Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review
Abstract
:1. Introduction
2. Nanomaterials in Wastewater Treatment
2.1. Carbon-Based Nano Adsorbents
2.2. Metal-Based Nano Adsorbents
2.3. Hydrogels
2.4. Nano-Sponges
2.5. Nanocomposites
2.6. Layered Double Hydroxide (LDH)-Based Materials
3. Nanomaterials Used for Heavy Metal Removal
3.1. Carbon-Based Nanostructures
3.1.1. Graphene
3.1.2. CNTs
3.1.3. Porous Carbon Adsorbents
3.2. Metal Nanoparticles
3.2.1. Silver Nanoparticles (AgNPs)
3.2.2. Gold Nanoparticles (AuNPs)
3.2.3. Iron Nanoparticles
3.3. Metal Oxide Nanoparticles
3.3.1. Copper Oxide (CuO) Nanoparticles
3.3.2. Titanium Dioxide (TiO2) Nanoparticles
3.3.3. Magnesium Oxide (MgO) Nanoparticles
3.3.4. Zinc Oxide (ZnO) Nanoparticles
3.3.5. Cerium Oxide (CeO2) Nanoparticles
3.3.6. Other Metal Oxide Nanoparticles
3.3.7. Biomass-Derived Nanomaterials
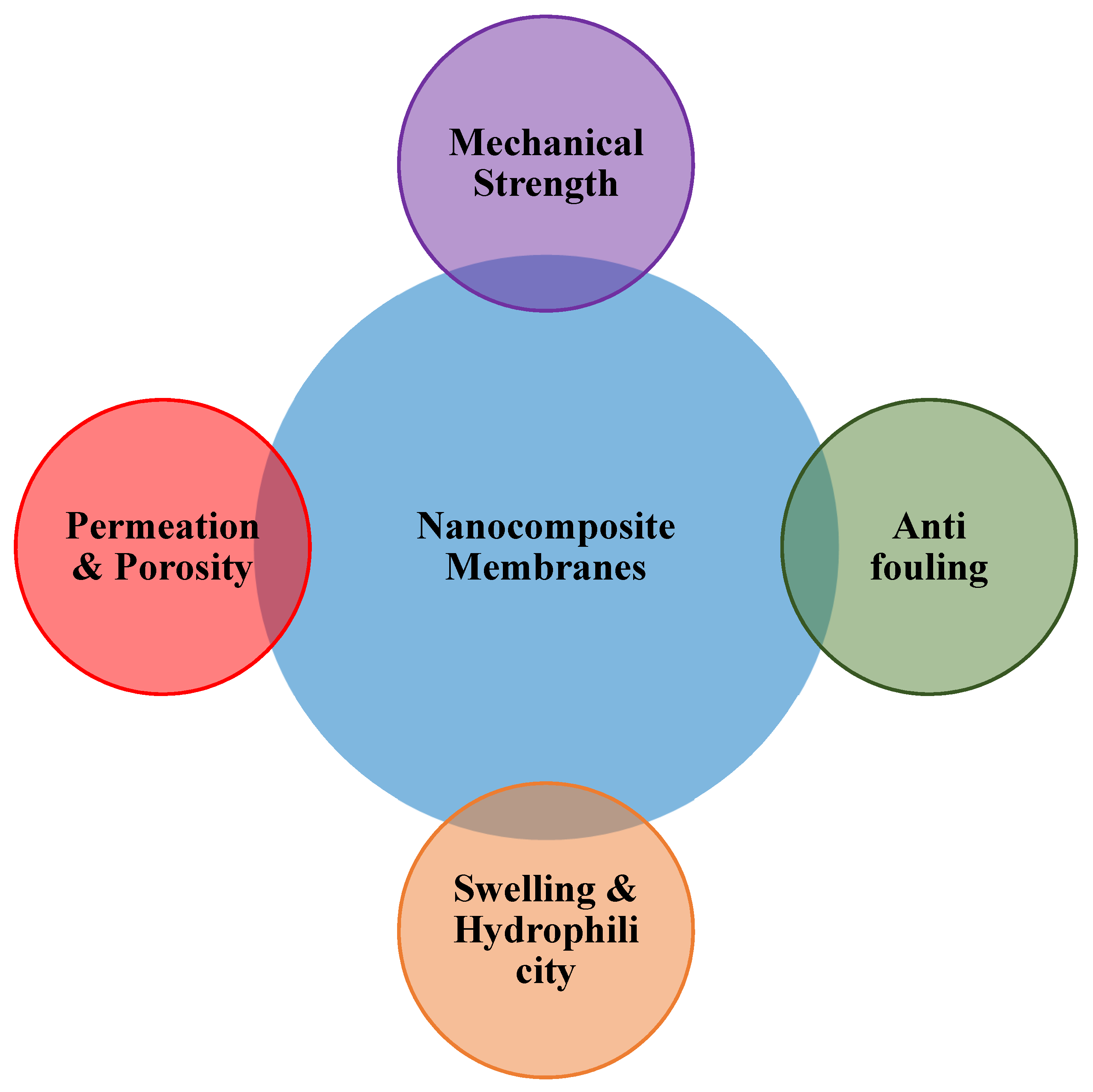
4. Role of Adsorptive Nanocomposite Membranes in Heavy Metal Removal
4.1. Mechanical Strength
4.2. Permeability
4.3. Surface Charge Alteration
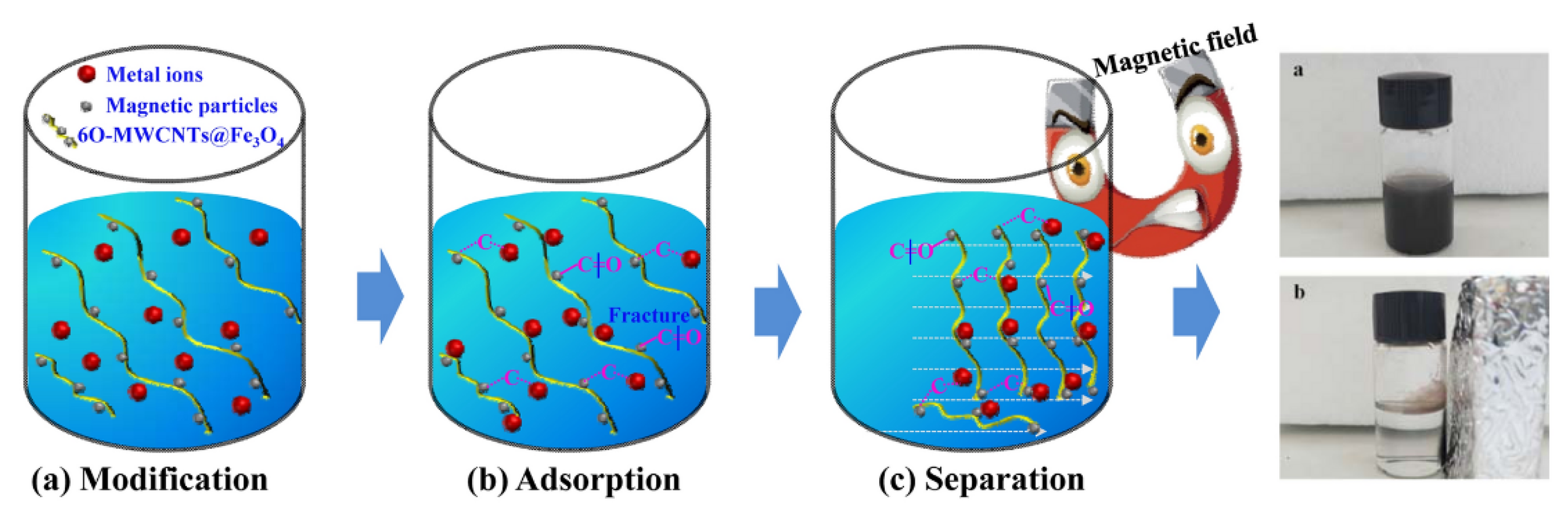
4.4. Mechanisms of Heavy Metal Ions via Adsorptive Nanocomposite Membranes
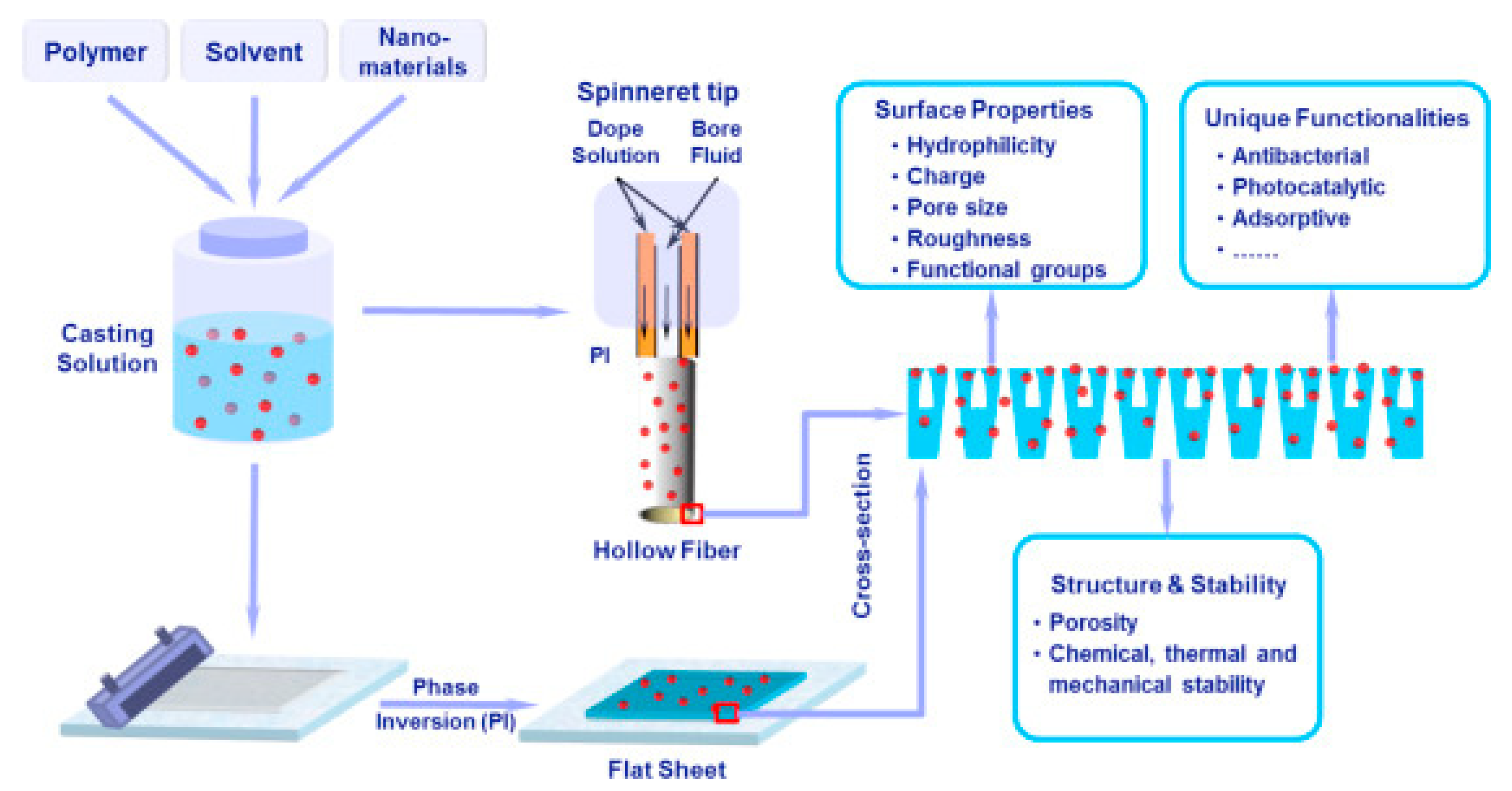
5. Fabrication of Nanocomposite Membranes
5.1. Phase Inversion
5.2. Stretching
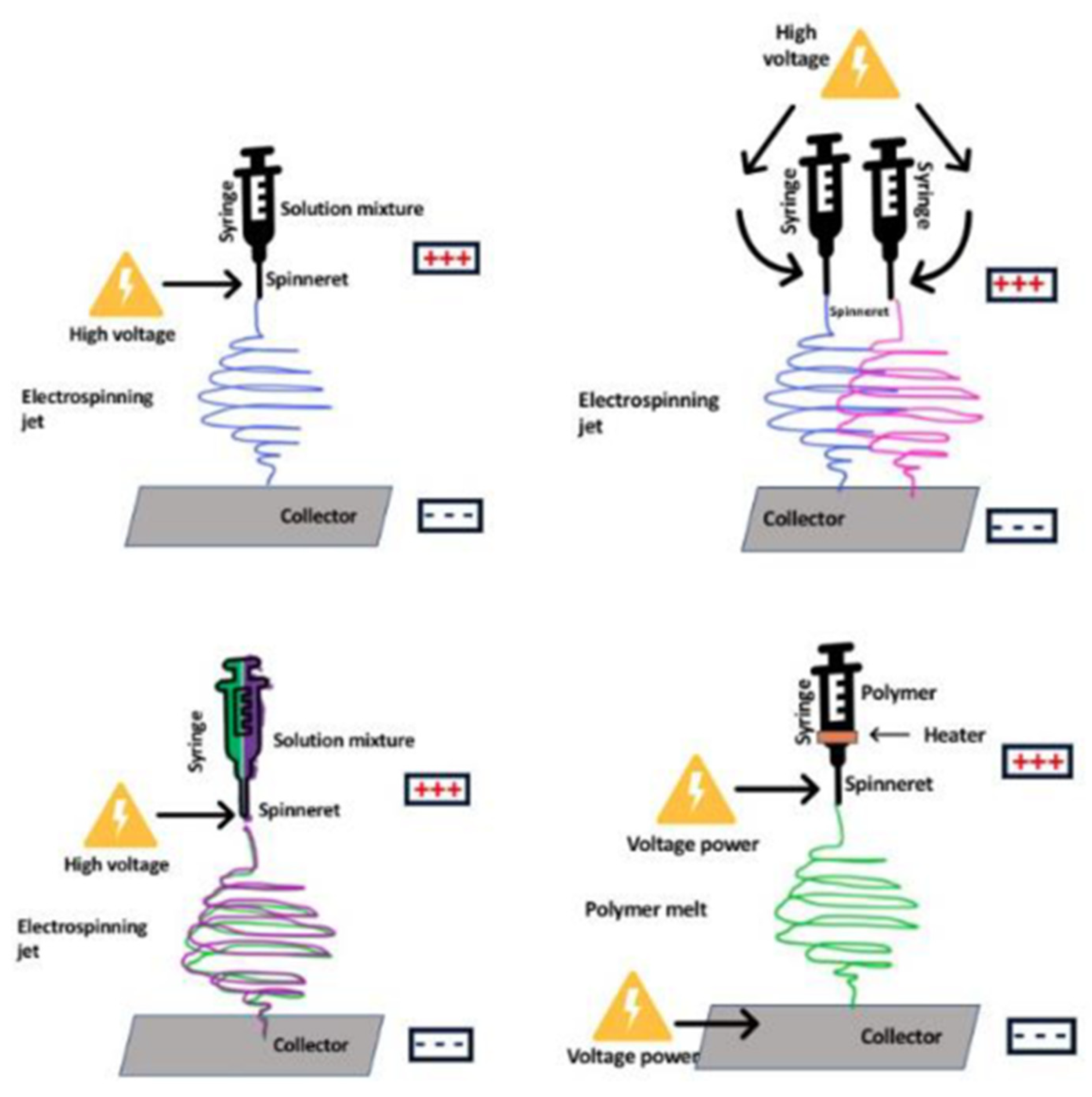
5.3. Electrospinning
6. Challenges and Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy Metal Pollution in Aquatic Ecosystems and its Phytoremediation using Wetland Plants: An ecosustainable approach. Int. J. Phytoremediat. 2008, 10, 133–160. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-Based Hydrogel Beads as a Biocompatible and Efficient Adsorbent for Dye Removal from Aqueous Solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Maity, J.; Ray, S.K. Chitosan based nano composite adsorbent—Synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr. Polym. 2018, 182, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jahani, D.; Nazari, A.; Ghourbanpour, J.; Ameli, A. Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents. Polymers 2020, 12, 2179. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Ciesielczyk, F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, M.; Belkasmi, A.I.; Kastoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Hussain, A.; Madan, S.; Madan, R. Removal of Heavy Metals from Wastewater by Adsorption. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021. [Google Scholar]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Soon, C.F.; Mohamed, R.M.S.R.; Morsin, M.; Zainal, N.; Nayan, N.; Zulkifli, C.Z.; Harun, N.H. A review of nanotechnological applications to detect and control surface water pollution. Environ. Technol. Innov. 2021, 24, 102032. [Google Scholar] [CrossRef]
- Borji, H.; Ayoub, G.M.; Bilbeisi, R.; Nassar, N.; Malaeb, L. How Effective Are Nanomaterials for the Removal of Heavy Metals from Water and Wastewater? Water Air Soil Pollut. 2020, 231, 330. [Google Scholar] [CrossRef]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the Treatment of Heavy Metal Contaminated Water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H.; Zhang, Y.; Zhao, X.; Chen, Y.; Kong, X. Effective removal of heavy metal ions by attapulgite supported sulfidized nanoscale zerovalent iron from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 640, 128192. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive nanocomposite membranes for heavy metal remediation: Recent progresses and challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Cost evaluation for Se(IV) removal, by applying common drinking water treatment processes: Coagulation/precipitation or adsorption. J. Environ. Chem. Eng. 2020, 8, 104209. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Xu, Y.; Cheng, X.; Li, G.; Liang, H. Hybrid UF/NF process treating secondary effluent of wastewater treatment plants for potable water reuse: Adsorption vs. coagulation for removal improvements and membrane fouling alleviation. Environ. Res. 2020, 188, 109833. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Su, J.; He, S.; Zhao, Z.; Liu, X.; Li, H. Efficient preparation of cetyltrimethylammonium bromide-graphene oxide composite and its adsorption of Congo red from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 227–236. [Google Scholar] [CrossRef]
- Yusuf, M.; Elfghi, F.M.; Zaidi, S.A.; Abdullah, E.C.; Khan, M.A. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: A systematic and comprehensive overview. RSC Adv. 2015, 5, 50392–50420. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Toro-Labbé, A. Improving As (iii) adsorption on graphene based surfaces: Impact of chemical doping. Phys. Chem. Chem. Phys. 2015, 17, 12056–12064. [Google Scholar] [CrossRef]
- Kulkarni, D.; Damiri, F.; Rojekar, S.; Zehravi, M.; Ramproshad, S.; Dhoke, D.; Musale, S.; Mulani, A.A.; Modak, P.; Paradhi, R.; et al. Recent Advancements in Microneedle Technology for Multifaceted Biomedical Applications. Pharmaceutics 2022, 14, 1097. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Khatri, O.P. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: Plausible adsorption pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Lee, H.-J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers 2022, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Magnetic Iron Oxide Nanoparticles and Nanohybrids for Advanced Water Treatment Technology. In Handbook of Magnetic Hybrid Nanoalloys and Their Nanocomposites; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–24. [Google Scholar]
