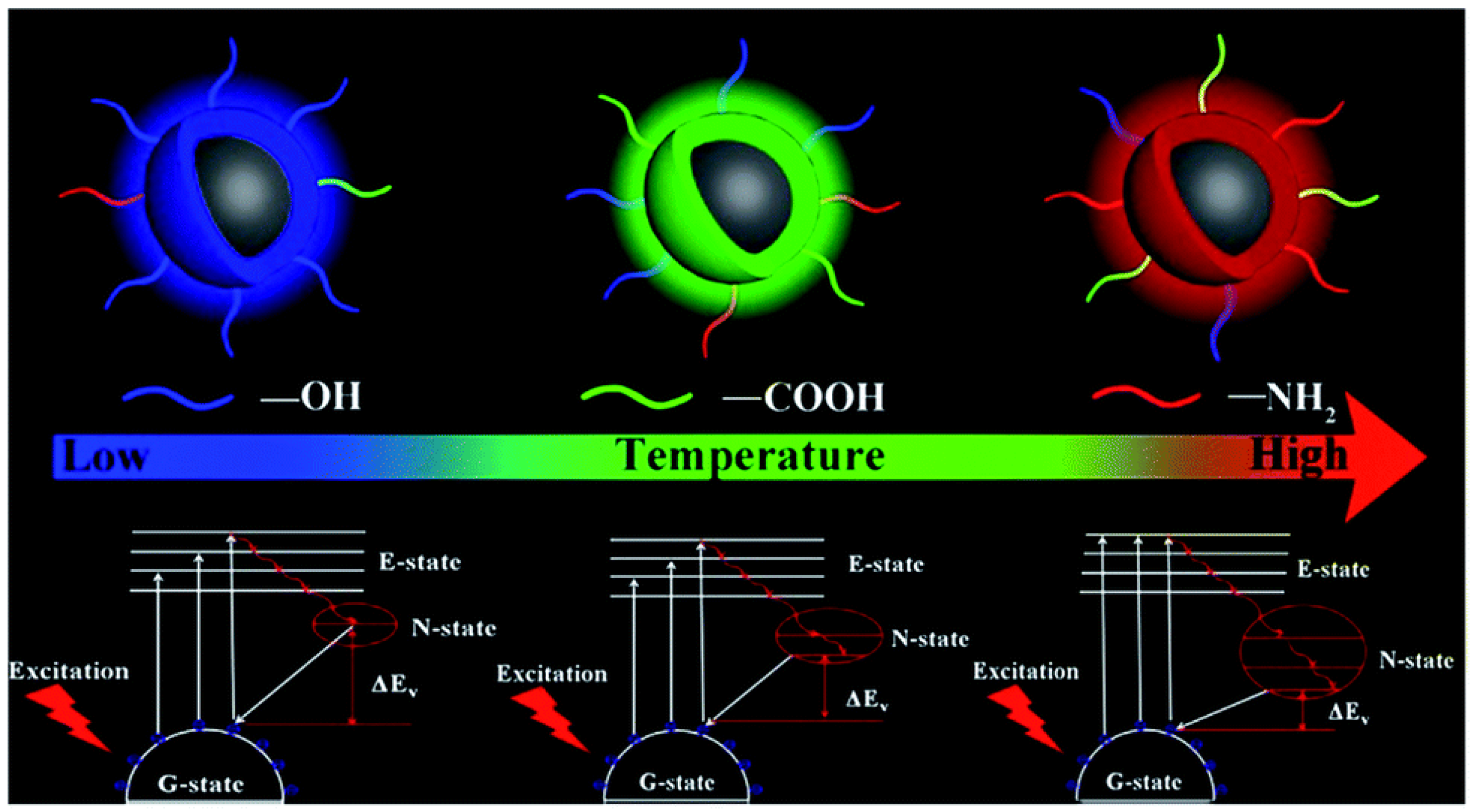
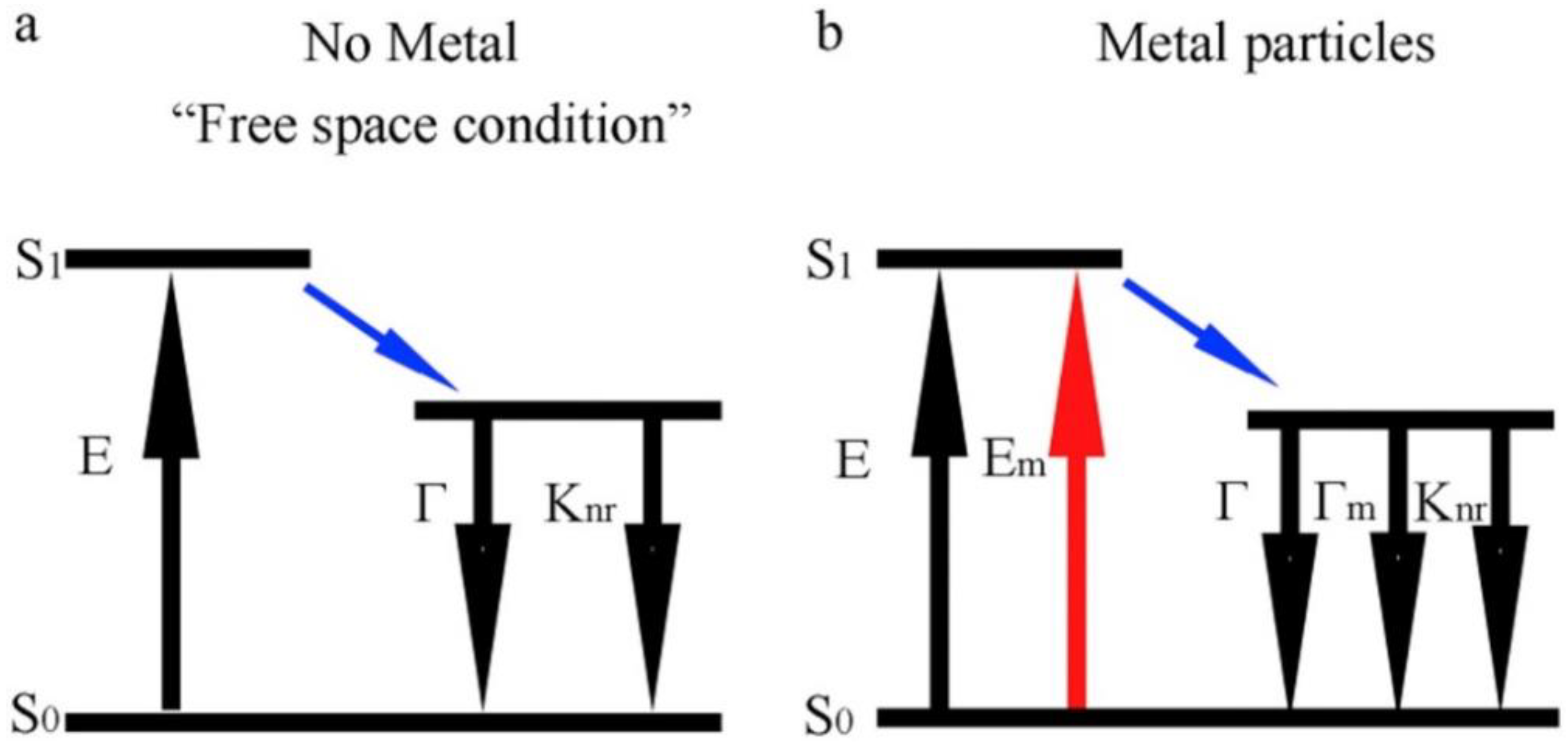
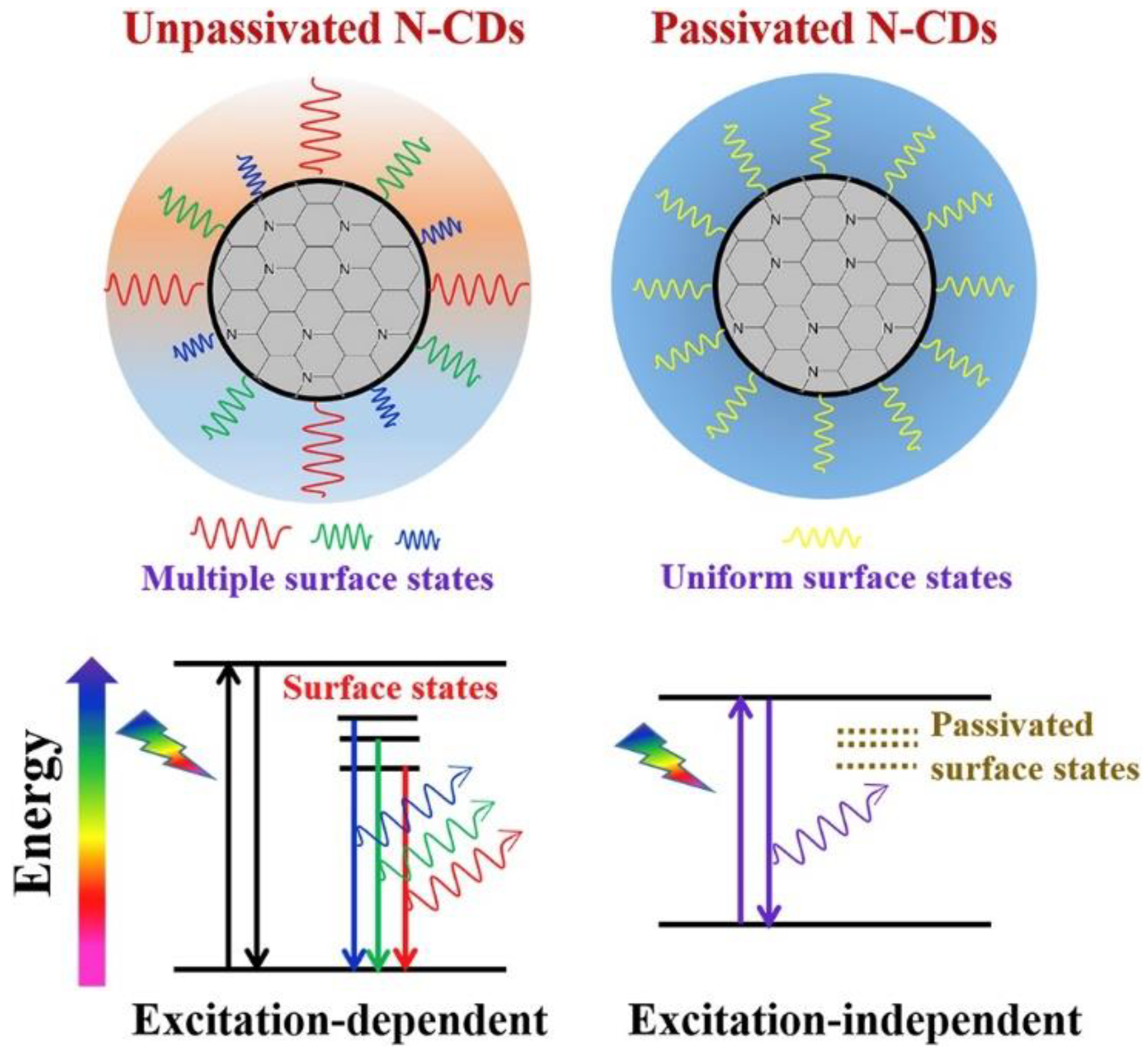
3. CD Photoluminescence Quenching
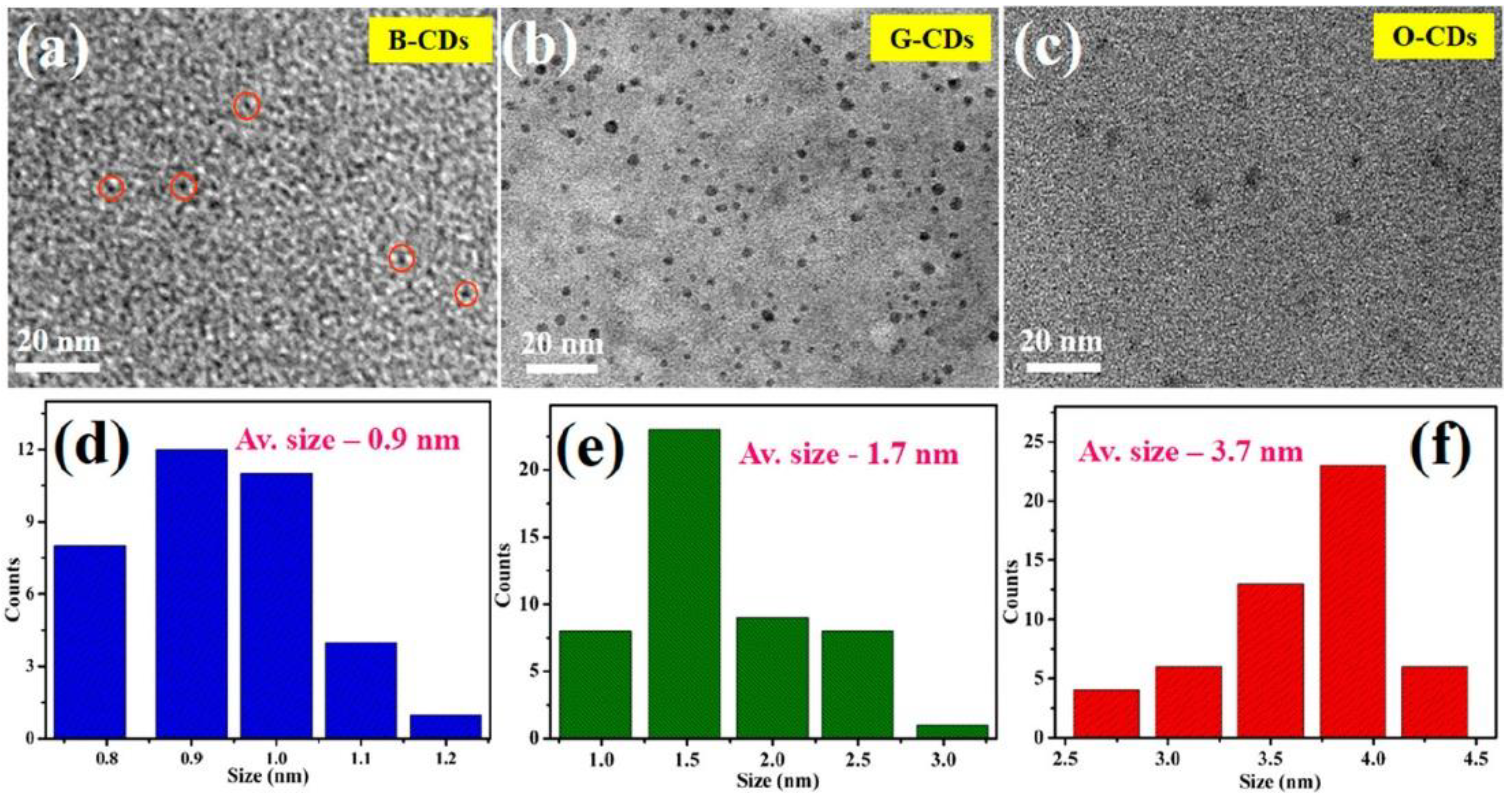
4. CD Preparation
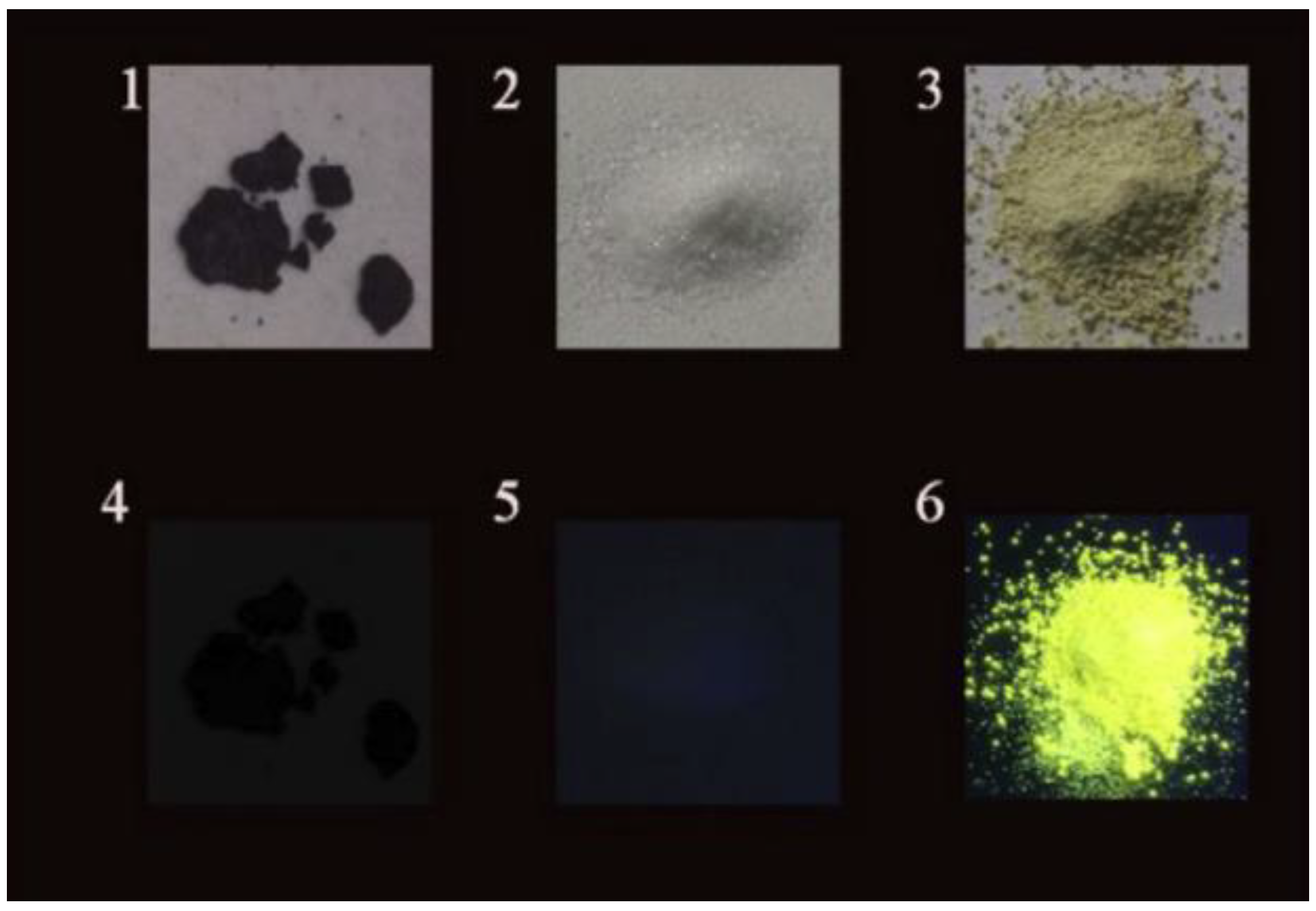
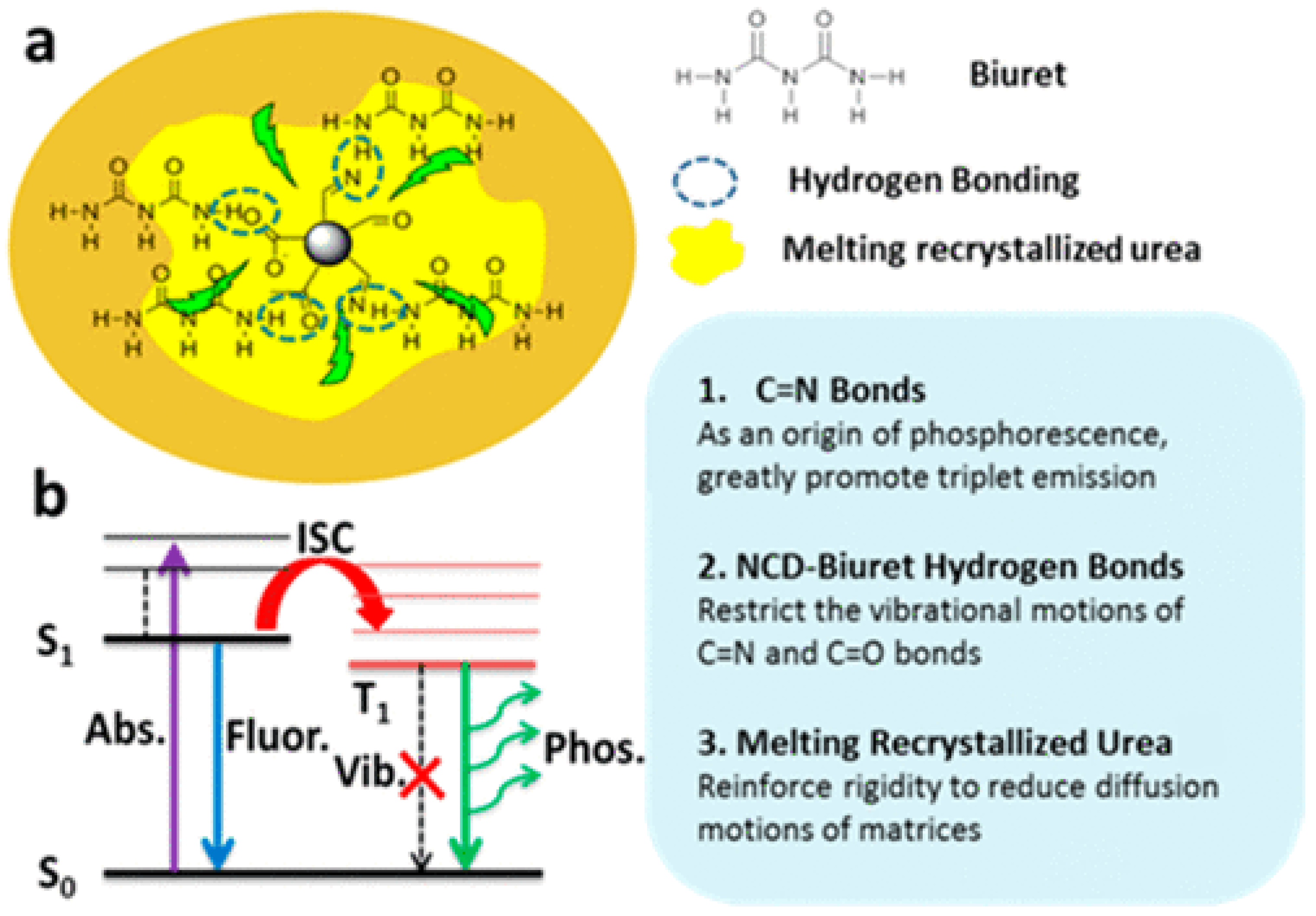
5. Fluorescent CDs in Solid-State Form
5.1. Solid-State Fluorescence in Matrices
5.2. Self-Quenching-Resistant CDs
6. Color Conversion CD-Based LEDs
6.1. AIQ and Possible Solutions
6.2. Chromaticity and Luminescence Properties
6.3. QY Improvement
6.4. Luminous Intensity and Luminous Efficiency
6.5. Stability
6.6. Biocompatibility
6.7. Applications of CD-Based LED in Telecommunications
6.8. Synoptic and Chronological Framework of Color Conversion CD-Based LEDs
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, N.; Rashid, S.A.; Tan, T. Chapter 5—Natural biomass as carbon sources for the synthesis of photoluminescent carbon dots. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–134. [Google Scholar]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, E.; Lee, H.A.; Sugnaux, C.; Shin, M.; Jeong, C.J.; Lee, J.; Messersmith, P.B.; Park, S.Y.; Lee, H. Phenolic condensation and facilitation of fluorescent carbon dot formation: A mechanism study. Nanoscale 2017, 9, 16596–16601. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Essner, J.B.; Baker, G.A. The emerging roles of carbon dots in solar photovoltaics: A critical review. Environ. Sci. Nano 2017, 4, 1216–1263. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Kong, D.; Jin, R.; Sun, C.; Du, D.; Lin, Y.; Lu, G. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 2020, 5, 218–234. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Pricilla, R.B.; Skoda, D.; Urbanek, P.; Urbanek, M.; Suly, P.; Domincova Bergerova, E.; Kuritka, I. Unravelling the highly efficient synthesis of individual carbon nanodots from casein micelles and the origin of their competitive constant-blue-red wavelength shift luminescence mechanism for versatile applications. RSC Adv. 2022, 12, 16277–16290. [Google Scholar] [CrossRef]
- Yuan, F.; He, P.; Xi, Z.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. Highly efficient and stable white LEDs based on pure red narrow bandwidth emission triangular carbon quantum dots for wide-color gamut backlight displays. Nano Res. 2019, 12, 1669–1674. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhuo, P.; Yin, H.; Fan, Y.; Liu, X.; Chen, Z. Carbon Dots Exhibiting Concentration-Dependent Full-Visible-Spectrum Emission for Light-Emitting Diode Applications. ACS Appl. Mater. Interfaces 2019, 11, 46054–46061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-Step Hydrothermal Synthesis of Nitrogen-Doped Conjugated Carbonized Polymer Dots with 31% Efficient Red Emission for In Vivo Imaging. Small 2018, 14, 1703919. [Google Scholar] [CrossRef] [PubMed]
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by Laser Irradiation in a Continuous Flow Jet of Carbon Quantum Dots for Fluorescence Imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of yellow-emitting carbon quantum dots from fullerene carbon soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Sciortino, A.; Marino, E.; van Dam, B.; Schall, P.; Cannas, M.; Messina, F. Solvatochromism Unravels the Emission Mechanism of Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3419–3423. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kwak, B.E.; Kim, D.H. Self-Quenching Origin of Carbon Dots and the Guideline for Their Solid-State Luminescence. J. Phys. Chem. C 2019, 123, 27124–27131. [Google Scholar] [CrossRef]
- Holonyak, N.; Bevacqua, S.F. Coherent (visible) light emission from Ga(As1−xPx) junctions. Appl. Phys. Lett. 1962, 1, 82–83. [Google Scholar] [CrossRef]
- Pearsall, T.P.; Miller, B.I.; Capik, R.J.; Bachmann, K.J. Efficient lattice-matched double-heterostructure LED’s at 1.1 μm from GaxIn1−xAsyP1−y. Appl. Phys. Lett. 1976, 28, 499–501. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Heo, J.; Chung, W.J. The effect of rare earth on color conversion properties of Cd–S–Se quantum dot embedded silicate glasses for white LED. Opt. Mater. 2021, 111, 110545. [Google Scholar] [CrossRef]
- Neikov, O.D.; Yefimov, N.A. Chapter 9 Nanopowders. In Handbook of Non-Ferrous Metal Powders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 271–311. [Google Scholar] [CrossRef]
- Edvinsson, T. Optical quantum confinement and photocatalytic properties in two-, one- and zero-dimensional nanostructures. R. Soc. Open Sci. 2018, 5, 180387. [Google Scholar] [CrossRef] [Green Version]
- Rama Krishna, M.V.; Friesner, R.A. Quantum confinement effects in semiconductor clusters. J. Chem. Phys. 1991, 95, 8309–8322. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Perspectives on the Physical Chemistry of Semiconductor Nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef] [Green Version]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Saleh, B.E.A.; Teich, M.C. Fundamentals of Photonics, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-58581-8. [Google Scholar]
- Hou, B.; Cho, Y.; Kim, B.-S.; Ahn, D.; Lee, S.; Park, J.B.; Lee, Y.-W.; Hong, J.; Im, H.; Morris, S.M.; et al. Red green blue emissive lead sulfide quantum dots: Heterogeneous synthesis and applications. J. Mater. Chem. C 2017, 5, 3692–3698. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bai, X.; Wang, T.; Yan, L.; Zhang, T.; Zhang, Y.; Yu, W.W. Efficient near-infrared light-emitting diodes based on liquid PbSe quantum dots. Nanotechnology 2017, 28, 215703. [Google Scholar] [CrossRef]
- Crouch, D.J.; O’Brien, P.; Malik, M.A.; Skabara, P.J.; Wright, S.P. A one-step synthesis of cadmium selenide quantum dots from a novel single source precursor. Chem. Commun. 2003, 12, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Chen, M.; Cooper, H.M.; Lu, G.Q.; Xu, Z.P. One-pot preparation of highly fluorescent cadmium telluride/cadmium sulfide quantum dots under neutral-pH condition for biological applications. J. Colloid Interface Sci. 2013, 390, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rajh, T.; Micic, O.I.; Nozik, A.J. Synthesis and characterization of surface-modified colloidal cadmium telluride quantum dots. J. Phys. Chem. 1993, 97, 11999–12003. [Google Scholar] [CrossRef]
- Franke, D.; Harris, D.K.; Chen, O.; Bruns, O.T.; Carr, J.A.; Wilson, M.W.B.; Bawendi, M.G. Continuous injection synthesis of indium arsenide quantum dots emissive in the short-wavelength infrared. Nat. Commun. 2016, 7, 12749. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, P.; Kim, N.; Kang, Y.-S.; Ramirez, O.; Lee, J.-S. Tunable, Bright, and Narrow-Band Luminescence from Colloidal Indium Phosphide Quantum Dots. Chem. Mater. 2017, 29, 6893–6899. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi, A.; Jilani, A.; Iqbal, J. Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light. Nanomaterials 2018, 8, 979. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef]
- Shiohara, A.; Hoshino, A.; Hanaki, K.-I.; Suzuki, K.; Yamamoto, K. On the cyto-toxicity caused by quantum dots. Microbiol. Immunol. 2004, 48, 669–675. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhao, J.; Zhang, L.; Li, L.; Zhu, Z. Intramolecular Hydrogen Bonds Quench Photoluminescence and Enhance Photocatalytic Activity of Carbon Nanodots. Chem. Eur. J. 2015, 21, 8561–8568. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Jia, Y.; Li, P.; Zhang, X.; Feng, X.; Zhu, L.; Sun, Y.; Hu, W.; Zhao, G. Tunable dual fluorescence emissions with high photoluminescence quantum yields modulated by Na ion dispersion method for purely solid state N-doped carbon dots. J. Photochem. Photobiol. Chem. 2020, 397, 112548. [Google Scholar] [CrossRef]
- Ma, P.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Green and Orange Emissive Carbon Dots with High Quantum Yields Dispersed in Matrices for Phosphor-Based White LEDs. ACS Sustain. Chem. Eng. 2020, 8, 3151–3161. [Google Scholar] [CrossRef]
- Zhu, S.; Tang, S.; Zhang, J.; Yang, B. Control the size and surface chemistry of graphene for the rising fluorescent materials. Chem. Commun. 2012, 48, 4527–4539. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Iqbal, A.; Tian, Y.; Wang, X.; Gong, D.; Guo, Y.; Iqbal, K.; Wang, Z.; Liu, W.; Qin, W. Carbon dots prepared by solid state method via citric acid and 1,10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sens. Actuators B Chem. 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Kimball, J.; Chavez, J.; Ceresa, L.; Kitchner, E.; Nurekeyev, Z.; Doan, H.; Szabelski, M.; Borejdo, J.; Gryczynski, I.; Gryczynski, Z. On the origin and correction for inner filter effects in fluorescence Part I: Primary inner filter effect-the proper approach for sample absorbance correction. Methods Appl. Fluoresc. 2020, 8, 033002. [Google Scholar] [CrossRef]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence Quenching by Photoinduced Electron Transfer: A Reporter for Conformational Dynamics of Macromolecules. Chem. Phys. Chem. 2009, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- So, Y.-H.; Zaleski, J.M.; Murlick, C.; Ellaboudy, A. Synthesis and Photophysical Properties of Some Benzoxazole and Benzothiazole Compounds. Macromolecules 1996, 29, 2783–2795. [Google Scholar] [CrossRef]
- Murphy, C.B.; Zhang, Y.; Troxler, T.; Ferry, V.; Martin, J.J.; Jones, W.E. Probing Förster and Dexter Energy-Transfer Mechanisms in Fluorescent Conjugated Polymer Chemosensors. J. Phys. Chem. B 2004, 108, 1537–1543. [Google Scholar] [CrossRef]
- Chen, Y.; O’Donoghue, M.B.; Huang, Y.-F.; Kang, H.; Phillips, J.A.; Chen, X.; Estevez, M.-C.; Yang, C.J.; Tan, W. A Surface Energy Transfer Nanoruler for Measuring Binding Site Distances on Live Cell Surfaces. J. Am. Chem. Soc. 2010, 132, 16559–16570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Li, J.; Xiao, X.; Yue, T.; Zhao, D. The one-step preparation of green-emission carbon dots based on the deactivator-reducing reagent synergistic effect and the study on their luminescence mechanism. RSC Adv. 2018, 8, 20016–20024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, Z.; Shao, H.; Jiang, X. Hydrothermal synthesis of highly fluorescent carbon nanoparticles from sodium citrate and their use for the detection of mercury ions. Carbon 2013, 52, 583–589. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Li, X.; Wang, J. Intrinsically fluorescent nitrogen-containing carbon nanoparticles synthesized by a hydrothermal process. Carbon 2011, 49, 5207–5212. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. Eur. J. Inorg. Chem. 2010, 2010, 4411–4414. [Google Scholar] [CrossRef]
- Li, H.; Ming, H.; Liu, Y.; Yu, H.; He, X.; Huang, H.; Pan, K.; Kang, Z.; Lee, S.-T. Fluorescent carbon nanoparticles: Electrochemical synthesis and their pH sensitive photoluminescence properties. New J. Chem. 2011, 35, 2666–2670. [Google Scholar] [CrossRef]
- He, C.; Yan, H.; Li, X.; Wang, X. Ultrafast preparation of polymer carbon dots with solid-state fluorescence for white light-emitting diodes. Mater. Res. Express 2019, 6, 065609. [Google Scholar] [CrossRef]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of Electrochemiluminescent Oxidized Carbon Quantum Dots from Activated Carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qu, S.; Hao, Z.; Ji, W.; Jing, P.; Zhang, H.; Zhang, L.; Zhao, J.; Shen, D. Towards efficient solid-state photoluminescence based on carbon-nanodots and starch composites. Nanoscale 2014, 6, 13076–13081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javanbakht, S.; Namazi, H. Solid state photoluminescence thermoplastic starch film containing graphene quantum dots. Carbohydr. Polym. 2017, 176, 220–226. [Google Scholar] [CrossRef]
- He, Y.; He, J.; Yu, Z.; Zhang, H.; Liu, Y.; Hu, G.; Zheng, M.; Dong, H.; Zhuang, J.; Lei, B. Double carbon dot assembled mesoporous aluminas: Solid-state dual-emission photoluminescence and multifunctional applications. J. Mater. Chem. C 2018, 6, 2495–2501. [Google Scholar] [CrossRef]
- He, Y.; He, J.; Zhang, H.; Liu, Y.; Lei, B. Luminescent properties and energy transfer of luminescent carbon dots assembled mesoporous Al2O3: Eu3+ co-doped materials for temperature sensing. J. Colloid Interface Sci. 2017, 496, 8–15. [Google Scholar] [CrossRef]
- Mosconi, D.; Mazzier, D.; Silvestrini, S.; Privitera, A.; Marega, C.; Franco, L.; Moretto, A. Synthesis and Photochemical Applications of Processable Polymers Enclosing Photoluminescent Carbon Quantum Dots. ACS Nano 2015, 9, 4156–4164. [Google Scholar] [CrossRef]
