Synthesis and Characterization of the Metal–Organic Framework CIM-80 for Organic Compounds Adsorption
Abstract
:1. Introduction
2. Materials and Methods
Characterization
3. Results and Discussion
3.1. Reaction Yield
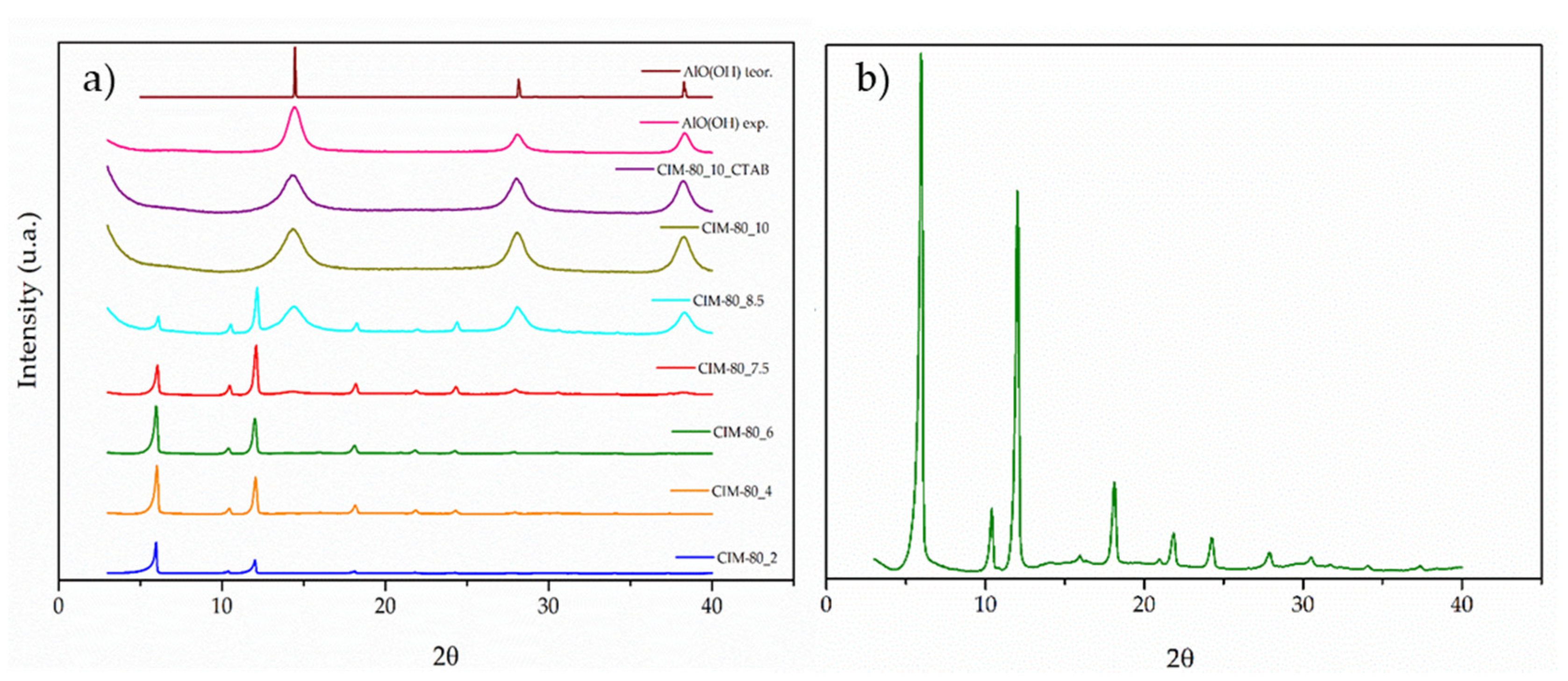
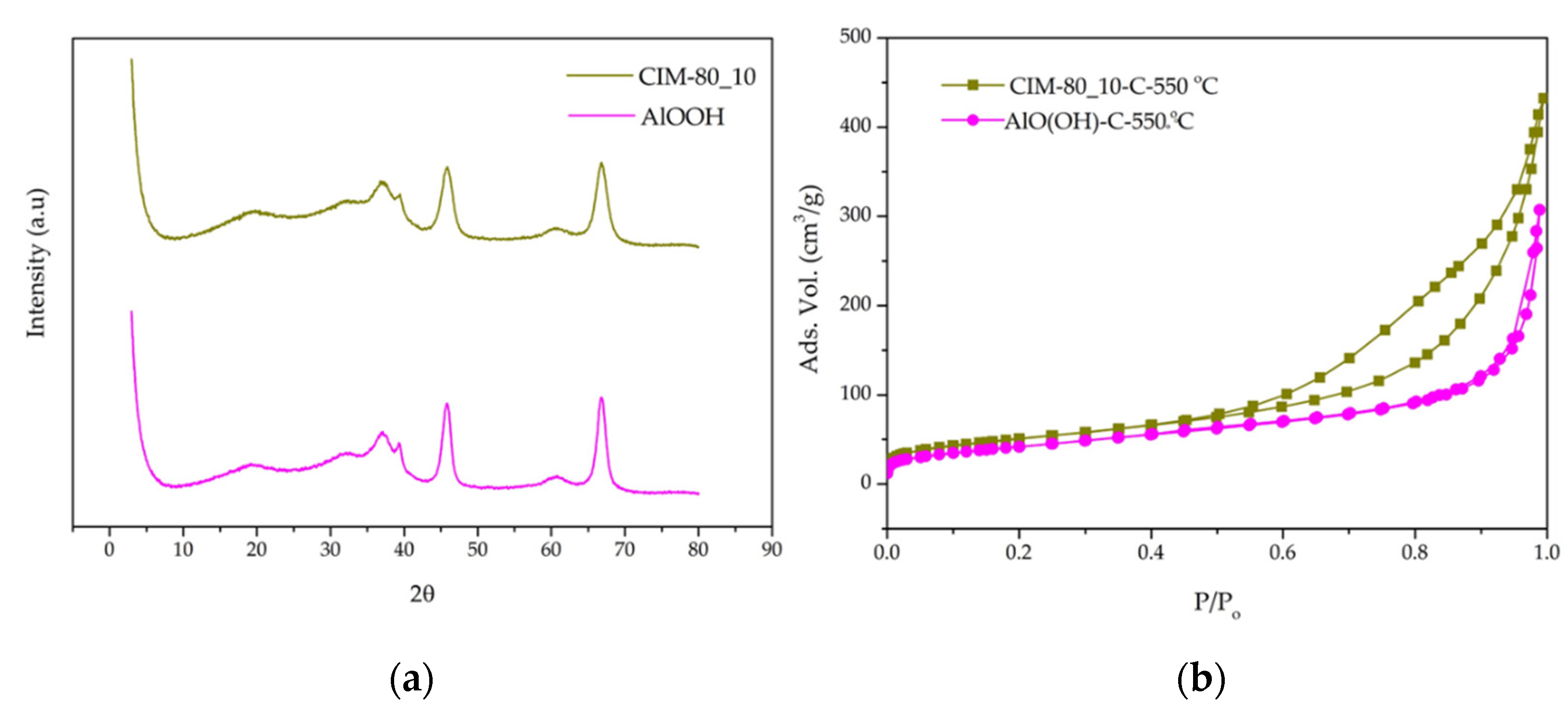
3.2. X-ray Diffraction
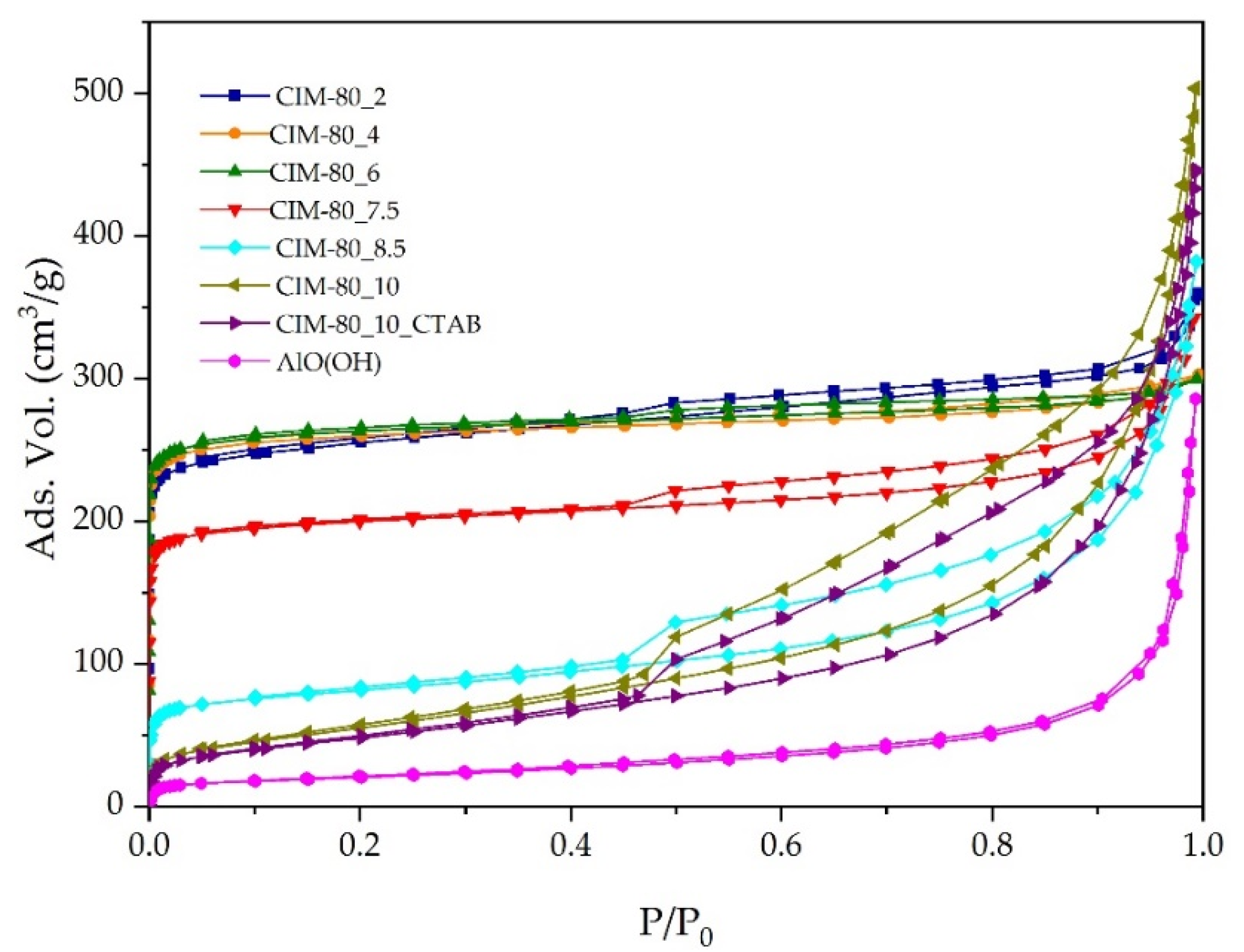
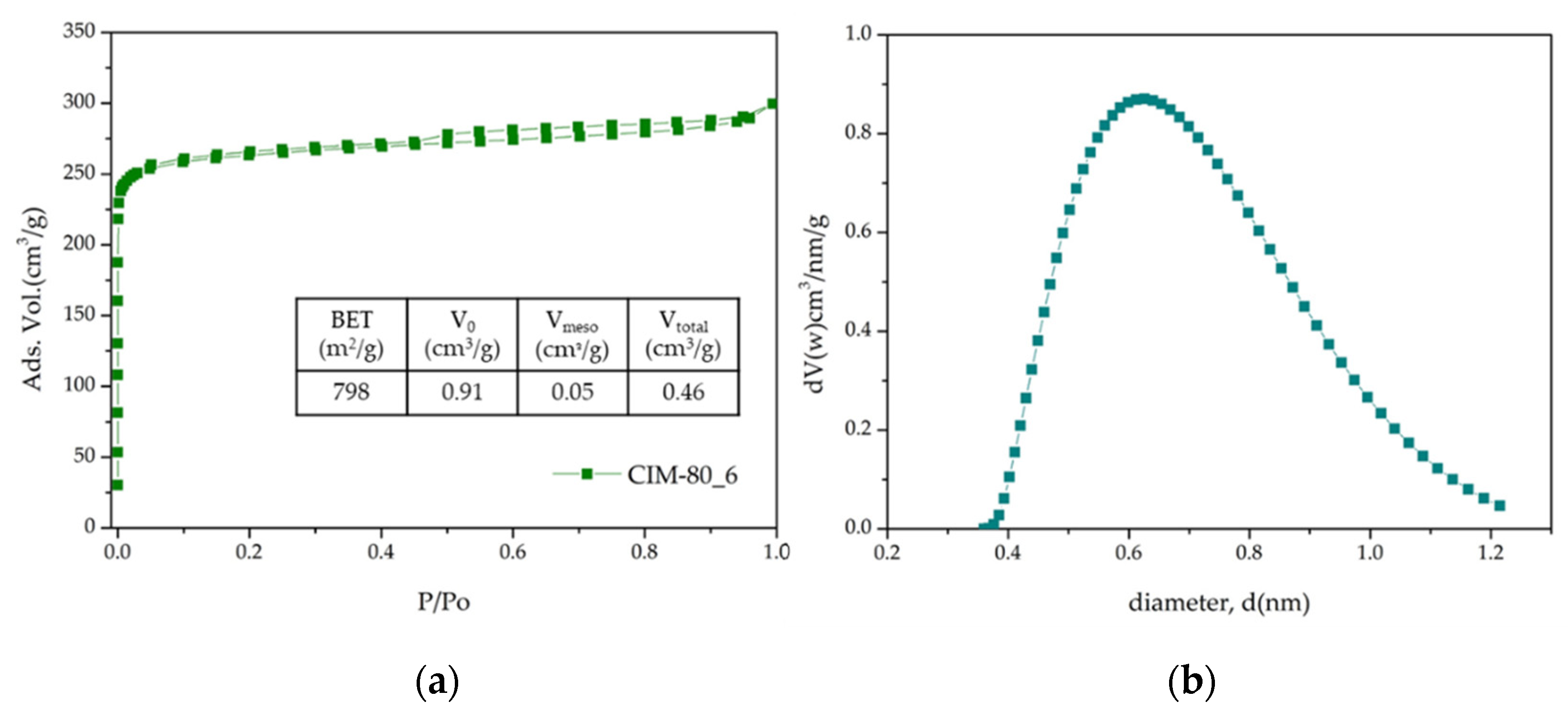
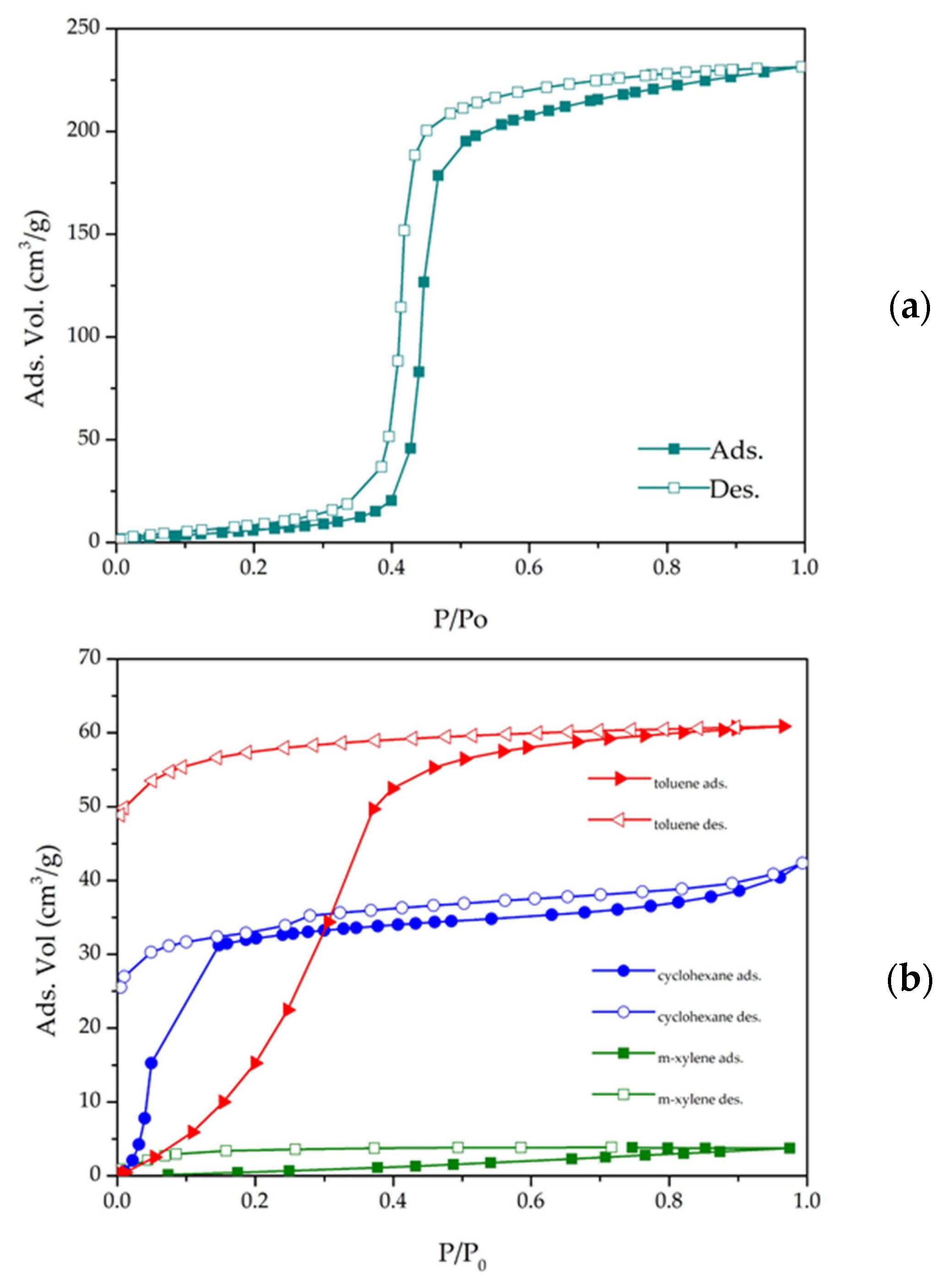
3.3. Textural Properties—N2 Adsorption
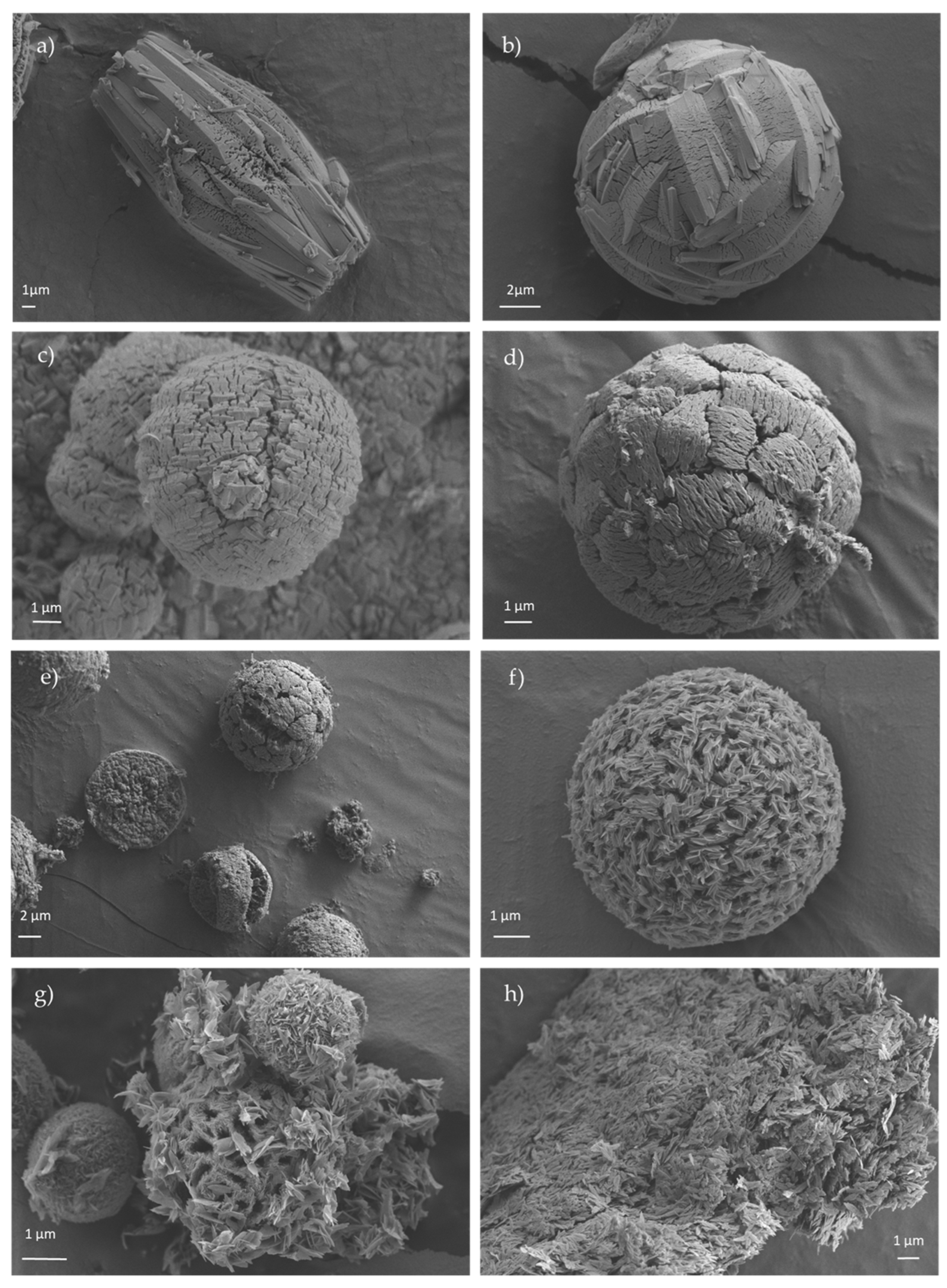
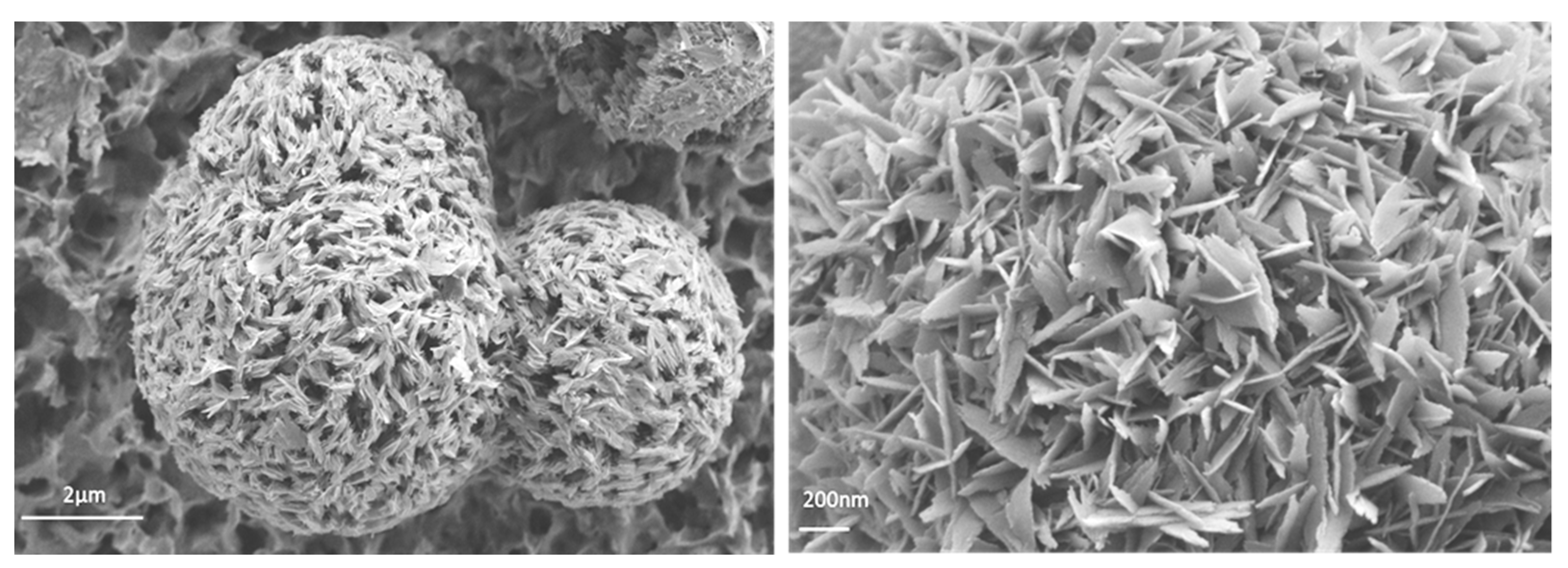
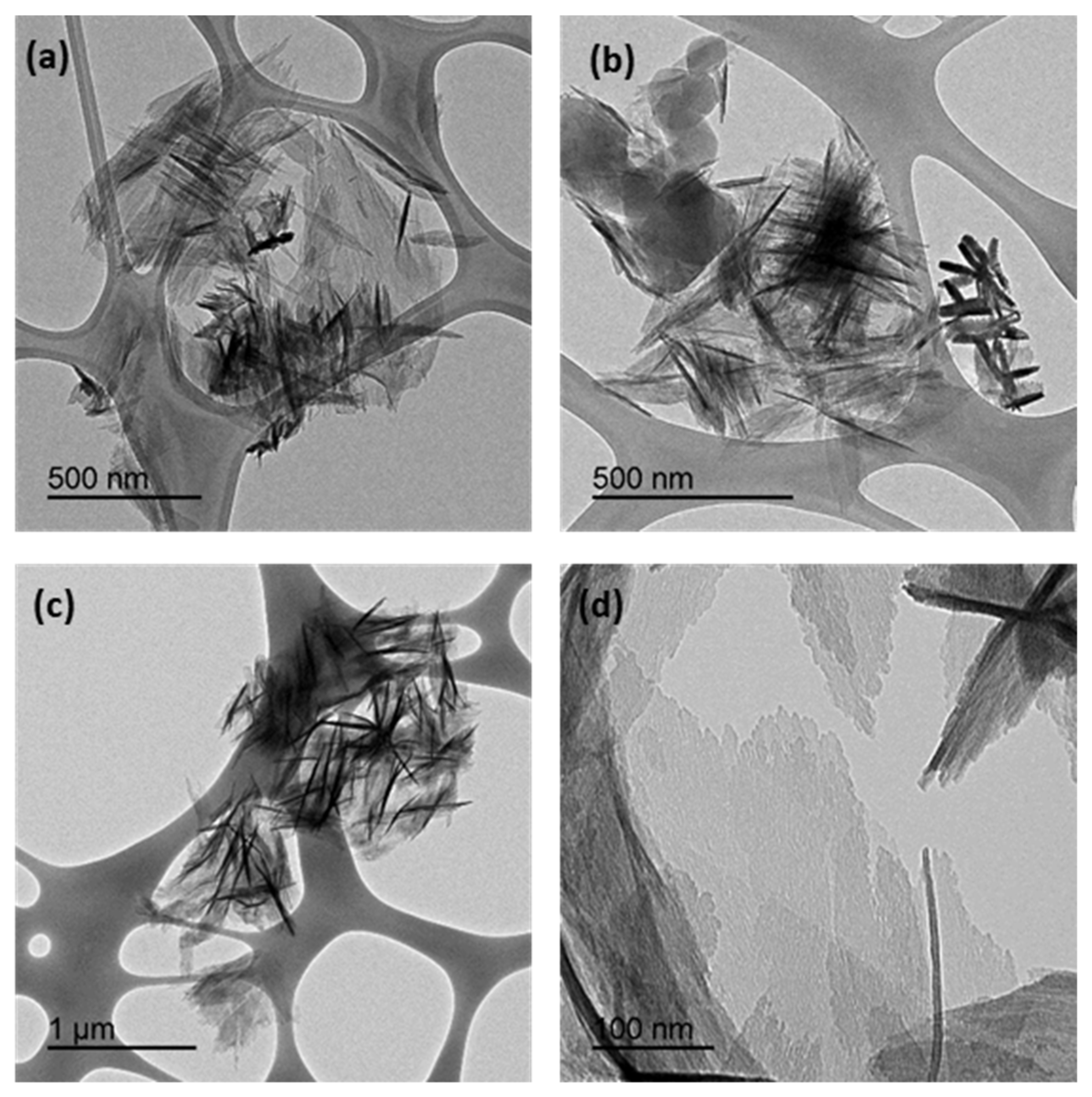
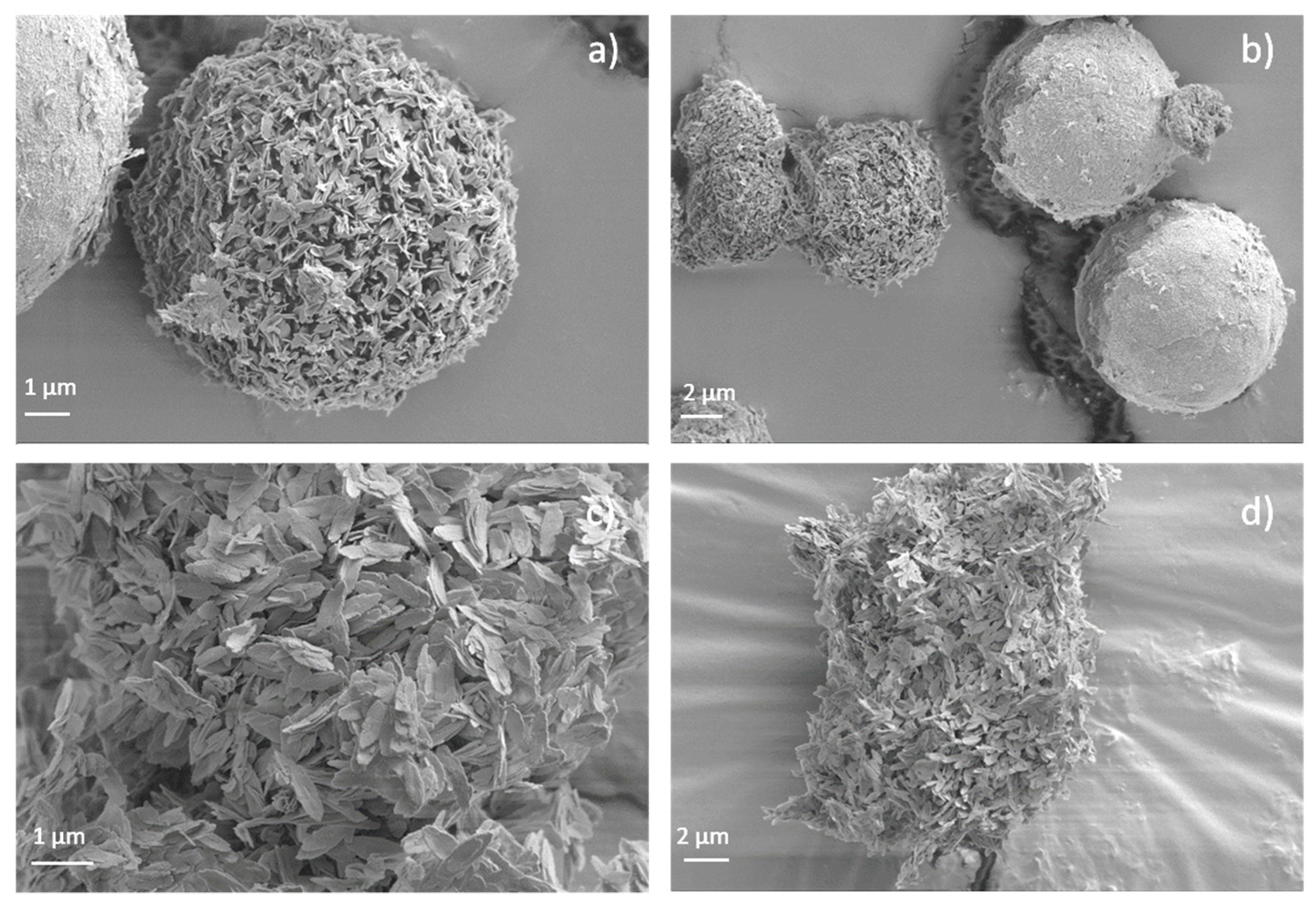
3.4. Scanning Electron Microscopy (SEM) and Transmision Electronic Microscopy (TEM)
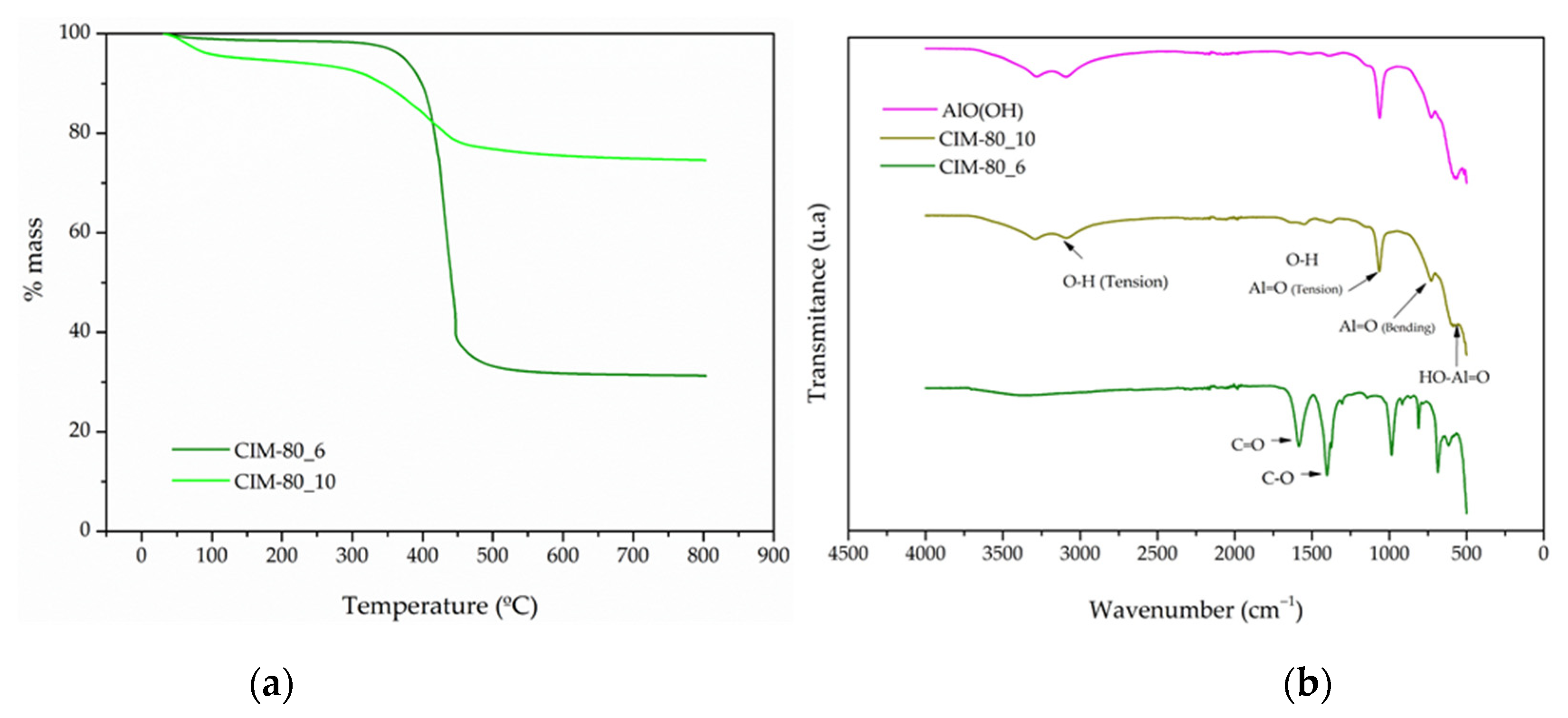
3.5. Thermogravimetric Analysis (TGA) and Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Characterization of Calcined Samples
3.7. Water and VOC Adsorption on MOF CIM-80_6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malika, K.; Kim, T.; Losic, D.; Thanh, T. Recent advances in engineered graphene and composites for detection of volatile organic compounds (VOCs) and non-invasive diseases diagnosis. Carbon N. Y. 2016, 110, 97–129. [Google Scholar]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; Kim, K.; Parise, J. Seeking the most powerful and practical real-world sorbents for gaseous benzene as a representative volatile organic compound based on performance metrics. Sep. Purif. Technol. 2019, 212, 980–985. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, X.; Chen, J.; Yang, Y.; Lu, G. Journal of Environmental Chemical Engineering The preparation of defective UiO-66 metal organic framework using MOF-5 as structural modifier with high sorption capacity for gaseous toluene. J. Environ. Chem. Eng. 2019, 7, 103405. [Google Scholar] [CrossRef]