- Fouad, D.; Bachra, Y.; Ayoub, G.; Ouaket, A.; Bennamara, A.; Knouzi, N.; Berrada, M. A Novel Drug Delivery System Based on Nanoparticles of Magnetite Fe3O4 Embedded in an Auto Cross-Linked Chitosan. In Chitin and Chitosan—Physicochemical Properties and Industrial Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Ghadimi, M.; Zangenehtabar, S.; Homaeigohar, S. An Overview of the Water Remediation Potential of Nanomaterials and Their Ecotoxicological Impacts. Water 2020, 12, 1150. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-F.; Qin, J.; Xia, P.-F.; Guo, B.-B.; Yang, C.-M.; Song, C.; Wang, S.-G. Graphene oxide–silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Ali Shah, L.; Ali Khan, S. Polymer Hydrogels for Wastewater Treatment. In Environmental Chemistry and Recent Pollution Control Approaches; IntechOpen: London, UK, 2019. [Google Scholar]
- Pardeshi, S.; Damiri, F.; Zehravi, M.; Joshi, R.; Kapare, H.; Prajapati, M.K.; Munot, N.; Berrada, M.; Giram, P.S.; Rojekar, S.; et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers 2022, 14, 3126. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper–poly(amidoxime) composite hydrogel for highly efficient uranium (vi) capture from seawater. Chem. Commun. 2020, 56, 3935–3938. [Google Scholar] [CrossRef]
- Liu, R.; Wen, S.; Sun, Y.; Yan, B.; Wang, J.; Chen, L.; Peng, S.; Ma, C.; Cao, X.; Ma, C.; et al. A nanoclay enhanced Amidoxime-Functionalized Double-Network hydrogel for fast and massive uranium recovery from seawater. Chem. Eng. J. 2021, 422, 130060. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Pillay, K.; Yangkou Mbianda, X. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Arkas, M.; Allabashi, R.; Tsiourvas, D.; Mattausch, E.-M.; Perfler, R. Organic/Inorganic Hybrid Filters Based on Dendritic and Cyclodextrin “Nanosponges” for the Removal of Organic Pollutants from Water. Environ. Sci. Technol. 2006, 40, 2771–2777. [Google Scholar] [CrossRef]
- El Hanache, L.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Daou, T.J. Performance of surfactant-modified *BEA-type zeolite nanosponges for the removal of nitrate in contaminated water: Effect of the external surface. J. Hazard. Mater. 2019, 364, 206–217. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for Water Treatment: Progress and Challenges. Appl. Sci. 2022, 12, 4182. [Google Scholar] [CrossRef]
- Mohamed, H.H. Rationally designed Fe2O3/GO/WO3 Z-Scheme photocatalyst for enhanced solar light photocatalytic water remediation. J. Photochem. Photobiol. A Chem. 2019, 378, 74–84. [Google Scholar] [CrossRef]
- Senathiraja, T.; Lolla, S.A.; Singh, Y.; Kollarahithlu, S.C.; Balakrishnan, R.M. Adsorption of selective fluoroquinolones by cysteine modified silane magnetic nanocomposite from the aqueous phase. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Taib, M.; Damiri, F.; Bachra, Y.; Berrada, M.; Bouyazza, L. Recent Advances in Micro- and Nanoencapsulation of Bioactive Compounds and Their Food Applications. In Nanotechnology in Intelligent Food Packaging; Wiley: Hoboken, NJ, USA, 2022; pp. 271–289. [Google Scholar]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Yan, L.; Yang, Y.; Yang, K.; Yu, S.; Yu, H.; Zhu, B.; Du, B. Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Zhou, J.Z.; Liu, J.; Chi, Y.; Xu, Z.P.; Qian, G. Effective removal of pyrophosphate by Ca–Fe–LDH and its mechanism. Chem. Eng. J. 2012, 179, 72–79. [Google Scholar] [CrossRef]
- Zong, Y.; Li, K.; Tian, R.; Lin, Y.; Lu, C. Highly dispersed layered double oxide hollow spheres with sufficient active sites for adsorption of methyl blue. Nanoscale 2018, 10, 23191–23197. [Google Scholar] [CrossRef]
- Mubarak, M.; Jeon, H.; Islam, M.S.; Yoon, C.; Bae, J.-S.; Hwang, S.-J.; Choi, W.S.; Lee, H.-J. One-pot synthesis of layered double hydroxide hollow nanospheres with ultrafast removal efficiency for heavy metal ions and organic contaminants. Chemosphere 2018, 201, 676–686. [Google Scholar] [CrossRef]
- Zhang, F.; Song, Y.; Song, S.; Zhang, R.; Hou, W. Synthesis of Magnetite–Graphene Oxide-Layered Double Hydroxide Composites and Applications for the Removal of Pb(II) and 2,4-Dichlorophenoxyacetic Acid from Aqueous Solutions. ACS Appl. Mater. Interfaces 2015, 7, 7251–7263. [Google Scholar] [CrossRef] [PubMed]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Rapid adsorption of toxic Pb(II) ions from aqueous solution using multiwall carbon nanotubes synthesized by microwave chemical vapor deposition technique. J. Environ. Sci. 2016, 45, 143–155. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Novel microwave-assisted multiwall carbon nanotubes enhancing Cu (II) adsorption capacity in water. J. Taiwan Inst. Chem. Eng. 2015, 53, 140–152. [Google Scholar] [CrossRef]
- Adegoke, H.I.; AmooAdekola, F.; Fatoki, O.S.; Ximba, B.J. Adsorption of Cr (VI) on synthetic hematite (α-Fe2O3) nanoparticles of different morphologies. Korean J. Chem. Eng. 2014, 31, 142–154. [Google Scholar] [CrossRef]