- Gong, X.; Ma, W.; Li, Y.; Zhong, L.; Li, W.; Zhao, X. Fabrication of high-performance luminescent solar concentrators using N-doped carbon dots/PMMA mixed matrix slab. Org. Electron. 2018, 63, 237–243. [Google Scholar] [CrossRef]
- Li, Y.; Miao, P.; Zhou, W.; Gong, X.; Zhao, X. N-doped carbon-dots for luminescent solar concentrators. J. Mater. Chem. A 2017, 5, 21452–21459. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Jia, K.; Zhang, D.; Shou, H.; Liu, X. Incorporation of polyethylene glycol into polyethylene terephthalate towards blue emitting co-polyester. Mater. Lett. 2016, 182, 367–371. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Y.; Zhang, H.; Xuan, Y.; Chen, G.; Ma, W.; Li, J.; Yu, J. Carbon Dots in a Matrix: Energy-Transfer-Enhanced Room-Temperature Red Phosphorescence. Angew. Chem. Int. Ed. 2019, 58, 18443–18448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A Self-Quenching-Resistant Carbon-Dot Powder with Tunable Solid-State Fluorescence and Construction of Dual-Fluorescence Morphologies for White Light-Emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Zang, J.-H.; Lou, Q.; Su, L.-X.; Li, Z.; Liu, Z.-Y.; Dong, L.; Shan, C.-X. In-situ embedding of carbon dots in a trisodium citrate crystal matrix for tunable solid-state fluorescence. Carbon 2018, 136, 359–368. [Google Scholar] [CrossRef]
- Song, H.; Liu, X.; Wang, B.; Tang, Z.; Lu, S. High production-yield solid-state carbon dots with tunable photoluminescence for white/multi-color light-emitting diodes. Sci. Bull. 2019, 64, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Wang, H.-J.; Hou, W.-Y.; Hao, Y.-W.; Jiang, W.-S.; Chen, H.-L.; Zhang, Q.-Q. Novel yellow solid-state fluorescent-emitting carbon dots with high quantum yield for white light-emitting diodes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119340. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, P.; Liu, X.; Mei, S.; Zhou, D.; Li, D.; Jing, P.; Zhang, W.; Guo, R.; Qu, S.; et al. Hydrogen Peroxide-Treated Carbon Dot Phosphor with a Bathochromic-Shifted, Aggregation-Enhanced Emission for Light-Emitting Devices and Visible Light Communication. Adv. Sci. 2018, 5, 1800369. [Google Scholar] [CrossRef]
- Li, C.-X.; Yu, C.; Wang, C.-F.; Chen, S. Facile plasma-induced fabrication of fluorescent carbon dots toward high-performance white LEDs. J. Mater. Sci. 2013, 48, 6307–6311. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Mao, L.-H.; Yin, Y.-J.; Wang, C.-F.; Chen, S. Synthesis of silica-based carbon dot/nanocrystal hybrids toward white LEDs. J. Mater. Sci. 2014, 49, 7391–7398. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, Y.; Liu, W.; Kalytchuk, S.; Kershaw, S.V.; Zhang, T.; Zhang, X.; Zhao, J.; Yu, W.W.; et al. High color rendering index white light emitting diodes fabricated from a combination of carbon dots and zinc copper indium sulfide quantum dots. Appl. Phys. Lett. 2014, 104, 261106. [Google Scholar] [CrossRef]
- Kim, T.H.; Wang, F.; McCormick, P.; Wang, L.; Brown, C.; Li, Q. Salt-embedded carbon nanodots as a UV and thermal stable fluorophore for light-emitting diodes. J. Lumin. 2014, 154, 1–7. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. Direct synthesis of highly stable nitrogen rich carbon dots toward white light emission. RSC Adv. 2015, 5, 101333–101337. [Google Scholar] [CrossRef]
- Ma, L.; Xiang, W.; Gao, H.; Pei, L.; Ma, X.; Huang, Y.; Liang, X. Carbon dot-doped sodium borosilicate gel glasses with emission tunability and their application in white light emitting diodes. J. Mater. Chem. C 2015, 3, 6764–6770. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Sun, K.; Reckmeier, C.; Zhang, T.; Zhang, X.; Zhao, J.; Wu, C.; Yu, W.W.; Rogach, A.L. Combination of carbon dot and polymer dot phosphors for white light-emitting diodes. Nanoscale 2015, 7, 12045–12050. [Google Scholar] [CrossRef]
- Othong, J.; Boonmak, J.; Promarak, V.; Kielar, F.; Youngme, S. Sonochemical Synthesis of Carbon Dots/Lanthanoid MOFs Hybrids for White Light-Emitting Diodes with High Color Rendering. ACS Appl. Mater. Interfaces 2019, 11, 44421–44429. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Song, X.; Wang, H.; Gu, H.; Zeng, H. Intercrossed Carbon Nanorings with Pure Surface States as Low-Cost and Environment-Friendly Phosphors for White-Light-Emitting Diodes. Angew. Chem. Int. Ed. 2015, 54, 1759–1764. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Wang, Z.; Zhang, Y.; Yuan, R.; Liang, X.; Xiang, W.; Zhou, Y. Construction of full-color light-emitting N-based carbon nanodots and their efficient solid-state materials via tape-casting technology for warm WLED. Chem. Eng. J. 2017, 324, 194–202. [Google Scholar] [CrossRef]
- Zheng, X.G.; Wang, H.L.; Ding, G.Q.; Cui, G.L.; Chen, L.; Zhang, P.H.; Gong, Q.; Wang, S.M. Facile synthesis of highly graphitized nitrogen-doped carbon dots and carbon sheets with solid-state white-light emission. Mater. Lett. 2017, 195, 58–61. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Y.; Yang, Y.; Liu, X. Efficient resistance against solid-state quenching of carbon dots towards white light emitting diodes by physical embedding into silica. Carbon 2018, 126, 426–436. [Google Scholar] [CrossRef]
- Zhu, J.; Bai, X.; Bai, J.; Pan, G.; Zhu, Y.; Zhai, Y.; Shao, H.; Chen, X.; Dong, B.; Zhang, H.; et al. Emitting color tunable carbon dots by adjusting solvent towards light-emitting devices. Nanotechnology 2018, 29, 085705. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly Emissive Carbon Dots in Solid State and Their Applications in Light-Emitting Devices and Visible Light Communication. ACS Sustain. Chem. Eng. 2019, 7, 9301–9308. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Du, Q.; Yang, Y.; Liu, X.; Xu, B. Orange-emissive carbon dot phosphors for warm white light-emitting diodes with high color rendering index. Opt. Mater. 2020, 109, 110346. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Ling, L.; Li, F.; Li, Q.; Wang, C.-F.; Wang, J.; Chen, S. Constructing honeycomb architectures from polymer carbon dot composites for luminous efficacy enhancement of LEDs. Appl. Phys. A 2019, 125, 91. [Google Scholar] [CrossRef]
- He, L.; Bai, Y.; Ge, C.; Yang, H.; Yu, X.; Zhang, X. Tunable luminescence and morphological evolution of facile synthesized zinc borate/carbon dots composites for NUV-WLEDs. J. Alloy. Compd. 2020, 834, 155021. [Google Scholar] [CrossRef]
- Mosca, M.; Macaluso, R.; Crupi, I. Hybrid Inorganic-Organic White Light Emitting Diodes. In Polymers for Light-Emitting Devices and Displays; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 197–262. ISBN 978-1-119-65464-3. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuo, P.; Yin, H.; Fan, Y.; Zhang, J.; Liu, X.; Chen, Z. Solid-State Fluorescent Carbon Dots with Aggregation-Induced Yellow Emission for White Light-Emitting Diodes with High Luminous Efficiencies. ACS Appl. Mater. Interfaces 2019, 11, 24395–24403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, S.F.; Fei, L.; Jin, L.; Pan, S.; Lin, P. Large-area color controllable remote carbon white-light light-emitting diodes. Carbon 2015, 85, 344–350. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Kalytchuk, S.; Wang, Y.; Zhang, X.; Gao, W.; Zhao, J.; Cepe, K.; Zboril, R.; Yu, W.W.; et al. Down-conversion monochromatic light-emitting diodes with the color determined by the active layer thickness and concentration of carbon dots. J. Mater. Chem. C 2015, 3, 6613–6615. [Google Scholar] [CrossRef]
- Chen, D.; Gao, H.; Chen, X.; Fang, G.; Yuan, S.; Yuan, Y. Excitation-Independent Dual-Color Carbon Dots: Surface-State Controlling and Solid-State Lighting. ACS Photonics 2017, 4, 2352–2358. [Google Scholar] [CrossRef]
- Lin, S.; Lin, C.; He, M.; Yuan, R.; Zhang, Y.; Zhou, Y.; Xiang, W.; Liang, X. Solvatochromism of bright carbon dots with tunable long-wavelength emission from green to red and their application as solid-state materials for warm WLEDs. RSC Adv. 2017, 7, 41552–41560. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Adv. Opt. Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Wang, K.; Yin, Z.; Du, F. Green light–emitting diodes with high efficiency organosilane-functionalized carbon dots. Integr. Ferroelectr. 2017, 181, 70–77. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, J.; Xiang, W.; Liang, X. Red-emitting carbon dots phosphors: A promising red color convertor toward warm white light emitting diodes. J. Mater. Sci. Mater. Electron. 2018, 29, 10453–10460. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Wang, Q. TiC2: A new two-dimensional sheet beyond MXenes. Nanoscale 2017, 8, 233–242. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, H.; Bai, X.; Zhai, Y.; Zhu, Y.; Chen, X.; Pan, G.; Dong, B.; Xu, L.; Zhang, H.; et al. Modulation of the photoluminescence in carbon dots through surface modification: From mechanism to white light-emitting diodes. Nanotechnology 2018, 29, 245702. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, F.; Li, L.; Qi, B.; Zhu, D.; Lü, J.; Lü, C. Tricolor White-Light-Emitting Carbon Dots with Multiple-Cores@Shell Structure for WLED Application. ACS Appl. Mater. Interfaces 2018, 10, 19796–19805. [Google Scholar] [CrossRef]
- Ren, J.; Sun, J.; Sun, X.; Song, R.; Xie, Z.; Zhou, S. Precisely Controlled Up/Down-Conversion Liquid and Solid State Photoluminescence of Carbon Dots. Adv. Opt. Mater. 2018, 6, 1800115. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Y.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S. Red carbon dots-based phosphors for white light-emitting diodes with color rendering index of 92. J. Colloid Interface Sci. 2018, 528, 281–288. [Google Scholar] [CrossRef]
- Wang, H.-J.; Yu, T.-T.; Chen, H.-L.; Nan, W.-B.; Xie, L.-Q.; Zhang, Q.-Q. A self-quenching-resistant carbon dots powder with tunable solid-state fluorescence and their applications in light-emitting diodes and fingerprints detection. Dye. Pigment. 2018, 159, 245–251. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; Wang, B.; Deng, Z.; Jin, Y.; Kang, Y.; Chen, J. Orange, yellow and blue luminescent carbon dots controlled by surface state for multicolor cellular imaging, light emission and illumination. Microchim. Acta 2018, 185, 539. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar Sahu, S. Synthesis of Longer-Wavelength-Emissive Carbon Quantum Dots for WLEDs and Investigation of Their Photoluminescence Properties. Chem. Sel. 2018, 3, 12998–13005. [Google Scholar] [CrossRef]
- Hu, T.; Wen, Z.; Wang, C.; Thomas, T.; Wang, C.; Song, Q.; Yang, M. Temperature-controlled spectral tuning of full-color carbon dots and their strongly fluorescent solid-state polymer composites for light-emitting diodes. Nanoscale Adv. 2019, 1, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Pal, K.; Karmakar, P.; Sahu, S.K. pH-Responsive Mn-Doped Carbon Dots for White-Light-Emitting Diodes, Fingerprinting, and Bioimaging. ACS Appl. Nano Mater. 2019, 2, 5900–5909. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Wu, P.; Ma, C.; Wu, X.; Luo, S.; Liu, S. Organosilane-functionalized carbon quantum dots and their applications to “on-off-on” fluorometric determination of chromate and ascorbic acid, and in white light-emitting devices. Microchim. Acta 2019, 186, 516. [Google Scholar] [CrossRef]
- Su, R.; Guan, Q.; Cai, W.; Yang, W.; Xu, Q.; Guo, Y.; Zhang, L.; Fei, L.; Xu, M. Multi-color carbon dots for white light-emitting diodes. RSC Adv. 2019, 9, 9700–9708. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Guo, Q.; Cai, Z.; Qiu, J.; Dong, G. Synthesis of multi-color fluorescent carbon quantum dots and solid state CQDs@SiO2 nanophosphors for light-emitting devices. Ceram. Int. 2019, 45, 17387–17394. [Google Scholar] [CrossRef]
- Kumari, R.; Sahu, S.K. Effect of Solvent-Derived Highly Luminescent Multicolor Carbon Dots for White-Light-Emitting Diodes and Water Detection. Langmuir 2020, 36, 5287–5295. [Google Scholar] [CrossRef]
