Investigation of the Implementation of Laser Surface Alloying of Cu with Cr–WC
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
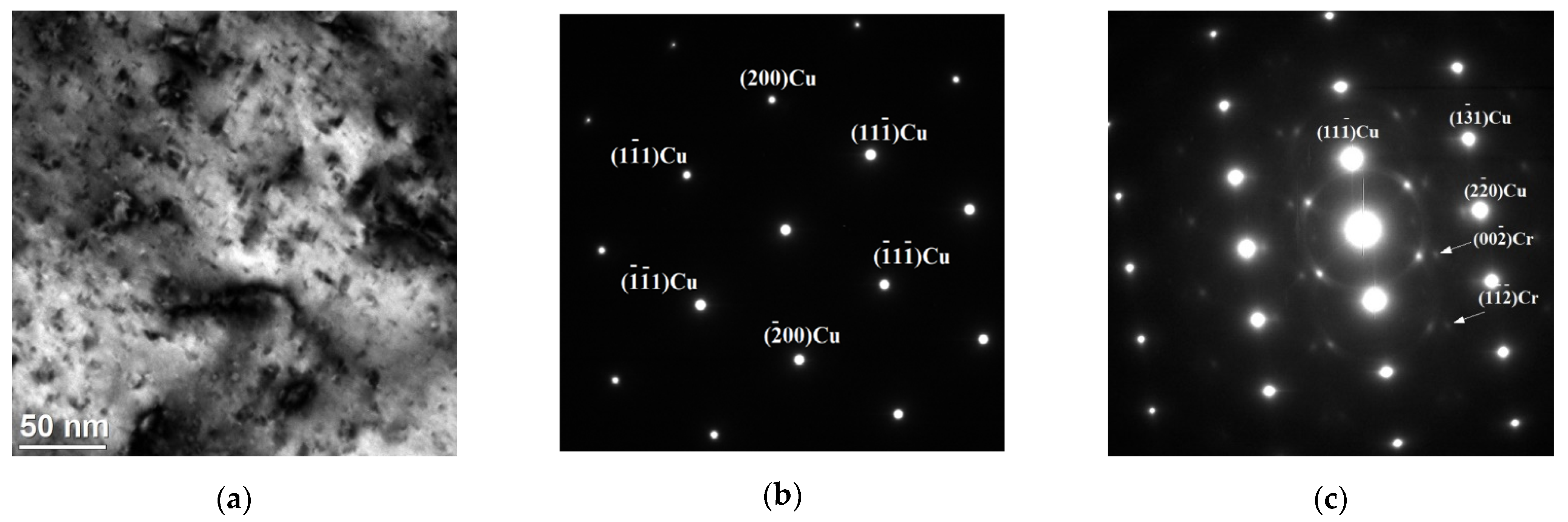
3.1. Microstructure Analysis
3.2. Microhardness and Wear Resistance
4. Conclusions
- The average thickness of the alloyed layer of copper with Cr–WC was about 900 µm.
- Absence of cracks and surface craters/ripples in the alloyed layer of copper with Cr–WC indicates that the process parameters are: laser power—2.0 kW, alloying rate—0.15 m/min, and the powder feed rate—2 g/min, representing the optimum conditions for LSA of cp copper.
- The copper top layer after laser treatment consists of the particles of used powders in the alloyed layer and nanoscale precipitates in the Cu matrix. Several characteristic types of precipitates were observed—different in terms of morphology, structure and chemical composition:
- Precipitates containing W, Cr and Mo have a diameter of about 100–180 nm and are located on spherical particles containing Cr and Mo.
- Coarse chromium (bcc Cr) precipitations with a rhombic shape and a size of about 100 nm.
- Precipitates rich in Cr and Ni with round shape and a size of about 50 nm are coherent with the Cu matrix.
- Oval precipitates—dominant components are Cr, Ni and probably Cu.
- Fine-dispersed particles below 2 nm—precipitates with coffee-bean contrast are probably a Cr phase with fcc structure.
- The surface layer of copper, after being alloyed with Cr75WC25 and Cr50WC50, shows areas with uniformly dispersed particles of applied powders. The surface layer of Cu after alloying with powder richest in WC (Cr25WC75) is characterized by the least homogeneous microstructure. The undissolved WC particles can be separated in the laser-alloyed layer.