- Awwad, A.; Amer, M.; Al-aqarbeh, M. TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: Adsorption and thermodynamics of Pb(II) and Cd(II) ions in aqueous solution. Chem. Int. 2020, 6, 168–178. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Qin, L.; Huang, D.; Yi, H.; Liu, X.; Liu, S.; Zhang, M.; Li, B.; Li, L.; et al. Recent progress of noble metals with tailored features in catalytic oxidation for organic pollutants degradation. J. Hazard. Mater. 2022, 422, 126950. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, O.; Hamed, M.G.; Breky, M.; Borai, E.H. Synthesis of magnetic nanoparticles-containing nanocomposite hydrogel and its potential application for simulated radioactive wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126613. [Google Scholar] [CrossRef]
- Kim, J.; Han, S.W.; Kim, J.-C.; Ryoo, R. Supporting Nickel to Replace Platinum on Zeolite Nanosponges for Catalytic Hydroisomerization of n-Dodecane. ACS Catal. 2018, 8, 10545–10554. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-Cross-Linked β-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef] [PubMed]
- Ranjan Rout, D.; Mohan Jena, H. Synthesis of novel reduced graphene oxide decorated β-cyclodextrin epichlorohydrin composite and its application for Cr(VI) removal: Batch and fixed-bed studies. Sep. Purif. Technol. 2021, 278, 119630. [Google Scholar] [CrossRef]
- Lujanienė, G.; Šemčuk, S.; Lečinskytė, A.; Kulakauskaitė, I.; Mažeika, K.; Valiulis, D.; Pakštas, V.; Skapas, M.; Tumėnas, S. Magnetic graphene oxide based nano-composites for removal of radionuclides and metals from contaminated solutions. J. Environ. Radioact. 2017, 166, 166–174. [Google Scholar] [CrossRef]
- Mubarak, M.; Islam, M.S.; Yoon, D.-Y.; Lee, J.H.; Park, H.J.; Bae, J.-S.; Lee, H.-J. Flower-like Mg/Fe-layered double oxide nanospheres with ultrahigh adsorption efficiency for anionic organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126446. [Google Scholar] [CrossRef]
- Parvin, F.; Rikta, S.Y.; Tareq, S.M. Application of Nanomaterials for the Removal of Heavy Metal from Wastewater. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–157. [Google Scholar]
- Kabir, M.T.; Ferdous Mitu, J.; Akter, R.; Akhtar, M.F.; Saleem, A.; Al-Harrasi, A.; Bhatia, S.; Rahman, M.S.; Damiri, F.; Berrada, M.; et al. Therapeutic potential of dopamine agonists in the treatment of type 2 diabetes mellitus. Environ. Sci. Pollut. Res. 2022, 29, 46385–46404. [Google Scholar] [CrossRef]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep. 2021, 11, 3790. [Google Scholar] [CrossRef]
- Biswas, S.; Fatema, J.; Debnath, T.; Rashid, T.U. Chitosan–Clay Composites for Wastewater Treatment: A State-of-the-Art Review. ACS ES&T Water 2021, 1, 1055–1085. [Google Scholar] [CrossRef]
- Kolluru, S.S.; Agarwal, S.; Sireesha, S.; Sreedhar, I.; Kale, S.R. Heavy metal removal from wastewater using nanomaterials-process and engineering aspects. Process Saf. Environ. Prot. 2021, 150, 323–355. [Google Scholar] [CrossRef]
- Jayan, N.; Metta, L.D.B. Process optimization by response surface methodology-central composite design for the adsorption of lead by green synthesized TiO2 using Phyllanthus acidus extract. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Sillanpää, M.; Makgwane, P.R.; Kumar, S.; Kumari, K. Carbon nano-structures and functionalized associates: Adsorptive detoxification of organic and inorganic water pollutants. Inorg. Chem. Commun. 2022, 141, 109579. [Google Scholar] [CrossRef]
- Sasidharan, V.; Damiri, F.; Talreja, N.; Chauhan, D.; Mangalaraja, R.V.; Berrada, M.; Ashfaq, M. Carbon-Based Nanomaterials: An Efficient Tool for Improving the Nutritional Quality of Crops. In Metabolic Engineering in Plants; Springer Nature: Singapore, 2022; pp. 375–389. [Google Scholar]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Drumond, G.P.; da Silva, M.M.; de Bernardes, R.O.; de Santos, L.V.S.; Jacob, R.S.; Viana, M.M.; de Vasconcelos, C.K.B. Graphene oxide for efficient treatment of real contaminated water by mining tailings: Metal adsorption studies to Paraopeba river and risk assessment. Chem. Eng. J. Adv. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Gao, C.; Dong, Z.; Hao, X.; Yao, Y.; Guo, S. Preparation of Reduced Graphene Oxide Aerogel and Its Adsorption for Pb(II). ACS Omega 2020, 5, 9903–9911. [Google Scholar] [CrossRef]
- Zunita, M.; Irawanti, R.; Koesmawati, T.A.; Lugito, G.; Wentena, I.G. Graphene Oxide (GO) Membrane in Removing Heavy Metals from Wastewater: A Review. Chem. Eng. Trans. 2020, 82, 415–420. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef] [Green Version]
- Damiri, F.; Rahman, M.H.; Zehravi, M.; Awaji, A.A.; Nasrullah, M.Z.; Gad, H.A.; Bani-Fwaz, M.Z.; Varma, R.S.; Germoush, M.O.; Al-malky, H.S.; et al. MXene (Ti3C2Tx)-Embedded Nanocomposite Hydrogels for Biomedical Applications: A Review. Materials 2022, 15, 1666. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Sep. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Jie, F.; Zhao, Z.; Zhou, K.; Liu, H. The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater. Sci. Rep. 2021, 11, 16878. [Google Scholar] [CrossRef]
- Adam, A.M.; Saad, H.A.; Atta, A.A.; Alsawat, M.; Hegab, M.S.; Altalhi, T.A.; Refat, M.S. An Environmentally Friendly Method for Removing Hg(II), Pb(II), Cd(II) and Sn(II) Heavy Metals from Wastewater Using Novel Metal–Carbon-Based Composites. Crystals 2021, 11, 882. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Feng, L.; Yan, B.; Zheng, J.; Chen, J.; Wei, R.; Jiang, S.; Yang, W.; Zhang, Q.; He, S. Soybean protein-derived N, O co-doped porous carbon sheets for supercapacitor applications. New J. Chem. 2022, 46, 10844–10853. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, Q.; Chen, J.; Ma, B.; Chen, S. Carbothermal preparation of porous carbon-encapsulated iron composite for the removal of trace hexavalent chromium. Chem. Eng. J. 2014, 253, 24–33. [Google Scholar] [CrossRef]