- Yang, X.; Sui, L.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, B.; Lu, S. Red-emitting, self-oxidizing carbon dots for the preparation of white LEDs with super-high color rendering index. Sci. China Chem. 2021, 64, 1547–1553. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Yang, Q.; Wu, Q.; Shi, J.; Gong, A.; Yang, M. Efficient Room-Temperature Phosphorescence from Nitrogen-Doped Carbon Dots in Composite Matrices. Chem. Mater. 2016, 28, 8221–8227. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhen, S.; Li, X.; Zhang, W.; Sun, X.; Xu, B.; Wang, X.; Gao, Z.; Meng, X. Gram-Scale Synthesis of 41% Efficient Single-Component White-Light-Emissive Carbonized Polymer Dots with Hybrid Fluorescence/Phosphorescence for White Light-Emitting Diodes. Adv. Sci. 2020, 7, 1902688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Cong, R.; Zhu, S.; Zhao, X.; Liu, J.; S.Tse, J.; Meng, S.; Yang, B. pH-Dependent Synthesis of Novel Structure-Controllable Polymer-Carbon NanoDots with High Acidophilic Luminescence and Super Carbon Dots Assembly for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Xia, C.; Xia, J.; Jiang, D.; Yu, C.; Li, H. A yellow carbon dots-based phosphor with high efficiency for white light-emitting devices. J. Lumin. 2019, 206, 97–104. [Google Scholar] [CrossRef]
- Li, L.; Zhao, F.; Zhang, T.; Lü, C. A facile method to prepare polymer functionalized carbon dots inspired by the mussel chemistry for LED application. Dye. Pigment. 2019, 162, 845–854. [Google Scholar] [CrossRef]
- Perikala, M.; Bhardwaj, A. Excellent color rendering index single system white light emitting carbon dots for next generation lighting devices. Sci. Rep. 2021, 11, 11594. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z.; Chang, Y.; Wang, H.; Yuan, N.; Li, G.; Yu, D.; Jiang, Y. Ammonium hydroxide modulated synthesis of high-quality fluorescent carbon dots for white LEDs with excellent color rendering properties. Nanotechnology 2016, 27, 295202. [Google Scholar] [CrossRef]
- Schubert, E.F. Light-Emitting Diodes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-86538-8. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Chen, C.-F.; Ying, S.-P.; Yeh, Y.-Y. The Effects of TiO2 Diffuser-Loaded Encapsulation on Corrected Color Temperature Uniformity of Remote Phosphor White LEDs. Appl. Sci. 2019, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-S.; Tang, Y.; Li, Z.-T.; Li, Z.; Ding, X.-R.; Rao, L.-S. Investigation of the Emission Spectral Properties of Carbon Dots in Packaged LEDs Using TiO2 Nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–7. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Z.; Zhang, Y.; Huang, Y.; Yuan, R.; Xiang, W.; Zhou, Y. Easy synthesis of silver nanoparticles-orange emissive carbon dots hybrids exhibiting enhanced fluorescence for white light emitting diodes. J. Alloy. Compd. 2017, 700, 75–82. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Chen, Y.; Qian, S.; Hu, P.; Li, W.; Deng, Y.; Fang, Y.; Han, L.; Luqman, M.; et al. Core-shell Ag@SiO2@mSiO2 mesoporous nanocarriers for metal-enhanced fluorescence. Chem. Commun. 2011, 47, 11618–11620. [Google Scholar] [CrossRef]
- Aslan, K.; Huang, J.; Wilson, G.M.; Geddes, C.D. Metal-Enhanced Fluorescence-Based RNA Sensing. J. Am. Chem. Soc. 2006, 128, 4206–4207. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Holley, P.; Geddes, C.D. Metal-enhanced fluorescence from silver nanoparticle-deposited polycarbonate substrates. J. Mater. Chem. 2006, 16, 2846–2852. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhang, J.; Lin, S.; Huang, Y.; Yuan, R.; Liang, X.; Xiang, W. Intense enhancement of yellow luminescent carbon dots coupled with gold nanoparticles toward white LED. Dye. Pigment. 2017, 140, 122–130. [Google Scholar] [CrossRef]
- Cui, S.; Ji, X.; Qin, J.; Yang, G.; Wang, Y.; Zhu, Y.; Pan, G. Enhanced luminescence of solid blue carbon dots by the “Islands” Ag films for highly efficient light-emitting diodes. Appl. Phys. Express 2020, 13, 082010. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Wang, J.; Yang, Y.; Liu, X. Rapid microwave-assisted synthesis of highly luminescent nitrogen-doped carbon dots for white light-emitting diodes. Opt. Mater. 2017, 73, 319–329. [Google Scholar] [CrossRef]
- An, J.; Liu, G.; Chen, M.; Hu, Y.; Chen, R.; Lyu, Y.; Zhang, C.; Liu, Y. One-step synthesis of fluorescence-enhanced carbon dots for Fe (III) on−off−on sensing, bioimaging and light-emitting devices. Nanotechnology 2021, 32, 285501. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Mosca, M.; Rinella, S.; Macaluso, R.; Calì, C.; Saiano, F.; Feltin, E. Frequency-Downconversion Stability of PMMA Coatings in Hybrid White Light-Emitting Diodes. J. Electron. Mater. 2016, 45, 682–687. [Google Scholar] [CrossRef]
- Mosca, M.; Caruso, F.; Zambito, L.; Seminara, B.; Macaluso, R.; Calì, C.; Feltin, E. Warm white LED light by frequency down-conversion of mixed yellow and red Lumogen. In Proceedings of the Integrated Photonics: Materials, Devices, and Applications II; Fédéli, J.-M., Vivien, L., Smit, M.K., Eds.; SPIE Microtechnologies: Grenoble, France, 2013; Volume 8767, pp. 140–149. [Google Scholar] [CrossRef]
- Caruso, F.; Mosca, M.; Macaluso, R.; Feltin, E.; Calì, C. Generation of white LED light by frequency downconversion using perylene-based dye. Electron. Lett. 2012, 48, 1417–1419. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, L.; Yang, L.; Wu, X.; Zhang, C.; Wei, K.; He, L.; Han, X.; Qiao, H.; Asiri, A.M.; et al. Orange-red, green, and blue fluorescence carbon dots for white light emitting diodes. J. Mater. Sci. Technol. 2020, 50, 184–191. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Yang, Y.; Liu, X.; Xu, B. Rapid and green synthesis of fluorescent carbon dots from starch for white light-emitting diodes. New Carbon Mater. 2018, 33, 276–288. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Yang, Y.; Chen, Y.; Liu, X. Preparation of N-doped carbon dots based on starch and their application in white LED. Opt. Mater. 2018, 86, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Li, T.; Su, H.; Ni, Y.; Yan, L. Transparent Wood Film Incorporating Carbon Dots as Encapsulating Material for White Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2018, 6, 9314–9323. [Google Scholar] [CrossRef]
- Isnaeni; Zufara, B.S.; Lewa, I.W.L.; Herbani, Y.; Shiddiq, M. Role of surface states on luminescence shift of caramelised sugar carbon dots for color conversion emitting devices. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015003. [Google Scholar] [CrossRef]
- Ge, M.; Huang, X.; Ni, J.; Han, Y.; Zhang, C.; Li, S.; Cao, J.; Li, J.; Chen, Z.; Han, S. One-step synthesis of self-quenching-resistant biomass-based solid-state fluorescent carbon dots with high yield for white lighting emitting diodes. Dye. Pigment. 2021, 185, 108953. [Google Scholar] [CrossRef]
- Meng, W.; Wang, B.; Ai, L.; Song, H.; Lu, S. Engineering white light-emitting diodes with high color rendering index from biomass carbonized polymer dots. J. Colloid Interface Sci. 2021, 598, 274–282. [Google Scholar] [CrossRef]
- Xie, Y.; Geng, X.; Gao, J.; Shi, W.; Zhou, Z.; Wang, H.; Zhang, D.; Deng, B.; Yu, R. Synthesis of carbon dots@Mg(OH)2 solid-state composites with blue, red emitting for horticultural application. J. Alloy. Compd. 2021, 873, 159663. [Google Scholar] [CrossRef]
- Sajjad, M.T.; Manousiadis, P.P.; Orofino, C.; Cortizo-Lacalle, D.; Kanibolotsky, A.L.; Rajbhandari, S.; Amarasinghe, D.; Chun, H.; Faulkner, G.; O’Brien, D.C.; et al. Fluorescent Red-Emitting BODIPY Oligofluorene Star-Shaped Molecules as a Color Converter Material for Visible Light Communications. Adv. Opt. Mater. 2015, 3, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Pathak, P.H.; Feng, X.; Hu, P.; Mohapatra, P. Visible Light Communication, Networking, and Sensing: A Survey, Potential and Challenges. IEEE Commun. Surv. Tutor. 2015, 17, 2047–2077. [Google Scholar] [CrossRef]
- Mao, L.-H.; Tang, W.-Q.; Deng, Z.-Y.; Liu, S.-S.; Wang, C.-F.; Chen, S. Facile Access to White Fluorescent Carbon Dots toward Light-Emitting Devices. Ind. Eng. Chem. Res. 2014, 53, 6417–6425. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, F.; Wang, Y.; Zhang, Y.; Yang, Y.; Liu, X. Fluorescent Carbon Quantum Dots as Single Light Converter for White LEDs. J. Electron. Mater. 2016, 45, 2784–2788. [Google Scholar] [CrossRef]
- Joseph, J.; Anappara, A.A. White light emission of carbon dots by creating different emissive traps. J. Lumin. 2016, 178, 128–133. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Xie, Z.; Zhao, X.; Zhou, C.; Zhou, S.; Chen, P. Polysiloxane Functionalized Carbon Dots and Their Cross-Linked Flexible Silicone Rubbers for Color Conversion and Encapsulation of White LEDs. ACS Appl. Mater. Interfaces 2016, 8, 9961–9968. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Zhou, X.; Liu, Y.; Lei, B. A dual-emitting core–shell carbon dot–silica–phosphor composite for LED plant grow light. RSC Adv. 2017, 7, 16662–16667. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhan, J.; Geng, B.; He, P.; Wu, K.; Wang, L.; Xu, G.; Li, Z.; Yin, L.; Pan, D. Scalable synthesis of organic-soluble carbon quantum dots: Superior optical properties in solvents, solids, and LEDs. Nanoscale 2017, 9, 13195–13202. [Google Scholar] [CrossRef]
- Xie, Z.; Yin, Z.; Wu, Y.; Liu, C.; Hao, X.; Du, Q.; Xu, X. White Light-Emitting Diodes Based on Individual Polymerized Carbon Nanodots. Sci. Rep. 2017, 7, 12146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Bai, X.; Zhai, Y.; Chen, X.; Zhu, Y.; Pan, G.; Zhang, H.; Dong, B.; Song, H. Carbon dots with efficient solid-state photoluminescence towards white light-emitting diodes. J. Mater. Chem. C 2017, 5, 11416–11420. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Zhu, Z.; Yu, S.; Zhang, Y.; Yu, D.; Jiang, Y. N-doped carbon dots from phenol derivatives for excellent colour rendering WLEDs. RSC Adv. 2018, 8, 4850–4856. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Zheng, J.; Wang, J.; Yang, Y.; Liu, X. The synthesis of green fluorescent carbon dots for warm white LEDs. RSC Adv. 2018, 8, 19585–19595. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Xie, Z.; Chen, P.; Zhou, S. Highly efficient carbon dots and their nanohybrids for trichromatic white LEDs. J. Mater. Chem. C 2018, 6, 5957–5963. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Gao, H.; Zhong, J. Red C-dots and C-dot films: Solvothermal synthesis, excitation-independent emission and solid-state-lighting. RSC Adv. 2018, 8, 29855–29861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, B.; Guan, S.; Sun, X.; Li, X.; Zeng, H.; Xie, Z.; Chen, P.; Zhou, S. Highly Efficient Carbon Dots with Reversibly Switchable Green–Red Emissions for Trichromatic White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 16005–16014. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, D.; Fang, G.; Yuan, S.; Chen, X.; Zhong, J. Excellent luminescence films of excitation-independent carbon quantum dots toward non-rare-earth phosphor-based white light-emitting diodes. J. Alloy. Compd. 2018, 764, 17–23. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Wang, Y.; Yang, Y.; Liu, X.; Xu, B. Facile and Rapid Synthesis of Yellow-Emission Carbon Dots for White Light-Emitting Diodes. J. Electron. Mater. 2018, 47, 7497–7504. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, T.; Xu, M.; Zhang, M.; Guo, Y.; Zhang, L.; Street, J.; Ong, W.-J.; Xu, Q. Full color carbon dots through surface engineering for constructing white light-emitting diodes. J. Mater. Chem. C 2019, 7, 2212–2218. [Google Scholar] [CrossRef]
- Guner, T.; Yuce, H.; Tascioglu, D.; Simsek, E.; Savaci, U.; Genc, A.; Turan, S.; Demir, M.M. Optimization and performance of nitrogen-doped carbon dots as a color conversion layer for white-LED applications. Beilstein J. Nanotechnol. 2019, 10, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Z.; Ding, J.; Xu, Y.; Chen, G.; Liu, J.; Zhao, L.; Huang, N.; He, Z.; Li, Y.; et al. Diamond-like carbon structure-doped carbon dots: A new class of self-quenching-resistant solid-state fluorescence materials toward light-emitting diodes. Carbon 2019, 149, 342–349. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wu, Y.; Zhang, R.; Cao, Y.; Xu, X.; Chen, X.; Cai, L.; Xu, Q. Multicolor tunable highly luminescent carbon dots for remote force measurement and white light emitting diodes. Chem. Commun. 2019, 55, 12164–12167. [Google Scholar] [CrossRef]
- Madhu, M.; Chen, T.-H.; Tseng, W.-L. White-light emission of single carbon dots prepared by hydrothermal carbonization of poly(diallyldimethylammonium chloride): Applications to fabrication of white-light-emitting films. J. Colloid Interface Sci. 2019, 556, 120–127. [Google Scholar] [CrossRef]
