Investigation of Structural, Physical, and Attenuation Parameters of Glass: TeO2-Bi2O3-B2O3-TiO2-RE2O3 (RE: La, Ce, Sm, Er, and Yb), and Applications Thereof
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. XRD, Physical Parameters, and UV–VIS–NIR Spectra
3.2. Structural Categorization of Glass Using FTIR Spectra
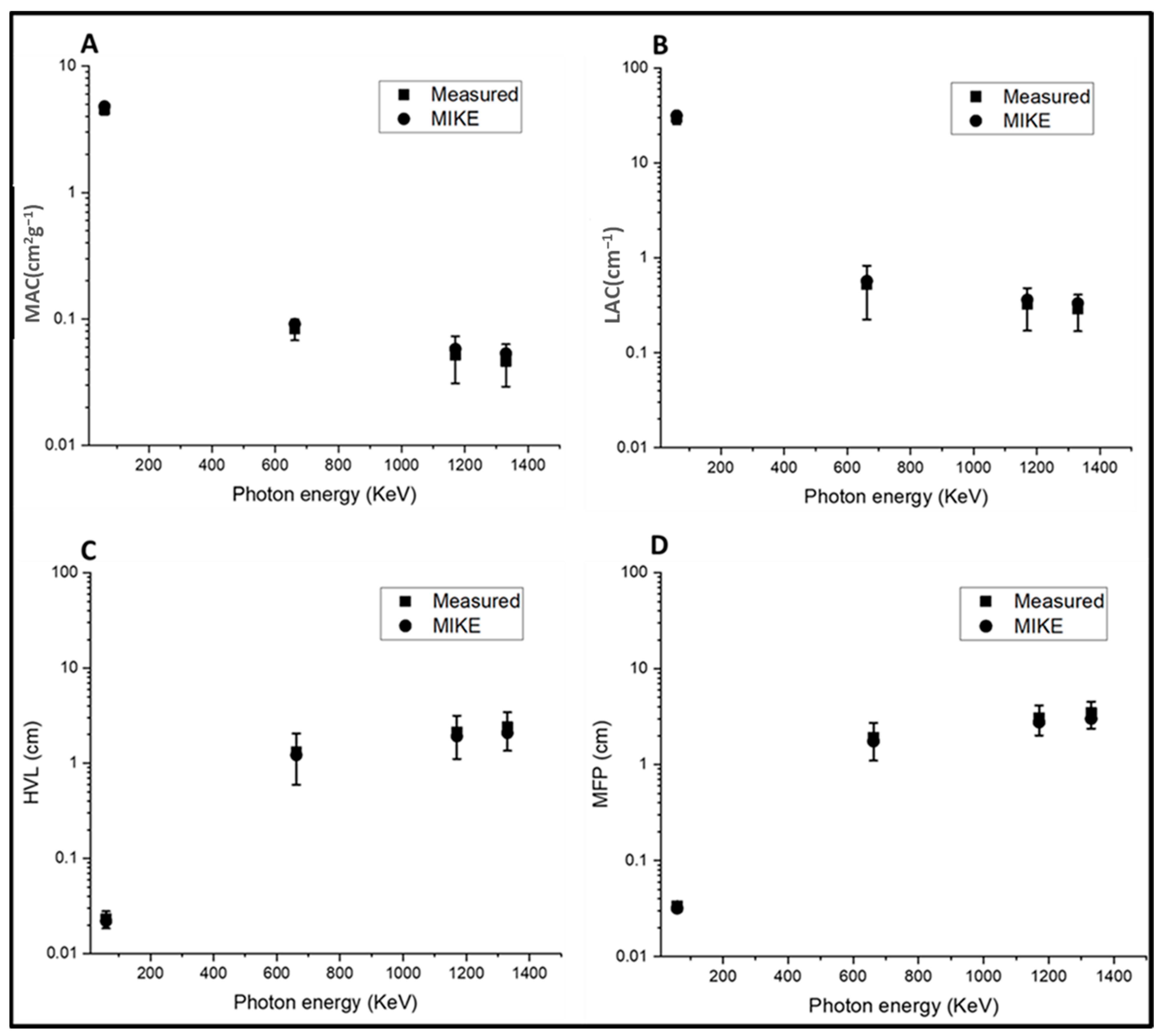
3.3. Attenuation Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xinyu, Z.; Xiaoli, W.; Hai, L.; Zhiqiang, W. Electronic polarizability and optical basicity of lanthanide oxides. Phys. B Condens. Matter 2017, 392, 132–136. [Google Scholar]
- Fudzi, F.M.; Kamari, H.M.; Latif, A.A.; Noorazlan, A.M. Linear Optical Properties of Zinc Borotellurite Glass Doped with Lanthanum Oxide Nanoparticles for Optoelectronic and Photonic Application. J. Nanomater. 2017, 2017, 4150802. [Google Scholar] [CrossRef]
- Selvi, S.; Marimuthu, K.; Muralidharan, G. Structural and luminescence behavior of Sm3+ ions doped lead boro-telluro-phosphate glasses. J. Lumin. 2015, 159, 207–218. [Google Scholar] [CrossRef]
- Hussein, K.I.; Alqahtani, M.S.; Alzahrani, K.J.; Alqahtani, F.F.; Zahran, H.Y.; Alshehri, A.M.; Yahia, I.S.; Reben, M.; Yousef, E.S. The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System. Materials 2022, 15, 1844. [Google Scholar] [CrossRef]
- Selvaraju, K.; Marimuthu, K. Structural and spectroscopic studies on concentration dependent Sm3+ doped boro-tellurite glasses. J. Alloys Compd. 2013, 553, 273–281. [Google Scholar] [CrossRef]
- Golis, E.; Yousef, E.S.; Reben, M.; Kotynia, K.; Filipecki, J. Measurements of defect structures by positron annihilation lifetime spectroscopy of the tellurite glass TeO2–P2O5–ZnO–LiNbO3 doped with ions of rare earth elements: Er3+, Nd3+ and Gd3+. Solid State Sci. 2015, 50, 81–84. [Google Scholar] [CrossRef]
- Hussain, N.S.; Hungerford, G.; El-Mallawany, R.; Gomes, M.J.M.; Lopes, M.A.; Ali, N.; Santos, J.D.; Buddhudu, S. Absorption and Emission Analysis of RE3+(Sm3+ and Dy3+): Lithium Boro Tellurite Glasses. J. Nanosci. Nanotechnol. 2009, 9, 3672–3677. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Abbas, R.; El-Mallawany, R.; Hathot, S.F. Optical properties and crystallization of bismuth boro-tellurite glasses. J. Non-Cryst. Solids 2017, 476, 15–24. [Google Scholar] [CrossRef]
- Boda, R.; Shareefuddin, M.D.; Chary, M.N.; Sayanna, R. FTIR and optical properties of europium doped lithium zinc bismuth borate glasses. Mater. Today 2016, 3, 1914–1922. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, A.; Khanna, A.; Gonzàlez, F.; Pesquera, C.; Iordanova, R.; Chen, B. Structure-property correlations in TiO2-Bi2O3-B2O3-TeO2 glasses. J. Non-Cryst. Solids 2017, 470, 168–177. [Google Scholar] [CrossRef]
