Evaluation of a Method to Determine Wear Resistance of Class I Tooth Restorations during Cyclic Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wear Resistance
2.2. Fracture Toughness
2.3. Statistical Analysis
3. Results
3.1. Wear Resistance
3.2. Fracture Toughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gryst, M.E.; Mount, G.J. The use of glass ionomer in special needs patients. Aust. Dent. J. 1999, 44, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, J.H.; Croll, T.P. Glass ionomer restorative cement systems: An update. Pediatr. Dent. 2015, 37, 116–124. [Google Scholar] [PubMed]
- World Health Organization. World Health Organization Model List of Essential Medicines—22nd List, 2021; World Health Organization: Geneva, Switzerland, 2021.
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, S.S.; Gosavi, S.Y.; Alla, R.K. Local and systemic effects of unpolymerised monomers. Dent. Res. J. 2010, 7, 82–87. [Google Scholar]
- El Wakeel, A.M.; Elkassas, D.W.; Yousry, M.M. Bonding of contemporary glass ionomer cements to different tooth substrates; microshear bond strength and scanning electron microscope study. Eur. J. Dent. 2015, 9, 176–182. [Google Scholar] [CrossRef]
- Yamakami, S.A.; Ubaldini, A.L.M.; Sato, F.; Neto, A.M.; Pascotto, R.C.; Baesso, M.L. Study of the chemical interaction between a high-viscosity glass ionomer cement and dentin. J. Appl. Oral Sci. 2018, 26, e20170384. [Google Scholar] [CrossRef]
- Powers, J.M.; Farah, J.W. Technique sensitivity in bonding to enamel and dentin. Compend. Contin. Educ. Dent. 2010, 31, 1–8. [Google Scholar]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Deery, C. Atraumatic restorative techniques could reduce discomfort in children receiving dental treatment. Evid. Based Dent. 2005, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Hatibovic-Kofman, S.; Suljak, J.P.; Koch, G. Remineralization of natural carious lesions with a glass ionomer cement. Swed. Dent. J. 1997, 21, 11–17. [Google Scholar]
- Ngo, H.C.; Mount, G.; Mc Intyre, J.; Tuisuva, J.; Von Doussa, R.J. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: An in vivo study. J. Dent. 2006, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, R.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Aguilera, F.S. In vitro mechanical stimulation facilitates stress dissipation and sealing ability at the conventional glass ionomer cement-dentin interface. J. Dent. 2018, 73, 61–69. [Google Scholar] [CrossRef] [PubMed]