- Wu, J.; Yan, X.; Li, L.; Gu, J.; Zhang, T.; Tian, L.; Su, X.; Lin, Z. High-efficiency adsorption of Cr(VI) and RhB by hierarchical porous carbon prepared from coal gangue. Chemosphere 2021, 275, 130008. [Google Scholar] [CrossRef]
- Kaur, S.; Roy, A. Bioremediation of heavy metals from wastewater using nanomaterials. Environ. Dev. Sustain. 2021, 23, 9617–9640. [Google Scholar] [CrossRef]
- El-Tawil, R.S.; El-Wakeel, S.T.; Abdel-Ghany, A.E.; Abuzeid, H.A.M.; Selim, K.A.; Hashem, A.M. Silver/quartz nanocomposite as an adsorbent for removal of mercury (II) ions from aqueous solutions. Heliyon 2019, 5, e02415. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.K.; Andra, S.; Damiri, F.; Sivaramalingam, A.; Sudandaradoss, M.V.; Kumarasamy, K.; Bhakthavachalam, K.; Ali, F.; Kundu, M.K.; Rahman, M.H.; et al. Size-Dependent Antibacterial, Antidiabetic, and Toxicity of Silver Nanoparticles Synthesized Using Solvent Extraction of Rosa indica L. Petals. Pharmaceuticals 2022, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Attatsi, I.K.; Nsiah, F. Application of silver nanoparticles toward Co(II) and Pb(II) ions contaminant removal in groundwater. Appl. Water Sci. 2020, 10, 152. [Google Scholar] [CrossRef]
- Ituen, E.; Yuanhua, L.; Verma, C.; Alfantazi, A.; Akaranta, O.; Ebenso, E.E. Synthesis and characterization of walnut husk extract-silver nanocomposites for removal of heavy metals from petroleum wastewater and its consequences on pipework steel corrosion. J. Mol. Liq. 2021, 335, 116132. [Google Scholar] [CrossRef]
- Negi, S.; Singh, V.; Rawat, J. Green synthesis of silver nanoparticles using microalgal extract and its application in metal ion removal from aqueous solution. J. Exp. Biol. Agric. Sci. 2021, 9, 214–230. [Google Scholar] [CrossRef]
- Hyder, A.; Buledi, J.A.; Nawaz, M.; Rajpar, D.B.; Shah, Z.-H.; Orooji, Y.; Yola, M.L.; Karimi-Maleh, H.; Lin, H.; Solangi, A.R. Identification of heavy metal ions from aqueous environment through gold, Silver and Copper Nanoparticles: An excellent colorimetric approach. Environ. Res. 2022, 205, 112475. [Google Scholar] [CrossRef]
- He, Z.; Yin, H.; Chang, C.-C.; Wang, G.; Liang, X. Interfacing DNA with Gold Nanoparticles for Heavy Metal Detection. Biosensors 2020, 10, 167. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Z.; Zhang, C.; Wei, X.; Lou, T.; Zhao, Y. Colorimetric Detection of Hg2+ Based on the Growth of Aptamer-Coated AuNPs: The Effect of Prolonging Aptamer Strands. Small 2017, 13, 1603370. [Google Scholar] [CrossRef]
- De Ruíz-Baltazar, Á.J. Environmentally friendly alternative for heavy metal adsorption based on doped diatoms with Au nanoparticles: A novel approach in green synthesis of adsorbents and kinetic adsorption study. Colloid Interface Sci. Commun. 2022, 46, 100559. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Liu, S.; Du, L.; Chen, Q.; Li, X.; Dong, J.; Zhang, Z.; Lu, S.; Gong, Y.; et al. Insights into the Synthesis, types and application of iron Nanoparticles: The overlooked significance of environmental effects. Environ. Int. 2022, 158, 106980. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Habib, F.; Darwish, N.; Shanableh, A. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 2021, 380, 219–228. [Google Scholar] [CrossRef]
- Saleh, M.; Isik, Z.; Aktas, Y.; Arslan, H.; Yalvac, M.; Dizge, N. Green synthesis of zero valent iron nanoparticles using Verbascum thapsus and its Cr (VI) reduction activity. Bioresour. Technol. Rep. 2021, 13, 100637. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100443. [Google Scholar] [CrossRef]
- Taman, R.; Ossman, M.E.; Mansour, M.S.; Farag, H.A. Metal Oxide Nano-particles as an Adsorbent for Removal of Heavy Metals. J. Adv. Chem. Eng. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 12547. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, M.S. Vanadium doped titania nanoparticles for photocatalytic removal of heavy metals from aqueous solutions. J. Exp. Nanosci. 2021, 16, 51–61. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Bitar-Castro, N.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; González-Delgado, Á.D. Nickel adsorption from aqueous solution using lemon peel biomass chemically modified with TiO2 nanoparticles. Sustain. Chem. Pharm. 2020, 17, 100299. [Google Scholar] [CrossRef]
- Fatehizadeh, A.; Rahimi, S.; Ahmadian, M.; Barati, R.; Yousefi, N.; Moussavi, S.; Rahimi, K.; Reshadat, S.; Ghasemi, S.; Gilan, N. Photocatalytic removal of cadmium (II) and lead (II) from simulated wastewater at continuous and batch system. Int. J. Environ. Health Eng. 2014, 3, 31. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of chromium (VI) ions from aqueous solutions using amine-impregnated TiO2 nanoparticles modified cellulose acetate membranes. Chemosphere 2018, 191, 673–684. [Google Scholar] [CrossRef]
- Madzokere, T.C.; Karthigeyan, A. Heavy Metal Ion Effluent Discharge Containment Using Magnesium Oxide (MgO) Nanoparticles. Mater. Today Proc. 2017, 4, 9–18. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Wu, D.; Wang, W.; Tan, F.; Wang, X.; Wong, P.K.; Qiao, X. Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. Chem. Eng. J. 2017, 312, 158–166. [Google Scholar] [CrossRef]
- Habiby, S.R.; Esmaeili, H.; Foroutan, R. Magnetically modified MgO nanoparticles as an efficient adsorbent for phosphate ions removal from wastewater. Sep. Sci. Technol. 2020, 55, 1910–1921. [Google Scholar] [CrossRef]