- Sapian, I.N.; Yusof, M.I.M.; Yahya, A.K. Elastic and Structural Properties of (95-x) TeO2-5La2O3-xTiO2 Lanthanum Tellurite Glass System. Chalcogenide Lett. 2014, 11, 471–484. [Google Scholar]
- Maheshvaran, K.; Linganna, K.; Marimuthu, K. Composition dependent structural and optical properties of Sm3+ doped boro-tellurite glasses. J. Lumin. 2011, 131, 2746–2753. [Google Scholar] [CrossRef]
- Jamalaiah, B.; Kumar, M.V.; Gopal, K.R. Fluorescence properties and energy transfer mechanism of Sm3+ ion in lead telluroborate glasses. Opt. Mater. 2011, 33, 1643–1647. [Google Scholar] [CrossRef]
- Singh, G.P.; Parvinder, K.; Simranpreet, K.; Singh, D.P. Investigation of structural, physical and optical properties of CeO2–Bi2O3–B2O3 glasses. Phys. B Condens. Matter 2012, 407, 4168–4172. [Google Scholar] [CrossRef]
- Pisarska, J.; Agnieszka, K.; Marta, S.; Agata, G.; Ewa, P.; Pisarski, W.A. Spectroscopy and energy transfer in Tb3+/Sm3+ co-doped lead borate glasses. J. Lumin. 2018, 195, 87–95. [Google Scholar] [CrossRef]
- Hegazy, H.H.; Almojadah, S.; Reben, M.; Yousef, E.S. Absorption spectra and Raman gain coefficient in near-IR region of Er3+ ions doped TeO2–Nb2O5–Bi2O3–ZnO glasses. Opt. Laser Technol. 2015, 74, 138–144. [Google Scholar]
- Sayyed, M.I.; Laariedh, F.; Kumr, A.; Al-Buriahi, M.S. Experimental studies on the gamma photons-shielding competence of TeO2–PbO–BaO–Na2O–B2O3 glasses. Appl. Phys. A 2020, 126, 4. [Google Scholar] [CrossRef]
- Kamislioglu, M.; Guclu, E.A.; Tekin, H.O. Comparative evaluation of nuclear radiation shielding properties of x TeO2+ (100 − x) Li2O glass system. Appl. Phys. A 2020, 126, 1–16. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Kavaz, E.; Perişanoğlu, U.; Kilic, G.; El-Agawany, F.I.; Tekin, H.O. Charged particles and gamma-ray shielding features of oxyfluoride semiconducting glasses: TeO2-Ta2O5-ZnO/ZnF2. Ceram. Int. 2020, 46, 25035–25042. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kaky, K.M.; Baki, S.O.; Ye, S.; Lira, A.; Kityk, I.V.; Mahdi, M.A. Concentration dependent structural, thermal, and optical features of Pr3+-doped multicomponent tellurite glasses. J. Alloys Compd. 2016, 686, 769–784. [Google Scholar] [CrossRef]
- Gerward, L.; Guilbert, N.; Jensen, K.; Levring, H. WinXCom—A program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 2004, 71, 653–654. [Google Scholar] [CrossRef]