- Wang, D.; Wu, G.; Zhao, Y.; Cui, L.; Shin, C.; Ryu, M.; Cai, J. Study on the copper (II)-doped MIL-101 (Cr) and its performance in VOCs adsorption. Environ. Sci. Pollut. Res. Int. 2018, 101, 28109–28119. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Mantri, K. Adsorption of aromatic hydrocarbons on highly siliceous MCM-41. Langmuir 2000, 16, 7031–7037. [Google Scholar] [CrossRef]
- Pires, J.; Carvalho, A.; De Carvalho, M.B. Adsorption of volatile organic compounds in Y zeolites and pillared clays. Microporous Mesoporous Mater. 2001, 43, 277–287. [Google Scholar] [CrossRef]
- Chou, M.-S.; Chiou, J.-H. Modeling Effects of Moisture on Adsorption Capacity of Activated Carbon for VOCs. J. Environ. Eng. 1997, 123, 437–443. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon N. Y. 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Aizpuru, A.; Malhautier, L.; Roux, J.C.; Fanlo, J.L. Biofiltration of a mixture of volatile organic compounds on granular activated carbon. Biotechnol. Bioeng. 2003, 83, 479–488. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Factors governing the adsorption of ethanol on spherical activated carbons. Carbon N. Y. 2015, 83, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbons for low concentration toluene adsorption. Carbon N. Y. 2010, 48, 2625–2633. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbons for the adsorption of a real multicomponent VOC mixture. Carbon N. Y. 2019, 148, 214–223. [Google Scholar] [CrossRef]
- Kutluay, S.; Temel, F. Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125848. [Google Scholar] [CrossRef]
