Template-Mediated Synthesis of Hierarchically Porous Metal–Organic Frameworks for Efficient CO2/N2 Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Characterizations
2.2. Synthesis of CuBTCMOF
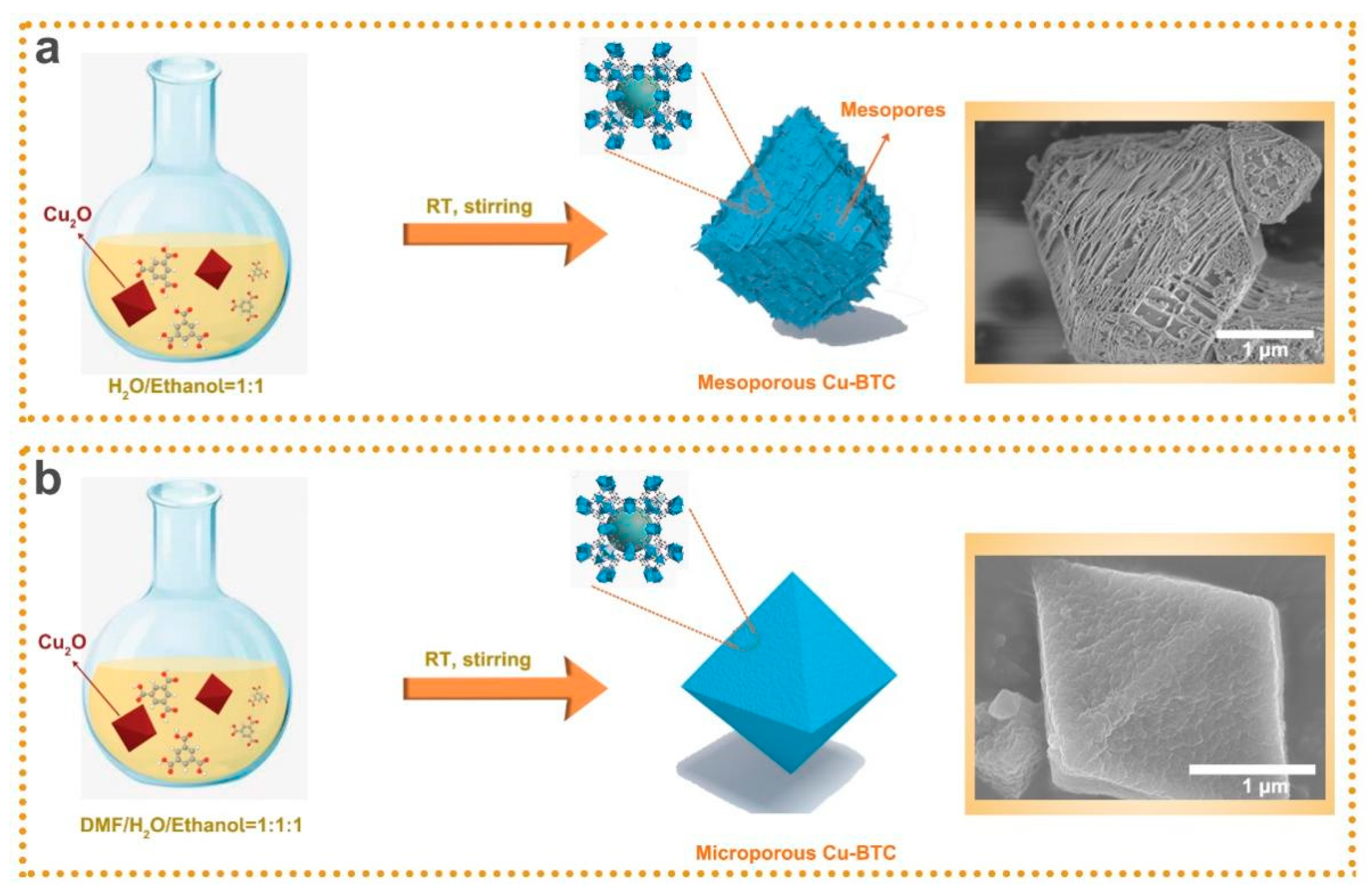
2.3. Synthesis of CuBTC-DMF
2.4. Synthesis of CuBTC-Water
2.5. Gas Adsorption Measurements
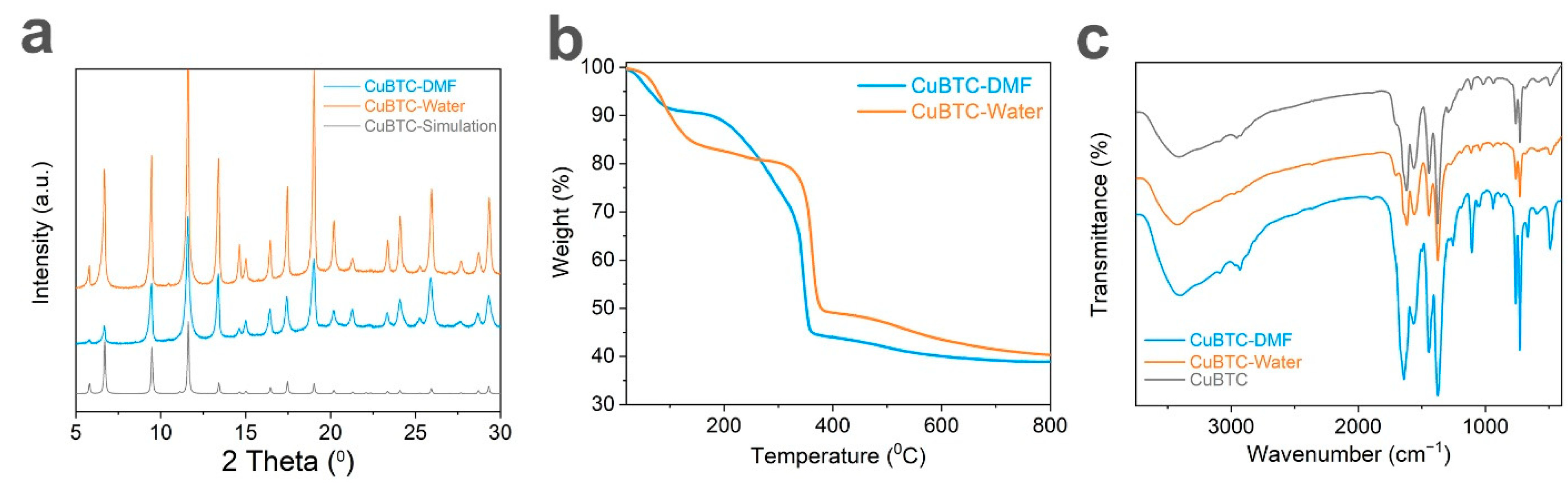
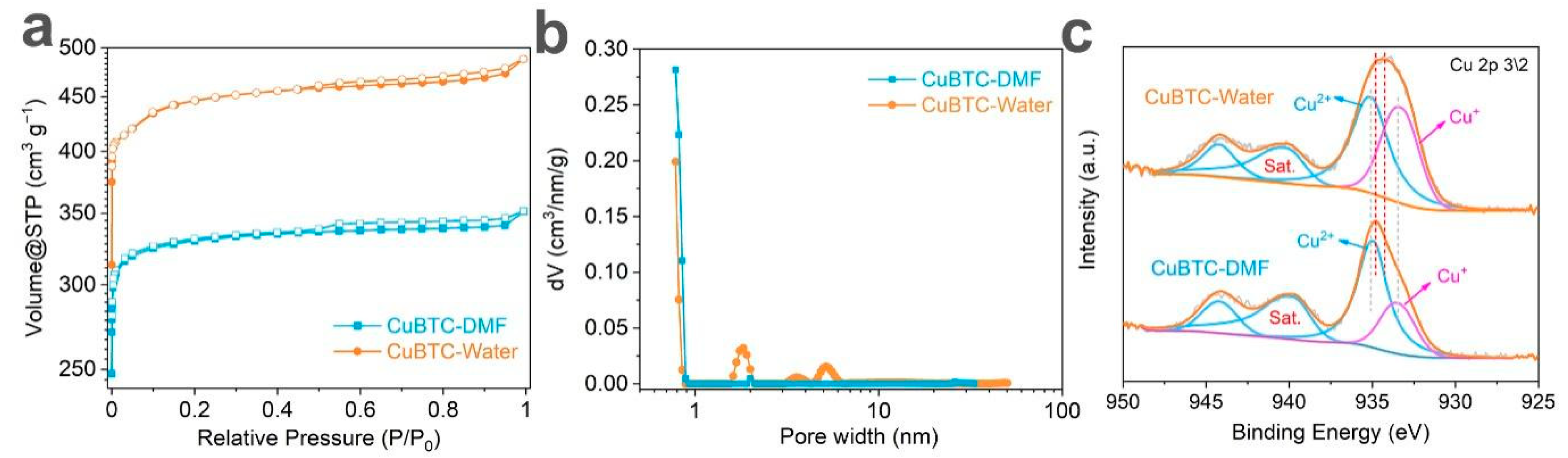
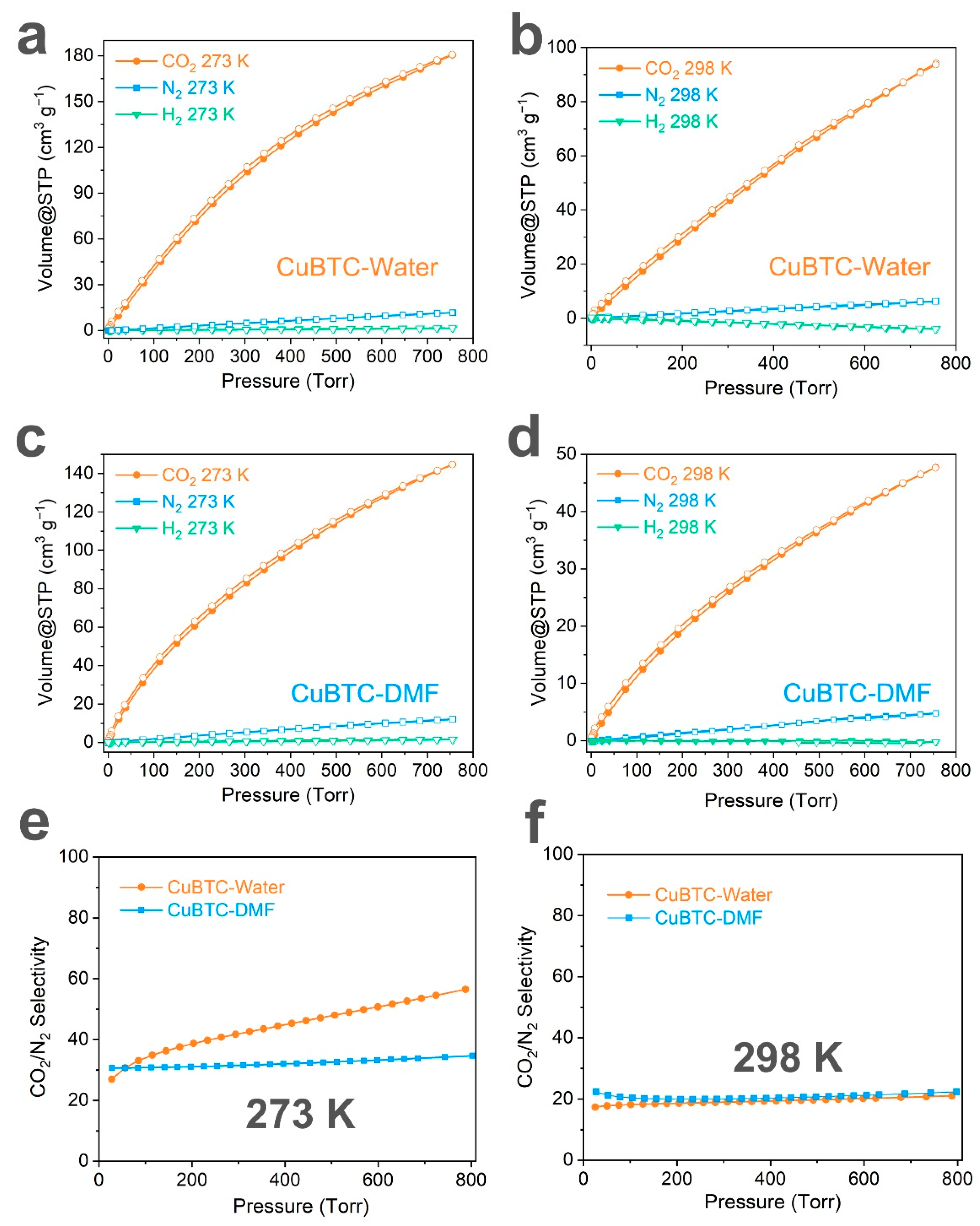
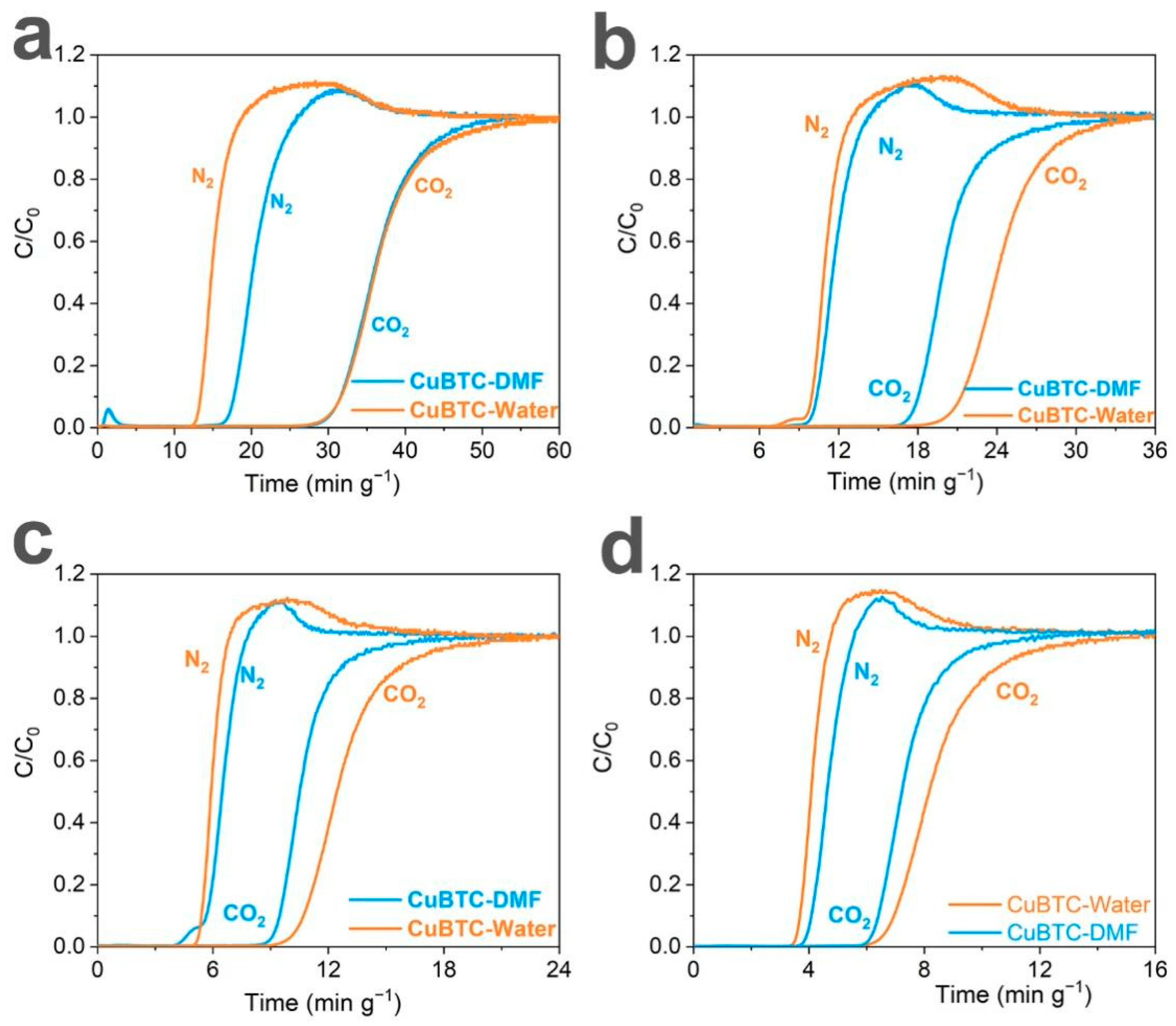
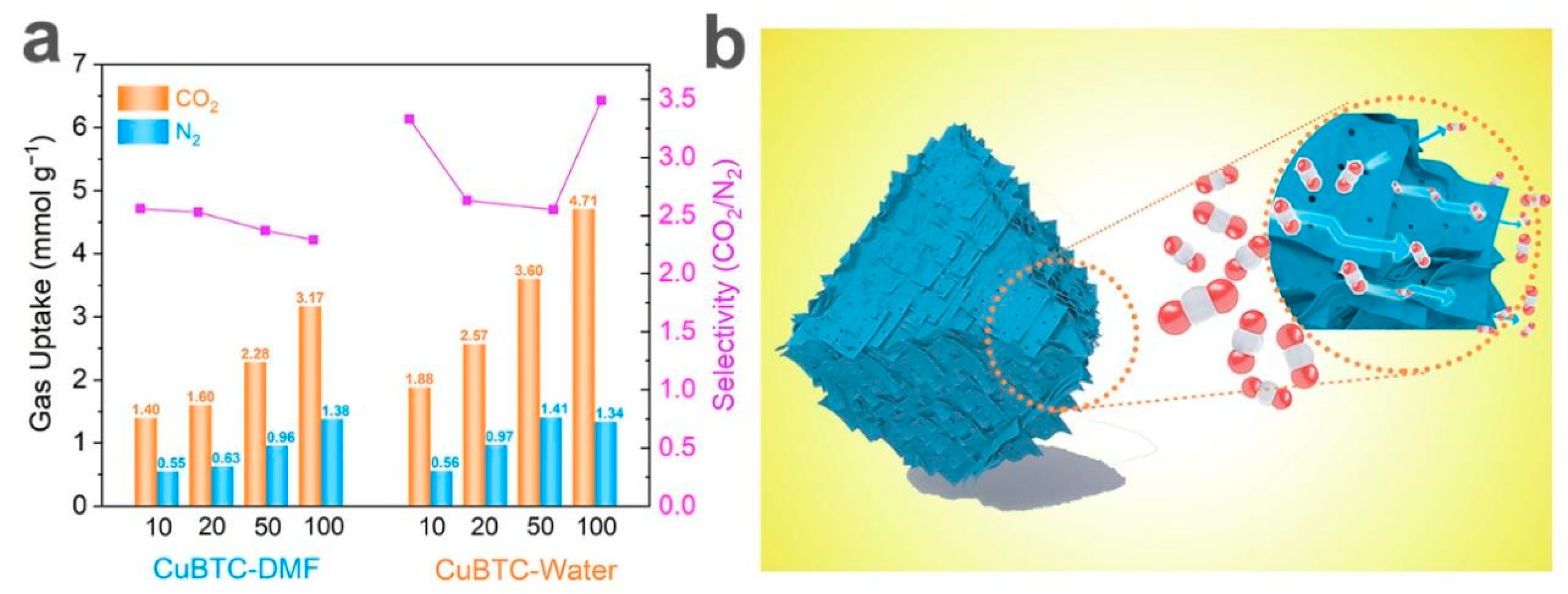
3. Results and Discussion
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, J.Z.; Zhong, Z.Y.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. A Porous Coordination Copolymer with over 5000 m2/g BET Surface Area. J. Am. Chem. Soc. 2009, 131, 4184–4185. [Google Scholar] [CrossRef]
- Farha, O.K.; Yazaydin, A.O.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal-Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.-Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.