- The surface layer of investigated samples was found to be composed primarily of metallic copper. The crystalline phases detected in the samples’ surface layer after laser-alloying process include mainly bcc chromium, tungsten, tungsten carbide WC and a second bcc phase, corresponding to solid solution of Cr, W and Mo.
- The surface layers of copper obtained as a result of laser alloying with Cr–WC powders are characterized by increased microhardness, which is 120–180 HV0.1 for Cu matrix with spherical particles of Cr and 200–400 HV0.1 for Cr particles while microhardness in the Cr-W-Mo precipitates is within a range 700–1715 HV0.1 compared to unalloyed pure copper (87 HV0.1). The large spread of the microhardness values in the laser-alloyed zone is caused by the presence of a number of chemical composition fluctuations. The increase in microhardness in the Cu surface layer after laser alloying with Cr–WC is attributed to solution and dispersion hardening. Due to the combination of non-equilibrium microstructures and the dispersion of hard phases, the surface properties are superior to that of copper not treated with a laser.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rdzawski, Z. Copper Alloy. Monograph 229; Silesian University of Technology: Gliwice, Poland, 2009. [Google Scholar]
- Tyler, D.; Black, D.E. Introduction to copper and copper Alloys. In ASM Handbook, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International, Metals Park: Almere, The Netherlands, 1990; Volume 2, pp. 216–240. [Google Scholar]
- Kusiński, J. Laser Applications in Material Engineering; Scientific Publisher “Akapit”: Cracow, Poland, 2000. (In Polish) [Google Scholar]
- Hirose, A.; Kobayashi, K.F. Surface alloying of copper with chromium by CO2 laser. Mater Sci. Eng. 1994, 174, 199–206. [Google Scholar] [CrossRef]
- Dubourg, L.; Pelletier, H.; Vaissiere, D.; Hlawka, F.; Cornet, A. Mechanical characterisation of laser surface alloyed aluminium–copper systems. Wear 2002, 253, 1077–1085. [Google Scholar] [CrossRef]
- Dutta Majumdar, J.; Manna, I. Laser material processing. Int. Mater. Rev. 2011, 56, 341–388. [Google Scholar] [CrossRef]
- Manna, I.; Abraham, S.; Reddy, G.; Bose, D.N.; Ghosh, T.B.; Pabi, S.K. Laser surface alloying of aluminum on copper substrate. Scr. Metall. Mater. 1994, 31, 713–718. [Google Scholar] [CrossRef]
- Dutta Majumdar, J.; Manna, I. Laser surface alloying of copper with chromium: I. Microstructural evolution I. Mater. Sci. Eng. A 1999, 268, 216–226. [Google Scholar] [CrossRef]
- Yang, Y.W.; Cristino, V.A.M.; Tam, L.M.; Lo, K.H.; Kwok, C.T. Laser surface alloying of copper with Cr/Ti/CNT for enhancing surface properties. J. Mater. Res. Technol. 2022, 17, 560–573. [Google Scholar] [CrossRef]
- Krupiński, M.; Smolarczyk, P.E.; Bonek, M. Microstructure and Properties of the Copper Alloyed with Ag and Ti Powders Using Fiber Laser. Materials 2020, 13, 2430. [Google Scholar] [CrossRef]
- Dias, M.; Pinhao, N.; Faustino, R.; Martins, R.M.S.; Ramos, A.S.; Vieira, M.T.; Correia, J.B.; Camacho, E.; Braz Fernandes, F.M.; Nunes, B.; et al. New WC-Cu composites for the divertor in fusion reactors. J. Nucl. Mater 2019, 521, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Huang, Y.; Zeng, X.; Hu, Q. Microstructure characteristics of Ni-based WC composite coatings by laser induction hybrid rapid cladding. Mater. Sci. Eng. A 2008, 480, 564–572. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, J.; Ye, N.; Zhou, W.; Wei, C.; Liu, D. Influence of additives and concentration of WC nanoparticles on properties of WC−Cu composite prepared by electroplating. Trans. Nonferrous Met. Soc. China 2020, 30, 1594–1604. [Google Scholar] [CrossRef]