- Chu, Y.H.; Kim, H.J.; Song, K.Y.; Shul, Y.G.; Jung, K.T.; Lee, K.; Han, M.H. Preparation of mesoporous silica fiber matrix for VOC removal. Catal. Today 2002, 74, 249–256. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Jafari, S.; Ghorbani-Shahna, F.; Bahrami, A.; Kazemian, H. Adsorptive removal of toluene and carbon tetrachloride from gas phase using Zeolitic Imidazolate Framework-8: Effects of synthesis method, particle size, and pretreatment of the adsorbent. Microporous Mesoporous Mater. 2018, 268, 58–68. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, J.H.; Lee, C.H. Adsorption and thermal regeneration of acetone and toluene vapors in dealuminated Y-zeolite bed. Sep. Purif. Technol. 2011, 77, 312–324. [Google Scholar] [CrossRef]
- Narciso, J.; Ramos-Fernandez, E.V.; Delgado-Marín, J.J.; Affolter, C.W.; Olsbye, U.; Redekop, E.A. New route for the synthesis of Co-MOF from metal substrates. Microporous Mesoporous Mater. 2021, 324, 111310. [Google Scholar] [CrossRef]
- Ramos-Fernandez, E.V.; Redondo-Murcia, A.; Grau-Atienza, A.; Sepúlveda-Escribano, A.; Narciso, J. Clean production of Zeolitic Imidazolate Framework 8 using Zamak residues as metal precursor and substrate. J. Clean. Prod. 2020, 260, 121081. [Google Scholar] [CrossRef]
- Delgado-Marín, J.J.; Izan, D.P.; Molina-Sabio, M.; Ramos-Fernandez, E.V.; Narciso, J. New Generation of MOF-Monoliths Based on Metal Foams. Molecules 2022, 27, 1968. [Google Scholar] [CrossRef]
