RETRACTED: The Effect of 600 keV Ag Ion Irradiation on the Structural, Optical, and Photovoltaic Properties of MAPbBr3 Films for Perovksite Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. XRD Analysis
3.1.1. Grain Size (D) and Dislocation Line Density ()
3.1.2. d-Spacing
3.1.3. Lattice Parameters and Volume
3.2. Optical Properties
3.2.1. Energy Band Gap
3.2.2. Extinction Coefficient (k) and Refractive Index (n)
3.2.3. Dielectric Constants (εr, εi)
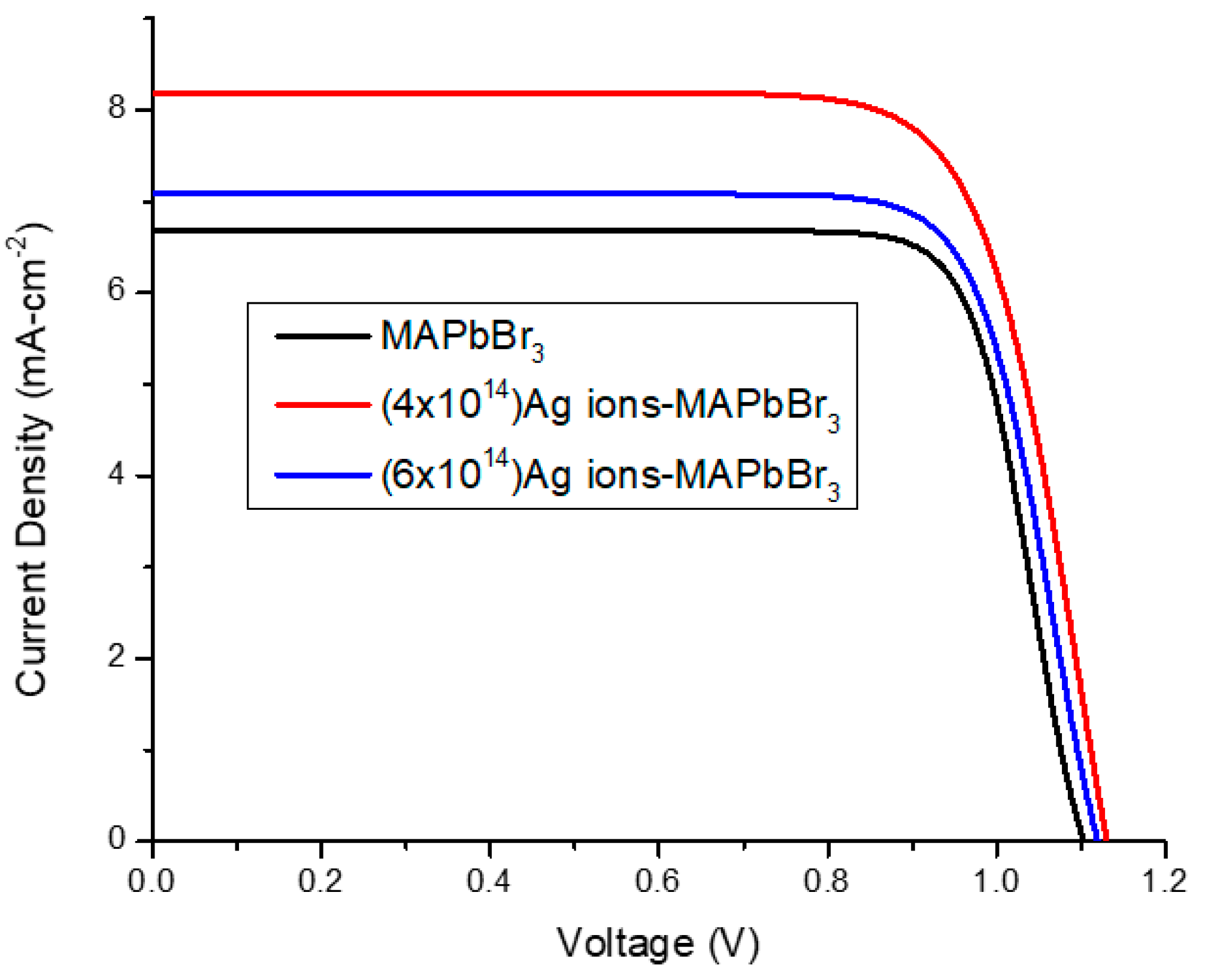
3.3. J-V Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amjad, M.; Khan, M.I.; Alwadai, N.; Irfan, M.; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Photovoltaic Properties of ZnO Films Co-Doped with Mn and La to Enhance Solar Cell Efficiency. Nanomaterials 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mukhtar, A.; Alwadai, N.; Irfan, M.; Haq, I.-u.; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Improving the Structural, Optical and Photovoltaic Properties of Sb-and Bi-Co-Doped MAPbBr3 Perovskite Solar Cell. Coatings 2022, 12, 386. [Google Scholar] [CrossRef]
- Lee, S.; Joung, Y.-H.; Yoon, Y.-K.; Choi, W. Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System. Nanomaterials 2022, 12, 215. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Ďurišová, Ľ.; Bujdoš, M.; Černý, I.; Chlpík, J.; Juriga, M.; et al. Effects of Foliar Application of ZnO Nanoparticles on Lentil Production, Stress Level and Nutritional Seed Quality under Field Conditions. Nanomaterials 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A. Perovskite solar cells: The birth of a new era in photovoltaics. ACS Energy Lett. 2017, 2, 822–830. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation observations of encapsulated planar CH 3 NH 3 PbI 3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Wu, C.; Priya, S. Room temperature fabrication of CH3NH3PbBr3 by anti-solvent assisted crystallization approach for perovskite solar cells with fast response and small J–V hysteresis. Nano Energy 2015, 17, 269–278. [Google Scholar] [CrossRef]
- Arora, N.; Orlandi, S.; Dar, M.I.; Aghazada, S.; Jacopin, G.; Cavazzini, M.; Mosconi, E.; Gratia, P.; De Angelis, F.; Pozzi, G.; et al. High open-circuit voltage: Fabrication of formamidinium lead bromide perovskite solar cells using fluorene–dithiophene derivatives as hole-transporting materials. ACS Energy Lett. 2016, 1, 107–112. [Google Scholar] [CrossRef]
- Kedem, N.; Brenner, T.M.; Kulbak, M.; Schaefer, N.; Levcenko, S.; Levine, I.; Abou-Ras, D.; Hodes, G.; Cahen, D. Light-induced increase of electron diffusion length in ap–n junction type CH3NH3PbBr3 perovskite solar cell. J. Phys. Chem. Lett. 2015, 6, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Ho-Baillie, A.; Huang, S.; Chen, S.; Wen, X.; Hao, X.; Green, M.A. Methylammonium lead bromide perovskite-based solar cells by vapor-assisted deposition. J. Phys. Chem. C 2015, 119, 3545–3549. [Google Scholar] [CrossRef]
