Impact of ZnO Modifier Concentration on TeO2 Glass Matrix for Optical and Gamma-Ray Shielding Capabilities
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
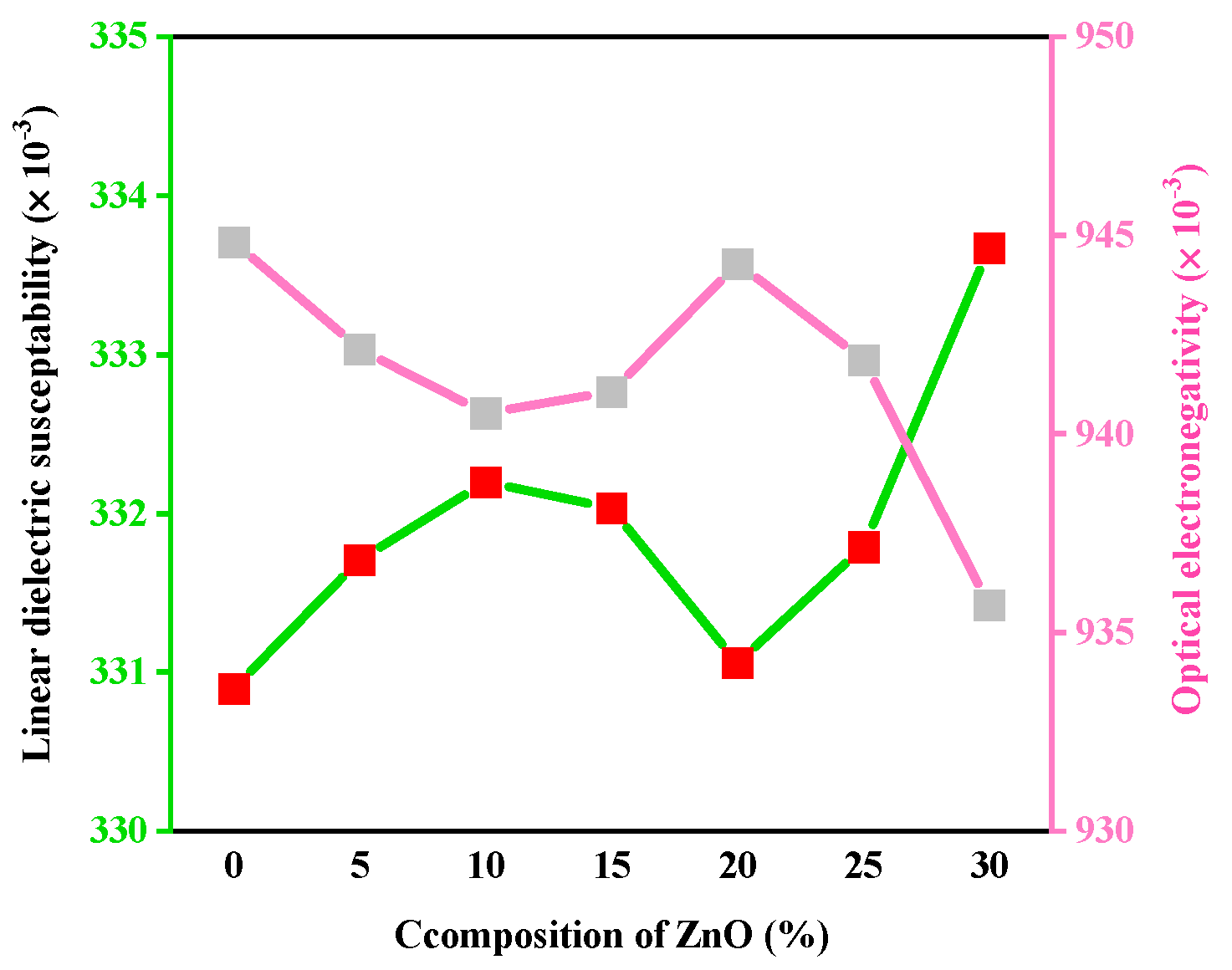
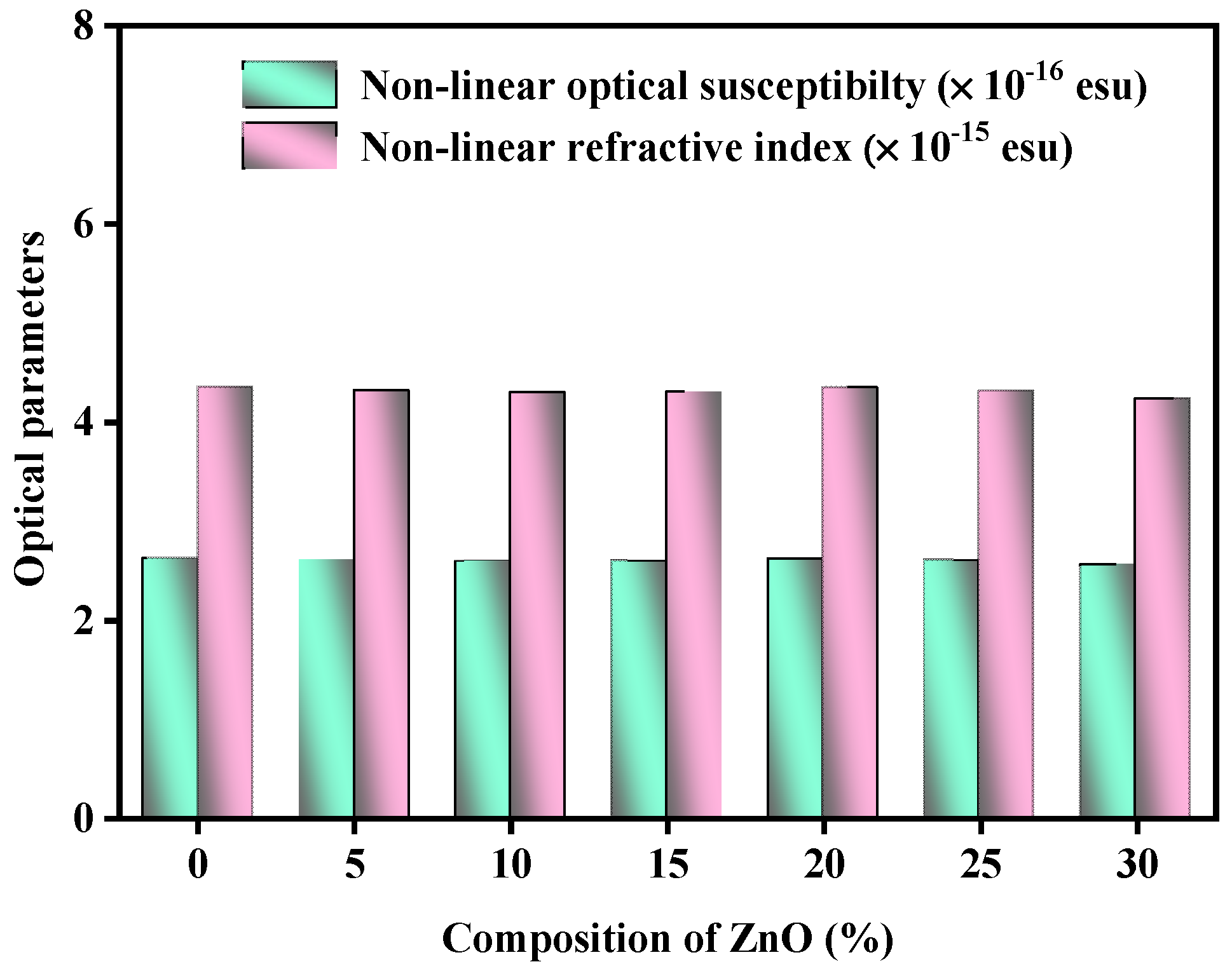
3.1. Optical Features
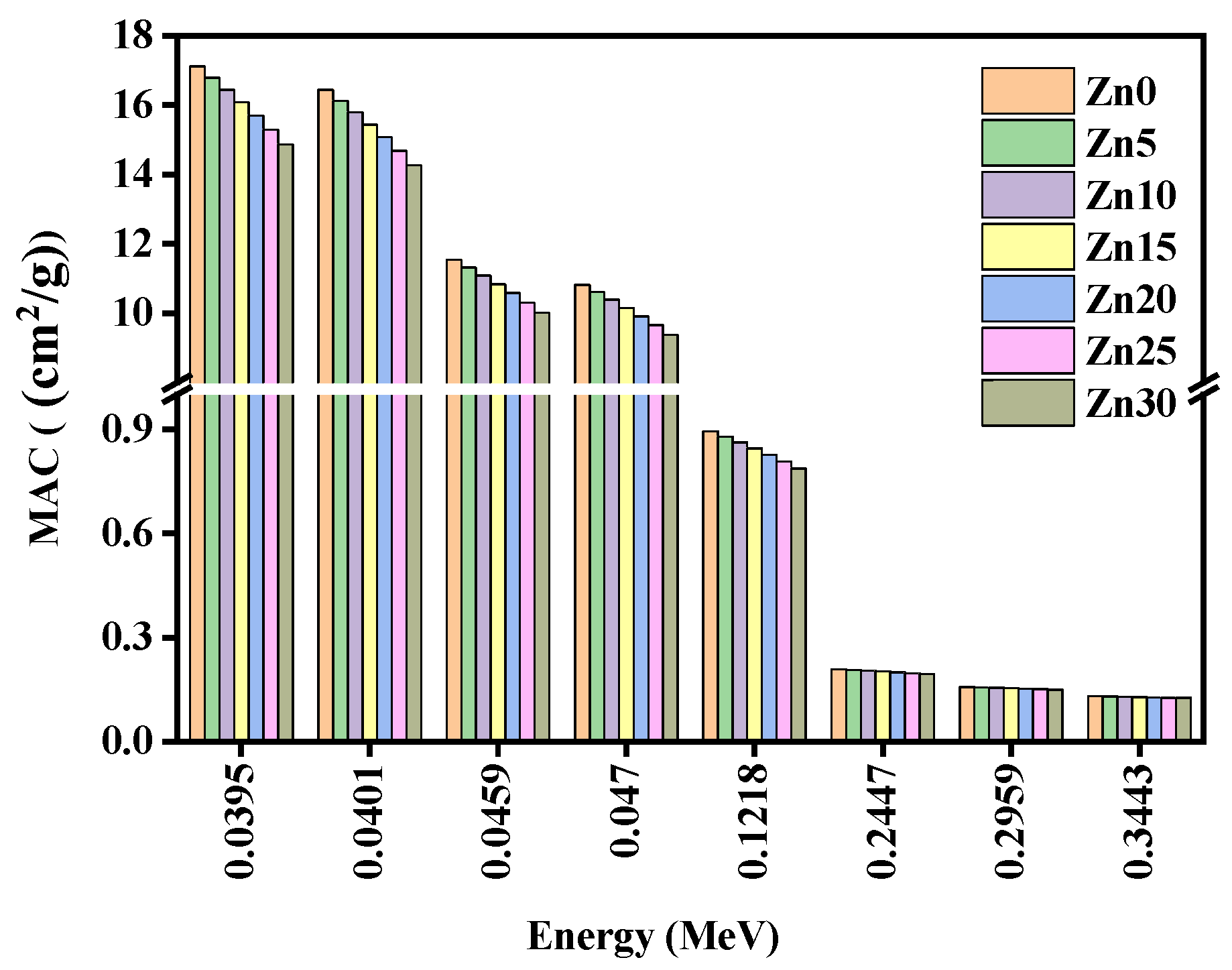
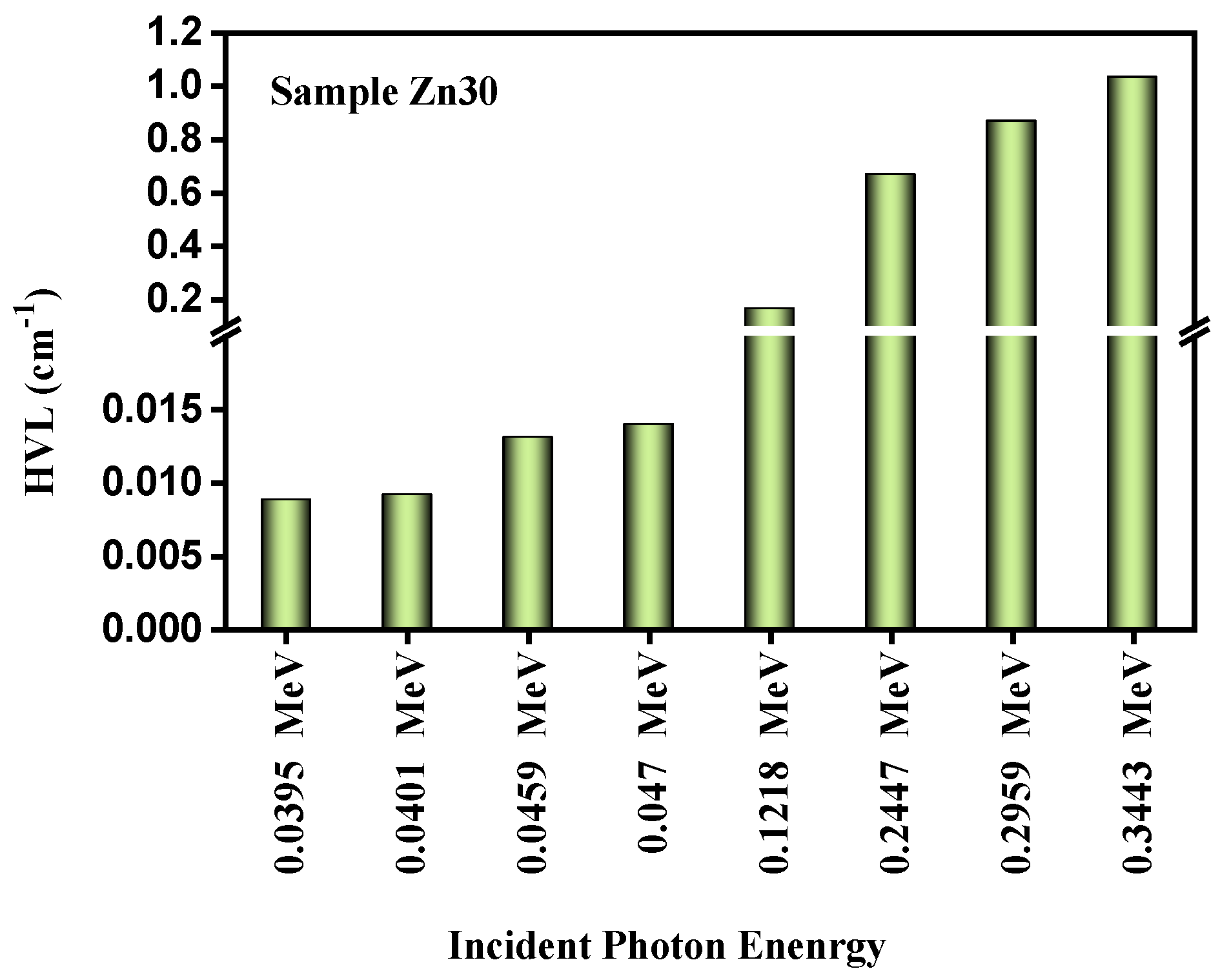
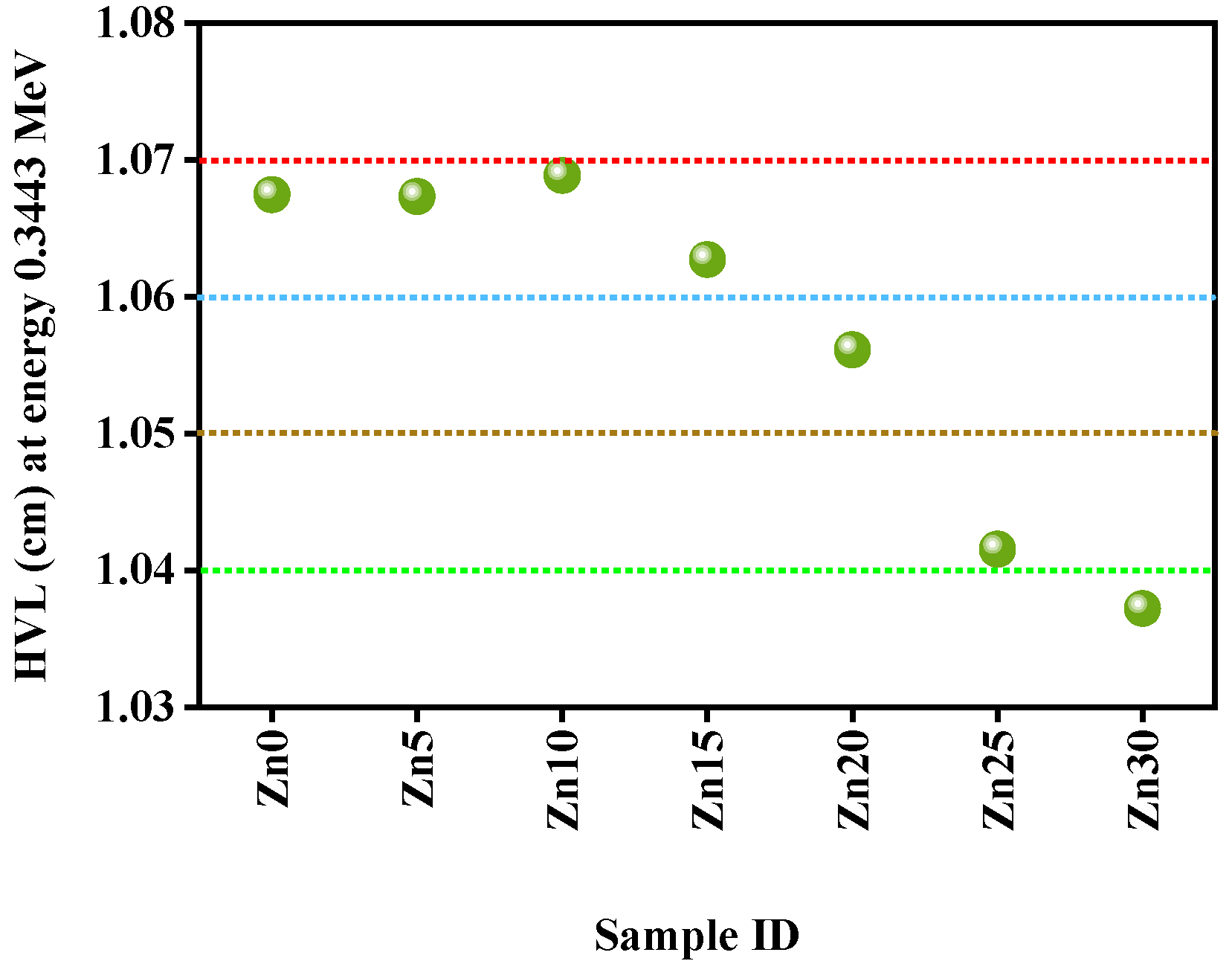
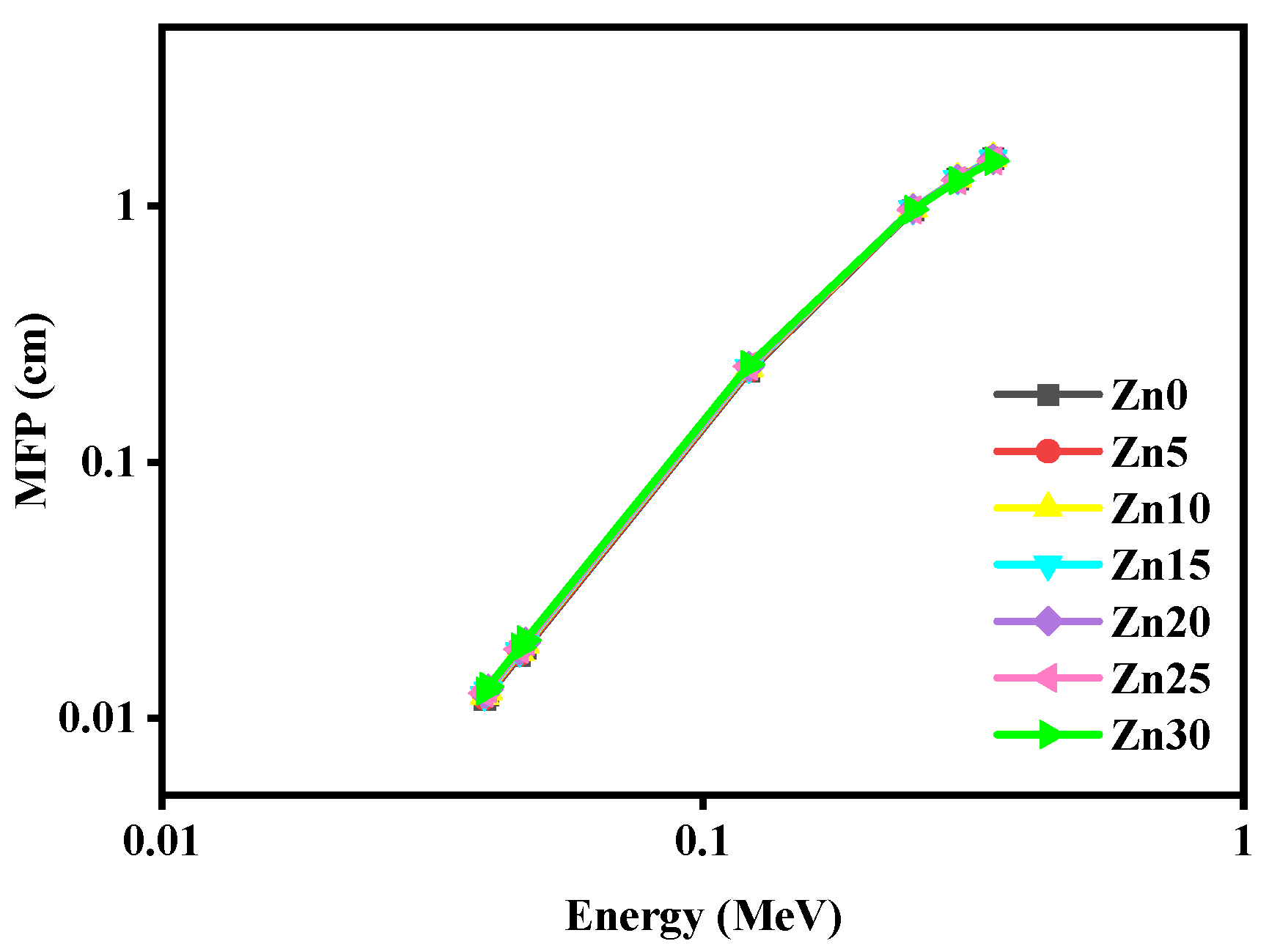
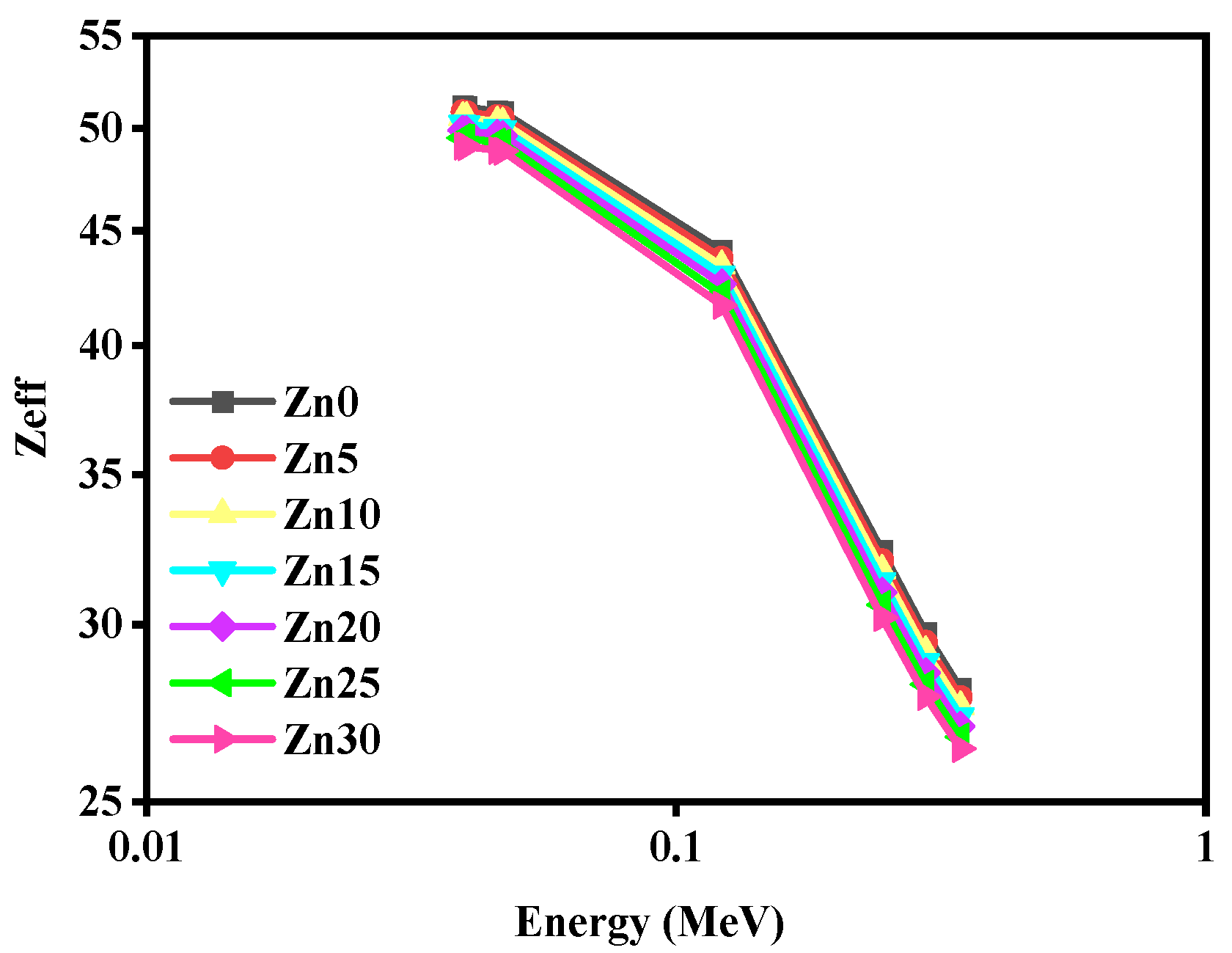
3.2. Radiation Shielding Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araz, A.; Kavaz, E.; Durak, R. Neutron and photon shielding competences of aluminum open-cell foams filled with different epoxy mixtures: An experimental study. Radiat. Phys. Chem. 2021, 182, 109382. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Kavaz, E. Bi2O3 effect on physical, optical, structural and radiation safety characteristics of B2O3-Na2O-ZnO-CaO glass system. J. Non-Cryst. Solids 2020, 535, 119993. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Yang, H.; Sayyed, M.; Tishkevich, D.; Trukhanov, A.; Almousa, N. Upcycling of boron bearing blast furnace slag as highly cost-effective shield for protection of neutron radiation hazard: An innovative way and proposal of shielding mechanism. J. Clean. Prod. 2022, 355, 131817. [Google Scholar] [CrossRef]