- Villalgordo-Hernández, D.; Grau-Atienza, A.; García-Marín, A.A.; Ramos-Fernández, E.V.; Narciso, J. Manufacture of Carbon Materials with High Nitrogen Content. Materials 2022, 15, 2415. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Pellicer-Carreño, I.; Grau-Atienza, A.; Boada, R.; Diaz-Moreno, S.; Narciso-Romero, J.; Serrano-Ruiz, J.C.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. Mixed-Valence Ce/Zr Metal-Organic Frameworks: Controlling the Oxidation State of Cerium in One-Pot Synthesis Approach. Adv. Funct. Mater. 2021, 31, 2102582. [Google Scholar] [CrossRef]
- Ramos-Fernández, E.V.; Serrano-Ruiz, J.C.; Sepúlveda-Escribano, A.; Narciso, J.; Ferrando-Soria, J.; Pardo, E. From material chemistry to catalytic applications. In Heterogeneous Catalysis for Energy Applications; Royal Society of Chemistry: London, UK, 2020; pp. 235–303. ISBN 978-1-78801-718-3. [Google Scholar]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Herm, Z.R.; Bloch, E.D.; Long, R. Hydrocarbon Separations in Metal—Organic Frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.; Hwang, Y.K.; Chang, J.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal—Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Int. Ed. Engl. 2010, 100, 5949–5952. [Google Scholar] [CrossRef]
- Yang, K.; Xue, F.; Sun, Q.; Yue, R.; Lin, D. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. J. Environ. Chem. Eng. 2013, 1, 713–718. [Google Scholar] [CrossRef]
- Chen, R.; Yao, Z.; Han, N.; Ma, X.; Li, L.; Liu, S.; Sun, H.; Wang, S.; Li, L. Insights into the Adsorption of VOCs on a Cobalt-Adeninate Metal-Organic Framework (Bio-MOF-11). ACS Omega 2020, 5, 15402–15408. [Google Scholar] [CrossRef]
- Wang, C.; Yin, H.; Tian, P.; Sun, X.; Pan, X.; Chen, K.; Chen, W.J.; Wu, Q.H.; Luo, S. Remarkable adsorption performance of MOF-199 derived porous carbons for benzene vapor. Environ. Res. 2020, 184, 109323. [Google Scholar] [CrossRef]