- Talbert, E.M.; Zarick, H.F.; Orfield, N.J.; Li, W.; Erwin, W.R.; DeBra, Z.R.; Reid, K.R.; McDonald, C.P.; McBride, J.R.; Valentine, J.; et al. Interplay of structural and compositional effects on carrier recombination in mixed-halide perovskites. RSC Adv. 2016, 6, 86947–86954. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Cahen, D.; Hodes, G. High open-circuit voltage solar cells based on organic–inorganic lead bromide perovskite. J. Phys. Chem. Lett. 2013, 4, 897–902. [Google Scholar] [CrossRef]
- López, C.A.; Martínez-Huerta, M.V.; Alvarez-Galván, M.C.; Kayser, P.; Gant, P.; Castellanos-Gomez, A.; Fernández-Díaz, M.T.; Fauth, F.; Alonso, J.A. Elucidating the methylammonium (MA) conformation in MAPbBr3 perovskite with application in solar cells. Inorg. Chem. 2017, 56, 14214–14219. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, A.; Deschler, F.; Thomas, T.H.; Dutton, S.E.; Goedel, K.C.; Hanusch, F.C.; Lai, M.L.; Steiner, U.; Bein, T.; Docampo, P.; et al. Preparation of single-phase films of CH3NH3Pb (I1–x Br x) 3 with sharp optical band edges. J. Phys. Chem. Lett. 2014, 5, 2501–2505. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Binek, A.; Hanusch, F.C.; Docampo, P.; Bein, T. Stabilization of the trigonal high-temperature phase of formamidinium lead iodide. J. Phys. Chem. Lett. 2015, 6, 1249–1253. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved phase stability of formamidinium lead triiodide perovskite by strain relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, J.-W.; Choi, C.; Tan, S.; Lee, C.; Zhao, Y.; Dai, Z.; De Marco, N.; Lee, S.-J.; Bae, S.-H.; et al. Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells. Nat. Commun. 2019, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, T.; Iqbal, M.; Abbas, M. Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. cerevisiae. Braz. J. Microbiol. 2017, 48, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0. 9Sn0. 1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M. Stabilizing cesium lead halide perovskite lattice through Mn (II) substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Mir, W.J.; Nag, A. Can B-site doping or alloying improve thermal-and phase-stability of all-inorganic CsPbX3 (X = Cl, Br, I) perovskites? ACS Energy Lett. 2018, 3, 286–289. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Z.; Hao, H.; Wang, G.; Gao, H.; Wang, J.; Pan, B.; Qi, Q. Efficient Ag-doped perovskite solar cells fabricated in ambient air. Crystals 2021, 11, 1521. [Google Scholar] [CrossRef]