- Wang, H.-J.; Hou, W.-Y.; Yu, T.-T.; Chen, H.-L.; Zhang, Q.-Q. Facile microwave synthesis of carbon dots powder with enhanced solid-state fluorescence and its applications in rapid fingerprints detection and white-light-emitting diodes. Dye. Pigment. 2019, 170, 107623. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Tang, Z.; Yang, B.; Lu, S. Near-infrared emissive carbon dots with 33.96% emission in aqueous solution for cellular sensing and light-emitting diodes. Sci. Bull. 2019, 64, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hu, T.; Chen, Y.; Xu, Y.; Song, Q. Polymer-Assisted Self-Assembly of Multicolor Carbon Dots as Solid-State Phosphors for Fabrication of Warm, High-Quality, and Temperature-Responsive White-Light-Emitting Devices. ACS Appl. Mater. Interfaces 2019, 11, 22332–22338. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.; Liu, S.; Ma, S.; Jing, G.; Hu, Y.; Wang, M.; Ye, Z.; Cheng, X. Facile one-pot synthesis of long-term thermally stable CDs@AlOOH toward white-light illumination. J. Mater. Chem. C 2019, 7, 14717–14724. [Google Scholar] [CrossRef]
- Yuan, T.; Yuan, F.; Li, X.; Li, Y.; Fan, L.; Yang, S. Fluorescence–phosphorescence dual emissive carbon nitride quantum dots show 25% white emission efficiency enabling single-component WLEDs. Chem. Sci. 2019, 10, 9801–9806. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, F.; Zhang, X.; Jing, P.; Li, D.; Yang, X.; Zhou, D.; Xu, X.; Qu, S. Synthesis of green emissive carbon dots@montmorillonite composites and their application for fabrication of light-emitting diodes and latent fingerprints markers. J. Colloid Interface Sci. 2019, 554, 344–352. [Google Scholar] [CrossRef]
- Zhao, K.; Zheng, X.; Zhang, H.; Xu, M.; Wang, S.; Yang, Q.; Xiong, C. Multi-color fluorescent carbon dots with single wavelength excitation for white light-emitting diodes. J. Alloy. Compd. 2019, 793, 613–619. [Google Scholar] [CrossRef]
- He, W.; Weng, W.; Sun, X.; Pan, Y.; Chen, X.; Liu, B.; Shen, J. Multifunctional Carbon Dots with Solid–Liquid State Orange Light Emission for Vitamin B12 Sensing, Cellular Imaging, and Red/White Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 7420–7427. [Google Scholar] [CrossRef]
- Lan, X.; Ren, H.; Yang, X.; Wang, J.; Gao, P.; Zhang, Y. A facile microwave-assisted synthesis of highly crystalline red carbon dots by adjusting the reaction solvent for white light-emitting diodes. Nanotechnology 2020, 31, 215704. [Google Scholar] [CrossRef]
- Lin, H.; Yang, J.; Liu, Y.; Zeng, F.; Tang, X.-S.; Yao, Z.; Guan, H.; Xiong, Q.; Zhou, J.; Wu, D.; et al. Stable and efficient hybrid Ag-In-S/ZnS@SiO2-carbon quantum dots nanocomposites for white light-emitting diodes. Chem. Eng. J. 2020, 393, 124654. [Google Scholar] [CrossRef]
- Meng, L.; Ushakova, E.V.; Zhou, Z.; Liu, E.; Li, D.; Zhou, D.; Tan, Z.; Qu, S.; Rogach, A.L. Microwave-assisted in situ large scale synthesis of a carbon dots@g-C3N4 composite phosphor for white light-emitting devices. Mater. Chem. Front. 2020, 4, 517–523. [Google Scholar] [CrossRef]
- Qiao, G.; Chen, G.; Wen, Q.; Liu, W.; Gao, J.; Yu, Z.; Wang, Q. Rapid conversion from common precursors to carbon dots in large scale: Spectral controls, optical sensing, cellular imaging and LEDs application. J. Colloid Interface Sci. 2020, 580, 88–98. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Wei, J.; Chen, L.; Zhang, Y. Multicolor carbon dots with concentration-tunable fluorescence and solvent-affected aggregation states for white light-emitting diodes. Nano Res. 2020, 13, 52–60. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, H.; Wang, Y.; Zhang, L.; Zhang, W.; Zhang, Y. Tri-chromatic quantum-dot synthesized sun-like white light-emitting diodes reaching maximum spectral similarity of 0.98. Opt. Laser Technol. 2020, 121, 105828. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Zhu, Z.; Luo, J.; Wu, Z.; Wang, Z. High-efficient, spherical and thermal-stable carbon dots@silica fluorescent composite as rare earth-free phosphors for white LED. Ceram. Int. 2020, 46, 14706–14712. [Google Scholar] [CrossRef]
- An, Y.; Liu, C.; Li, Y.; Chen, M.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Application of high-efficiency green fluorescent carbon dots prepared by acid catalysis in multicolour LEDs. RSC Adv. 2021, 11, 38033–38039. [Google Scholar] [CrossRef]
- An, Y.; Lin, X.; Guo, Z.; Yin, Q.; Li, Y.; Zheng, Y.; Shi, Z.; Zhang, W.; Liu, C. Red Emission Carbon Dots Prepared by 1,4-Diaminonaphthalene for Light-Emitting Diode Application and Metal Ion Detection. Materials 2021, 14, 4716. [Google Scholar] [CrossRef]
- Park, S.J.; Yang, H.K.; Moon, B.K. Correlated color temperature alteration with changing the position of carbon dot film for warm WLEDs. Dye. Pigment. 2021, 186, 109063. [Google Scholar] [CrossRef]
- Han, B.; Jiang, J.; Yan, Q.; Xin, Z.; Yan, Q. One-step straightfoward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes. Chin. Chem. Lett. 2021, 32, 591–593. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Hu, Y.; Han, L. Solid-state N,P-doped carbon dots conquer aggregation-caused fluorescence quenching and couple with europium metal-organic frameworks toward white light-emitting diodes. Dye. Pigment. 2021, 187, 109090. [Google Scholar] [CrossRef]
- He, L.; Liu, R.; Ge, C.; Yang, L.; Jia, K.; Zhang, X. Carbon dots optimize the luminescence and morphological of Tm3+ doped zinc borate composites for NUV-WLEDs. J. Alloys Compd. 2021, 872, 159665. [Google Scholar] [CrossRef]
- Hernández-Rivera, D.; Torres-Landa, S.D.; Rangel-Ayala, M.; Agarwal, V. Fluorescent films based on PVDF doped with carbon dots for evaluation of UVA protection of sunscreens and fabrication of cool white LEDs. RSC Adv. 2021, 11, 32604–32614. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chu, B.; Wang, Y.-L.; Hu, L.-F.; Hu, S.; Zhang, X.-H. Carbon dioxide derived carbonized polymer dots for multicolor light-emitting diodes. Green Chem. 2021, 23, 422–429. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Yu, K.; Yu, J.; Zhao, D.; Li, C. Encapsulating carbon quantum dot and organic dye in multi-shell nanostructured MOFs for use in white light-emitting diode. Microporous Mesoporous Mater. 2021, 319, 111062. [Google Scholar] [CrossRef]
- Vidya, T.; Anupama, M.; Muhammed, S.; Joseph, J.; Anappara, A.A. Multi-functional carbon dots for visual detection of picric acid and white-light emission. Mater. Res. Bull. 2021, 138, 111223. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, Q.; Wen, M.; Mao, Z.; Wei, H.; Chang, H.-J.; Niu, X. Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes. Nanotechnol. Rev. 2021, 10, 465–477. [Google Scholar] [CrossRef]
- Xavier, M.M.; Adarsh, N.N.; Nair, P.R.; Mathew, S. Carbon Nitride Quantum Dot-Embedded Poly(vinyl alcohol) Transparent Thin Films for Greenish-Yellow Light-Emitting Diodes. ACS Omega 2021, 6, 22840–22847. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, H.; Xu, J.; Wu, Y.; Zang, Y.; Sun, J. Color Emission Carbon Dots with Quench-ResixAstant Solid-State Fluorescence for Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2021, 9, 3901–3908. [Google Scholar] [CrossRef]
- Yin, L.; Yan, Y.; Guo, H.; Wang, L.; Xiu, H.; Zhang, J. Solid-State Carbon Dots-Based White Light-Emitting Diodes with Ultrahigh Color Rendering Index and Good Thermal Stability. IEEE Trans. Electron Devices 2021, 68, 3901–3906. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Y.; Cao, M.; Zhu, M.; Chen, R.; Li, H. Multi-color carbon dots from cis-butenedioic acid and urea and highly luminescent carbon dots@Ca(OH)2 hybrid phosphors with excellent thermal stability for white light-emitting diodes. J. Lumin. 2021, 237, 118202. [Google Scholar] [CrossRef]
- Bao, S.; Yu, H.; Gao, G.; Zhu, H.; Wang, D.; Zhu, P.; Wang, G. Rare-earth single atom based luminescent composite nanomaterials: Tunable full-color single phosphor and applications in WLEDs. Nano Res. 2022, 15, 3594–3605. [Google Scholar] [CrossRef]
- Cao, W.; Wu, Y.; Li, X.; Jiang, X.; Zhang, Y.; Zhan, Y.; Sun, Z. One-pot synthesis of double silane-functionalized carbon dots with tunable emission and excellent coating properties for WLEDs application. Nanotechnology 2022, 33, 115703. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, W.; Zhang, X.; Ma, C.; Xia, Z.; Lei, B. Carbon dots embedded in lead-free luminescent metal halide crystals toward single-component white emitters. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; An, Y.; Li, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Red, green, and blue light-emitting carbon dots prepared from gallic acid for white light-emitting diode applications. Nanoscale Adv. 2022, 4, 14–18. [Google Scholar] [CrossRef]
- Da, X.; Han, Z.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation of multicolor carbon dots with high fluorescence quantum yield and application in white LED. Chem. Phys. Lett. 2022, 794, 139497. [Google Scholar] [CrossRef]
- Dai, R.; Chen, X.; Ouyang, N.; Hu, Y. A pH-controlled synthetic route to violet, green, and orange fluorescent carbon dots for multicolor light-emitting diodes. Chem. Eng. J. 2022, 431, 134172. [Google Scholar] [CrossRef]
- Fang, M.; Neto, A.N.C.; Fu, L.; Ferreira, R.A.S.; deZeaBermudez, V.; Carlos, L.D. A Hybrid Materials Approach for Fabricating Efficient WLEDs Based on Di-Ureasils Doped with Carbon Dots and a Europium Complex. Adv. Mater. Technol. 2022, 7, 2100727. [Google Scholar] [CrossRef]
- Kaushal, N.; Sharma, A.L.; Saha, A. Visible LED-based photo-redox properties of sulfur and nitrogen-doped carbon dots designed by solid-state synthesis. Mater. Adv. 2022, 3, 355–361. [Google Scholar] [CrossRef]
- Kumari, R.; Sahu, S.K. A new insights into multicolor emissive carbon dots using Trachelospermum jasminoides leaves for the application of WLEDs. Colloids Surf. Physicochem. Eng. Asp. 2022, 647, 128959. [Google Scholar] [CrossRef]
- Lei, X.; Li, D.; Chen, Y.; Liu, Q.; Yan, Q.; Wang, J.; Han, B.; He, G.; An, B. RGB-multicolor fluorescent carbon dots by changing the reaction solvent type for white light-emitting diodes. New J. Chem. 2022, 46, 4979–4982. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, H.; Wang, Z.; Jia, M.; Wei, Y.; Fu, Z. Tunable KLa(MoO4)2:Eu3+@CDs composite materials for white LED and multi-mode information encryption technology. J. Alloys Compd. 2022, 894, 162298. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, F.; Xu, J.; Zhang, H.; Chen, L. Solvent-controlled synthesis strategy of multicolor emission carbon dots and its applications in sensing and light-emitting devices. Nano Res. 2022, 15, 414–422. [Google Scholar] [CrossRef]
- Wang, B.; Song, H.; Tang, Z.; Yang, B.; Lu, S. Ethanol-derived white emissive carbon dots: The formation process investigation and multi-color/white LEDs preparation. Nano Res. 2022, 15, 942–949. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Yang, Y.; Liu, X.; Qiu, J.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, L.; Guo, H.; Wang, L.; Zhang, J. High Stability Carbon Dots Phosphor and Ultra-High Color Rendering Index White Light-Emitting Diodes. IEEE Photonics J. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, Y.; Yu, C.; Xuan, T. Highly stable carbon nanodot-based phosphor as a color converter for WLED. J. Lumin. 2022, 246, 118836. [Google Scholar] [CrossRef]





| Year | Precursors | Preparation Method | QY (%) | LED λexc (nm) | LED λemis (nm) | CIE (x,y) | CCT (K) | CRI | Luminous Efficacy (lm/W) | Encapsulant | LED Emission Color | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | acrylamide, cadmium chloride, N-acetyl- L-cysteine (NAC), sodium borohydride, tellurium powder, sodium hydroxide | plasma-induced method (150 W) | ~6% | 380 | 490 (FWHM: 110 nm) | (0.20, 0.18) CDs (0.38, 0.36) mix | NR | 87 | 30 (@350 mA) | silicone (OE-6550A: OE-6550B) | blue (CDs), white (mix) | CDs mixed with CdTe QDs | [83] |
| 2014 | citric acid, (3-aminopropyl) triethoxysilane (APTES) (1:1) | hydrothermal method (180 °C, 4 h) | 20 | 380 | broadband (two peaks at 470 and 550) | (0.27, 0.32) mix | 9051 | 71 | NR | silicone | white (mix) | CDs mixed with CDs nanocrystals | [84] |
| citric acid, AAPMS | heating from a solution (240 °C, 1 min) | NR | 400 | 490 | (0.22–0.24, 0.38–0.43) | NR | NR | NR | silicone | bluish white | CDs embedded in silica, KCl, KBr, NaCl | [86] | |