- Reinsch, H.; Homburg, T.; Heidenreich, N.; Fröhlich, D.; Hennninger, S.; Wark, M.; Stock, N. Green Synthesis of a New Al-MOF Based on the Aliphatic Linker Mesaconic Acid: Structure, Properties and In Situ Crystallisation Studies of Al-MIL-68-Mes. Chem.-A Eur. J. 2018, 24, 2173–2181. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Ruiz-Pérez, C.; Vallcorba, O.; Afonso, A.M.; Pasán, J. A green metal-organic framework to monitor water contaminants. RSC Adv. 2018, 8, 31304–31310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Serpa, A.; Jiménez-Abizanda, A.I.; Jiménez-Moreno, F.; Pasán, J.; Pino, V. Core-shell microparticles formed by the metal-organic framework CIM-80(Al) (Silica@CIM-80(Al)) as sorbent material in miniaturized dispersive solid-phase extraction. Talanta 2020, 211, 120723. [Google Scholar] [CrossRef] [PubMed]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders Porous Solids: Principles, Methodology and Applications; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bustos, H.F.; Lucio-Ortiz, C.J.; de la Rosa, J.R.; de Haro del Río, D.A.; Sandoval-Rangel, L.; Martínez-Vargas, D.X.; Maldonado, C.S.; Rodriguez-González, V.; Garza-Navarro, M.A.; Morales-Leal, F.J. Synthesis and characterization of bimetallic catalysts Pd-Ru and Pt-Ru supported on Γ-alumina and zeolite FAU for the catalytic transformation of HMF. Fuel 2019, 239, 191–201. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds. In Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts; Elsevier: Amsterdam, The Netherlands, 1971; pp. 1–18. [Google Scholar]