- Shahbazi, S.; Tsai, C.-M.; Narra, S.; Wang, C.-Y.; Shiu, H.-S.; Afshar, S.; Taghavinia, N.; Diau, E.W.-G. Ag doping of organometal lead halide perovskites: Morphology modification and p-type character. J. Phys. Chem. C 2017, 121, 3673–3679. [Google Scholar] [CrossRef]
- Chen, R.; Ye, Q.-L.; He, T.; Ta, V.D.; Ying, Y.; Tay, Y.Y.; Wu, T.; Sun, H. Exciton localization and optical properties improvement in nanocrystal-embedded ZnO core–shell nanowires. Nano Lett. 2013, 13, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Asadpour, R.; Blancon, J.-C.; Stoumpos, C.C.; Durand, O.; Strzalka, J.W.; Chen, B.; Verduzco, R.; Ajayan, P.M.; Tretiak, S.; et al. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Science 2018, 360, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Khan, M.; Bhatti, K.; Qindeel, R.; Althobaiti, H.S.; Alonizan, N. Structural, electrical and optical properties of multilayer TiO2 thin films deposited by sol–gel spin coating. Results Phys. 2017, 7, 1437–1439. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Kiasari, N.M.; Servati, P. An efficient inverted organic solar cell with improved ZnO and gold contact layers. Org. Electron. 2012, 13, 945–953. [Google Scholar] [CrossRef]
- Yan, N.; Yin, H.; Wang, Z.; Yuan, H.; Xin, Y.; Tang, Y. Role of Ammonium Derivative Ligands on Optical Properties of CH3NH3PbBr3 Perovskite Nanocrystals. Langmuir 2019, 35, 15151–15157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Shu, Y.; Xin, Y.; Ma, B. Highly luminescent nanoscale quasi-2D layered lead bromide perovskites with tunable emissions. Chem. Commun. 2016, 52, 3887–3890. [Google Scholar] [CrossRef]
- Jeon, Y.P.; Woo, S.J.; Kim, T.W. Transparent and flexible photodetectors based on CH3NH3PbI3 perovskite nanoparticles. Appl. Surf. Sci. 2018, 434, 375–381. [Google Scholar] [CrossRef]
- Mehmood, B.; Khan, M.; Iqbal, M.; Mahmood, A.; Al-Masry, W. Structural and optical properties of Ti and Cu co-doped ZnO thin films for photovoltaic applications of dye sensitized solar cells. Int. J. Energy Res. 2021, 45, 2445–2459. [Google Scholar] [CrossRef]
- Khan, M.; Bhatti, K.; Qindeel, R.; Bousiakou, L.G.; Alonizan, N. Investigations of the structural, morphological and electrical properties of multilayer ZnO/TiO2 thin films, deposited by sol–gel technique. Results Phys. 2016, 6, 156–160. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, L.; Lu, T.; Liu, X.; Lv, T.; Xu, T.; Sun, Z. Electrophoretic deposition of carbon nanotubes films as counter electrodes of dye-sensitized solar cells. Electrochim. Acta 2011, 56, 10288–10291. [Google Scholar] [CrossRef]