- De Gee, A.J.; van Duinen, R.N.; Werner, A.; Davidson, C.L. Early and long-term wear of conventional and resin-modified glass ionomers. J. Dent. Res. 1996, 75, 1613–1619. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Mechanical behavior of glass ionomer cements as a function of loading condition and mixing procedure. Dent. Mater. J. 2007, 26, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Moberg, M.; Brewster, J.; Nicholson, J.; Roberts, H. Physical property investigation of contemporary glass ionomer and resin-modified glass ionomer restorative materials. Clin. Oral Investig. 2019, 23, 1295–1308. [Google Scholar] [CrossRef]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. J. Oral Biol. Craniofac. Res. 2019, 9, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.G.; Jaiswal, J.; Murthy, R.; Pandey, R. Estimation of fluoride release from various dental materials in different media-an in vitro study. Int. J. Clin. Pediatr. Dent. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Berzins, D.W.; Abey, S.; Costache, M.C.; Wilkie, C.A.; Roberts, H.W. Resin-modified glass-ionomer setting reaction competition. J. Dent. Res. 2010, 89, 82–86. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of polyelectrolyte modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2012, 22, 2824–2833. [Google Scholar] [CrossRef]
- Souza, J.C.; Silva, J.B.; Aladim, A.; Carvalho, O.; Nascimento, R.M.; Silva, F.S.; Martinelli, A.E.; Henriques, B. Effect of Zirconia and Alumina Fillers on the Microstructure and Mechanical Strength of Dental Glass Ionomer Cements. Open Dent. J. 2016, 10, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Park, E.Y.; Kang, S. Current aspects and prospects of glass ionomer cements for clinical dentistry. Yeungnam Univ. J. Med. 2020, 37, 169–178. [Google Scholar] [CrossRef]
- Alatawi, R.A.S.; Elsayed, N.H.; Mohamed, W.S. Influence of hydroxyapatite nanoparticles on the properties of glass ionomer cement. J. Mater. Res. Technol. 2019, 8, 344–349. [Google Scholar] [CrossRef]
- Pelleg, J. Fracture. In Mechanical Properties of Ceramics; Springer Science & Business: Berlin/Heidelberg, Germany, 2014; Volume 213. [Google Scholar] [CrossRef]
- Rubinstein, A.E.; Wang, P. The fracture toughness of a particulate-reinforced brittle matrix. J. Mech. Phys. Solids 1998, 46, 1139–1154. [Google Scholar] [CrossRef]
- Ferracane, J.L. Is the wear of dental composites still a clinical concern? Is there still a need for in vitro wear simulating devices? Dent. Mater. 2006, 22, 689–692. [Google Scholar] [CrossRef]