- Ram, S. Infrared spectral study of molecular vibrations in amorphous, nanocrystalline and AlO(OH) · αh2O bulk crystals. Infrared Phys. Technol. 2001, 42, 547–560. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. CO and CO2 methanation over Ni catalysts supported on alumina with different crystalline phases. Korean J. Chem. Eng. 2017, 34, 3085–3091. [Google Scholar] [CrossRef]
- Canivet, J.; Bonnefoy, J.; Daniel, C.; Legrand, A.; Coasne, B.; Farrusseng, D. Structure-property relationships of water adsorption in metal-organic frameworks. New J. Chem. 2014, 38, 3102–3111. [Google Scholar] [CrossRef]
- Coudert, F.X.; Ortiz, A.U.; Haigis, V.; Bousquet, D.; Fuchs, A.H.; Ballandras, A.; Weber, G.; Bezverkhyy, I.; Geoffroy, N.; Bellat, J.P.; et al. Water adsorption in flexible gallium-based MIL-53 metal-organic framework. J. Phys. Chem. C 2014, 118, 5397–5405. [Google Scholar] [CrossRef] [Green Version]
- Jahandar Lashaki, M.; Fayaz, M.; Niknaddaf, S.; Hashisho, Z. Effect of the adsorbate kinetic diameter on the accuracy of the Dubinin-Radushkevich equation for modeling adsorption of organic vapors on activated carbon. J. Hazard. Mater. 2012, 241–242, 154–163. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef]
- Wang, C.M.; Chang, K.S.; Chung, T.W.; Wu, H. Adsorption equilibria of aromatic compounds on activated carbon, silica gel, and 13X zeolite. J. Chem. Eng. Data 2004, 49, 527–531. [Google Scholar] [CrossRef]
- Oh, K.-J.; Park, D.-W.; Kim, S.-S.; Park, S.-W. Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon. Korean J. Chem. Eng. 2010, 27, 632–638. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Sun, C.; Li, H.; Sun, J.; Liu, X. Adsorption performance and kinetic study of hierarchical porous Fe-based MOFs for toluene removal. Sci. Total Environ. 2021, 793, 148622. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.R.; Kim, H.Y.; Ahn, W.S. Adsorption of volatile organic compounds over MIL-125-NH2. Polyhedron 2018, 154, 343–349. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