- Hussein, K.I.; Alqahtani, M.S.; Algarni, H.; Zahran, H.; Yaha, I.S.; Grelowska, I.; Reben, M.; Yousef, E.S. MIKE: A new computational tool for investigating radiation, optical and physical properties of prototyped shielding materials. J. Instrum. 2021, 16, T07004. [Google Scholar] [CrossRef]
- Swapna; Upender, G.; Prasad, M. Vibrational, Optical and EPR studies of TeO2-Nb2O5-Al2O3-V2O5 glass system doped with vanadium. Opt. Int. J. Light Electron. Opt. 2016, 127, 10716–10726. [Google Scholar] [CrossRef]
- Yousef, E.S.; Elokr, M.; AbouDeif, Y. Optical, elastic properties and DTA of TNZP host tellurite glasses doped with Er3+ ions. J. Mol. Struct. 2016, 1108, 257–262. [Google Scholar] [CrossRef]
- El-Moneim, A.A.; El-Latif, L.A. Theoretically calculated elastic moduli of tellurite, phosphate and silicate glasses. Phys. Chem. Glasses 2003, 44, 446–453. [Google Scholar]
- Sidkey, M.A.; El-Moneim, A.A. Ultrasonic studies on ternary TeO2–V2O5–Sm2O3 glasses. Mater. Chem. Phys. 1999, 61, 103–109. [Google Scholar] [CrossRef]
- Laila, S.; Suraya, A.K.; Yahya, A.K. Effect of glass network modification on elastic and structural properties of mixed electronicionic 35V2O5-(65-x) TeO2-(x) Li2O glass system. Chalcogenide Lett. 2014, 11, 91–104. [Google Scholar]
- Luo, M.; Sha, X.; Chen, B.; Zhang, X.; Yu, H.; Li, X.; Zhang, J.; Xu, S.; Cao, Y.; Wang, Y.; et al. Optical transition properties, internal quantum efficiencies, and temperature sensing of Er3+ doped BaGd2O4 phosphor with low maximum phonon energy. J. Am. Ceram. Soc. 2022, 105, 3353–3363. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 179. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Marzouk, S.Y.; Shahin, S. Synthesis and optical properties of new fluoro-tellurite glass within (TeO2-ZnO-LiF-Nb2O5-NaF) system. J. Non-Cryst. Solids 2017, 472, 39–45. [Google Scholar] [CrossRef]
- Charfi, B.; Damak, K.; Alqahtani, M.S.; Hussein, K.I.; Alshehri, A.M.; Elkhoshkhany, N.; Assiri, A.L.; Alshehri, K.F.; Reben, M.; Yousef, E.S. Luminescence and Gamma Spectroscopy of Phosphate Glass Doped with Nd3+/Yb3+ and Their Multifunctional Applications. Photonics 2022, 9, 406. [Google Scholar] [CrossRef]
- Ahmadi, F.; Hussin, R.; Ghoshal, S. Spectroscopic attributes of Sm3+ doped magnesium zinc sulfophosphate glass: Effects of silver nanoparticles inclusion. Opt. Mater. 2017, 73, 268–276. [Google Scholar] [CrossRef]
- Komatsu, T.; Dimitrov, V. Features of electronic polarizability and approach to unique properties in tellurite glasses. Int. J. Appl. Glas. Sci. 2019, 11, 253–271. [Google Scholar] [CrossRef]
- Arunkumar, S.; Marimuthu, K. Concentration effect of Sm3+ ions in B2O3–PbO–PbF2–Bi2O3–ZnO glasses–structural and luminescence investigations. J. Alloy Compd. 2013, 565, 104–114. [Google Scholar] [CrossRef]