- Rajesh, M.; Kavaz, E. Deva Prasad Raju, Photoluminescence, radiative shielding properties of Sm3+ ions doped fluoroborosilicate glasses for visible (reddish-orange) display and radiation shielding applications. Mater. Res. Bull. 2021, 142, 111383. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Öztürk, B.C.; Çam, N.F.; Andiç-Çakır, Ö. Gamma-ray attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cem. Concr. Compos. 2018, 92, 56–69. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A. The potential use of boron containing resources for protection against nuclear radiation. Radiat. Phys. Chem. 2021, 188, 109601. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Sayyed, M.; Tishkevich, D.; Trukhanov, A.; Wang, C. Study of comprehensive shielding behaviors of chambersite deposit for neutron and gamma ray. Prog. Nucl. Energy 2022, 146, 104155. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Li, Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 2017, 141, 239–244. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Vasin, D.S.; Zubar, T.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Sivakov, V.A.; Muradyan, T.R.; et al. Function composites materials for shielding applications: Correlation between phase separation and attenuation properties. J. Alloys Compd. 2019, 771, 238–245. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Kamislioglu, M. An investigation into gamma radiation shielding parameters of the (Al:Si) and (Al+Na):Si-doped international simple glasses (ISG) used in nuclear waste management, deploying Phy-X/PSD and SRIM software. J. Mater. Sci. Mater. Electron. 2021, 32, 12690–12704. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Issa, S.A.M.; Tekin, H.O.; Saddeek, Y.B. Comparative study of gamma-ray shielding and elastic properties of BaO–Bi2O3–B2O3 and ZnO–Bi2O3–B2O3 glass systems. Mater. Chem. Phys. 2018, 217, 11–22. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Akyildirim, H.; Al-Buriahi, M.S. Eloic Lacomme, Rachid Ayad, Giovanni Bonvicini, Oxyfluoro-tellurite-zinc glasses and the nuclear-shielding ability under the substitution of AlF3 by ZnO. Appl. Phys. A 2020, 126, 88. [Google Scholar] [CrossRef]
- Kamislioglu, M. Research on the effects of bismuth borate glass system on nuclear radiation shielding parameters. Result Phys. 2021, 22, 103844. [Google Scholar] [CrossRef]