- Gayathri, V.; Rameshbabu, M.; Sasiflorence, S.; Ravichandran, K.; Ramachandran, K.; Mohan, C.R.; Prabha, K. Influence of La on nano titanium dioxide (TiO2) based solar cell. Mater. Today: Proc. 2019, 35, 2–5. [Google Scholar] [CrossRef]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: The role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef]
- Khan, M.; Mehmood, B.; Naeem, M.; Younis, M.; Mahmoud, K.H.; El-Bahy, Z.M.; Subhani, W.S.; Hussain, S.; Alwadai, N.; Albalawi, H. Investigations the structural, optical and photovoltaic properties of La doped TiO2 photoanode based dye sensitized solar cells. Opt. Mater. 2021, 122, 111610. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Peng, X.; Liu, L.; Dong, S.; Lin, L.; Chen, S.; Meng, S.; Feng, Y. Preparation of dye-sensitized solar cells with high photocurrent and photovoltage by using mesoporous titanium dioxide particles as photoanode material. Nano Res. 2015, 8, 3830–3841. [Google Scholar] [CrossRef]
- Mehdi, H.; Mhamdi, A.; Hannachi, R.; Bouazizi, A. MAPbBr3 perovskite solar cells via a two-step deposition process. RSC Adv. 2019, 9, 12906–12912. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.; Subhani, W.S.; Mustafa, G.M.; Saleem, M.; Abubshait, S.A.; Abubshait, H.A.; Saleh, D.I.; Mahmoud, S.F. Decorating wide band gap CH3NH3PbBr3 perovskite with 4AMP for highly efficient and enhanced open circuit voltage perovskite solar cells. Sol. Energy 2021, 230, 501–508. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature-and component-dependent degradation of perovskite photovoltaic materials under concentrated sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef]
- Joseph, D.P.; Venkateswaran, C. Bandgap engineering in ZnO by doping with 3d transition metal ions. J. At. Mol. Phys. 2011, 2011, 270540. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kobayashi, K.; Takahashi, Y.; Harada, J.; Inabe, T. Effective band gap tuning by foreign metal doping in hybrid tin iodide perovskites. J. Mater. Chem. C 2017, 5, 4048–4052. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Liang, P.-W.; Chueh, C.-C.; Yang, Z.; Jen, A.K.-Y.; Wang, H.-L. Large grained perovskite solar cells derived from single-crystal perovskite powders with enhanced ambient stability. ACS Appl. Mater. Interfaces 2016, 8, 14513–14520. [Google Scholar] [CrossRef] [PubMed]
- Pourdavoud, N.; Mayer, A.; Buchmüller, M.; Brinkmann, K.; Haeger, T.; Hu, T.; Heiderhoff, R.; Shutsko, I.; Görrn, P.; Chen, Y.; et al. Distributed feedback lasers based on MAPbBr3. Adv. Mater. Technol. 2018, 3, 1700253. [Google Scholar] [CrossRef]
- Shan, F.; Liu, G.; Lee, W.; Lee, G.; Kim, I.; Shin, B.; Kim, Y. Transparent conductive ZnO thin films on glass substrates deposited by pulsed laser deposition. J. Cryst. Growth 2005, 277, 284–292. [Google Scholar] [CrossRef]