- Hager, I.Z.; El-Mallawany, R. Preparation and structural studies in the (70 − x) TeO2–20WO3–10Li2O–xLn2O3 glasses. J. Mater. Sci. 2010, 45, 897. [Google Scholar] [CrossRef]
- Berwal, N.; Kundu, R.S.; Nanda, K.; Punia, R.; Kishore, N. Physical, structural and optical characterizations of borate modified bismuth–silicate–tellurite glasses. J. Mol. Struct. 2015, 1097, 37–44. [Google Scholar] [CrossRef]
- Berwal, N.; Dhankhar, S.; Sharma, P.; Kundu, R.; Punia, R.; Kishore, N. Physical, structural and optical characterization of silicate modified bismuth-borate-tellurite glasses. J. Mol. Struct. 2017, 1127, 636–644. [Google Scholar] [CrossRef]
- Rada, S.; Culea, M.; Culea, E. Structure of TeO2·B2O3 glasses inferred from infrared spectroscopy and DFT calculations. J. Non-Cryst. Solids 2008, 354, 5491–5495. [Google Scholar] [CrossRef]
- Kundu, R.; Dhankhar, S.; Punia, R.; Nanda, K.; Kishore, N. Bismuth modified physical, structural and optical properties of mid-IR transparent zinc boro-tellurite glasses. J. Alloys Compd. 2014, 587, 66–73. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Aleksandrov, L.I. Structural, thermal, optical and photo-luminescent properties of barium tellurite glasses doped with rare-earth ions. J. Non-Cryst. Solids 2017, 476, 67–74. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth borate glasses doped with europium ions evidenced by FT-IR spectroscopy. J. Mater. Sci. Mater. Electron. 2007, 19, 424–428. [Google Scholar] [CrossRef]
- Hung, W.-C.; Fu, S.-H.; Tseng, J.-J.; Chu, H.; Ko, T.-H. Study on photocatalytic degradation of gaseous dichloromethane using pure and iron ion-doped TiO2 prepared by the sol–gel method. Chemosphere 2007, 66, 2142–2151. [Google Scholar] [CrossRef]
- Abdelghany, A.M. Combined DFT, deconvolution analysis for structural investigation of copper–doped lead borate glasses. Open Spectrosc. J. 2012, 6, 9–14. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Khatab, M.; Kabary, M.A. Thermal, FTIR and UV spectral studies on tellurite glasses doped with cerium oxide. Ceram. Int. 2018, 44, 2789–2796. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; El-Mallawany, R.; Syala, E. Mechanical and thermal properties of TeO2–Bi2O3–V2O5–Na2O–TiO2 glass system. Ceram. Int. 2016, 42, 19218–19224. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; González, F.; Pesquera, C.; Chen, B. Structural, optical, dielectric and thermal properties of molybdenum tellurite and borotellurite glasses. J. Non-Cryst. Solids 2016, 444, 1–10. [Google Scholar] [CrossRef]