- Aktas, B.; Acikgoz, A.; Yilmaz, D.; Yalcin, S.; Dogru, K.; Yorulmaz, N. The role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses, the role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses. J. Nucl. Mater. 2022, 563, 153619. [Google Scholar] [CrossRef]
- Ersundu, A.E.; Büyükyıldız, M.; Ersundu, M.Ç.; Şakar, E.; Kurudirek, M. The heavy metal oxide glasses within the WO3-MoO3-TeO2 system to investigate the shielding properties of radiation applications. Prog. Nucl. Energy 2018, 104, 280–287. [Google Scholar] [CrossRef]
- Aşkın, A. Gamma and neutron shielding characterizations of the Ag2O–V2O5–MoO3–TeO2 quaternary tellurite glass system with the Geant4 simulation toolkit and Phy-X software. Ceram. Int. 2020, 46, 6040–6051. [Google Scholar] [CrossRef]
- Sharma, A.; Nazrin, S.; Humaira, S.A.; Boukhris, I.; Kebaili, I. Impact of neodymium oxide on optical properties and X-ray shielding competence of Nd2O3–TeO2–ZnO glasses. Radiat. Phys. Chem. 2022, 195, 110047. [Google Scholar] [CrossRef]
- Bektasoglua, M.; Ali Mohammad, M. Investigation of radiation shielding properties of TeO2–ZnO–Nb2O5–Gd2O3 glasses at medical diagnostic energies. Ceram. Int. 2020, 46, 16217–16223. [Google Scholar] [CrossRef]
- Nazrin, S.; Sharma, A.; Muhammad, S.; Alghamdi, N.A.; Wageh, S. Mechanical and radiation shielding properties of CuO doped TeO2–B2O3 glass system. Radiat. Phys. Chem. 2022, 198, 110222. [Google Scholar] [CrossRef]