- Abuhatab, S.; El-Qanni, A.; Al-Qalaq, H.; Hmoudah, M.; Al-Zerei, W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manag. 2020, 268, 110713. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater. Colloid Interface Sci. Commun. 2021, 41, 100380. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Gu, M.; Hao, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Rodríguez, A.R.C.; McCarthy, J.E.; Alonso, A.; Moral-Vico, J.; Font, X.; Gun’ko, Y.K.; Sánchez, A. Cerium oxide nanoparticles anchored onto graphene oxide for the removal of heavy metal ions dissolved in water. Desalin. Water Treat. 2018, 124, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Dave, P.N.; Chopda, L.V. Application of Iron Oxide Nanomaterials for the Removal of Heavy Metals. J. Nanotechnol. 2014, 2014, 398569. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, Y.; Ma, X.; Guo, H.; Hu, J.; Zhang, K.; Jiang, S.; Yang, W.; Duan, G. Excellent fluoride removal performance by electrospun La–Mn bimetal oxide nanofibers. New J. Chem. 2022, 46, 490–497. [Google Scholar] [CrossRef]
- Jian, S.; Shi, F.; Hu, R.; Liu, Y.; Chen, Y.; Jiang, W.; Yuan, X.; Hu, J.; Zhang, K.; Jiang, S.; et al. Electrospun magnetic La2O3–CeO2–Fe3O4 composite nanofibers for removal of fluoride from aqueous solution. Compos. Commun. 2022, 33, 101194. [Google Scholar] [CrossRef]
- Ali, K.; Javaid, M.U.; Ali, Z.; Zaghum, M.J. Biomass-Derived Adsorbents for Dye and Heavy Metal Removal from Wastewater. Adsorpt. Sci. Technol. 2021, 2021, 9357509. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef] [PubMed]
- Sachan, D.; Ramesh, A.; Das, G. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Chen, L.; Ji, T.; Mu, L.; Shi, Y.; Brisbin, L.; Guo, Z.; Khan, M.A.; Young, D.P.; Zhu, J. Facile synthesis of mesoporous carbon nanocomposites from natural biomass for efficient dye adsorption and selective heavy metal removal. RSC Adv. 2016, 6, 2259–2269. [Google Scholar] [CrossRef] [Green Version]
- Darweesh, M.A.; Elgendy, M.Y.; Ayad, M.I.; Ahmed, A.M.M.; Kamel Elsayed, N.M.; Hammad, W.A. A unique, inexpensive, and abundantly available adsorbent: Composite of synthesized silver nanoparticles (AgNPs) and banana leaves powder (BLP). Heliyon 2022, 8, e09279. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, Z.; Marahel, F.; Hamoule, T.; Mombeni Goodajdar, B. Removal of Ni(II) and Co(II) ions from aqueous solutions utilizing Origanum majorana-capped silver nanoparticles. Desalin. Water Treat. 2021, 213, 381–394. [Google Scholar] [CrossRef]
- Hua, J. Synthesis and characterization of gold nanoparticles (AuNPs) and ZnO decorated zirconia as a potential adsorbent for enhanced arsenic removal from aqueous solution. J. Mol. Struct. 2021, 1228, 129482. [Google Scholar] [CrossRef]
- Zaidi, R.; Khan, S.U.; Azam, A.; Farooqi, I.H. A study on effective adsorption of lead from an aqueous solution using Copper Oxide nanoparticles. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1058, p. 012074. [Google Scholar] [CrossRef]
- Elumalai, P.; Shanmugavel, S. Performance of copper oxide nanoparticles treated polyaniline-itaconic acid based magnetic sensitive polymeric nanocomposites for the removal of chromium ion from industrial wastewater. Polym. Technol. Mater. 2021, 60, 2042–2056. [Google Scholar] [CrossRef]
- Kumar K, A.; Yeshwanth, M.; Kumar B, K.; Panwar, J.; Gupta, S. Functionalized Cu-based metal oxide nanoparticles with enhanced Cd+2 adsorption capacity and their ecotoxicity assessment by molecular docking. J. Environ. Manag. 2022, 307, 114523. [Google Scholar] [CrossRef]
- Venkatraman, Y.; Priya, A.K. Removal of heavy metal ion concentrations from the wastewater using tobacco leaves coated with iron oxide nanoparticles. Int. J. Environ. Sci. Technol. 2022, 19, 2721–2736. [Google Scholar] [CrossRef]
- Farhana, A.; Jenifer Selvarani, A.; Samrot, A.V.; Alsrhani, A.; Raji, P.; Sahithya, C.S.; Jane Cypriyana, P.J.; Senthilkumar, P.; Ling, M.P.; Yishak, S. Utilization of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Impregnated Activated Carbon for Removal of Hexavalent Chromium. J. Nanomater. 2022, 2022, 4326939. [Google Scholar] [CrossRef]
- Park, J.; Shin, J.-H.; Oh, W.; Choi, S.-J.; Kim, J.; Kim, C.; Jeon, J. Removal of Hexavalent Chromium(VI) from Wastewater Using Chitosan-Coated Iron Oxide Nanocomposite Membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.U.; Man, H.C.; Azis, R.S.; Isma Idris, A.; Hazwan Hamzah, M.; Yunos, K.F.; Katibi, K.K. Novel PVDF-PVP Hollow Fiber Membrane Augmented with TiO2 Nanoparticles: Preparation, Characterization and Application for Copper Removal from Leachate. Nanomaterials 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Ajala, M.A.; Abdulkareem, A.S.; Tijani, J.O.; Kovo, A.S. Adsorptive behaviour of rutile phased titania nanoparticles supported on acid-modified kaolinite clay for the removal of selected heavy metal ions from mining wastewater. Appl. Water Sci. 2022, 12, 19. [Google Scholar] [CrossRef]
- Badawy, A.A.; Ghanem, A.F.; Yassin, M.A.; Youssef, A.M.; Abdel Rehim, M.H. Utilization and characterization of cellulose nanocrystals decorated with silver and zinc oxide nanoparticles for removal of lead ion from wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100501. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Khalid, M. Optimization of fluoride removal by Al doped ZnO nanoparticles using response surface methodology from groundwater. Chemosphere 2021, 284, 131317. [Google Scholar] [CrossRef]