- Heintze, S.D.; Reichl, F.X.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods—A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Soriano-Valero, S.; Román-Rodriguez, J.; Agustín-Panadero, R.; Bellot-Arcís, C.; Fons-Font, A.; Fernández-Estevan, L. Systematic review of chewing simulators: Reality and reproducibility of in vitro studies. J. Clin. Exp. Dent. 2020, 12, e1189–e1195. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Characterization of fluoride releasing restorative dental materials. Dent. Mater. J. 2018, 37, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Heintze, S.D.; Zellweger, G.; Peschke, A. Wear of an ion-releasing powder/liquid polymer resin in relation to that of glass-ionomer and conventional resin composites. Am. J. Dent. 2020, 33, 171–177. [Google Scholar]
- Brzović Rajić, V.; Ivanišević Malčić, A.; Bilge Kütük, Z.; Gurgan, S.; Jukić, S.; Miletić, I. Compressive Strength of New Glass Ionomer Cement Technology based Restorative Materials after Thermocycling and Cyclic Loading. Acta Stomatol. Croat. 2019, 53, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.A.; Stamboulis, A. Nanoclay-Reinforced Glass-Ionomer Cements: In Vitro Wear Evaluation and Comparison by Two Wear-Test Methods. Dent. J 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heintze, S.D.; Ilie, N.; Hickel, R.; Reis, A.; Loguerico, A.; Rousson, V. Laboratory mechanical parameters of composite resins and their relation to fractures and wear in clinical trials—A systematic review. Dent. Mater. 2017, 33, e101–e114. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Sander, A.; Wu, Y.; Felber, R.; Lauer, H.; Rosentritt, M. In-vitro performance and fracture strength of thin monolithic zirconia crowns. J. Adv. Prosthodont. 2018, 10, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, R.; Dudek, M.; Winter, J.; Braun, A.; Krämer, N.; von Stein-Lausnitz, M.; Roggendorf, M.J. Amalgam Alternatives Critically Evaluated: Effect of Long-term Thermomechanical Loading on Marginal Quality, Wear, and Fracture Behavior. J. Adhes. Dent. 2020, 22, 107–116. [Google Scholar] [CrossRef]
- Baumgart, P.; Kirsten, H.; Haak, R.; Olms, C. Biomechanical properties of polymer-infiltrated ceramic crowns on one-piece zirconia implants after long-term chewing simulation. Int. J. Implant Dent. 2018, 4, 16. [Google Scholar] [CrossRef]
- Wassel, R.; McCabe, J.F.; Walls, A.W.G. A two-body frictional wear test. J. Dent. Res. 1994, 73, 1546–1553. [Google Scholar] [CrossRef] [Green Version]
- Rosentritt, M.; Preis, V.; Behr, M.; Hahnel, S.; Handel, G.; Kolbeck, C. Two-body wear of dental porcelain and substructure oxide ceramics. Clin. Oral Investig. 2012, 16, 935–943. [Google Scholar] [CrossRef]
- Nawafleh, N.; Hatamleh, M.; Elshiyab, S.; Mack, F. Lithium Disilicate Restorations Fatigue Testing Parameters: A Systematic Review. J. Prosthodont. 2016, 25, 116–126. [Google Scholar] [CrossRef]