| poly(acrylic acid) (PAA), glycerol | one-step pyrolysis | 9 | 380 | broadband (two peaks at 440 and 470) | (0.27, 0.32) | NR | NR | NR | silicone | white | CDs dispersed in a thermocurable resin (silicone) | [156] | |
| [3–(2-aminoethylamino)propyl]trimethoxysilane (AEATMS), citric acid | pyrolysis (240 °C, 2 min) | NR | 385 | 450 | (0.321, 0.312) at 10 mA (0.351, 0.322) at 30 mA | 3825–6452 | 93 | NR | PMMA | cold and warm white | CDs with zinc copper indium sulfide core–shell QDs | [85] | |
| 2015 | EDA | exothermic reaction between P2O5 and H2O | 28.5 | 360 (48 W) | broad band | (0.22, 0.33) at 360 nm of excitation | NR | NR | NR | PVA | bluish white | single precursor; stability after 16 h of irradiation | [87] |
| PVA | one-step hydrothermal process | NR | 460 | 550 | (0.28, 0.27) | NR | NR | NR | bluish white | intercrossed carbon nanorings (with relatively pure hydroxy surface states); AIQ is reduced | [91] | ||
| N-(β-aminoethyl)-γ-aminopropyl methyldimethoxy silane (AEAPMS), citric acid | pyrolysis (220 °C, 10 min) | 78 | 460 | 570 | (0.32, 0.33) | ~6190 | 78.9 | 58.1 | epoxy-resin | white | CD-doped sodium borosilicate gel (CD-NBS gel) | [88] | |
| citric acid, EDA | hydrothermal method | NR | 385 | ~460 | (0.326, 0.343) at 50 mA | 2805–7786 | 85–96 | 4.9 | silicone | high CRI WLEDs | phosphors based on the combination of CDs and polymer dots | [89] | |
| organic acid, silane | decomposing organic acid in silane coupling agent | NR | 460 (10 W) | broad band (two main peaks at 550 and 600) | (0.376, 0.374) for thickness of 400 μm | 2500–10,000 | NR | NR | epoxy matrix deposited on polystyrene substrate | white | different colors depends on the thickness of the film where CDs are dispersed | [102] | |
| AEAPMS, citric acid | pyrolysis (240 °C, 1 min) | 47 | 385 | 455, 550, 575, 585, 610 | (0.22, 0.22) (0.34, 0.43) (0.42, 0.51) (0.46, 0.50) (0,52, 0.46) | NR | NR | 1.21 cd/A | PMMA | blue, green, yellow, orange, red | five monochrome LEDs obtained by varying the CD concentration | [103] | |
| 2016 | glucose, polyethylene glycol (PEG) 200 | one-step hydrothermal method | 3.5 | 365 | broad band centered at 544 | (0.32, 0.37) | 5584 | NR | NR | epoxy resin | cool white light | glucose is first used as a carbon source | [157] |
| EDA, tetra-acetic acid (EDTA), ethylene glycol(EG) | microwave-assisted hydrothermal method | NR | 365 | broad band centered at 550 | (0.34, 0.38) | 5078 | 84 | NR | PMMA | white | CDs with different functional groups | [158] | |
| folic acid, chloridric acid | hydrothermal treatment | 36 (phosphorescence + fluorescence) | 360 | NR | (0.213, 0.204) (0.338, 0.363) | NR | NR | NR | melting recrystallization urea and biuret from the heating urea | blue white | phosphorescent N-doped CDs (NCDs) | [124] | |
| L-serine and L-tryptophan (molar ratio 3:1) at different pH values | one-pot aqueous synthesis controlled by pH | 16.3 (pH ≈ 8.1) 26.99 (3 < pH < 7) 46.83 (1 < pH < 3) | 365 | broad band (410−700) | (0.29, 0.31) | 6786 | 81 | NR | DMMA | cool white | crosslinked polymer carbon film (pH > 7, 200 °C) polymer carbon nanosheets (3 < pH < 7, 200 °C), amorphous carbon structures (1 < pH < 3, 200 °C) supersmall CDs (pH = 0.5, 300 °C) | [126] | |
| citric acid, ammonium hydroxide | ammonium hydroxide modulated hydrothermal method | 40 | 360 (1 W) | broad band centered at 450 (only CDs) | (0.18, 0.19) CDs’ LED (0.33, 0.37) RGB white | 5447 (at 100 mA) | 95.1 (at 100 mA) | NR | silicone, ethanol | white | mix of blue emission CDs, green emission SrSi2O2N2:Eu, red emission Sr2Si5N8:Eu | [130] | |
| aminopropyl methyl polysiloxane (AMS), citric acid | one-step solvothermal method | 16 | 460 | 590 | (0.33, 0.28) | NR | 66.6 | 14 | AMS-CDs crosslinked silicone rubbers (SRs) | white | AMS-CDs have a dual role of luminescence and encapsulation layer | [159] | |
| 2017 | p-phenylenediamine (p-PD), formamide (FA) | solvothermal method (200 °C, 1 h) | 10–15 (orange CDs) 20–30 (blue CDs) | 465 (350 mA) | 480−780 | (0.283, 0.246) - (0.470, 0.358) only CDs (0.323, 0.326) - (0.419, 0.376) CDs + Ce:YAG | 14,570 - 2158 only CDs 5977 - 3089 CDs + Ce:YAG | 73.3 (only CDs) 85 (CDs + Ce:YAG) | 60–80 | polyvinylpyrrolidone (PVP) | white | mix of orange-emitting CDs and Ce:YAG | [104] |
| NR (CDs produced by Nanjing JANUS) | NR | 40 (reported by CD manufacturer) | UV (λ NR) | 433–600 | vary in a large gamut with TiO2 nanoparticles concentrations (at a fixed CD concentration of 10 wt%) | from 3000 to 19,000 | from 40 to 85 | ~1.4 (increased by 31% with TiO2 nanoparticles) | silicone (mixed in a CDs-chloroform solution) | from cool to warm white | TiO2 nanoparticles used for enhancing the light scattering ability of the encapsulant | [133] | |
| citric acid, urea (dissolved in N,N-dimethylformamide -DMF) | hydrothermal method | 48.5 | 460 | broad band | (0.4595, 0.3925) (0.03 g CDs in 4 g OSi) | 2561 (0.03 g CDs in 4 g OSi) | 92.6 (0.03 g CDs in 4 g OSi) | 30.6 (0.03 g CDs in 4 g Osi) | N-(b-aminoethyl)-c-amin-o-propyl methyl dimethoxy silane (Osi) | warm white | red emission CDs (casted on Ce3+: YAG phosphor in glass (Ce: PiG) | [92] | |
| citric acid, AEAPMS, nitrogen | one-pot hot injecting method | 24.5 | 460 | broad band 500–700 | (0.33, 0.35) (0.6 mm-thick) | 5435 (0.6 mm-thick) | 74.6 (0.6 mm-thick) | 41.26 (0.6 mm-thick) | Ag nanoparticle solution | from bluish to neutral white | surface plasmon resonance from Ag nanoparticles enhances CD fluorescence | [134] | |
| o-phenylenediamine, pPD, N,N-dimethyl formamide (DMF) (solvent) | hydrothermal method | 52.4 | 460 | broad band | (0.3943, 0.3869) (CDs + Ce:PiG) | 3722 (CDs + Ce:PiG) | 83 (CDs + Ce:PiG) | 66.17 (CDs + Ce:PiG) | polyvinyl butyral (PVB) | warm white (CDs + Ce:PiG) | CDs shift gradually from 520 nm to 630 nm increasing their concentration in solution or changing the solvent polarity (solvatochromism) | [105] | |
| citric acid, urea (solvents: water, glycerol, DMF) | solvothermal synthesis | 30–40 | 395, 440 | 448, 515, 540, 570, 603, 622, 638 | (0.34, 0.31) (WLED) | 5048 (WLED) | 82.4 (WLED) | 8.34 (WLED) | silica | blue, cyan, green, yellow, orange, red, white | realization of full-color emissive CDs by exploiting the solvatochromism of three different (water, glycerol, DMF) | [106] | |
| 1,3-Dihydroxynaphthalene, KIO4 (dissolved in ethanol) | dehydrative condensation and dehydrogenative planarization (DCDP) method and solvothermally treatment | 53 | 365 | 430, 510, 620 | (0.3924, 0.3912) | 3875 | 97 | 31.3 (at 20 mA) | PMMA | warm white | three layers of blue-, green-, and red-emitting CDs | [145] | |
| citric acid, Silane Coupler KH-602, nitrogen | one-pot method using CTAB as the cationic surfactant, NaSal as the structure-directing agent, TEA as a catalyst, and BTEE and TEOS as the silica source | 29.8 | 362 | 470, 612 | (0.294, 0.280) (0.356, 0.343) | NR | 85–86 | NR | silica | white | dual-emitting CDs/CaAlSiN3:Eu2+-silica powder; applicability as a lighting source for plant growth | [160] | |
| citric acid, N-(2-aminoethyl)-3- aminopropyltrimethoxysilane (KH792) | one-step solvothermal method | 450 | 530 | 60,96 | polymer | green | CDs converting blue light to green light | [107] | |||||
| citric acid, EDA | one-step microwave- assisted hydrothermal method | 75.96 | 365 | weak peak at 442, strong peak at 572 | (0.42, 0.40) | 3416 | NR | NR | silicone | warm white | N-passivated CDs show record QY values | [140] | |
| oil-soluble 1,3,6-trinitropyrene (TNP) (as C and N sources), various solvents: toluene, acetone, EA, DMF) | solvothermal synthesis | 65.93 | 460 | broad band between 540 and 610 | (0.32, 0.31) | 6300 | NR | NR | PMMA | white | mix of long-wavelength emitting CDs and green phosphors; effect of the solvents on the CD emission | [161] | |
| citric acid, organosilane | one-pot pyrolysis method | 25–55 | ~450 | ~550 | (0.24, 0.28)–(0.31, 0.43) | 5030 (for x = 0.33 and y = 0.21) | 74 (for x = 0.33 and y = 0.21) | 79.4 (at 350 mA) | polymerized silane prefunctionalized carbon dots (SiCDs) | white | SiCDs individually polymerized one-component system, drip-coated and bulk polymerized on GaN LEDs | [162] | |
| citric acid, AEAPMS, nitrogen | pyrolysis | 39 | 460 | broad band centered at 560 | (0.33, 0.36) | 5653 | 78.2 | 43.75 | colloidal Au nanoparticles | white | gold-carbon dots (GCDs) with high luminescence | [138] | |
| ammonium citrate, EDTA; solvent: DMF | solvothermal syn-thesis | 67 | 460 | from 450 to 600 | (0.32, 0.33) (white) | 6565 (white) | 68.4 (white) | 32.26 (white) | Polyamideresin-650 (P-650) | red, orange, yellow, white, cyan, blue, and dark blue | white and multicolor N-doped CDs; color depends on molar ratio between the precursors | [109] | |
| ammonium citrate (in DMF), ethyl alcohol or ammonium citrate (in DMF), AEAPMS | solvothermal syn-thesis | 51 | 395 | ~560 | (0.33, 0.34) | 6735 | 51 | 25.63 | glass matrix | white | white CD-based glass thin films fabricated by screen printing technology | [160] | |
| glucose, ammonia | hydrothermal synthesis | 10.2 | ~395 | ~590 | (0.28, 0.37) | NR | NR | NR | PVA | white | synthesis of nitrogen-doped carbon dots (NCDs) and carbon sheets (NCSs); PVA used to prevent AIQ | [93] | |
| hexadecyltrimethyl ammonium bromide | 38.7 | ~385 | two broad bands at 495 and 660 | 3623–8121 | PVP | white | modulation of the emitting states of colloidal CDs | [163] | |||||
| 2018 | p-PD, ethanol, N-(3-(trimethoxysilyl) propyl) ethylenediamine (KH-792) | solvothermal syn-thesis | 41.72 | 400 | 480–720 | (0.44, 0.42) | 2951 | NR | NR | silica | warm white | embedding of CDs into a silica matrix overcomes the AIQ | [94] |
| phenol derivative, EDA | hydrothermal method | 24.4 in water; 53.3 in ethanol | 360 | broad band | (0.3316, 0.3373) | 5538 | 93.3 | NR | transparent epoxy JH-6800MA and JH-6800MB | yellow–green, white | WLEDs fabricated by mixing yellow–green N-doped CDs, blue CDs, and red emission (Sr, Ca) AlSiN3:Eu powders | [164] | |
| pyrogallic acid, DMF | solvothermal syn-thesis | 8 (in KH-792); 16.82 (in DMF solution) | 365 | 560 | (0.38, 0.48) (at 3.5 V) | 4503 (at 3.5 V) | NR | NR | KH-792 | white | green CD emission due to large conjugated sp2-domain promoted by DMF; high-stability | [165] | |
| amino silane (red CDs), N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (green CDs) | alkali-induced method | 80 (red); 49 (green) | 90.2 | 68.58 | trichromatic warm white | optical properties of red-emitting CDs modulated by the alkali-induced surface electronic states; mix of red- and green-emitting CDs | [166] | ||||||
| p-PD; solvent: isopropanol | solvothermal synthesis | 20.6 | 450 | broad band (peak at 580–600) | (0.335, 0.319) 0.5 nm-thick; (0.348, 0.324) 1.0 nm-thick; (0.371, 0.341) 1.5 nm-thick | 5359 (0.5 nm) 4772 (1.0 nm) 3994 (1.5 nm) | 81 (0.5 nm) 84 (1.0 nm) 85 (1.5 nm) | NR | PVA | white | combination of red CD solid film and yellow Ce:YAG PiG (0.5, 1.0, 1.5 nm film thickness) | [167] | |
| citric acid, urea; solvents: water, DMF, ethanol, NaOH | hydrothermal method | 34 (blue); 19 (green); 47 (red) | 365 | 442 (blue); 545 (green); 620 (red) | (0.38, 0.34) | 3913 | 91 | 10.2 | PVA | warm white | tunable emissions from blue, green, and red CDs (by changing the reaction solvent) | [95] | |
| Substituted derivatives from perylene (3,4,9,10-nitroperylene) (refluxed with HNO3) | solvothermal treatment in an alkaline solution | 81 (green); 80 (red) | 460 | 508 615 | 92.9 | 71.75 | methyltriethoxysilane (MTES) and APTES | trichromatic white | photoluminescent CDs with green and red emission switching using perylene as the precursor | [168] | |||
| 1,2,4-triaminobenzene, polyethylene glycol 200 (PEG 200); solvents: ethyl acetate, ethanediamine, oleylamine, DMSO | solvothermal method | from 10.8 to 25 | 460 | from 473 to 624 | (0.4557, 0.3840) (YAG/red CDs: 0.50) | 2514 (YAG/red CDs: 0.50) | 89.6 (YAG/red CDs: 0.50) | NR | silica | warm white (red CDs mixed with Ce3+:YAG) | CDs can be well-tuned from 473 to 624 nm in different solvents | [108] | |
| potato starch, EDS | microwave-assisted hydrothermal method | 2.46 (only starch; non-doped CDs); 5.71 (starch + EDS; N-doped CDs) | 375 | 560 (non-doped CDs); 430, 560 (N-doped CDs) | (0.38, 0.45) non-doped CDs; (0.33, 0.35) N-doped CDs | 4329 (non-doped CDs); 5437 (N-doped CDs) | NR | NR | starch | white | potato starch is used as a carbon source for CD and as an encapsulant | [147] | |