- Mondal, R.; De, S. Removal of copper(II) from aqueous solution using zinc oxide nanoparticle impregnated mixed matrix hollow fiber membrane. Environ. Technol. Innov. 2022, 26, 102300. [Google Scholar] [CrossRef]
- Uko, C.A.; Tijani, J.O.; Abdulkareem, S.A.; Mustapha, S.; Egbosiuba, T.C.; Muzenda, E. Adsorptive properties of MgO/WO3 nanoadsorbent for selected heavy metals removal from indigenous dyeing wastewater. Process Saf. Environ. Prot. 2022, 162, 775–794. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Gran, S.; Aziz, R.; Rafiq, M.T.; Abbasi, M.; Qayyum, A.; Elnaggar, A.Y.; Elganzory, H.H.; El-Bahy, Z.M.; Hussein, E.E. Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals. Polymers 2021, 13, 4464. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, K.; Khan, F.; Verma, D.K.; Agrawal, S. Effective removal of uranium from aqueous solution by using cerium oxide nanoparticles derived from citrus limon peel extract. J. Radioanal. Nucl. Chem. 2022. [Google Scholar] [CrossRef]
- Ramadan, R.; El-Masry, M.M. Comparative study between CeO2/Zno and CeO2/SiO2 nanocomposites for (Cr6+) heavy metal removal. Appl. Phys. A 2021, 127, 876. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Kishore Chand, A.A.; Bajer, B.; Schneider, E.S.; Mantel, T.; Ernst, M.; Filiz, V.; Glass, S. Modification of Polyacrylonitrile Ultrafiltration Membranes to Enhance the Adsorption of Cations and Anions. Membranes 2022, 12, 580. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Jia, Z.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Progress in adsorptive membranes for separation—A review. Sep. Purif. Technol. 2021, 255, 117772. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Ma, H.; Yu, M.; Li, L.; Li, J. Electrospun nanofibrous polyethylenimine mat: A potential adsorbent for the removal of chromate and arsenate from drinking water. RSC Adv. 2016, 6, 30739–30746. [Google Scholar] [CrossRef]
- Kampalanonwat, P.; Supaphol, P. Preparation and Adsorption Behavior of Aminated Electrospun Polyacrylonitrile Nanofiber Mats for Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2010, 2, 3619–3627. [Google Scholar] [CrossRef]
- Glass, S.; Mantel, T.; Appold, M.; Sen, S.; Usman, M.; Ernst, M.; Filiz, V. Amine-Terminated PAN Membranes as Anion-Adsorber Materials. Chem. Ing. Tech. 2021, 93, 1396–1400. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Bae, T.-H.; Tak, T.-M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Ghaemi, N.; Zereshki, S.; Heidari, S. Removal of lead ions from water using PES-based nanocomposite membrane incorporated with polyaniline modified GO nanoparticles: Performance optimization by central composite design. Process Saf. Environ. Prot. 2017, 111, 475–490. [Google Scholar] [CrossRef]
- Abdullah, N.; Gohari, R.J.; Yusof, N.; Ismail, A.F.; Juhana, J.; Lau, W.J.; Matsuura, T. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: Preparation, characterization and its adsorptive removal of lead (II) from aqueous solution. Chem. Eng. J. 2016, 289, 28–37. [Google Scholar] [CrossRef]
- Sankır, M.; Bozkır, S.; Aran, B. Preparation and performance analysis of novel nanocomposite copolymer membranes for Cr(VI) removal from aqueous solutions. Desalination 2010, 251, 131–136. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Lakhotia, S.R.; Ghosh, A.K.; Bindal, R.C. Removal of arsenic from aqueous media using zeolite/chitosan nanocomposite membrane. Sep. Sci. Technol. 2019, 54, 282–288. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Mostofi, M.; Alimohammadi, M.; McKay, G.; Yetilmezsoy, K.; Albadarin, A.B.; Heibati, B.; AlGhouti, M.; Mubarak, N.M.; Sahu, J.N. High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: Kinetics, adsorption, mechanism and optimization studies. J. Ind. Eng. Chem. 2016, 35, 63–74. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Liu, M.; Tian, J.; Zeng, G.; Li, Z.; Wang, K.; Zhang, Q.; Wan, Q.; Deng, F.; et al. Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2+ removal. Appl. Surf. Sci. 2015, 343, 19–27. [Google Scholar] [CrossRef]
- Ahmed, M.; Elektorowicz, M.; Hasan, S.W. GO, SiO2, and SnO2 nanomaterials as highly efficient adsorbents for Zn2+ from industrial wastewater—A second stage treatment to electrically enhanced membrane bioreactor. J. Water Process Eng. 2019, 31, 100815. [Google Scholar] [CrossRef]
- Kettum, W.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Chanlek, N.; Samart, C. Heavy metal sequestration with a boronic acid-functionalized carbon-based adsorbent. J. Environ. Chem. Eng. 2018, 6, 1147–1154. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalt. Trans. 2013, 42, 5682. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Y.; Gong, Y.; Lyu, H.; Wang, Q.; Ma, J. Preparation of a novel graphene oxide/Fe-Mn composite and its application for aqueous Hg(II) removal. J. Hazard. Mater. 2016, 316, 151–158. [Google Scholar] [CrossRef]
- Mondal, M.; Dutta, M.; De, S. A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: Spinning, characterization and application in heavy metal removal. Sep. Purif. Technol. 2017, 188, 155–166. [Google Scholar] [CrossRef]
- Rowley, J.; Abu-Zahra, N.H. Synthesis and characterization of polyethersulfone membranes impregnated with (3-aminopropyltriethoxysilane) APTES-Fe3O4 nanoparticles for As(V) removal from water. J. Environ. Chem. Eng. 2019, 7, 102875. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Walter, R.; Friedrich, K. Structure–property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites. Polymer 2001, 42, 167–183. [Google Scholar] [CrossRef]