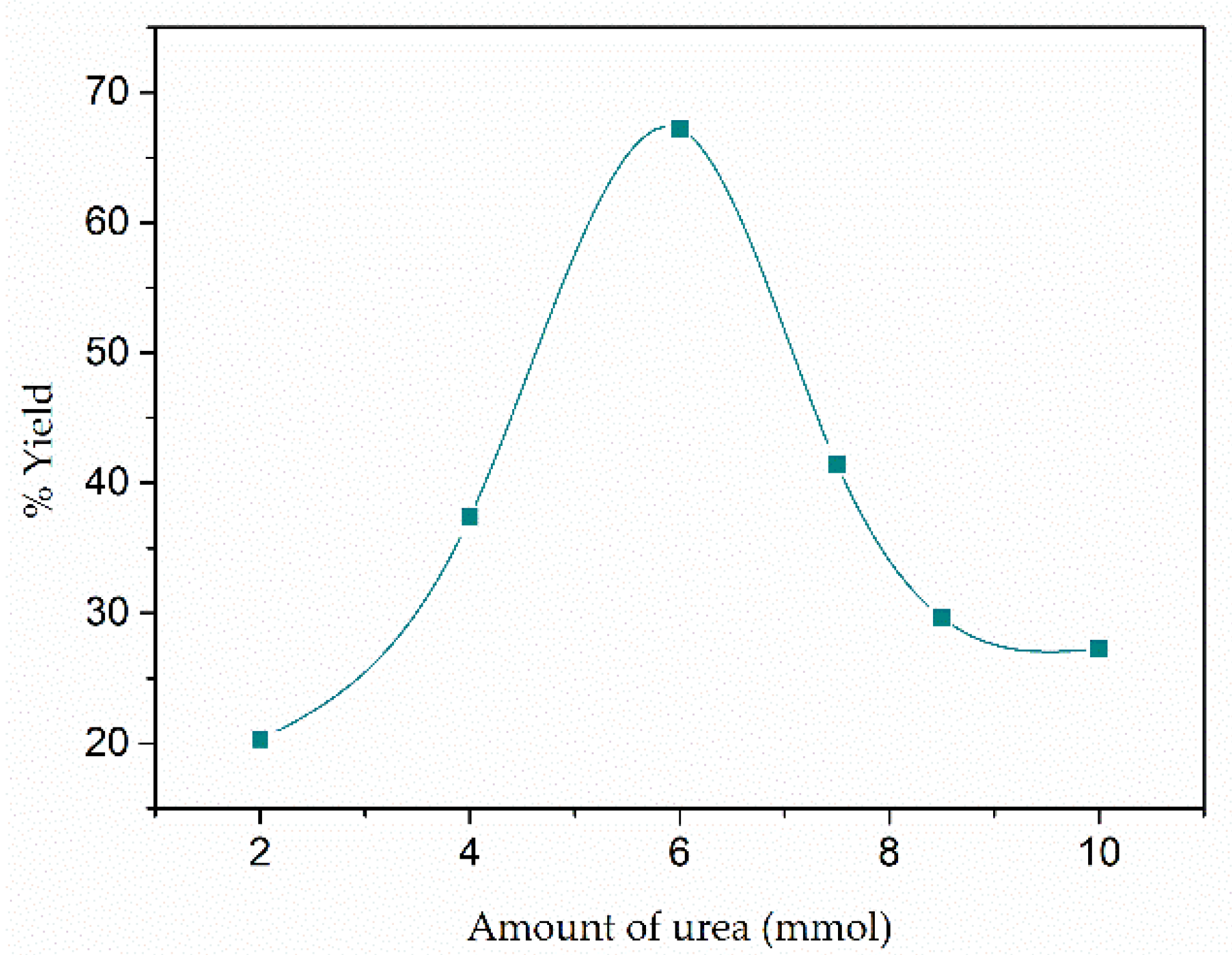

| Sample | Amount of Urea | % Yield |
|---|---|---|
| CIM-80_2 | 2 mmol | 20 |
| CIM-80_4 | 4 mmol | 37 |
| CIM-80_6 | 6 mmol | 67 |
| CIM-80_7.5 | 7.5 mmol | 41 |
| CIM-80_8.5 | 8.5 mmol | 30 |
| CIM-80_10 | 10 mmol | 27 |
| CIM-80_10_CTAB | 10 mmol + CTAB | 27 |
| AlO(OH) | 10 mmol | 98 |
| Sample | Specific Surface BET (m2/g) | V0 (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) |
|---|---|---|---|---|
| CIM-80_2 | 787 | 0.39 | 0.17 | 0.56 |
| CIM-80_4 | 787 | 0.40 | 0.06 | 0.46 |
| CIM-80_6 | 798 | 0.41 | 0.05 | 0.46 |
| CIM-80_7.5 | 610 | 0.31 | 0.22 | 0.53 |
| CIM-80_8.5 | 265 | 0.12 | 0.47 | 0.59 |
| CIM-80_10 | 205 | 0.08 | 0.70 | 0.78 |
| CIM-80_10_CTAB | 177 | 0.07 | 0.62 | 0.69 |
| AlO(OH) | 72 | 0.03 | 0.41 | 0.44 |
| Sample | BET (m2/g) | Vo (cm3/g) | Vmeso | Vtotal |
|---|---|---|---|---|
| CIM-80_10-C-550 °C | 181 | 0.08 | 0.59 | 0.67 |
| AlOOH-C-550 °C | 150 | 0.06 | 0.42 | 0.47 |
| VOCs | Density, ρ (g/mL, 25 °C) | M (g/mol) | D (nm) | σ (nm2) | Q0 (mg/g) | V (mL/g) |
|---|---|---|---|---|---|---|
| Toluene | 0.866 | 92.15 | 0.585 | 0.344 | 250.26 | 0.289 |
| Cyclohexane | 0.779 | 84.16 | 0.600 | 0.347 | 159 | 0.204 |
| m-xylene | 0.860 | 106.16 | 0.680 | 0.379 | 18.29 | 0.021 |
| Material | Precursor | B.E.T. (m2/g) | Vtotal (cm3/g) | V0 (cm3/g) | Pore Size (nm) | Green Synthesis | Q0(mg/g) Toluene | Ref |
|---|---|---|---|---|---|---|---|---|
| AC | Anthracite | 2746 | 0.97 | 0.8 | - | ↓ B | 640 | [16] |
| AC | Organic biomass | 990 | - | 0.09 | 2.7 | ↓ B | 109 | [45] |