-S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Yu, X.F.; Meng, W.; Han, S.B.; Liu, J.; Ye, Y.X.; Sun, C.Y.; Chen, G.J.; Zou, R.Q. A solvent ‘squeezing’ strategy to graft ethylenediamine on Cu3(BTC)2 for highly efficient CO2/CO separation. Chem. Eng. Sci. 2018, 184, 85–92. [Google Scholar] [CrossRef]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.G.; Yaghi, O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. PNAS 2009, 106, 20637–20640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buragohain, A.; Van der Voort, P.; Biswas, S. Facile synthesis and gas adsorption behavior of new functionalized Al-MIL-101-X (X = –CH3, –NO2, –OCH3, –C6H4, –F2, –(CH3)2, –(OCH3)2) materials. Micropor. Mesopor. Mat. 2015, 215, 91–97. [Google Scholar] [CrossRef]
- Yeon, J.S.; Lee, W.R.; Kim, N.W.; Jo, H.; Lee, H.; Song, J.H.; Lim, K.S.; Kang, D.W.; Seo, J.G.; Moon, D.; et al. Homodiamine-functionalized metal–organic frameworks with a MOF-74-type extended structure for superior selectivity of CO2 over N2. J. Mater. Chem. A 2015, 3, 19177–19185. [Google Scholar] [CrossRef]