- Fotooh Abadi, L.; Damiri, F.; Zehravi, M.; Joshi, R.; Pai, R.; Berrada, M.; Massoud, E.E.S.; Rahman, M.H.; Rojekar, S.; Cavalu, S. Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs. Polymers 2022, 14, 3090. [Google Scholar] [CrossRef]
- Murphy, T.M.; Offord, G.T.; Paul, D.R. Fundamentals of Membrane Gas Separation. In Membrane Operations; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 63–82. [Google Scholar]
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Membr. Sci. 2008, 320, 363–371. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wei, X.; Tian, X.; Wang, J.; Yang, S.; Wang, S. Comparison study of the effect of PVP and PANI nanofibers additives on membrane formation mechanism, structure and performance. J. Membr. Sci. 2011, 385–386, 110–122. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415–416, 250–259. [Google Scholar] [CrossRef]
- Moradihamedani, P.; Kalantari, K.; Abdullah, A.H.; Morad, N.A. High efficient removal of lead(II) and nickel(II) from aqueous solution by novel polysulfone/Fe3O4–talc nanocomposite mixed matrix membrane. Desalin. Water Treat. 2016, 57, 28900–28909. [Google Scholar] [CrossRef]
- Moradihamedani, P.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Yusof, N.A. Separation of CO2 from CH4 by pure PSF and PSF/PVP blend membranes: Effects of type of nonsolvent, solvent, and PVP concentration. J. Appl. Polym. Sci. 2013, 130, 1139–1147. [Google Scholar] [CrossRef]
- Castejón, P.; Habibi, K.; Saffar, A.; Ajji, A.; Martínez, A.; Arencón, D. Polypropylene-Based Porous Membranes: Influence of Polymer Composition, Extrusion Draw Ratio and Uniaxial Strain. Polymers 2017, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Microporous membranes obtained from PP/HDPE multilayer films by stretching. J. Membr. Sci. 2009, 345, 148–159. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Abdrashitov, E.F.; Kritskaya, D.A.; Bokun, V.C.; Ponomarev, A.N.; Novikova, K.S.; Sanginov, E.A.; Dobrovolsky, Y.A. Synthesis and properties of stretched polytetrafluoroethylene–sulfonated polystyrene nanocomposite membranes. Solid State Ion. 2016, 286, 135–140. [Google Scholar] [CrossRef]
- Sun, H.; Rhee, K.B.; Kitano, T.; Mah, S.I. High-density polyethylene (HDPE) hollow fiber membrane via thermally induced phase separation. I. Phase separation behaviors of HDPE-liquid paraffin (LP) blends and its influence on the morphology of the membrane. J. Appl. Polym. Sci. 1999, 73, 2135–2142. [Google Scholar] [CrossRef]
- Lin, T. (Ed.) Nanofibers—Production, Properties and Functional Applications; InTech: London, UK, 2011; ISBN 978-953-307-420-7. [Google Scholar]
- Gopal, R.; Kaur, S.; Ma, Z.; Chan, C.; Ramakrishna, S.; Matsuura, T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006, 281, 581–586. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Berrada, M. Synthesis and characterization of 4-formylphenylboronic acid cross-linked chitosan hydrogel with dual action: Glucose-sensitivity and controlled insulin release. Chin. J. Anal. Chem. 2022, 50, 100092. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; Akisin, C.J.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric Biomaterials for Wound Healing Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022. [Google Scholar] [CrossRef]
- Hota, G.; Kumar, B.R.; Ng, W.J.; Ramakrishna, S. Fabrication and characterization of a boehmite nanoparticle impregnated electrospun fiber membrane for removal of metal ions. J. Mater. Sci. 2008, 43, 212–217. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.; Tijing, L. Progress on the Fabrication and Application of Electrospun Nanofiber Composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef]
- Pant, H.R.; Bajgai, M.P.; Nam, K.T.; Seo, Y.A.; Pandeya, D.R.; Hong, S.T.; Kim, H.Y. Electrospun nylon-6 spider-net like nanofiber mat containing TiO2 nanoparticles: A multifunctional nanocomposite textile material. J. Hazard. Mater. 2011, 185, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.A.; Singh, G.; Rana, D.; Matsuura, T.; Anbharasi, V.; Shanmugasundaram, T.S. Preparation and characterization of highly hydrophobic poly(vinylidene fluoride)—Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 2012, 397–398, 80–86. [Google Scholar] [CrossRef]
- Park, S.-W.; Bae, H.-S.; Xing, Z.-C.; Kwon, O.H.; Huh, M.-W.; Kang, I.-K. Preparation and properties of silver-containing nylon 6 nanofibers formed by electrospinning. J. Appl. Polym. Sci. 2009, 112, 2320–2326. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Vossoughi, M.; Mahmoodi, N.M.; Sadrzadeh, M. Clay-based electrospun nanofibrous membranes for colored wastewater treatment. Appl. Clay Sci. 2019, 168, 77–86. [Google Scholar] [CrossRef]

| Nanomaterial | Example | Particle Size | Absorption Capacity | Pollutants | Ref |
|---|---|---|---|---|---|
| (A) Carbon-Based Nanomaterials | |||||
| Graphene-based nanomaterials (GNMs): | Graphene oxide (GO) and TiO2@rGO nanohybrids | 25 nm | Organic solvent | [63] | |
| Carbon nanotubes (CNTs) | Microwave-heated MWCNTs | 10–23 nm 18 to 28 nm | 104.2 mg/g 99 mg/g | Pb (II) Cu (II) | [64,65] |
| (B) Metal and Metal Oxide-Based Nanoparticles | |||||
| Nanosized iron oxide | Fe3O4, α-Fe2O3, μ-Fe2O3 | 15.69–85.84 nm | 6.33–200 mg/g | Cr(VI) and As(V) | [66] |
