A Clinical Risk Assessment of a 3D-Printed Patient-Specific Scaffold by Failure Modes and Effects Analysis
Abstract
:1. Introduction
- The Therapeutic Goods Act (1989)
- The Therapeutic Goods (Medical Devices) Regulations 2002
- The Therapeutic Goods Regulations 1990
2. Materials and Methods
2.1. Survey Design
2.2. Survey Validity and Reliability Assessments
- Not relevant
- Somewhat relevant
- Relevant
- Very relevant
2.3. Participant Selection and Survey Distribution
2.4. Data Analysis
3. Results
3.1. Survey Validity and Reliability
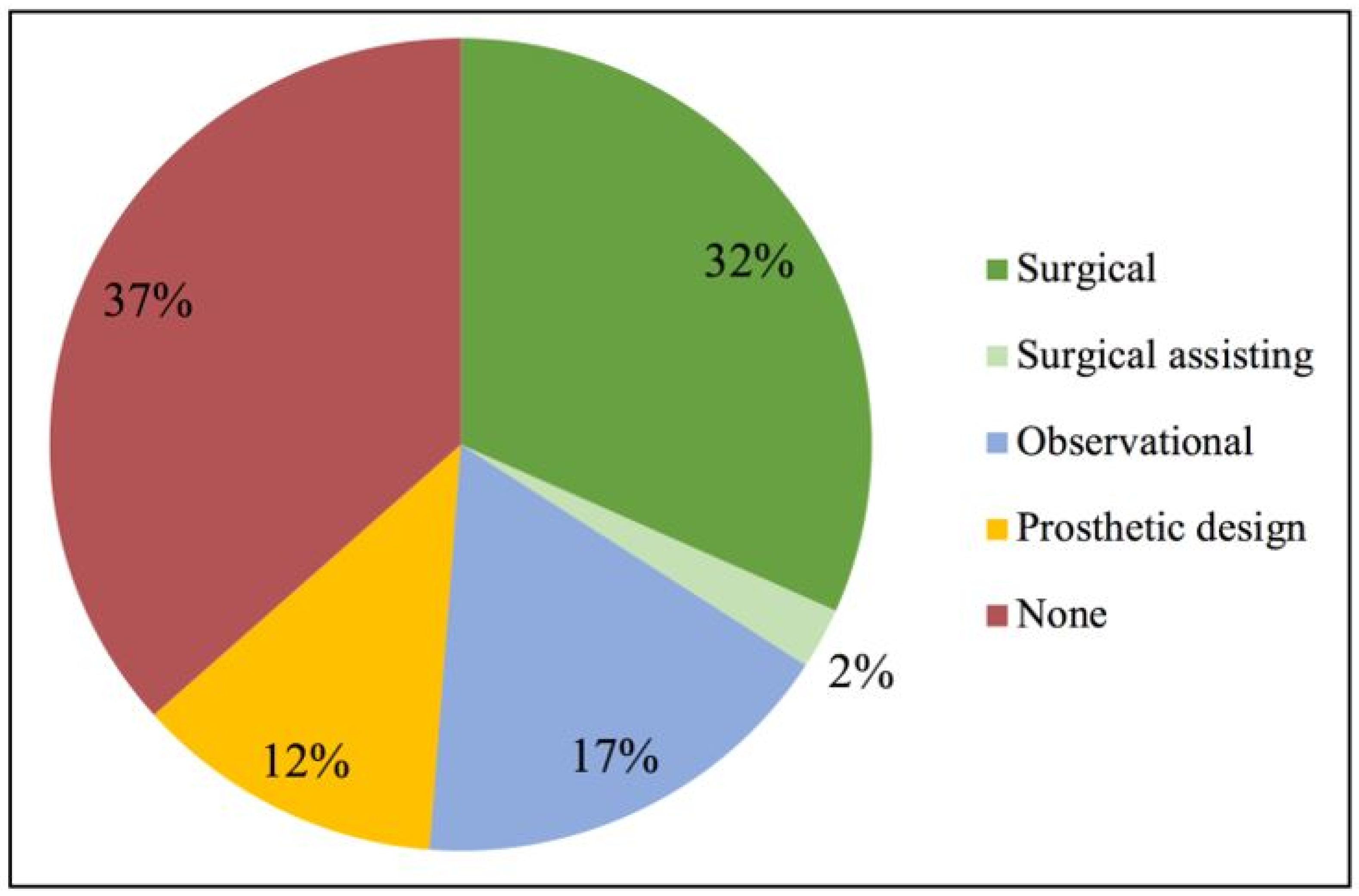
3.2. Demographics and Self-Rated Confidence in Using Scaffold Technologies
3.3. Satisfaction with Current Titanium-Based Scaffolds
3.4. Perceived Performance and Advantages of 3D-Printed Biodegradable Scaffolds and Common Post-Operative Complications with the Use of Scaffolds
3.5. FMEA Results
3.6. High-Priority Failure Modes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, P.; Duvina, M.; Barbato, L.; Biondi, E.; Nuti, N.; Brancato, L.; Delle Rose, G. Bone regeneration in dentistry. Clin. Cases Miner. Bone Metab. 2011, 8, 24. [Google Scholar] [PubMed]
- Kassebaum, N.J.; Smith, A.G.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global burden of severe tooth loss: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 20S–28S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.L.; Boyapati, L. Periodontal regeneration. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Wiley: Hoboken, NJ, USA, 2007; pp. 239–264. [Google Scholar]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Rios, H.F.; Cochran, D.L. Emerging regenerative approaches for periodontal reconstruction: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S134–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Kumar, S.; Ivanovski, S. Resorbable additively manufactured scaffold imparts dimensional stability to extraskeletally regenerated bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.-H.; Goh, B.-T.; Lim, J. Three-dimensional printed polycaprolactone scaffolds for bone regeneration success and future perspective. Tissue Eng. Part A 2019, 25, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Administration, T.G. Guidance on Conducting Clinical Trials in Australia Using ‘Unapproved’ Therapeutic Goods. 2020. Available online: https://www.tga.gov.au/resource/australian-clinical-trial-handbook (accessed on 21 February 2021).