- Fidan, M.; Acikgoz, A.; Demircan, G.; Yilmaz, D.; Aktas, B. Optical, structural, physical, and nuclear shielding properties, and albedo parameters of TeO2–BaO–B2O3–PbO–V2O5 glasses. J. Phys. Chem. Solids 2022, 163, 110543. [Google Scholar] [CrossRef]
- Geidam, I.G.; Matori, K.A.; Halimah, M.K.; Chan, K.T.; Muhammad, F.D.; Ishak, M.; Umar, S.A. Oxide ion polarizabilities and gamma radiation shielding features of TeO2–B2O3–SiO2 glasses containing Bi2O3 using Phy-X/PSD software. Mater. Today Commun. 2022, 31, 103472. [Google Scholar] [CrossRef]
- Ersundu, M.Ç.; Ersundu, A.E.; Gedikoğlu, N.; Şakar, E.; Büyükyıldız, M.; Kurudirek, M. Physical, mechanical and gamma-ray shielding properties of highly transparent ZnO-MoO3-TeO2 glasses. J. Non-Cryst. Solids 2019, 524, 119648. [Google Scholar] [CrossRef]
- Effendy, N.; Sidek, H.A.A.; Halimah, M.K.; Zaid, M.H.M. Enhancement on thermal, elastic and optical properties of new formulation tellurite glasses: Influence of ZnO as a glass modifier. Mater. Chem. Phys. 2021, 273, 125156. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.; Kumar, A. Dielectric constant, polarizability, susceptibility and gamma ray shielding behavior of the Li2O-Li2MoO4-TiO2-P2O5 glasses. Optik 2021, 245, 167639. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Sayyed, M.I.; Kumar, A.; Alotiby, M.; Sharma, A.; Al-Yousef, H.A.; Alsaif, N.A.; Al-Hadeethi, Y. Optical and gamma-ray shielding effectiveness of a newly fabricated P2O5–CaO–Na2O–K2O–PbO glass system. Prog. Nucl. Energy 2021, 138, 103798. [Google Scholar] [CrossRef]
- Agar, O.; Sayyed, M.; Tekin, H.; Kaky, K.M.; Baki, S.; Kityk, I. An investigation on shielding properties of BaO, MoO3 and P2O5 based glasses using MCNPX code. Results Phys. 2019, 12, 629–634. [Google Scholar] [CrossRef]
- Effendy, N.; Zaid, M.; Sidek, H.; Matori, K.; Mahmoud, K.; Sayyed, M. Influence of ZnO to the physical, elastic and gamma radiation shielding properties of the tellurite glass system using MCNP-5 simulation code. Radiat. Phys. Chem. 2021, 188, 109665. [Google Scholar] [CrossRef]
- Alsaif, N.A.M.; Alotiby, M.; Hanfi, M.Y.; Sayyed, M.I.; Mahmoud, K.A.; Alotaibi, B.M.; Alyousef, H.A.; Al-Hadeethi, Y. A comprehensive study on the optical, mechanical, and radiation shielding properties of the TeO2–Li2O– GeO2 glass system. J. Mater. Sci. Mater. Electron. 2021, 32, 15226–15241. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Kavaz, E. A novel B2O3-Na2O-BaO-HgO glass system: Synthesis, physical, optical and nuclear shielding features. Ceram. Int. 2020, 46, 16166–16177. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Alotiby, M.; Hanfi, M.Y.; Alotaibi, B.M.; Mahmoud, K.A.; Sayyed, M.I.; Al-Hadeethi, Y. Effect of the Fe2O3 addition on the elastic and gammaray shielding features of bismuth sodium-borate glass system. J. Mater. Sci. Mater. Electron. 2021, 32, 6942–6954. [Google Scholar] [CrossRef]




| Properties | Glass Samples | ||||||
|---|---|---|---|---|---|---|---|
| Zn0 | Zn5 | Zn10 | Zn15 | Zn20 | Zn25 | Zn30 | |
| The indirect band gap (eV) | 3.515 | 3.505 | 3.499 | 3.501 | 3.513 | 3.504 | 3.481 |
| n | 2.271 | 2.273 | 2.274 | 2.274 | 2.271 | 2.273 | 2.278 |
| ε | 5.156 | 5.166 | 5.172 | 5.170 | 5.158 | 5.167 | 5.191 |
| 4.156 | 4.166 | 4.172 | 4.170 | 4.158 | 4.167 | 4.191 | |
| RL | 0.151 | 0.151 | 0.151 | 0.151 | 0.151 | 0.151 | 0.152 |
| T | 0.738 | 0.737 | 0.737 | 0.737 | 0.738 | 0.737 | 0.736 |
| Rm (cm3/mol) | 18.767 | 18.223 | 17.698 | 17.033 | 16.352 | 15.593 | 15.018 |
| αm × 10−24 cm3 | 7.444 | 7.228 | 7.0203 | 6.756 | 6.486 | 6.185 | 5.957 |
| αe × 1024 | 8.244 | 8.252 | 8.257 | 8.256 | 8.246 | 8.253 | 8.273 |
| M | 0.151 | 0.151 | 0.151 | 0.151 | 0.151 | 0.151 | 0.152 |