| citric acid, 5-amino-1,10- phenanthroline (Aphen) | one-pot hydrothermal method | 67 (red); 29 (white) | 400 | triple emission bands: 430 (blue), 500 (green), 630 (red) | (0.33, 0.33) | NR | 92 | 30.5 | poly(2-hydroxyethyl methacrylate) (PHEMA) | pure white | multicolor emissive CDs with multiple core@shell structure | [111] | |
| citric acid, urea | hydrothermal method | 53.82 (blue CDs); 36.18 (green CDs); 12.73 (red CDs) | 365 | 445 (blue CDs); 510 (green CDs); 600 (red CDs); from 400 to 800 (white) | (0.33, 0.32) | 5237 | 83 | NR | transparent wood (lignin removed by oxalic acid and choline chloride), PAA | trichromatic pure white | green preparation of transparent wood as encapsulant; stability tested for 7 days | [149] | |
| p-PD, 3-isocyanatopropyltriethoxysilane (IPTS) | pyrolysis | NR | 460 | 570 (dichromatic LED); 500, 605 (trichromatic LED); @50 mA | (0.397, 0.428) (dichromatic LED); (0.385, 0.345) (trichromatic LED); @50 mA | 3949 (dichromatic LED); 4494 (trichromatic LED); @50 mA | 70 (dichromatic LED); 85 (trichromatic LED); @50 mA | 15.88 (dichromatic LED); 22.00 (trichromatic LED); @50 mA | PMMA (dichromatic LED); APTES-gel (trichromatic LED) | dichromatic and trichromatic white | a novel approach to achieve up/down-conversion photoluminescence of CDs based on polarity dependence | [112] | |
| o-phenylenediamine (o-PD); solvent: water | hydrothermal method | 25–35 | InGaN blue LED (λ NR) | broad band | (0.353,0.371) @100–150 mA | ~5400 @100–150 mA | ~78 @100–150 mA | ~45 @100–150 mA | PVA, silica gel | white | yellow-emitting N-doped CDs | [169] | |
| citric acid, urea | microwave-assisted heating method | 25 (red CDs); 36 (green CDs) | 450 | broad band (peaks at 532, 630) | (0.33, 0.33) | 5610 | 92 | 12 | PVP (for red CDs); starch (for green CDs) | pure white | enhanced red emissive CDs-based phosphors with high QY | [113] | |
| citric acid, piperazine; (different mass ratio: 1:0.5, 1:1, 1:2 w/w) | microwave-assisted heating method | NR | 395 | broad band centered at ~560 | (0.25, 0.28); mass ratio: 1:1 w/w | 13601 | NR | NR | silicone | bluish white | the mass ratio of the precursors not only has a great influence on the CD sizes, but can also affect their luminescence properties | [114] | |
| o-PD, urea | one-pot microwave-assisted hydrothermal method | 4.23 | 420 | 440–700 (peak: 563) | (0.30, 0.30) | 7915 | NR | NR | PVA | white | rapid synthesis of yellow fluorescent CDs | [170] | |
| starch, EDA | one-step hydrothermal method | 9.65 | 365 | two peaks at 420 and 555 | (0.33, 0.37) | 5462 | NR | NR | starch, PVA | white | starch used as a carbon source for CDs | [148] | |
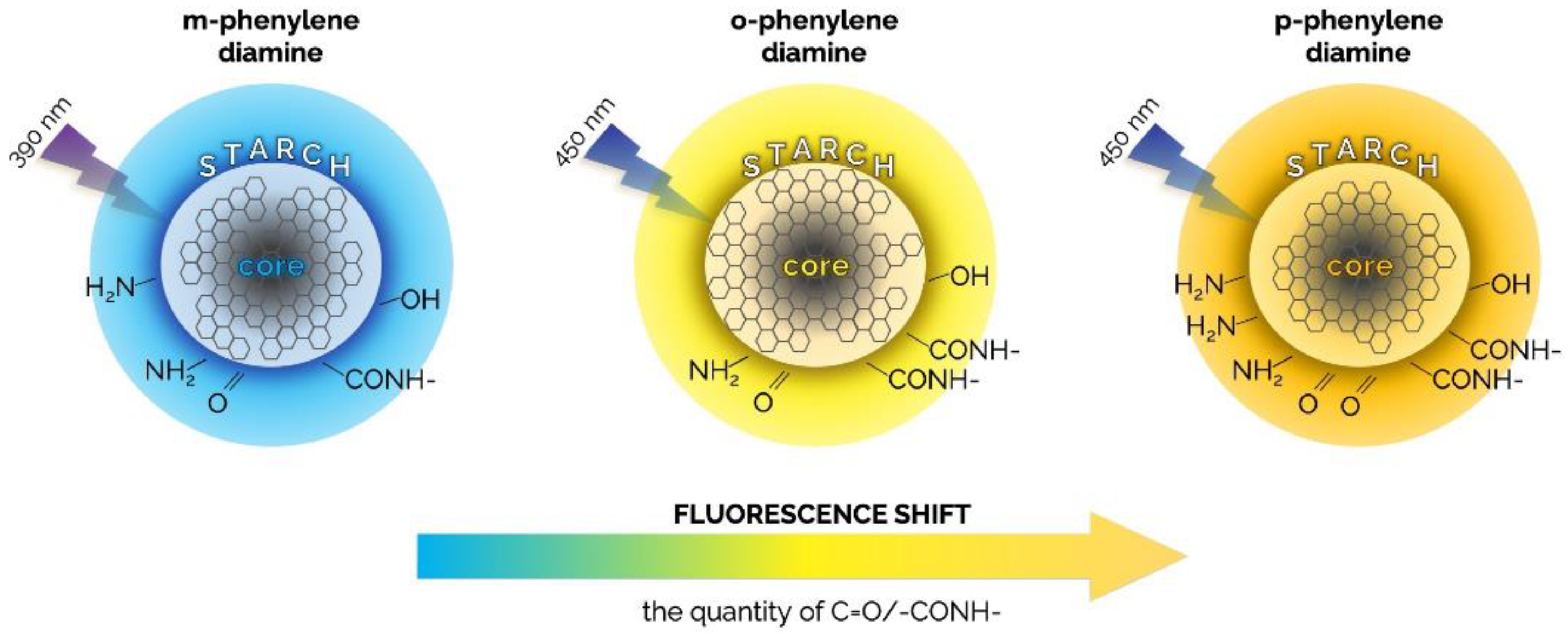
| phenylenediamine isomers (o-PD, m-PD, p-PD) formamide solution | microwave heating | in ethanol: 14 m-CDs; 45 o-CDs; 8 p-CDs; in water: 11 m-CDs; 38 o-CDs; 6 p-CDs | 390 (m-CDs); 450 (o-CDs, or p-CDs) | 470 m-CDs; 550 o-CDs; 600 p-CDs | (0.2678, 0.2945) m-CDs; (0.3613, 0.3851) o-CDs; (0.2423, 0.1283) p-CDs | 10,967 m-CDs; 4589 o-CDs; 3247 p-CDs | 83 m-CDs; 87 o-CDs; 81 p-CDs | 18.3 m-CDs; 21.0 o-CDs; 17.7 p-CDs | starch, silicone | cool, neutral, and warm white | phenylenediamine isomers (oPD, mPD, and pPD) used as precursors for producing multicolor emissive CDs | [115] | |
| pyromellitic acid (PA), diethylenetriamine (DETA) and thiourea. | one-pot solvothermal method | 16.7 | InGaN blue LED (λ NR) | 611 | (0.57, 0.42) (orange LED) | 1745 (orange LED) | 56 (orange LED) | NR | PMMA chloroform solution | orange, white | solvothermal route for the synthesis of nitrogen and sulfur co-doped CDs; orange emissive CDs | [116] | |
| 2019 | p-PD, amino acetic acid, ethanol, EDA | solvothermal method | 24.7 (red CDs) | 360 | broad band (peaks at 400, 465, 600) | (0.33, 0.33) | 5612 | 89 | NR | PVP | pure white | synthesis of blue-, green-, and red-emitting CDs with high dispersity both in aqueous and organic solvent; WLED obtained by mixing the three types of CDs | [171] |
| o-PD, starch | one-step hydrothermal method | 66.9 | 455 | broad band centered at around 600 | (0.3429, 0.2817) | 4613 | 83 | 30.54 | silicone | daylight white | highly efficient solid-state yellow-emitting CDs phosphors | [127] | |
| diammonium hydrogen citrate, urea | pyrolysis | NR | 450 | broad band | (0.31, 0.36) (composite fibers); (0.33, 0.34) (mix red phosphor and CDs) | 6000 (composite fibers) | 90 (composite fibers) | 63.5 (mix red phosphor and CDs) | PVP | white | WLED fabricated by a commercial red phosphor (Sr2Si5N8:Eu2+) and N-doped CDS embedded in PVP; fabrication of electrospun composite fibers | [172] | |
| citric acid, urea | one-step gaseous detonation method (within milliseconds) | 11.2 | 365 | broad band centered at 534 | (0.31, 0.42) | 6249 | NR | NR | water solution dripped on an optical lens and dried | white | rapid CD preparation by a one-step gaseous detonation approach | [63] | |
| citric acid, urea; solvent: DMF | one-step solvothermal treatment | 5.3 (blue CD); 12.4 (green CD); 8.9 (yellow CD); 6.9 (orange-red) | 365 | 450 (blue); 550 (green); 575 (yellow); 610 (orange-red); 440, 540–590 flat band (white) | (0.18, 0.21) blue; (0.34, 0.54) green; (0.49, 0.46) yellow; (0.58, 0.38) orange-red; (0.32, 0.33) white | 4820 | 82.7 | NR | PVA | blue, green, yellow, orange–red, white | study of temperature on the evolution of CD surface states and on the emissive properties of CD-based LEDs | [117] | |
| pyromellitic acid, pentaethylenehexamine (PEHA); (solvent: DMF; dopant: manganese chloride tetrahydrate) | solvothermal method | 28.5 (orange Mn-doped CDs); 83 (green CDs); 70 (blue CDs) | 365 | NR | (0.15, 0.19) blue; (0.25, 0.50) green; (0.55, 0.44) orange; (0.32, 0.31) white | 6216 | NR | NR | PVA | blue, green, orange, white | orange, green, and blue emissive CDs have synthesized; orange CDs doped with Mn to improve QY | [118] | |
| citric acid, tri(hydroxymethyl) amino methane hydrochloride (Tris-HMA) | one-step pyrolysis | 15 (CD@PS); 23 (CD@PEGMA) | 365 | ~440 (only blue CD@PS) | (0.32, 0.31) (mix of blue CD@PS, green 8-quinolinol, red CdSe/ZnS QDs) | NR | NR | NR | styrene, azobisisobutyronitrile (AIBN) | white | CDs prepared via mussel-inspired chemistry; CDs decorated by catechol-terminated hydrophilic poly(poly(ethylene glycol) methyl ether methacrylate (PPEGMA) and hydrophobic polystyrene (PS) | [128] | |
| KHP, NaN3, boric acid (BA); solvent: formaldehyde | one-step microwave-assisted pyrolysis | 67.8 | 365 | broad band centered at 432 | (0.17, 0.14) (15% mass ratio of D-CDs and resin) | >100,000 | 37 (15% mass ratio of D-CDs and resin) | 1.37 (15% mass ratio of D-CDs and resin) | epoxy silicone resin | bluish white | preparation of diamond-like carbon (sp3C) structure-doped carbon dots (D-CDs) powder for WLEDs | [173] | |
| citric acid, branched poly(ethylenimine) (b-PEI; molecular weight: 2000) | one-step hydrothermal method | 26 | 450 | 565 (WLED); 590 (yellow LED) | (0.34, 0.34) white; (0.56, 0.43) yellow | 4850 (white); 1849 (yellow) | 70.5 (white) | 8.9 (white) | NR | yellow, white | 4 ns of CD luminescence lifetime enabling the fabrication of WLEDs and high-performance visible light communication system | [96] | |
| Tobias acid, o-PD; solvents: formamide (blue CDs), ethanol (yellow CDs), sulfuric acid (red CDs) | one-step solvothermal method | 50.8 (red); 25.4 (yellow); 65.1 (blue); 50 (white) | 365 | 410–460 flat band, 560 | (0.31, 0.32) | 6135 | NR | NR | hydrogel | white | multicolor tunable highly luminescent CDs; N, S-CDs with red dual emission | [174] | |
| glucosamine, 3-[2-(2- aminoethylamino)ethylamino] propyl-trimethoxysilane (NQ-62); solvent: acetone | one-pot solvothermal treatment | 460 | broad band 380–780, centered at 600 | (0.269, 0.184) (CD concentration: 50 g/L); (0.340, 0.255) (CD concentration: 100 g/L); (0.355, 0.268) (CD concentration: 120 g/L); (0.427, 0.327) (CD concentration: 200 g/L); (0.547, 0.383) (CD concentration: 250 g/L) | 100,000 (CD concentration: 50 g/L); 4615 (CD concentration: 100 g/L); 3647 (CD concentration: 120 g/L); 2345 (CD concentration: 200 g/L); 1675 (CD concentration: 250 g/L) | 42.3 (CD concentration: 50 g/L); 67.5 (CD concentration: 100 g/L); 68.1 (CD concentration: 120 g/L); 78.0 (CD concentration: 200 g/L); 81.9 (CD concentration: 250 g/L) | NR | no | white | synthesis of organosilane-functionalized carbon quantum dots (Si-CDs) | [119] | ||
| poly(diallyldimethylammonium chloride) (PDDA) | microwave-assisted hydrothermal carbonization | ~11.0 in water; 7.3 in acetonitrile; 8.1 in DMF; 7.3 in methanol; 1.4 in acetone; 1.3 in PVA | 350 | broad band | (0.303, 0.332) in PVA; (0.307, 0.354) in water | 7023 in PVA; 5999 in water | NR | NR | PVA | white | CDs prepared starting from hydrothermal carbonization of PDDA; WLEDs fabricated by CD dispersion in water solution or in PVA | [175] | |
| citric acid, EDA | sonochemical synthesis | 9–11 | 365 | 450−850 | (0.334, 0.334) (optimized) | 4290–6606 | 88–94 | NR | lanthanoid metal–organic frameworks (Ln-MOFs) | white | fabrication of CDs/Ln-MOFs hybrids for WLEDs and as luminescent security inks | [90] | |
| poly(methyl methacrylate-co-dimethyl diallyl ammonium chloride) (PMMA-co-DMDAAC) | pyrolysis | NR | 360 | 450 | (0.1506, 0.0290) 1:5 molar ratios of DMDACC to MMA | NR | NR | 11.24 (1:5 DMDACC:MMA) | patterned PMMA-co-DMDAAC composite | blue | honeycomb-patterned films of different pore sizes as a matrix increases luminous efficiency | [98] | |
| citric acid, urea; solvents: water, ethyl alcohol, DMF | hydrothermal method | NR | 365 | broad band (peaks located at 441, 536, and 622) | (0.3497, 0.3045) (white) | 4878 | 85.2 | NR | silica | blue, green, yellow, orange, red, white | multicolor emission CDs synthesized by varying the ratio of precursors, the solvents, the temperature, and reaction time | [121] | |
| p-PD, ZnCl2 solvent: ethanol | one-step solvothermal method | 5.97 (pPD: ZnCl2 = 1:0—red); 6.24 (pPD: ZnCl2 = 1:0.1—purplish red); 6.92 (pPD: ZnCl2 = 1:0.5—purplish blue); 19.81 (pPD: ZnCl2 = 1:1—blue) | 365 | 400–670 (two peaks located at 440, and 580) | (0.3301, 0.3367) | 5606 | 89 | NR | PVP | white | facile preparation of single metal-doped CDs with color-tunable properties | [120] | |
| phthalic acid and piperazine | microwave-assisted method | 20.5 (in solid-state form and in aqueous solution) | 450 | 520 | (0.25, 0.32) CDs only; (0.33, 0.33) CDs + (Sr, Ca)AlSiN3:Eu | 11,229 CDs only; 5618 CDs + (Sr, Ca)AlSiN3:Eu | 62.5 CDs only; 88 CDs + (Sr, Ca)AlSiN3:Eu | 87.7 CDs only; 64.4 CDs + (Sr, Ca)AlSiN3:Eu | silicone | bluish-white, pure white | fluorescent CDs in solid state form (using phthalic acid and piperazine as precursors) | [176] | |
| o-PD, dopamine | hydrothermal method | 33.96 | 365 | 560–780 centered at 745 (red emission); broad band with three peaks at 400, 490, 600, 740 (multicolor white emission) | (0.68, 0.31) (red emission); (0.34, 0.37) (white emission) | 1000 (red emission); 4957 (white emission) | 71.8 (red emission); 81.9 (white emission) | NR | PMMA | red, white | preparation of red-emitting CDs, quenching in the presence of Fe3+; the CDs can be used as Fe3+ detectors in living cells | [177] | |
| citric acid, urea; solvent: DMF | pyrolysis | 25.0 | 380 | 450−750 | (0.35, 0.36) at 20 °C; (0.32, 0.23) at 80 °C | 4075 | 93.2 | 14.8 | polystyrene (PS) | warm and cool white | combination of blue and orange emissive CDs for WLEDs; temperature-dependent emission performance (emission spectrum turns from white (400−730 nm) at 20 °C to blue (∼440 nm) at 80 °C | [178] | |
| aluminum glycine (Al-Gly) | one-step decomposition route | 15.6 λexc: 400; 14.5 λexc: 450 | 400, or 450, or 465 | broad band with peak between 560 and 580 | (0.3466, 0.3493) λexc: 400; (0.3484, 0.3578 λexc: 450; (0.3553, 0.3547) λexc: 465 | 4935 λexc: 400; 4898 λexc: 450; 4637 λexc: 465 | 66.9 λexc: 400; 79.3 λexc: 450; 80.6 λexc: 465 | 1.0 λexc: 400; 6.9 λexc: 450; 10.4 λexc: 465 | AlOOH | white | preparation of CD-doped boehmite composite (CDs@AlOOH) alleviates the self-quenching effect | [179] | |
| urea | pyrolysis | 25 (fluorescence); 6 (phosphorescence) | 380 | 380–780 (peaks at 450, 570) | (0.35, 0.39) | 4935 | 85 | NR | cyanoacrylate (Super Glue) | white | blue–yellow fluorescence; phosphorescence dual emission | [180] | |
| resorcinol | solvothermal treatment | 72 (pure red); 75 (pure green) | 450 (FWHM: 18) | 522 (FWHM: 32); 615 (FWHM: 33) | (0.35, 0.33) at 20 mA | NR | 56.9 (at 20 mA) | 86.5 (at 20 mA) | PMMA | pure red, pure green, white | wide color gamut CD-based LEDs for backlight displays (color gamut: 110% NTSC); high color purity narrow bandwidth emission (30 nm) triangular CDs | [12] | |