| AC | Organic biomass | 805 | - | 0.47 | 2.35 | ↓ B | 59.2 | [46] |
| Zeolite 13× | Si/Al | 440 | 0.12 | - | 9.9 | ↓ B | 16.02 | [45] |
| MIL-100 | Fe | 1398 | 1.08 | 0.46 | 1.36 | ↑ WS | 663 | [47] |
| MIL-101 | Cr | 3980 | 1.85 | - | 2.6 | ↑ WS | 1067 | [44] |
| MOF-177 | Zn | 2970 | 1.11 | - | 0.94 | ↓ OS | 585 | [28] |
| MIL-125-NH2 | Ti | 1280 | - | 0.56 | 0.4 | ↓ OS | 293 | [48] |
| UiO-66-NH2 | Zr | 1250 | 0.62 | - | 1.98 | ↓ OS | 147 | [49] |
| UiO-66 | Zr | 1414 | 0.68 | - | 1.93 | ↓ OS | 64.2 | [49] |
| ZIF-67 | Co | 1401 | 1.22 | - | 3.49 | ↑ WS | 50.8 | [49] |
| MOF-5 | Zn | 424 | 0.22 | - | 2.07 | ↓ OS | 32.9 | [49] |
| CIM-80 | Al | 798 | 0.46 | 0.41 | 0.63 | ↑ WS | 250.6 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Quintero, L.; Ramos-Fernandez, E.V.; Narciso, J. Synthesis and Characterization of the Metal–Organic Framework CIM-80 for Organic Compounds Adsorption. Materials 2022, 15, 5326. https://doi.org/10.3390/ma15155326
Figueroa-Quintero L, Ramos-Fernandez EV, Narciso J. Synthesis and Characterization of the Metal–Organic Framework CIM-80 for Organic Compounds Adsorption. Materials. 2022; 15(15):5326. https://doi.org/10.3390/ma15155326
Chicago/Turabian StyleFigueroa-Quintero, Leidy, Enrique Vicente Ramos-Fernandez, and Javier Narciso. 2022. "Synthesis and Characterization of the Metal–Organic Framework CIM-80 for Organic Compounds Adsorption" Materials 15, no. 15: 5326. https://doi.org/10.3390/ma15155326
APA StyleFigueroa-Quintero, L., Ramos-Fernandez, E. V., & Narciso, J. (2022). Synthesis and Characterization of the Metal–Organic Framework CIM-80 for Organic Compounds Adsorption. Materials, 15(15), 5326. https://doi.org/10.3390/ma15155326