- Xue, S.; Zu, X.; Zheng, W.; Deng, H.; Xiang, X. Effects of Al doping concentration on optical parameters of ZnO: Al thin films by sol–gel technique. Phys. B Condens. Matter 2006, 381, 209–213. [Google Scholar] [CrossRef]
- Xie, G.; Fanga, L.; Peng, L.; Liu, G.; Ruan, H.; Wu, F.; Kong, C. Effect of In-doping on the optical constants of ZnO thin films. Phys. Procedia 2012, 32, 651–657. [Google Scholar] [CrossRef]
- Gadallah, A.-S.; El-Nahass, M.M. Structural, optical constants and photoluminescence of ZnO thin films grown by sol-gel spin coating. Adv. Condens. Matter Phys. 2013, 2013, 234546. [Google Scholar] [CrossRef]
- Khan, M.; Farooq, W.; Saleem, M.; Bhatti, K.; Atif, M.; Hanif, A. Phase change, band gap energy and electrical resistivity of Mg doped TiO2 multilayer thin films for dye sensitized solar cells applications. Ceram. Int. 2019, 45, 21436–21439. [Google Scholar] [CrossRef]
- Narang, R.; Saxena, M.; Gupta, M. Investigation of dielectric modulated (DM) double gate (DG) junctionless MOSFETs for application as a biosensors. Superlattices Microstruct. 2015, 85, 557–572. [Google Scholar]
- Aydin, C. Synthesis of Pd: ZnO nanofibers and their optical characterization dependent on modified morphological properties. J. Alloys Compd. 2019, 777, 145–151. [Google Scholar] [CrossRef]
- Tepehan, F.; Özer, N. A simple method for the determination of the optical constants, n and k of cadmium sulfide films from transmittance measurements. Sol. Energy Mater. Sol. Cells 1993, 30, 353–365. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Fatima, N.; Mustafa, G.M.; Sabir, M.; Abubshait, S.A.; Abubshait, H.A.; Alshahrani, T.; Iqbal, M.; Laref, A.; Baig, M. Improved photovoltaic properties of dye sensitized solar cell by irradiations of Ni2+ ions on Ag-doped TiO2 photoanode. Int. J. Energy Res. 2021, 45, 9685–9693. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, N.; Schlipf, J.; Oesinghaus, L.; Binek, A.; Bein, T.; Müller-Buschbaum, P.; Docampo, P. Synthesis of perfectly oriented and micrometer-sized MAPbBr3 perovskite crystals for thin-film photovoltaic applications. ACS Energy Lett. 2016, 1, 150–154. [Google Scholar] [CrossRef]
- de Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef]
- Wu, X.; Trinh, M.T.; Niesner, D.; Zhu, H.; Norman, Z.; Owen, J.S.; Yaffe, O.; Kudisch, B.J.; Zhu, X.-Y. Trap states in lead iodide perovskites. J. Am. Chem. Soc. 2015, 137, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shehzad, A.; Farooq, W.; Arfan, M.; Hasan, M.; Atif, M.; Hanif, A. 900 keV Au ions implantation effect on the efficiency of dye sensitized solar cells. Results Phys. 2019, 14, 102425. [Google Scholar] [CrossRef]