- Eevon, C.; Halimah, M.; Zakaria, A.; Azurahanim, C.; Azlan, M.; Faznny, M. Linear and nonlinear optical properties of Gd3+ doped zinc borotellurite glasses for all-optical switching applications. Results Phys. 2016, 6, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cheng, J.; Li, M. Effect of rare earths on viscosity and thermal expansion of soda-lime-silicate glass. J. Rare Earths 2010, 28, 308–311. [Google Scholar] [CrossRef]
- Divina, R.; Naseer, K.A.; Marimuthu, K.; Alajerami, Y.S.M.; Al-Buriahi, M.S. Effect of different modifier oxides on the synthesis, structural, optical, and gamma/beta shielding properties of bismuth lead borate glasses doped with europium. J. Mater. Sci. Mater. Electron. 2020, 31, 21486–21501. [Google Scholar] [CrossRef]
- Chen, Q.; Naseer, K.; Marimuthu, K.; Kumar, P.S.; Miao, B.; Mahmoud, K.; Sayyed, M. Influence of modifier oxide on the structural and radiation shielding features of Sm3+-doped calcium telluro-fluoroborate glass systems. J. Aust. Ceram. Soc. 2020, 57, 275–286. [Google Scholar] [CrossRef]
- Sathiyapriya, G.; Naseer, K.A.; Marimuthu, K.; Kavaz, E.; Alalawi, A.; Al-Buriahi, M.S. Structural, optical and nuclear radiation shielding properties of strontium barium borate glasses doped with dysprosium and niobium. J. Mater. Sci. Mater. Electron. 2021, 32, 8570–8592. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J. Investigation on radiation shielding parameters of bismuth borosilicate glass from 1 keV to 100 GeV. Ann. Nucl. Energy 2013, 55, 23–28. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Sayyed, M.I.; Kothan, S.; Kim, H.J.; Kaewkhao, J. High density of tungsten gadolinium borate glasses for radiation shielding material: Effect of WO3 concentration. Radiat. Phys. Chem. 2022, 192, 109926. [Google Scholar] [CrossRef]







| Sample Name | Glass Composition (mol%) | Sample Color | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TeO2 | B2O3 | Bi2O3 | TiO2 | La2O3 | Ce2O3 | Sm2O3 | Er2O3 | Yb2O3 | ||
| BBTTLa | 45 | 25 | 20 | 7 | 3 | ― | ― | ― | ― |  |
| BBTTCe | 45 | 25 | 20 | 7 | ― | 3 | ― | ― | ― |  |
| BBTTSm | 45 | 25 | 20 | 7 | ― | ― | 3 | ― | ― |  |
| BBTTEr | 45 | 25 | 20 | 7 | ― | ― | ― | 3 | ― |  |
| BBTTYb | 45 | 25 | 20 | 7 | ― | ― | ― | ― | 3 |  |
| Sample Name | (g/cm3) ± 0.001 | Vm (cm3/mol) ± 0.0056 | Oxygen Molar Volume, Vo (cm3/mol) ± 0.2980 | nb × 1022 (m−3) | (Nm−1) | OPD (mol/L) ± 0.078 |
|---|---|---|---|---|---|---|
| BBTTLa | 5.67 | 35 | 14.1 | 6.5 | 301.21 | 71.1 |
| BBTTCe | 6.083 | 32.5 | 13.1 | 7.05 | 300.2 | 76.2 |
| BBTTSm | 6.17 | 32.13 | 12.95 | 7.08 | 301.79 | 77.1 |