- Deshpande, P.K.; Lin, R.Y. Wear resistance of WC particle reinforced copper matrix composites and the effect of porosity. Mater. Sci. Eng. A 2006, 418, 137–145. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Olmos, L.; Vergara-Hernández, H.J.; Jiménez, O.; Garnica, P.; Bouvard, D.; Flore, M. Constrained sintering and wear properties of Cu−WC composite coatings. Trans. Nonferrous Met. Soc. China 2017, 27, 2214–2224. [Google Scholar] [CrossRef]
- Cao, W.; Liang, S.; Zhang, X.; Wang, X.; Yang, X. Effect of Mo addition on microstructure and vacuum arc characteristics of CuCr50 alloy. Vacuum 2011, 85, 943–948. [Google Scholar]
- Dehm, G.; Medres, B.; Shepeleva, L.; Scheu, C.; Bamberger, M.; Mordike, B.L.; Mordike, S.; Ryk, G.; Halperin, G.; Etsion, I. Microstructure and tribological properties of Ni-based claddings on Cu substrates. Wear 1999, 225–229, 18–26. [Google Scholar] [CrossRef]
- Ng, K.W.; Man, H.C.; Cheng, F.T.; Yue, T.M. Laser cladding of copper with molybdenum for wear resistance enhancement inelectrical contacts. Appl. Surf. Sci. 2007, 253, 6236–6241. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Gao, Y.L.; Dong, C.; Wang, S.B. Microstructure and properties of Ni-Co composite cladding coating on mould copper plate. Materials 2019, 12, 2782. [Google Scholar] [CrossRef] [Green Version]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Match!—Phase Identification from Powder Diffraction, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR; Kreuzherrenstr: Bonn, Germany; Available online: https://www.crystalimpact.de/match (accessed on 29 August 2018).
- Muvvala, G.; Karmakar, D.P.; Nath, A.K. Online assessment of TiC decomposition in laser cladding of metal matrix composite coating. Mater. Des. 2017, 121, 310–320. [Google Scholar] [CrossRef]
- Zeng, D.W.; Xie, C.S.; Hu, M.L.; Wang, A.H.; Song, W.L. In situ laser synthesis of Co/Cu composite coating on copper substrate and its microstructural evolution. Surf. Coat. Technol. 2006, 200, 4065–4071. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. The Cr-Cu (Chromium-Copper) system. BULL APD 1984, 5, 59–68. [Google Scholar]
- Cooper, K.P.; Ayers, J.D.; Kampe, J.C.M.; Feng, C.R. Microstructural evolution and thermal stability in rapidly solidified high-chromium-containing copper alloys. Mater. Sci. Eng. A 1991, 142, 221–233. [Google Scholar] [CrossRef]
- Si, S.-H.; Zhang, H.; He, Y.-Z.; Li, M.-X.; Guo, S. Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys. Metals 2015, 5, 2119–2127. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.A.; Morris, D.G. Microstructures and mechanical properties of rapidly solidified Cu-Cr alloys. Acta Metall. 1987, 35, 2511–2522. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, C.; Zhu, Y.; Yang, Z.; Ding, B.; Song, X. Microstructures of melt-spun Cu100− x–Crx (x= 3.4–25) ribbons. J. Alloys Compd. 2003, 361, 165–168. [Google Scholar] [CrossRef]
- Wu, P.; Du, H.M.; Chen, X.L.; Li, Z.Q.; Bai, H.L.; Jiang, E.Y. Influence of WC particle behavior on the wear resistance properties of Ni–WC composite coatings. Wear 2004, 257, 142–147. [Google Scholar] [CrossRef]