- Basu, K.; Benetti, D.; Zhao, H.; Jin, L.; Vetrone, F.; Vomiero, A.; Rosei, F. Enhanced photovoltaic properties in dye sensitized solar cells by surface treatment of SnO 2 photoanodes. Sci. Rep. 2016, 6, 23312. [Google Scholar] [CrossRef]
- Sanda, M.D.A.; Badu, M.; Awudza, J.A.; Boadi, N.O. Development of TiO2-based dye-sensitized solar cells using natural dyes extracted from some plant-based materials. Chem. Int. 2021, 7, 9–20. [Google Scholar]
- Aal, R.M.A.; Gitru, M.A.; Essam, Z.M. Novel synthetized near infrared cyanine dyes as sensitizer for dye sensitized solar cells based on nano-TiO2. Chem. Int. 2017, 3, 358–367. [Google Scholar]

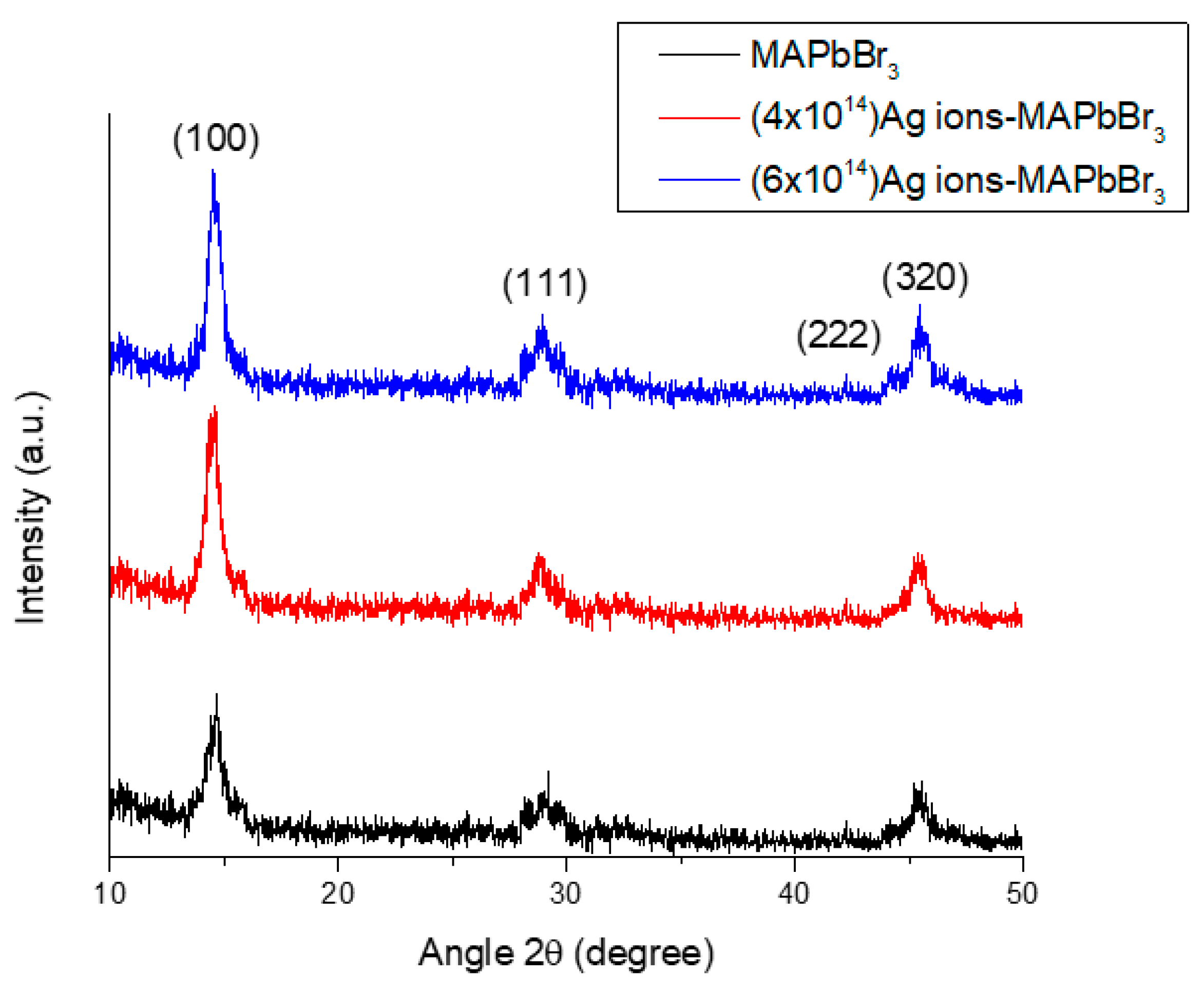






| Sample | D (nm) | a (Å) | ||
|---|---|---|---|---|
| PureMAPbBr3 | 19 | 3.52 | 6.11 | 2.28 |
| m2) | 26 | 3.44 | 6.10 | 2.20 |
| 22 | 4.42 | 6.04 | 2.27 |
| Sample | Real Dielectric Constant | Imaginary Dielectric Constant |
|---|---|---|
| εr | εi | |
| MAPbBr3 | 1.43 | 10.58 |
| 4 × 1014 Ag ion MAPbBr3 | 1.79 | 11.60 |
| 6 × 1014 Ag ion MAPbBr3 | 1.67 | 11.32 |
| Sample | Jsc | Voc | FF | Efficiency (η) |
|---|---|---|---|---|
| MAPbBr3 | 6.69 | 1.1 | 0.798 | 5.87 |
| Ag-irradiated MAPbBr3 | 8.18 | 1.13 | 0.759 | 7.01 |
| Ag-irradiated MAPbBr3 | 7.08 | 1.11 | 0.790 | 6.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Alwadai, N.; Khan, M.I.; Irfan, M.; Ikram-ul-Haq; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. RETRACTED: The Effect of 600 keV Ag Ion Irradiation on the Structural, Optical, and Photovoltaic Properties of MAPbBr3 Films for Perovksite Solar Cell Applications. Materials 2022, 15, 5299. https://doi.org/10.3390/ma15155299
Hussain S, Alwadai N, Khan MI, Irfan M, Ikram-ul-Haq, Albalawi H, Almuqrin AH, Almoneef MM, Iqbal M. RETRACTED: The Effect of 600 keV Ag Ion Irradiation on the Structural, Optical, and Photovoltaic Properties of MAPbBr3 Films for Perovksite Solar Cell Applications. Materials. 2022; 15(15):5299. https://doi.org/10.3390/ma15155299
Chicago/Turabian StyleHussain, Saddam, Norah Alwadai, Muhammad I. Khan, Muhammad Irfan, Ikram-ul-Haq, Hind Albalawi, Aljawhara H. Almuqrin, Maha M. Almoneef, and Munawar Iqbal. 2022. "RETRACTED: The Effect of 600 keV Ag Ion Irradiation on the Structural, Optical, and Photovoltaic Properties of MAPbBr3 Films for Perovksite Solar Cell Applications" Materials 15, no. 15: 5299. https://doi.org/10.3390/ma15155299
APA StyleHussain, S., Alwadai, N., Khan, M. I., Irfan, M., Ikram-ul-Haq, Albalawi, H., Almuqrin, A. H., Almoneef, M. M., & Iqbal, M. (2022). RETRACTED: The Effect of 600 keV Ag Ion Irradiation on the Structural, Optical, and Photovoltaic Properties of MAPbBr3 Films for Perovksite Solar Cell Applications. Materials, 15(15), 5299. https://doi.org/10.3390/ma15155299