| BBTTEr | 6.21 | 32.11 | 12.94 | 7.09 | 301.48 | 77.2 |
| BBTTYb | 6.31 | 31.63 | 12.7 | 7.14 | 303.04 | 78.4 |
| Sample Name | Eopt (eV) ±0.01 | n ±0.0001 | Rm (cm3) ±0.0466 | αm, (Ă3) ±0.0182 | (Ă3) ± 0.0065 | M ± 0.0008 | Λ ± 0.0025 |
|---|---|---|---|---|---|---|---|
| BBTTLa | 1.71 | 2.69 | 23.6 | 9.35 | 3.22 | 0.324 | 1.15 |
| BBTTCe | 2.47 | 2.61 | 21.5 | 8.52 | 2.88 | 0.339 | 1.09 |
| BBTTSm | 2.57 | 2.49 | 20.4 | 8.1 | 2.71 | 0.365 | 1.05 |
| BBTTEr | 2.68 | 2.46 | 20.2 | 8.008 | 2.68 | 0.371 | 1.04 |
| BBTTYb | 2.8 | 2.45 | 19.77 | 7.84 | 2.61 | 0.375 | 1.03 |
| Symbol | IR Bands Wavenumber (cm−1) | Assignments |
|---|---|---|
| a | 370–400 | Stretching mode of vibration of Bi–O–Bi linkages |
| b | 430 440 458 425 430 | Stretching vibration of La–O Stretching vibration of Ce–O Stretching vibration of Sm–O Stretching vibration of Er–O Stretching vibration of Yb–O |
| c | 463–480 | Bi–O–Bi vibration in distorted BiO6 octahedral units |
| b | 494–512 | Symmetrical stretching or bending vibrations of Te–O–Te or O–Te–O linkages |
| e | 552–563 | Bending vibration of Bi–O− in BiO6 units |
| f | 576–600 | Vibration of the continuous network consisting of TeO4 tbp |
| g | 616–623 | Ti–O bending vibration |
| h | 648–654 | Symmetrical stretching vibration of Te–Oax in TeO4 tetrahedral units |
| i | 671–674 | Stretching vibrations tellurium with BO of TeO3/TeO3+1 units |
| j | 692–695 | Bending vibrations of B–O–B linkages in the borate network |
| k | 712–721 | Stretching modes of NBO found on TeO3 and TeO3+1 units |
| l | 759–772 | Symmetrical and asymmetrical vibration of (Teeq–O) in TeO3+1 polyhedra or trigonal pyramid TeO3 (tp) units |
| m | 912–925 | Stretching vibrations of B–O bond in BO4 units from diborate groups |
| n | 990–1001 | Stretching vibrations of B–O–Bi linkages |
| o p | (1023–1028), (1062–1067) | Stretching vibrations of B–O bond in BO4 units from tri-, tetra- and penta-borate groups |
| q r | (1118–1120), (1162–1168) | TiO4 |
| s t | (1247–1248), (1280–1285) | Asymmetric stretching vibrations of B–O bond in BO3 triangular units from meta-, pyro-, and ortho-borate groups |
| u v | (1317–1321), (1348–1349) | Symmetrical stretching vibrations of B–O bond in BO3 triangular units from meta-, pyro-, and ortho-borate groups |
| w | 1375 | Asymmetrical stretching vibrations of B–O bond in BO3 triangular units |
| x | 1401–1404 | Asymmetrical stretching vibrations of B–O triangle with BO3, B2O− and stretching vibration of borate triangle with (NBO) in various borate groups |
| y | 1428–1430 | Stretching vibration of B–O bond in BO3 units from varied types of borate groups |