- Rosentritt, M.; Siavikis, G.; Behr, M.; Kolbeck, C.; Handel, G. Approach for valuating the significance of laboratory simulation. J. Dent. 2008, 36, 1048–1053. [Google Scholar] [CrossRef]
- Ruse, N.D.; Troczynski, T.; MacEntee, M.I.; Feduik, D. Novel fracture toughness test using a notchless triangular prism (NTP) specimen. J. Biomed. Mater. Res. 1996, 31, 457–463. [Google Scholar] [CrossRef]
- Fisher, J.; Varenne, B.; Narvaez, D.; Vickers, C. The Minamata Convention and the phase down of dental amalgam. Bull. World Health Organ. 2018, 96, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Krejci, I.; Reich, T.; Lutz, F.; Albertoni, M. In-vitro-Testverfahren zur Evaluation dentaler Restaurationssysteme. 1. Computergesteuerter Kausimulator. Quintessence Int. 1990, 100, 953–960. [Google Scholar]
- Turssi, C.P.; Faraoni, J.J.; de Menezes, M.; Serra, M.C. Analysis of potential lubricants for in vitro wear testing. Dent. Mater. 2006, 22, 77–83. [Google Scholar] [CrossRef]
- Alvanforoush, N.; Wong, R.; Burrow, M.; Palamara, J. Fracture toughness of glass ionomers measured with two different methods. J. Mech. Behav. Biomed. Mater. 2019, 90, 208–216. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Zheng, J. Tribology of dental materials: A review. J. Phys. D Appl. Phys. 2008, 41, 113001. [Google Scholar] [CrossRef]
- Klinke, T.; Daboul, A.; Turek, A.; Frankenberger, R.; Hickel, R.; Biffar, R. Clinical performance during 48 months of two current glass ionomer restorative systems with coatings: A randomized clinical trial in the field. Trials 2016, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Clegg, R.E.; Paterson, G.D. Ductile Particle Toughening of Hydroxyapatite Ceramics Using Platinum Particles. In Proceedings of the Structural Integrity and Fracture International Conference (SIF’04), Brisbane, Australia, 26–29 September 2004; pp. 47–53. [Google Scholar]
- Brzovic-Rajic, V.; Miletić, I.; Gurgan, S.; Peroš, K.; Verzak, Ž.; Ivanišević-Malčić, A. Fluoride Release from Glass Ionomer with Nano Filled Coat and Varnish. Acta Stomatol. Croat. 2018, 52, 307–313. [Google Scholar] [CrossRef]
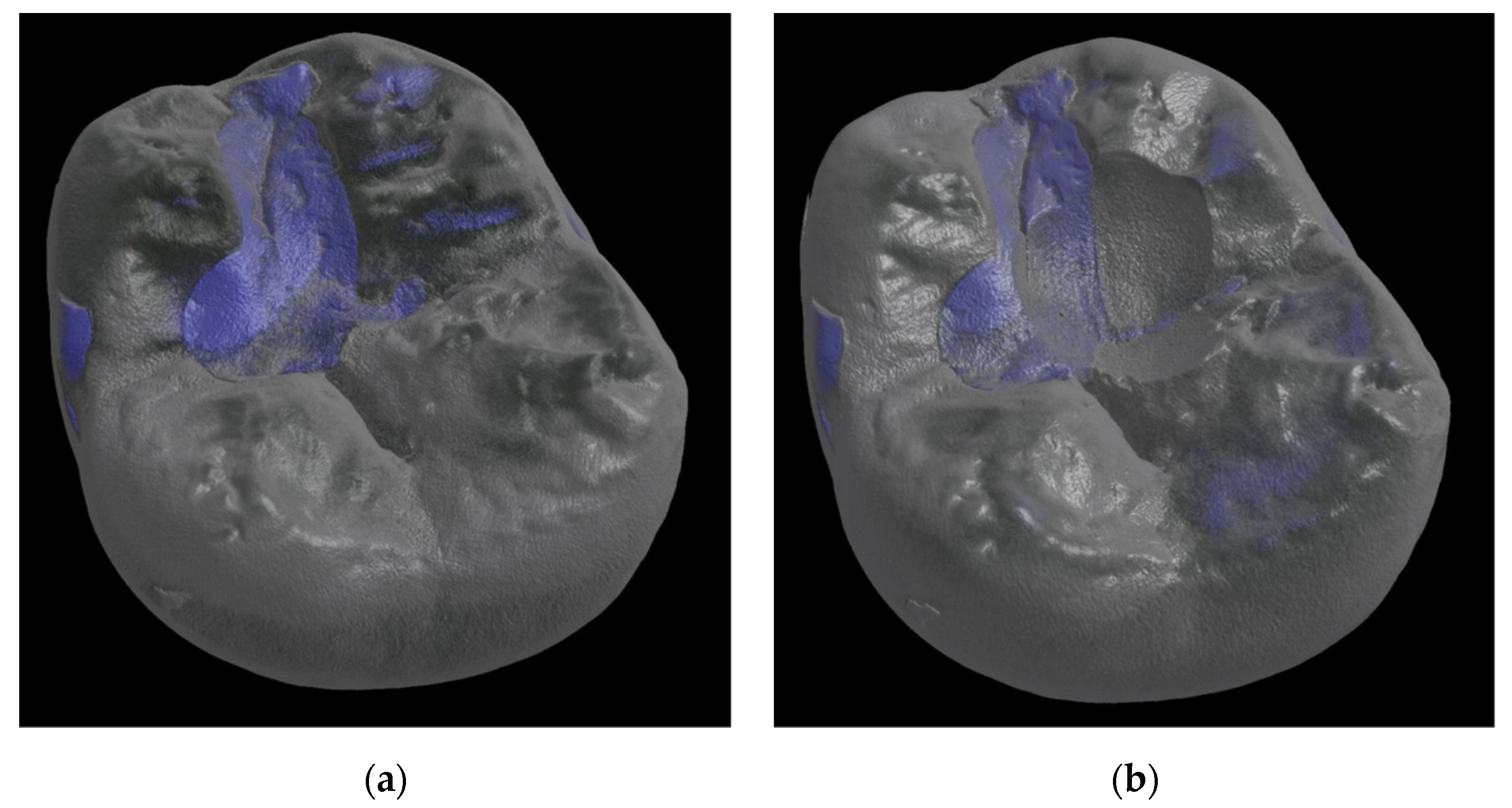



| Material | Composition | Powder/Liquid |
|---|---|---|
| DeltaFil (DMG) | Powder: fluoroaluminosilicate glass, polyacrylic acid Liquid: polyacrylic acid, tartaric acid, PEG-PU micelles, water | 370 mg/75 mg (ratio: 4.9/1.0) |
| Ketac Universal (3M) | Powder: oxide glass Liquid: copolymer of acrylic acid—maleic acid, tartaric acid, benzoic acid, water | 339 mg/106 mg (ratio: 3.2/1.0) |