| Nanosized titanium dioxide | TiO2 | 18 nm | 333.33 mg/g 250 mg/g | Pb(II) Cd(II) | [67] |
| Noble metal-based nanoparticles | Au, TiO2 NBsa/Au NPs | 5–15 | - | Tetrabromobisphenol A | [68] |
| (C) Hydrogels | |||||
| Magnetite in polystyrene-co-polymethacrylic acid (PS-co-PMAA) | Fe3O4, PS-co-PMAA | ~100 nm | 8.49 to 53.37, 11.17–80.69, and 10.75–65.35 mg/g | Cs+, Co2+, and Sr2+ | [69] |
| (D) Nano-sponges | |||||
| Zeolite nanosponges | Ni | 3–5 nm | - | Nitrate in contaminated water | [70] |
| Cyclodextrin-based nanosponges | EDTA-cross-linked β-cyclodextrin | - | 1.241 and 1.106 mmol.g–1 | Cu(II) and Cd(II) | [71] |
| β-cyclodextrin covalently cross-linked tannic acid | Reduced graphene oxide (RGO), beta-cyclodextrin (βCD), and epichlorohydrin | - | 1321.01 mg/g | Cr(VI) | [72] |
| (E) Nanocomposites | |||||
| Magnetic nanocomposites | - | <10 nm | 29 to 641 mg/g 333–362 mg/g | Co(II), Ni(II), Cu(II), and Pb(II) Cs(I) | [73] |
| Mineral-based nanocomposites | Nickel ferrite nanocomposite functionalized with L-cysteine-attached 3-glycidyloxypropyltrimethoxysilane | 10–15 nm | 122 mg/g, 135 mg/g, and 150 mg/g | Remove the fluoroquinolone class of antibiotics (lomefloxacin, ciprofloxacin, and norfloxacin) | [54] |
| (F) Layered Double Hydroxide (LDH)-Based Materials | |||||
| Mg/Fe-LDO hollow nanospheres | Flower-like Mg/Fe-layered double oxide | 17.1 nm | 1250 mg/g and 2000 mg/g | Organic dyes: Congo red and methylene blue | [74] |
| Fe3O4/graphene oxide/LDH | - | 20 nm | - | Pd (II) and 2,4-dichlorophenoxyacetic acid from an aqueous system | [62] |
| Type of Adsorbent | Shape and Size | Specific Surface Area | Heavy Metals | Removal Rate/Adsorption Capacity | Isotherm Model | pH | Dosage | Reference |
|---|---|---|---|---|---|---|---|---|
| AgNPs | Spherical, 46.2 nm | - | Pb2+, Cr6+, Cd2+ | 72.6% 81.3% 88.1% | Freundlich model | 8.68 | 0.75 g | [102] |
| AgNPs/banana leaf powder composite | Semi-spherical | - | Zn2+, Pb2+, Fe3+ | 79% 88% 91% | Langmuir model | 5 and 6 | 0.05 to 0.25 g | [134] |
| AgNPs | Spherical | 250 m2/g | Ni2+, Co2+ | 88% | Langmuir model | 9 and 7 | 40 and 50 mg | [135] |
| AuNPs/ZnO-ZrO2 composite | Granular particles | 115.03 m2/g | As5+ | 88% | Gunary model | 10 | 0.01 g | [136] |
| CuO nanoparticles | 21.6 nm | - | Pb2+ | 84.2% | Freundlich model | 2–6 | 0.1–1.0 g/L | [137] |
| Polyaniline/itaconic acid/copper oxide nanocomposite | Oval shape, 20 nm | - | Cr6+ | 75–96% | Langmuir and Freundlich models | 2–6 | 0.2–1.0 g | [138] |
| CuO nanoparticles | Spherical, 150 nm | 20 m2/g | Pb2+, Ni2+, Cd2+ | 18% 52% 84% | Freundlich model | 6 | 0.33 g/L | [113] |
| Diethylene glycol-functionalized Cu2O NPs | Quasi-spherical, 57.4 nm | 5.35 m2/g | Cd2+ | 98% | Langmuir model | 6.3 | 1.0 g/L | [139] |
| Fe2O3 nanoparticles | Spherical, 23 nm | - | Cr6+, Pb2+, Zn2+ | 92.26% 75.57% 89.36% | Thomas, Yoon–Nelson and BDST kinetic models | 6 | - | [140] |
| Superparamagnetic Fe2O3/activate carbon | Spherical, 23–35 nm | - | Cr6+ | 99.7% | Freundlich isotherm model | 3 | 10 g/L | [141] |
| Chitosan/Fe2O3/PVDF composite membrane | - | - | Cr6+ | 90.45% | Langmuir model | 4 | - | [142] |
| PVDF/PVP/TiO2 membrane | - | - | Cu2+ | 96.36% | Freundlich isotherm model | 10 | 1 wt.% TiO2 | [143] |
| Acid-activated kaolinite clay/titanium oxide nanocomposite | - | 32.98 m2/g | Mn2+, Fe3+, Pb2+, Cu2+ | 89.37% 91.99% 81.95% 32.39% | Langmuir model | 10 | 0.5 g | [144] |
| Cellulose nanocrystals/Ag or ZnO | - | - | Pb2+ | 94% | Langmuir model | 2–8 | 0.05 g | [145] |
| Al doped ZnO nanoparticles | - | 20.76 m2/g | F– | 98% | Temkin isotherm model | 7 | 0.005 g | [146] |
| ZnO hollow fiber membrane | 35–85 nm | - | Cu2+ | 92% | Langmuir model | 8 | 2 wt.% | [147] |
| MgO/WO3 nanocomposites | Spherical | 104.2 m2/g | Cu2+ Fe3+ Cr6+ | 98.1% 100% 100% | Langmuir model | - | - | [148] |
| MgO nanoparticles | Rod shape 30–85 nm (length) and 8.3–16.4 nm (width) | - | Cr6+, Co2+, Pb2+, Cd2+, Ni2+ | 94.2%, 63.4%, 72.7%, 74.1%, 70.8% | - | - | 100 mg | [149] |
| MgO nanoparticles | Cubic shape, 25–39 nm | - | PO43- | 72% | Freundlich isotherm models | 12 | 0.01 g | [148] |
| Cerium oxide/corncob nanocomposite | - | - | Cd2+ Cr6+ | 95% 88% | Intra-particle diffusion model | 9 | 20 mg | [150] |
| CeO2 nanoparticles | Spherical, 10 nm | - | UO22+ | 96% | Langmuir and Freundlich models | 4 | 0.003 mg | [151] |
| Ce2O3/SiO2/and Ce2O3/ZnO | Spherical shape | 84.62 m2/g and 46.12 m2/g | Cr6+ | 55% and 50% | Langmuir isotherm model | 7 | 0.02 g | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. https://doi.org/10.3390/ma15155392
Damiri F, Andra S, Kommineni N, Balu SK, Bulusu R, Boseila AA, Akamo DO, Ahmad Z, Khan FS, Rahman MH, et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials. 2022; 15(15):5392. https://doi.org/10.3390/ma15155392
Chicago/Turabian StyleDamiri, Fouad, Swetha Andra, Nagavendra Kommineni, Satheesh Kumar Balu, Raviteja Bulusu, Amira A. Boseila, Damilola O. Akamo, Zubair Ahmad, Farhat S. Khan, Md. Habibur Rahman, and et al. 2022. "Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review" Materials 15, no. 15: 5392. https://doi.org/10.3390/ma15155392
APA StyleDamiri, F., Andra, S., Kommineni, N., Balu, S. K., Bulusu, R., Boseila, A. A., Akamo, D. O., Ahmad, Z., Khan, F. S., Rahman, M. H., Berrada, M., & Cavalu, S. (2022). Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials, 15(15), 5392. https://doi.org/10.3390/ma15155392