- ISO 14971:2019 Medical Devices—Application of Risk Management to Medical Devices. 2019. Available online: https://www.iso.org/standard/72704.html (accessed on 1 July 2021).
- Cristea, G.; Constantinescu, D.M. A comparative critical study between FMEA and FTA risk analysis methods. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012046. [Google Scholar]
- Liu, H.C.; Zhang, L.J.; Ping, Y.J.; Wang, L. Failure mode and effects analysis for proactive healthcare risk evaluation: A systematic literature review. J. Eval. Clin. Pract. 2020, 26, 1320–1337. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shuai, M.; Wang, Z.; Li, P. Use-related risk analysis for medical devices based on improved FMEA. Work 2012, 41, 5860–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulino, M.F.; Patel, T.; Fisher, S.P. The application of failure modes and effects analysis methodology to intrathecal drug delivery for pain management. Neuromodulation 2017, 20, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Standardisation. IOf. IEC 60812:2018; Failure Modes and Effects Analysis (FMEA and FMECA). BSI Standards Limited: Brussels, Belgium, 2018.
- Polit, D.F.; Beck, C.T. The content validity index: Are you sure you know what’s being reported? Critique and recommendations. Res. Nurs. Health 2006, 29, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, R.E.; Mikulak, R.J.; Beauregard, M.R. FMEA; Taylor & Francis Group: New York, NY, USA, 2009. [Google Scholar]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Miccoli, G.; Reda, R.; Mazzoni, A.; Di Nardo, D.; Testarelli, L. Sulcus fluid volume, IL-6, and Il-1b concentrations in periodontal and peri-implant tissues comparing machined and laser-microtextured collar/abutment surfaces during 12 weeks of healing: A split-mouth RCT. Clin. Oral Implants Res. 2022, 33, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V. Rasperini G. 3D-printed scaffolds and biomaterials: Review of alveolar bone augmentation and periodontal regeneration applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Medical Device Classification | Level of Potential Harm | Examples of Devices (Dental) |
|---|---|---|
| Class I | Lowest | Forceps, dental; Light, dental, polymerisation activator; |
| Class Is-sterile Class Im-measuring Class Ir-reusable | Low | Dental examination kit; Dental implant template set; |
| Class IIa | Low to Moderate | Artificial crown, custom-made, all ceramic; Surgical procedure kit, dental, non- medicated, reusable; |
| Class IIb | Moderate to High | Dental implant system; Dental implant, transgingival/intramucosal; |
| Class III | High | Dental bone matrix implant, synthetic -Q-Oss+ |
| Frequency | |
|---|---|
| Post-operative complications rated as most common in the treatment of horizontal and/or vertical bony defects Pain and discomfort (N, %) Suture dehiscence (N, %) Early graft exposure (N, %) Sire infection (N, %) Biomaterial resorption (N, %) Poor stability of the newly formed bone once scaffold is removed (N, %) | 15 (13%) 26 (23%) 25 (22%) 11 (10%) 20 (18%) 15 (13%) |
| Advantages of 3D-preinted scaffolds rated as most significant in the treatment of horizontal and/or vertical bony defects Prosthetic guided planning (N, %) High adaptability to a specific bony defect (N, %) Soft tissue adaptation (N, %) Acceleration of the surgical procedure (N, %) No need for surgical removal (N, %) | 28 (23%) 27 (22%) 21 (17%) 17 (14%) 30 (24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi Lim, P.; Huey Lim, S.; Sherilyn, M.; Fernandez-Medina, T.; Ivanovski, S.; Hosseinpour, S. A Clinical Risk Assessment of a 3D-Printed Patient-Specific Scaffold by Failure Modes and Effects Analysis. Materials 2022, 15, 5442. https://doi.org/10.3390/ma15155442
Qi Lim P, Huey Lim S, Sherilyn M, Fernandez-Medina T, Ivanovski S, Hosseinpour S. A Clinical Risk Assessment of a 3D-Printed Patient-Specific Scaffold by Failure Modes and Effects Analysis. Materials. 2022; 15(15):5442. https://doi.org/10.3390/ma15155442
Chicago/Turabian StyleQi Lim, Ping, Sue Huey Lim, Maria Sherilyn, Tulio Fernandez-Medina, Sašo Ivanovski, and Sepanta Hosseinpour. 2022. "A Clinical Risk Assessment of a 3D-Printed Patient-Specific Scaffold by Failure Modes and Effects Analysis" Materials 15, no. 15: 5442. https://doi.org/10.3390/ma15155442
APA StyleQi Lim, P., Huey Lim, S., Sherilyn, M., Fernandez-Medina, T., Ivanovski, S., & Hosseinpour, S. (2022). A Clinical Risk Assessment of a 3D-Printed Patient-Specific Scaffold by Failure Modes and Effects Analysis. Materials, 15(15), 5442. https://doi.org/10.3390/ma15155442