| Fuji IX GP (GC) | Powder: fluoroaluminosilicate glass, polyacrylic acid Liquid: polyacrylic acid, polybasic carboxylic acid, water | 400 mg/110 mg (ratio: 3.6/1.0) |
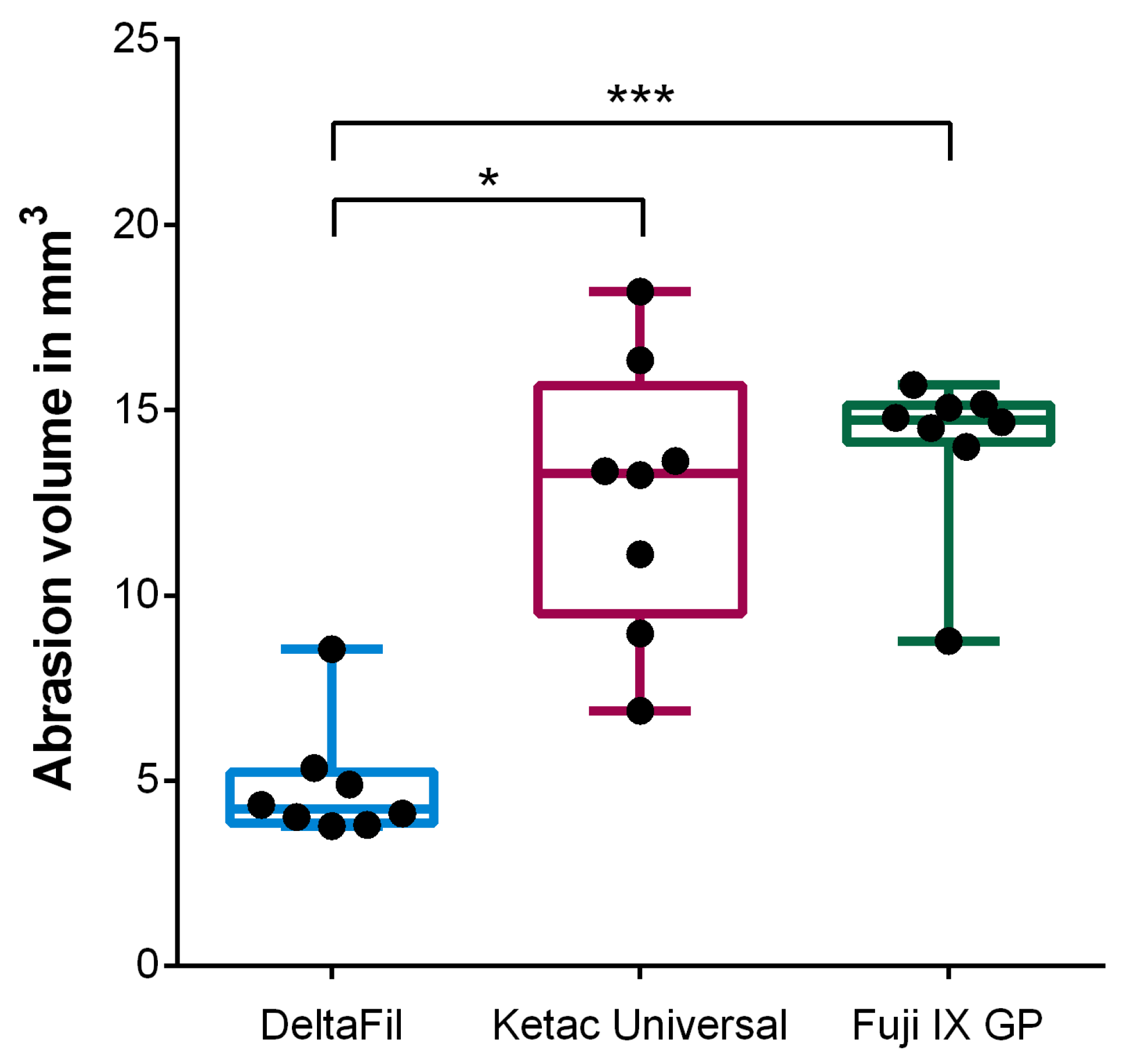
| Material | Probe | Abrasion Volume in mm3 | Cycle Number |
|---|---|---|---|
| DeltaFil | 1 | 4.34 | 1,200,000 |
| 2 | 3.81 | 1,200,000 | |
| 3 | 8.55 | 1,200,000 | |
| 4 | 4.02 | 1,200,000 | |
| 5 | 5.34 | 1,200,000 | |
| 6 | 4.89 | 1,200,000 | |
| 7 | 3.77 | 1,200,000 | |
| 8 | 4.11 | 1,200,000 | |
| Median (IQR) | 4.23 (3.86–5.23) | 1,200,000 (1,200,000–1,200,000) | |
| Ketac Universal | 1 | 8.97 | 668,629 |
| 2 | 6.89 | 1,200,000 | |
| 3 | 16.35 | 902,660 | |
| 4 | 11.11 | 1,200,000 | |
| 5 | 18.20 | 1,200,000 | |
| 6 | 13.63 | 668,629 | |
| 7 | 13.35 | 565,098 | |
| 8 | 13.25 | 565,098 | |
| Median (IQR) | 13.30 (9.51–15.67) | 785,645 (590,981–1,200,000) | |
| Fuji IX GP | 1 | 8.77 | 1,200,000 |
| 2 | 15.68 | 1,200,000 | |
| 3 | 14.52 | 1,200,000 | |
| 4 | 15.16 | 518,602 | |
| 5 | 14.79 | 419,892 | |
| 6 | 14.67 | 419,892 | |
| 7 | 15.07 | 518,602 | |
| 8 | 14.01 | 737,351 | |
| Median (IQR) | 14.73 (14.14–15.14) | 627,977 (444,570–1,200,000) |
| Material | Fracture Toughness in MPa∙m0.5 (Mean ± SD) |
|---|---|
| DeltaFil (n = 16) | 0.333 ± 0.066 |
| Ketac Universal (n = 12) | 0.278 ± 0.059 |
| Fuji IX GP (n = 17) | 0.277 ± 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messer-Hannemann, P.; Samadi, M.; Böttcher, H.; Duy, S.; Duy, D.; Albrecht, N.; Schwendicke, F.; Effenberger, S. Evaluation of a Method to Determine Wear Resistance of Class I Tooth Restorations during Cyclic Loading. Materials 2022, 15, 5440. https://doi.org/10.3390/ma15155440
Messer-Hannemann P, Samadi M, Böttcher H, Duy S, Duy D, Albrecht N, Schwendicke F, Effenberger S. Evaluation of a Method to Determine Wear Resistance of Class I Tooth Restorations during Cyclic Loading. Materials. 2022; 15(15):5440. https://doi.org/10.3390/ma15155440
Chicago/Turabian StyleMesser-Hannemann, Philipp, Mariam Samadi, Henrik Böttcher, Sebastian Duy, Daniela Duy, Niclas Albrecht, Falk Schwendicke, and Susanne Effenberger. 2022. "Evaluation of a Method to Determine Wear Resistance of Class I Tooth Restorations during Cyclic Loading" Materials 15, no. 15: 5440. https://doi.org/10.3390/ma15155440
APA StyleMesser-Hannemann, P., Samadi, M., Böttcher, H., Duy, S., Duy, D., Albrecht, N., Schwendicke, F., & Effenberger, S. (2022). Evaluation of a Method to Determine Wear Resistance of Class I Tooth Restorations during Cyclic Loading. Materials, 15(15), 5440. https://doi.org/10.3390/ma15155440