- Wang, G.F.; Graham, E.; Zheng, S.L.; Zhu, J.X.; Zhu, R.L.; He, H.P.; Sun, Z.M.; Mackinnon, I.D.R.; Xi, Y.F. Diatomite-Metal-Organic Framework Composite with Hierarchical Pore Structures for Adsorption/Desorption of Hydrogen, Carbon Dioxide and Water Vapor. Materials 2020, 13, 4700. [Google Scholar] [CrossRef]
- Duan, C.; Zou, W.; Du, Z.J.; Mi, J.G.; Han, J.X.; Zhang, C. Construction of Oxygen-Rich Carbon Foams for Rapid Carbon Dioxide Capture. Materials 2020, 14, 173. [Google Scholar] [CrossRef]
- Hwang, S.; Chi, W.S.; Lee, S.J.; Im, S.H.; Kim, J.H.; Kim, J. Hollow ZIF-8 nanoparticles improve the permeability of mixed matrix membranes for CO2/CH4 gas separation. J. Membr. Sci. 2015, 480, 11–19. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chuah, C.Y.; Dong, P.P.; Cha, Y.-H.; Bae, T.-H.; Song, M.-K. Hierarchically Porous Co-MOF-74 Hollow Nanorods for Enhanced Dynamic CO2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 43316–43322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.Q.; Yu, X.F.; Meng, W.; Liu, J.; Zhi, C.X.; Zou, R.Q. Amine-Grafted MIL-101(Cr) via Double-Solvent Incorporation for Synergistic Enhancement of CO2 Uptake and Selectivity. ACS Sustain. Chem. Eng. 2018, 6, 16493–16502. [Google Scholar] [CrossRef]
- Rieth, A.J.; Dinca, M. Controlled Gas Uptake in Metal-Organic Frameworks with Record Ammonia Sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef]
- Fonseca, J.; Choi, S. Synthesis of a novel amorphous metal organic framework with hierarchical porosity for adsorptive gas separation. Micropor. Mesopor. Mat. 2021, 310, 110600. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically porous metal–organic frameworks: Synthetic strategies and applications. Natl. Sci. Rev. 2020, 7, 1743–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, T.J.; Liang, Z.B.; Guo, W.H.; Tabassum, H.; Gao, S.; Zou, R.Q. Metal-Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Q.; Kujawski, W.; Knozowska, K.; Kujawa, J. Thin