- Fujii, T.; Nakazawa, H.; Kato, M.; Dahmen, U. Crystallography and morphology of nanosized Cr particles in a Cu–0.2% Cr alloy. Acta Mater. 2000, 48, 1033–1045. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Xu, G.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 609, 4575–4585. [Google Scholar] [CrossRef] [Green Version]
- Komem, Y.; Rezek, J. Precipitation at coherency loss in Cu-0.35 wt pct Cr. Metall. Trans. A 1975, 6, 549–551. [Google Scholar] [CrossRef]
- Pearson, W.B. (Ed.) Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon Press: Belfast, Northern Ireland, 1964. [Google Scholar]
- Manna, I.; Dutta Mazumdar, J.; Chatterjee, U.K.; Nath, A.K. Laser Surface Engineering of copper with chromium for enhanced wear resistance. Scr. Mater. 1996, 35, 405. [Google Scholar] [CrossRef]





















| Parameters | Value |
|---|---|
| Wavelength, nm | 808–940 |
| Power range, W | 100–2000 |
| Focal length, mm | 82 |
| Power density range, kW/cm² | 0.8–36.5 |
| Laser beam spot dimensions, mm | 1.8 × 6.8 |
| Specimen | Powder Composition | Laser Parameters | ||
|---|---|---|---|---|
| Cr (wt%) | WC (wt%) | Power, kW | Scanning Speed, m/min | |
| Cr75WC25 | 75 | 25 | 2.0 | 0.15 |
| Cr50WC50 | 50 | 50 | 2.0 | 0.15 |
| Cr25WC75 | 25 | 75 | 2.0 | 0.15 |
| Elements | Composition in Weight, % | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Cu | 3.7 | 3.3 | 2.8 | 1.8 | 51.6 | 87.0 | 7.9 | |
| Cr | - | - | - | 44.8 | 35.8 | 6.8 | 2.8 | 55.8 |
| Ni | - | - | - | 21.3 | 8.7 | 20.0 | 6.3 | 2.3 |
| W | 87.2 | 87.6 | 88.6 | 13.7 | 35.2 | 3.4 | 1.2 | 14.4 |
| C | 9.1 | 9.1 | 11.4 | 11.4 | 13.1 | 0.3 | 2.7 | 11.4 |
| Mo | - | - | - | 6.0 | 5.4 | 17.9 | 5.7 | |
| O | - | - | - | - | - | - | - | 2.5 |
| Lattice Parameters, a, Å, c, Å | ||||
|---|---|---|---|---|
| 75Cr25WC | 50Cr50WC | 25Cr75WC | Literature [21,34] | |
| Cu (γ1 phase) (cubic Fm-3m #225) | a = 3.6173 ± 0.00005 | a = 3.6174 ± 0.0001 | a = 3.6194 ± 0.0002 | a = 3.6146 (20 °C) |
| Cr (α phase) (cubic Im-3m #229) | a = 2.8868 ± 0.0007 | a = 2.886 ± 0.001 | a = 2.888 ± 0.005 | a = 2.8846 (20 °C) |
| (W)C qusongite (hexagonal, P-6m2 #187) | a = 2.925 ± 0.002 c = 2.9475 ± 0.05 | a = 2.9244 ± 0.003 c = 3.0258 ± 0.3 | a = 2.9293 ± 0.002 c = 2.9781 ± 0.007 | a = 2.90 c = 2.93 (25 °C) |
| W (cubic Im-3m #229) | a = 3.188 ± 0.01 | a = 3.185 ± 0.016 | a = 3.207 ± 0.013 | a = 3.1650 (25 °C) |
| Cr0.2W0.2Mo0.6 (cubic Im-3m #229) | a = 3.1159 ± 0.007 | a = 3.111 ± 0.011 | a = 3.1158 ± 0.002 | a = 3.11 (25 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domagała-Dubiel, J.; Bilewska, K.; Pawlyta, M.; Kulasa, J.; Janicki, D. Investigation of the Implementation of Laser Surface Alloying of Cu with Cr–WC. Materials 2022, 15, 5396. https://doi.org/10.3390/ma15155396
Domagała-Dubiel J, Bilewska K, Pawlyta M, Kulasa J, Janicki D. Investigation of the Implementation of Laser Surface Alloying of Cu with Cr–WC. Materials. 2022; 15(15):5396. https://doi.org/10.3390/ma15155396
Chicago/Turabian StyleDomagała-Dubiel, Justyna, Katarzyna Bilewska, Mirosława Pawlyta, Joanna Kulasa, and Damian Janicki. 2022. "Investigation of the Implementation of Laser Surface Alloying of Cu with Cr–WC" Materials 15, no. 15: 5396. https://doi.org/10.3390/ma15155396
APA StyleDomagała-Dubiel, J., Bilewska, K., Pawlyta, M., Kulasa, J., & Janicki, D. (2022). Investigation of the Implementation of Laser Surface Alloying of Cu with Cr–WC. Materials, 15(15), 5396. https://doi.org/10.3390/ma15155396