| citric acid, urea | microwave-assisted heating method | 11 | 450 | broad band (peak centered between 540 and 570) | (0.22, 0.23) volume ratio CDs@MMT: epoxy = 4:3; (0.36, 0.38) volume ratio CDs@MMT: epoxy = 5:3; (0.46, 0.49) volume ratio CDs@MMT: epoxy = 8:3 | 53131 volume ratio CDs@MMT: epoxy = 4:3; 4598 volume ratio CDs@MMT: epoxy = 5:3; 3232 volume ratio CDs@MMT: epoxy = 8:3 | NR | NR | montmorillonite (MMT) clays, epoxy-silicone resin | bluish-white, yellowish | preparation of green emissive CDs@montmorillonite (CDs@MMT) composites | [181] | |
| citric acid, L-cysteine (CYS), KCl | one-pot microwave heating method | 65 | 460 | broad band (peak at 550) | (0.29, 0.38) 63.6% CD concentration; (0.32, 0.42) 70% CD concentration | NR | NR | 97.8 (63.6% CD concentration); 93.9 (70% CD concentration) | PDMS | from cool to warm white | enhancement of yellow CDs fluorescence by AIE | [101] | |
| 3,5-diaminobenzoic acid (DABA); 3,4-DABA; phosphoric acid—not blue CDs | one-pot solvothermal method | B-CDs, G-CDs and 29.2 (blue); 69.2 (green); 24.8 (red) | 365 | 400–700 | (0.2963, 0.3225), | 7452 | NR | NR | transparent silicone resin | cool white | preparation of high-emitting RGB-CDs with excitation-independent; fabrication of WLEDs by multicolor RGB-CDs | [182] | |
| citric acid, various hydroxyl-containing amino compounds | microwave-assisted heating method | from 56.9 to 87.0 | 405 | 472, 503, 527, 578, 629, 698 | (0.332, 0.335) neutral WLED; (0.430, 0.405) warm WLED | 5475.4 neutral WLED; 3058.7 warm WLED | 96.6 neutral WLED; 96.4 warm WLED | 46.8 neutral WLED; 41.2 warm WLED | epoxy resin | multicolor; warm and neutral white | preparation of tunable fluorescent CDs over the whole visible region; warm and neutral WLEDs are produced by coating cyan- and red-emitting CD layers on 405 nm LED chips | [13] | |
| expanded polystyrene (WEPS), dichloromethane (DCE), HNO3 | one-step solvothermal method | 5.2 (white solid-state CDs); 3.4 (yellow solid-state CDs); 3.1 (orange solid-state CDs) | 365 | broad band (peaks at 440, 550, 730) | (0.34, 0.39) white | 5199 (white) | 80.0 (white) | NR | PDMS | yellow, orange, red, warm-white, | preparation of CDs based on WEPS as the precursor to fabricate LEDs | [79] | |
| 2020 | 2,7- dihydroxynaphthalene (C10H6(OH)2); N, N-dimethylformamide (DMF, C3H7NO); solvent: ethanol | solvothermal method | 26.03 | 360 | 480–680 | (0.41, 0.39) | 3330 | 91 | NR | silane coupling agent (KH-792) | warm white | preparation of wide-spectrum orange-emitting CDs; KH-792 suppresses quenching | [97] |
| citric acid, EDA | pyrolysis | 56.6 (CDs/ PVP); 79.2 Ag/CDs/PVP | 380 | 435 | (0.156, 0.110) | NR | NR | 39.2 | PVP, PMMA photonic crystal; AgNO3 (for “islands” Ag film) | blue | blue CDs embedded in PVP and coupled with “island” Ag film result in a fluorescence enhancement | [139] | |
| citric acid, safranine T (ST) | one-step hydrothermal methodology | 15.2 (liquid); 39.9 (solid) | 365 | 599 (red); ~450, ~510, ~590, | (0.62, 0.36) red CDs; (0.33, 0.34) white CDs (with BMA:Eu2+ and BaSrSi:Eu2+) | 5347 (white) | 81 (white) | 19.11 (white) | hardener (not specified) | red, white | red emissive host−guest CDs using citric acid (precursor and host) and ST (guest) as precursors; vitamin B12 quenches the fluorescence of CDs | [183] | |
| o-PD, NH3 | hydrothermal method | NR | 395 | broad band with two peaks: 448 (zinc borate), 553 (CDs) | from (0.2366, 0.2550) to (0.4563, 0.5089) | NR | NR | NR | zinc borate matrix | blue, yellow, white | preparation of zinc borate/yellow N-doped CDs composite for WLEDs | [99] | |
| caramelized sugar (sucrose), | microwave-assisted heating method | ~5 | 460 (blue LED), 520 (green LED) | broad yellow emission at 582 (blue exc.); broad red emission at ~630 (green exc.) | (0.31, 0.32) blue exc.; (0.4, 054) green exc. | NR | NR | NR | caramelized sugar | white, yellow | caramelized sugar CDs for color conversion applications | [150] | |
| L-tyrosine (for blue CDs), o-PD (for green CDs), L-tyrosine/o-PD mixture (for orange-red CDs) | hydrothermal method | 8.6 (blue CDs); 12.6 (green CDs); 20.9 (orange-red CDs) | 370 | 450 (blue); 545 (green); 580 (orange); 380−750 (white) | (0.23, 0.26) blue LED; (0.34, 0.43) green LED; (0.41, 0.42) orange LED; (0.30, 0.33) white LED | 6293 (white) | 83 (white) | NR | PVA | blue, green, orange, cool white | tunable emission colors in CDs by changing the molar ratio of suitable carbon sources | [146] | |
| 1,2,4-triaminobenzene dihydrochloride, urea; (solvents: water, ethanol, tetrahydrofuran (THF), ethyl acetate (EtOAc), acetone) | solvothermal method | 42 (blue CDs—acetone); 70 (green CDs—EtOAc); 79 (yellow CDs—THF); 64 (orange CDs—ethanol); 55 (red CDs—water) | UV (λ NR) | NR | (0.33, 0.45) white LED | 5440 (white LED) | NR | NR | PVA | green, yellow, orange, white | multicolor emissive CDs based on solvent-controlled and solvent-responsive approaches | [122] | |
| ammonium citrate tribasic, formamide, glycerol, ethylene glycol | microwave-assisted method (at atmospheric pressure) | 37.4 | 365 | broad band (Ce:YAG/CDs @MPS) | (0.344, 0.333) (Ce:YAG/CDs@MPS) | 4962 (Ce: YAG/CDs @MPS) | 90.9 (Ce: YAG/ CDs @MPS) | 67.5 (Ce: YAG/ CDs@ MPS) | mesoporous silica (MPS), epoxy AB glue | warm white | synthesis of red CDs; glycerol and formamide promote the carbonization precursor and enhance the crystallinity | [184] | |
| citric acid, EDA | electrostatic adsorption between positively charged QDs and negatively charged CDs | 35 | 365 | 450–700 | (0.32, 0.33) cool white; (0.37, 0.39) warm white | 6338 (cool white); 4248 (warm white) | 91 (warm white) | 16.8 (warm white) | PMMA | cool and warm white | WLED prepared by core–shell structure nanocomposites based on Ag-In-S /ZnS@SiO2 QDs (AIS@SiO2) and CDs | [185] | |
| citric acid, urea; (solvent: water) | microwave-assisted heating method | 62 | 450 | broad band (peak at 540) | (0.29, 0.33) | 7557 | NR | 42 | epoxy silicone resin | cool white | a composite phosphor (CDs@g-C3N4) comprising carbon dots (CDs) and graphitic carbon nitride (g-C3N4) is prepared in water on a large scale | [186] | |
| maleic acid, m-phenylenediamine (m-PD) | room-temperature synthesis | 42 (blue CDs); 35 (green CDs) | 365 | 460 (blue LED); 520 (green LED) | (0.1993, 0.2423) blue LED; (0.3199, 0.5027) green LED; (at 80 mA) | NR | NR | 16.6 (blue LED); 17.1 (green LED); (at 80 mA) | ethanol dropped on an optical lens | blue, green | no external energy or irradiations, reactants or high temperature are required to prepare CDs | [187] | |
| phosphoric acid, urea | one-step process involving polymerization, deamination, and dehydration reactions | ~41 (white fluorescence) 23 (green phosphorescence) | 370 | 380–580 | (0.268, 0.346) | 8756 | 85.3 | 18.7 (at 20 mA) | silicone | cool white | carbonized polymer dots (CPDs) white light with dual components consisting of simultaneous fluorescence (S1→S0) and phosphorescence (T1→S0) | [125] | |
| citric acid; 1-(2-pyridylazo)-2-naphthol (PAN); solvents: DMSO, DMF, ethanol, (THF), dichloromethane (DCM) | one-step solvothermal method | 46.5 (purple CDs); 32.3 (blue CDs); 31.6 (red CDs) | 365 | 375, 455, 570 | (0.29, 0.31) in epoxy resin | NR | NR | NR | epoxy resin; PMMA | purple, blue, red, white | multicolor emission CDs synthesized by one-step solvothermal treatment using citric acid and PAN with concentration-tunable fluorescence and solvent-affected aggregation states | [188] | |
| citric acid, nitric acid, 1-octadecene, oleylamine (OLA), methanol, nitrogen | microemulsion process | NR | 360 | 450, 500, 590, 690 (D65) | (0.35, 0.37) D50; (0.32, 0.33) D65 | 6354 (D65; at 120 mA) | 95.3 (max) | 17.68 (D65; at 20 mA); 7.93 (D65; at 120 mA) | PMMA/toluene solution | D50, D65 white | synthesis of a sun-like light source (D50, D65) with tri-chromatic broad spectra (435-nm CDs), 695-nm perovskite QDs, and dual peak 510- and 590-nm Ag-doped InP QDs | [189] | |
| citric acid, EDA (blue CDs); citric acid, urea (green CDs) | pyrolysis (blue CDs); microwave-assisted heating method (green CDs) | >60 (blue, green) | 390 | 450–700 | (0.3514, 0.3715) CD@SiO2 + red CdTe@CaCO3 | 4850 (CD@ SiO2 + red CdTe@ CaCO3) | 89.1 (CD@SiO2 + red CdTe@ CaCO3) | NR | spherical SiO2 matrix, cetyltrimethylammonium bromide (CTAB) | blue, green, red (CdTe@ CaCO3), white | blue/green-emitting N-doped carbon dots embedded into silica nanospheres (CD@SiO2) with spherical morphology | [190] | |
| 2021 | potassium bisulfate, acetic acid, hydrochloric acid; m-PD | solvothermal method | NR | 365 (for green and yellow LEDs); 460 (for white LEDs) | 500 (green); 550 (yellow); 560 (white) | (0.29, 0.49) green; (0.39, 0.49) yellow; (0.26, 0.27) white | NR | NR | NR | PVA | green, yellow, white | acid catalyst induces a fluorescence red shift and improved the QY of green emissive CDs. | [191] |
| citric acid, EDA | hydrothermal method | 4.57 (blue CDs only) 40.1 (CS composites) | 365 | 445 | (0.15, 0.13) at 35 mA | NR | 79.1 | NR | silicone | deep blue | synthesis of CD-silica (SiO2) spheres composites (CS composites) with 10-fold fluorescence enhancement | [141] | |
| 1,4-diaminonaphthalene (solvents: octane, toluene, ethanol, acetone, DMF) | solvothermal method | 26.4 (in octane); 17.9 (in acetone); 13.9 (in toluene); 12.1 (in ethanol); 7.5 (in DMF) | 365 | 427–679 (peak at 618) | (0.4175, 0.2936) | NR | NR | NR | epoxy resin | red | preparation of red CDs using different solvents for carbonization; CDs prepared in octane have the high QY and fluorescence intensity | [192] | |
| citric acid, urea (solvent: DMF) | solvothermal method | 22.7 (exc. 340); 28.1 (exc. 360); 24.0 (exc. 380); 23.8 (exc. 400); 15.6 (exc. 420); 14.2 (exc. 440); 10.0 (exc. 460); 9.8 (exc. 480) | 460 | broad band (up to 600) centered at 540 (CDs only); two large peaks at 470 and 535 (CDs + Ce:YAG) | (0.33, 0,45) CDs + Ce:YAG (CCT: 5602) | 5602–9242 | NR | NR | PVA (MW: 1500) | cool and warm white | WLEDs fabricated with an adjustable CCT by combining Ce:YAG and green-emitting CD films | [193] | |
| dehydroabietic acid, ethanolamine | one-pot hydrothermal reaction | 10 | 450 | 500–675 | (0.3304, 0.3055) | 5608 | 88.6. | NR | epoxy resin | white | steric hindrance is exploited to prepare biomass-based solid-state emissive CDs with high yield | [151] | |
| 1,6-dihydroxynaphthalene (1,6-DHN); L-asparagine (L-Asn) | solvent-free carbonization method | NR | 365 | 451, 518 | (0.32, 0.31) | NR | NR | NR | polyvinyl butyral (PVB) | pure white | white light-emitting CDs prepared through a solvent-free method | [194] | |
| polyethyleneimine (PEI), phosphoric acid, ethanol | solvothermal method | 4.4 (in aqueous solution or in powder form) | 365 | broad band | (0.33, 0.33) | 3319.6 | 76.3 | NR | AB glue | pure white | novel kind of self-quenching-resistant N,P-doped CDs; blue-emitting in solid state form and mixed with a red emitting Eu-MOF powder (CD@Eu-MOF) | [195] | |
| o-PD, ammonia water (NH3·H2O, 25 wt%), | hydrothermal synthesis method | NR | 365 | broad band | from (0.2798, 0.2916) to (0.3246, 0.3305) (ZBH:9%Tm3+/yNCDs) | NR | NR | NR | ZBH:9% Tm3+ | white | incorporation of yellow N-doped CDs in a Tm3+-doped zinc borate (4ZnO·B2O3· H2O, ZBH) with a flake-like morphology | [196] | |
| Pithecellobium dulce leaves (solvent: DMF) | muffle furnace carbonization method | NR | 365 | 530 | (0.32, 0.43) PVDF/CDs film dried at room temperature (white); (0.33, 0.42) PVDF/CDs film dried at 65 °C (greenish-white) | 5863 (white); 5576 (greenish-white) | NR | NR | polyvinylidene fluoride (PVDF) | white, greenish white | films doped with plant derived photoluminescent CDs | [197] | |
| EDA, trimethylolpropane tri(cyclic carbonate)ether (TPTE) (synthesized from the reaction of CO2 and trimethylolpropane triglycidyl ether in ethanol) | solvothermal treatment | 46.2 (in solution) 11.3 (as a solid) | 365 (orange); 420 (white, yellow); 460 (red) | 620 (orange); 605 (yellow); 675 (red); ~610 (white) | (0.582, 0.413) orange; 0.549, 0.443) yellow; (0.545, 0.309) red; (0.376, 0.288) white | 3161 (white) | 85 (white) | NR | concentrated CPDs optical lens (no encapsulant) | orange, yellow, red, warm white | new type of carbon dioxide (CO2) derived CPD; SSF due to the self-passivation of poly(hydroxyurethane) chains on the surface of the carbon core | [198] | |