| z | 1461 | Anti-symmetric stretching vibrations with 3 NBO of B–O–B linkages |
| Energy (keV) | Mass Attenuation Coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BBTTLa | BBTTCe | BBTTSm | |||||||
| Exp | WinXCom | MIKE | Exp | WinXCom | MIKE | Exp | WinXCom | MIKE | |
| 59.5 | 4.528 | 4.717 | 4.7924 | 4.6070 | 4.741 | 4.8151 | 4.654 | 4.831 | 4.9056 |
| 662 | 0.084 | 0.090 | 0.0912 | 0.0839 | 0.091 | 0.0913 | 0.0841 | 0.0907 | 0.0914 |
| 1170 | 0.052 | 0.057 | 0.0580 | 0.0521 | 0.058 | 0.0580 | 0.0522 | 0.0579 | 0.0580 |
| 1330 | 0.046 | 0.053 | 0.0534 | 0.0463 | 0.053 | 0.0534 | 0.0464 | 0.0534 | 0.0535 |
| Energy (keV) | Mass Attenuation Coefficient | |||||
|---|---|---|---|---|---|---|
| BBTTEr | BBTTYb | |||||
| Exp | WinXCom | MIKE | Exp | WinXCom | MIKE | |
| 59.5 | 4.8245 | 4.988 | 5.0682 | 4.3090 | 4.4710 | 4.5282 |
| 662 | 0.0844 | 0.091 | 0.0917 | 0.0845 | 0.0912 | 0.0918 |
| 1170 | 0.0524 | 0.058 | 0.0581 | 0.0526 | 0.0580 | 0.0581 |
| 1330 | 0.0467 | 0.053 | 0.0535 | 0.0468 | 0.0535 | 0.0535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkhoshkhany, N.; Marzouk, S.; El-Sherbiny, M.; Ibrahim, H.; Burtan-Gwizdala, B.; Alqahtani, M.S.; Hussien, K.I.; Reben, M.; Yousef, E.S. Investigation of Structural, Physical, and Attenuation Parameters of Glass: TeO2-Bi2O3-B2O3-TiO2-RE2O3 (RE: La, Ce, Sm, Er, and Yb), and Applications Thereof. Materials 2022, 15, 5393. https://doi.org/10.3390/ma15155393
Elkhoshkhany N, Marzouk S, El-Sherbiny M, Ibrahim H, Burtan-Gwizdala B, Alqahtani MS, Hussien KI, Reben M, Yousef ES. Investigation of Structural, Physical, and Attenuation Parameters of Glass: TeO2-Bi2O3-B2O3-TiO2-RE2O3 (RE: La, Ce, Sm, Er, and Yb), and Applications Thereof. Materials. 2022; 15(15):5393. https://doi.org/10.3390/ma15155393
Chicago/Turabian StyleElkhoshkhany, Nehal, Samir Marzouk, Mohammed El-Sherbiny, Heba Ibrahim, Bozena Burtan-Gwizdala, Mohammed S. Alqahtani, Khalid I. Hussien, Manuela Reben, and El Sayed Yousef. 2022. "Investigation of Structural, Physical, and Attenuation Parameters of Glass: TeO2-Bi2O3-B2O3-TiO2-RE2O3 (RE: La, Ce, Sm, Er, and Yb), and Applications Thereof" Materials 15, no. 15: 5393. https://doi.org/10.3390/ma15155393
APA StyleElkhoshkhany, N., Marzouk, S., El-Sherbiny, M., Ibrahim, H., Burtan-Gwizdala, B., Alqahtani, M. S., Hussien, K. I., Reben, M., & Yousef, E. S. (2022). Investigation of Structural, Physical, and Attenuation Parameters of Glass: TeO2-Bi2O3-B2O3-TiO2-RE2O3 (RE: La, Ce, Sm, Er, and Yb), and Applications Thereof. Materials, 15(15), 5393. https://doi.org/10.3390/ma15155393