| M (Eg) | 0.088 | 0.088 | 0.087 | 0.088 | 0.088 | 0.088 | 0.087 |
| M (n) | 0.419 | 0.419 | 0.418 | 0.418 | 0.419 | 0.419 | 0.417 |
| χ(1) | 0.331 | 0.332 | 0.332 | 0.332 | 0.331 | 0.332 | 0.334 |
| χ* | 0.945 | 0.942 | 0.941 | 0.941 | 0.944 | 0.942 | 0.936 |
| χ3 × 10−16 (esu) | 2.620 | 2.602 | 2.591 | 2.594 | 2.616 | 2.600 | 2.558 |
| n2optical × 10−15 (esu) | 4.348 | 4.313 | 4.292 | 4.299 | 4.341 | 4.310 | 4.230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloraini, D.A.; Sayyed, M.I.; Kumar, A.; Yasmin, S.; Almuqrin, A.H. Impact of ZnO Modifier Concentration on TeO2 Glass Matrix for Optical and Gamma-Ray Shielding Capabilities. Materials 2022, 15, 5342. https://doi.org/10.3390/ma15155342
Aloraini DA, Sayyed MI, Kumar A, Yasmin S, Almuqrin AH. Impact of ZnO Modifier Concentration on TeO2 Glass Matrix for Optical and Gamma-Ray Shielding Capabilities. Materials. 2022; 15(15):5342. https://doi.org/10.3390/ma15155342
Chicago/Turabian StyleAloraini, Dalal A., M. I. Sayyed, Ashok Kumar, Sabina Yasmin, and Aljawhara H. Almuqrin. 2022. "Impact of ZnO Modifier Concentration on TeO2 Glass Matrix for Optical and Gamma-Ray Shielding Capabilities" Materials 15, no. 15: 5342. https://doi.org/10.3390/ma15155342