| citric acid, urea | microwave-assisted heating method (700 W, 5 min) | 5.78 | 460 | broad band | (0.42, 0.51) | NR | NR | NR | zeolitic imidazolate framework 8 (ZIF-8), epoxy resin | white | green-emitting CDs and red-emitting rhodamine B (RhB) molecules, encapsulated into porous ZIF-8 to obtain a yellow-emitting composite phosphor | [199] | |
| avocado peel (CPDs-P); sarcocarp (CPDs-S) | hydrothermal method | 9.56 (CPDs-P); 8.97 (CPDs-S) | 365 | broad band centered at 600 (warm white); broad band centered at 485 (cool white) | (0.38, 0.39) warm white; (0.29, 0.34) cool white | 4088 (cool white) | 90.47 (warm white); 84.54 (cool white) | NR | epoxy resin | warm and cool white | blue-emitting CPDs-P (peel) and blue–green-emitting CPDs-S (sarcocarp) prepared using the peel and sarcocarp of avocado, respectively | [152] | |
| 2-aminoterephthalic acid (ATA), polyethylene glycol, orthophosphoric acid (H3PO4). | microwave-assisted pyrolysis | 67 | 365 | broad band centered at 578 | (0.35, 0.33) | 5246 | 92 | NR | PVA | pure white | synthesis of multifunctional CDs for WLEDs, ultrasensitive to the nitroaromatic explosive picric acid | [200] | |
| citric acid, octadecene, hexadecyl amine (HDA) (solvent: chloroform) | open-air atmosphere carbonization synthesis | 5–13 (in colloidal state); 16 (in solid state) | 350 | 360–700 (peak: 430, FWHM: ~154) | (0.31, 0.33) | 6412 | ~ 96 | NR | PDMS | pure white | ecofriendly open-air atmosphere synthesis of highly luminescent CPDs for high CRI WLEDs | [129] | |
| ammonium citrate (red CDs); AEAPMS (+red CDs) (white CDs); solvent: DMF | pyrolysis | NR | 395 | broad band (peaks: 540, 645) | (0.357, 0.359) | 4271 | 89 | 1.3 | cellulose acetate | white | novel solvent engineering by controlling the dilution ratios between the solvent (DMF) and pristine red CDs solution | [201] | |
| citric acid; 1,4,7,10-tetraazacyclododecane (cyclen) | microwave-assisted synthesis method | 48 | 450 | 545 (CDs only); 545, 595 (CDs + (Sr, Ca) AlSiN3: Eu) | (0.30, 0.34) (CDs only); (0.33, 0.32) (CDs + (Sr, Ca) AlSiN3:Eu) | 7001 (CDs only); 5557 (CDs + (Sr, Ca) AlSiN3: Eu) | 57.7 (CDs only); 82.1 (CDs + (Sr, Ca) AlSiN3: Eu) | 25.5 (CDs only); 31.0 (CDs + (Sr, Ca) AlSiN3: Eu) | silicone | white | bright yellow fluorescence from cyclen-based CDs powder | [81] | |
| citric acid, urea, oleic acid | microwave-assisted solvothermal reaction | 0.99 (CDs embedded in PVA) | 420 | broad band (peak: 510–530) | (0.39, 0.46) | 4105 | NR | NR | PVA | white | PVA polymer encapsulated with N-doped CDs sustaining the emission in its solid state | [202] | |
| spinach after soaking in ethanol/water (4:1 vol ratio) | one-step hydrothermal method | NR | 395 | 447, 677 | (0.185, 0.104) blue; (0.352, 0.339) white; (0.433, 0.375) orange | NR | 65–82 | 12.4–19.5 | Mg(OH)2 nanosheets, ethylene vinyl acetate copolymer (EVA) | blue, white, orange | blue/red CDs@Mg(OH)2 anti-self-quenching luminescent composites for plant growth applications | [153] | |
| maleic acid, APTES | one-step solvothermal method | 34.06 (green CDs); 38.07 (yellow CDs); 20.3 (orange CDs) | 365 | 475 (blue LED); 460, 510 (yellow LED); 560 (orange LED) | (0.22, 0.41) green LED; (0.38, 0.47) yellow LED; (0.50, 0.43) orange LED | 9885 (blue LED); 6772 (yellow LED); 2379 (orange LED) | 70.2 (blue LED); 72.6 (yellow LED); 74.5 (orange LED) | NR | epoxy resin | blue, yellow, orange | SSF Si-doped CDs prepared using maleic acid and APTES as precursors | [203] | |
| citric acid, thiourea, ammonium fluoride (solvent: DMF) | solvothermal method | 22.64 | 525 (for red LEDs); UV (λ NR) (for white LEDs) | 695 (red LEDs); 425, 530, 650 (white LEDs) | (0.72, 0.28) red LEDs; (0.36, 0.36) white LEDs | 1000 (red LEDs); 4705 (white LEDs) | 57.7 (red LEDs); 93.8 (white LEDs) | NR | epoxy resin | red, white | preparation of highly stable near-IR CDs (emission at 714 nm) using citric acid as the carbon source, thiourea and ammonium fluoride as the dopant source | [123] | |
| 2-amino-1-naphthol, EDA (solvent: ethanol) | one-pot hydrothermal method | NR | 452 | 550 (yellow–green CDs), 628 (red Cd2+-based QDs) | (0.3669, 0.3671) | 4329 | 95.1 | NR | BaSO4, silica gel | white | improvement of yellow–green CDs by encapsulation in BaSO4 | [204] | |
| cis-butenedioic acid (C-BA), urea | one-step solvothermal method | 35.12 | 460 | broad band centered at 580 | (0.3341, 0.3075) | 5388 | 86.9 | 15.12 | Ca(OH)2, transparent silicone (YD65-5A), anhydride curing agent (YD65-5B) | pure white | multicolor CDs obtained from cis-butenedioic acid (C-BA) and urea; solid-state luminescent CD obtained by adding alkaline Ca(OH)2 | [205] | |
| citric acid, EDA, Ln(NO3)3∙6H2O | solvothermal and hydrothermal methods | NR | 275 | narrow bands: ~420, ~475, ~530, ~580, ~610, ~680 | (0.337.0.339) neutral white | 5319 (neutral white) | 93 (neutral white) | 322 (warm white) | NR | cool, neutral, warm white | rare-earth single-atom-based NaGdF4:Tb3+/ Eu3+@CDs:N/ Eu3+ composite with tunable full-color luminescence | [206] | |
| citric acid; (3-aminopropyl)triethoxysilane; N-[3-(trimethoxysilyl)propyl]ethylenediamine (silanes molar ratio = 7:3) | one-step solvothermal method | ~20 | 365, 450 | 550 (exc. 365, yellow emission); 575 (exc. 450, white emission) | (0.41, 0.52) yellow; (0.33, 0.31) white | 5774 (white) | 81.6 (white) | NR | no encapsulation | yellow, white | double silane-functionalized carbon dots (DSi-CDs) with emission at longer wavelengths | [207] | |
| citric acid, tris(hydroxymethyl)methyl aminomethane | microwave-mediated heating; liquid-liquid diffusion-assisted crystallization method | 92.7 (CDs only) | 380 | 430, 600 | (0.3580, 0.3611) | 4255 | 93.6 | 12.64 | NR | white | assembly of blue-emitting CDs and yellow-emitting Cs2InCl5·H2O: Sb3+ metal halide crystals for WLEDs (CDs@Cs2InCl5·H2O: Sb3+) | [208] | |
| gallic acid, o-PD (solvents: methanol, ethanol, acetone, octane, toluene, THF, DMF) | solvothermal method | 10 (exc. 340, blue CDs); 11 (exc. 500, green CDs); 23 (exc. 500, red CDs) | purple (λ NR) | 425 (blue), 510 (green), 585 (red); broad band (white) | (0.16, 0.12) blue; (0.27, 0.45) green; (0.53, 0.38) red; (0.32, 0.34) white | NR | NR | NR | epoxy resin | blue, green, red, white | blue, green, and red CDs prepared using gallic acid as the raw material | [209] | |
| tartaric acid, triammonium citrate | one-step solvothermal method | 43.6 (blue CDs); 41.2 (green CDs); 44.1 (red CDs) | 365 | 465, 530, 570 | (0.17, 0.15) blue; (0.31, 0.53) green; (0.58, 0.40) red; (0.34, 0.35) white | 5336 (white) | 83.1 (white) | NR | KH-792 silane coupling agent | multicolors, warm white | synthesis of multicolor fluorescent CDs with adjustable emission wavelength and high QY, using ecofriendly precursors | [210] | |
| 2,3-diaminopyridine (solvents: NaOH, pure water, HCl) | pH-controlled hydrothermal method | 8.4 (violet); 9.3 (green); 8.3 (orange) | 365 | ~400, ~540, ~600 | (0.22, 0.17) bluish; (0.30, 0.33) cool white; (0.41, 0.37) warm white | 7466 (cool white); 3265 (warm white) | 78 (cool white); 90 (warm white) | NR | starch, AB glue | bluish, cool white, warm white | single precursor to synthesize colorful CDs in different pH conditions | [211] | |
| citric acid, urea | microwave-assisted pyrolysis | 15 (exc. 395); 14 (exc. 410) | 400 | ~500, ~610 | (0.321, 0.367) cool white; (0.390, 0.455) warm white | 5796 (cool white); 4228 (warm white) | 89 (cool white); 84 (warm white) | 13.2 | d-U(600) di-ureasil hybrid matrix | cool and warm white | assembly of a commercial LED and flexible films of a di-ureasil hybrid comprising a mixture of a cyan component originating from CDs and a red one resulting from the Eu(tta)3(bpyO2) complex | [212] | |
| citric acid, urea, thiourea | microwave-assisted solvent-free synthesis method | NR | 410, 455 | 605 | NR | NR | NR | NR | NR | NR | synthesis of graphitized N-doped and S,N-doped CDs | [213] | |
| Star Jasmine leaves (Trachelospermum jasminoides) | solvothermal treatment | 54.8 (blue CDs); 15 (green CDs); 19.8 (red CDs) | NR | 466 (blue); 521 (green); 625 (red) | (0.15, 0.18) blue; (0.26, 0.60) green; (0.58, 0.31) red; (0.36, 0.32) white | 4283 (white) | NR | NR | polyurethane | blue, green, red, white | synthesis of RGB CDs with high optical tuning using sustainable green precursors | [214] | |
| 2022 | o-PD, phenylalanine | one-pot solvothermal method | 52.34 (blue CDs); 65.67 (green CDs); 12.87 (red CDs) | 365 | 400–750 (peaks: 400, 500) | (0.30, 0.35) | 7127 | 86 | NR | epoxy resin, tetraethylenepentamine | pure white | a RGB-multicolor CDs prepared by adjusting the type of reaction solvent | [215] |
| EDA, nitrogen | hydrothermal method | NR | 395 | broad band (peaks: 450, 475, 580, 610, 700) | (0.18, 0.16) (KLM: Eu3+: CDs = 0:1, blue LED); (0.19, 0.21) (KLM: Eu3+: CDs = 1:2, cyan LED); (0.21, 0.22) (KLM: Eu3+: CDs = 1:1, cool white LED); (0.29, 0.25) (KLM: Eu3+: CDs = 2:1, neutral white LED); (0.38, 0.28) (KLM: Eu3+: CDs = 10:1, warm white LED); (0.56, 0.32) (KLM: Eu3+: CDs = 1:0, red LED); | NR | NR | NR | silica sol | blue, cyan, cool, neutral, and warm white, red | fabrication of polychromatic nanoplatform KLa(MoO4)2: Eu3+@CDs (KLM: Eu3+ @CDs) by encapsulating CDs on the surface of KLM: Eu3+ with silicon shell | [216] | |
| citric acid, Nile blue A (NBA) | one-pot solvothermal method | 64 (blue CDs); 57 (yellow CDs); 51 (red CDs) | 395 | 475, ~550, 625 | (0.15, 0.21) blue LED; (0.43, 0.54) yellow LED; (0.46, 0.16) red LED; (0.31, 0.29) white LED | 5643 (white) | 87.2 (white) | NR | epoxy resin, curing agent EDA, | blue, yellow, red, white | synthesis of tunable multicolor emission CDs, covering the entire visible spectrum | [217] | |
| ethanol, H2SO4 | one-step carbonization process | 14.88 (blue CDs); 4.85 (cyan CDs); 17.54 (yellow CDs) | UV (λ NR) | ~450, ~475, ~590, | (0.17, 0.21) blue LED; (0.24, 0.33) cyan LED; (0.50, 0.47) yellow LED; (0.37, 0.39) white LED | ~4500 | 87.8 (white) | NR | epoxy resin | blue, cyan, yellow, white | white light-emitting CDs using ethanol as the carbon source and H2SO4 as the carbonizing agent with three emission centers (blue, cyan, yellow) | [218] | |
| phloroglucinol, urea | one-step microwave method | 48.2 (blue CDs); 26.0 (green CDs); 18.5 (yellow CDs); 13.7 (orange); 5.7 (red) | UV (λ NR) | ~450 (blue); ~520 (green) ~580 (yellow); ~600 (orange); ~670 (red); 430–650 (white) | (0.20, 0.18) blue LED; (0.31, 0.44) green LED; (0.48, 0.50) yellow LED; (0.52, 0.45) orange LED; (0.68, 0.31) red LED; (0.29, 0.31) cool white LED; (0.32, 0.36) pure white LED; (0.39, 0.40) warm white LED | 8588 (cool white LED); 6104 (pure white LED); 3937 (warm white LED) | 88 (cool white LED); 82 (pure white LED); 80 (warm white LED) | NR | optical sealant OE6250 A, and B on optical lens | blue, green, yellow, orange, red, cool, pure, and warm white | self-quenching-resistant solid-state fluorescent CDs displaying tunable full-color emission | [219] | |
| 2-amino-1-naphthol; EDA (solvent: ethanol) | pyrolysis | NR | 460 | ~545 ~625 | (0.332, 0.336) | 5498 | 93.9 | 32.19 (before ageing) | starch, epoxy resin | white | CD synthesized by adsorbing yellow–green CDs to starch particles and mixed with CdZnSeS/ZnS phosphor | [220] | |
| Polyethylene glycol (Mw = 4000) (PEG-4K); 1,2-diaminobenzene | hydrothermal method | 62.5 | 455 | 400–700 (peak at 541) | (0.3003, 0.3731) | 5117 | 80.9 | 40.6 | mesoporous silica nanosphere-stellate (MSNS), silicone resin | pure white | CD@monodisperse mesoporous silica nanosphere-stellate (CD@MSNS) hybrid phosphor with highly concentrated emitting centers | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, D.; Macaluso, R.; Crupi, I.; Mosca, M. Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review. Materials 2022, 15, 5450. https://doi.org/10.3390/ma15155450
Trapani D, Macaluso R, Crupi I, Mosca M. Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review. Materials. 2022; 15(15):5450. https://doi.org/10.3390/ma15155450
Chicago/Turabian StyleTrapani, Danilo, Roberto Macaluso, Isodiana Crupi, and Mauro Mosca. 2022. "Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review" Materials 15, no. 15: 5450. https://doi.org/10.3390/ma15155450
APA StyleTrapani, D., Macaluso, R., Crupi, I., & Mosca, M. (2022). Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review. Materials, 15(15), 5450. https://doi.org/10.3390/ma15155450