Film Mixed Matrix Hollow Fiber Membrane Fabricated by Incorporation of Amine Functionalized Metal-Organic Framework for CO2/N2 Separation. Materials 2021, 14, 3366. [Google Scholar] [CrossRef]
- Li, P.; Vermeulen, N.A.; Malliakas, C.D.; Gomez-Gualdron, D.A.; Howarth, A.J.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; O’Keeffe, M.; Farha, O.K. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 2017, 356, 624–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.W.; Liu, T.F.; Su, J.; Bosch, M.; Wei, Z.W.; Wan, W.; Yuan, D.Q.; Chen, Y.P.; Wang, X.; Wang, K.C.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, T.J.; Gao, S.; Liang, Z.B.; Wang, D.G.; Tabassum, H.; Zhong, R.Q.; Zou, R.Q. Pristine Hollow Metal-Organic Frameworks: Design, Synthesis and Application. Angew. Chem. Int. Ed. 2021, 60, 17314–17336. [Google Scholar] [CrossRef]
- Cai, G.R.; Jiang, H.L. A Modulator-Induced Defect-Formation Strategy to Hierarchically Porous Metal–Organic Frameworks with High Stability. Angew. Chem. Int. Ed. 2017, 56, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Zhang, L.; Chen, X.D.; Liu, L.M.; Zhang, D.L.; Han, Y.; Chen, J.Y.; Long, J.L.; Luque, R.; Li, Y.W.; et al. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, M.M.; Zhang, X.L.; Li, M.Y.; Chen, B.; Yin, J.; Jin, H.C.; Lin, L.; Chen, C.; Zhang, N. Hollow Pd/MOF Nanosphere with Double Shells as Multifunctional Catalyst for Hydrogenation Reaction. Small 2017, 13, 1701395. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Arque, X.; Patino, T.; Guillerm, V.; Blersch, P.-R.; Perez-Carvajal, J.; Imaz, I.; Maspoch, D.; Sanchez, S. Enzyme-Powered Porous Micromotors Built from a Hierarchical Micro- and Mesoporous UiO-Type Metal-Organic Framework. J. Am. Chem. Soc. 2020, 142, 20962–20967. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Huang, J.J.; Yang, Q.; Wang, S.J.; Sun, X.M.; Zhang, W.N.; Liu, J.F.; Huo, F.W. Multi-shelled Hollow Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 5512–5516. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Zou, W.X.; Wan, H.; Dong, L.; Guan, G.F. Structural modulation of UiO-66-NH2 metal-organic framework via interligands cross-linking: Cooperative effects of pore diameter and amide group on selective CO2 separation. Appl. Surf. Sci. 2021, 553, 149547. [Google Scholar] [CrossRef]
- Liu, Y.L.; Di, Y.Y.; Qiao, C.F.; Liu, M.B.; Zhou, C.S. A novel microporous metal-organic framework with Lewis basic sites and open O donor sites: Crystal structure and adsorption properties. J. Solid State Chem. 2020, 292, 121688. [Google Scholar] [CrossRef]
- Du, L.T.; Miao, Y.C.; Zheng, B.S.; Ma, M.T.; Zhang, J.C. Honeycomb-like 2D metal-organic polyhedral framework exhibiting selectively adsorption of CO2. J. Solid State Chem. 2021, 300, 122230. [Google Scholar] [CrossRef]
- Zhou, X.J.; Liu, L.L.; Kou, H.; Zheng, S.M.; Song, M.J.; Lu, J.T.; Tai, X.S. A Multifunctional 3D Supermolecular Co Coordination Polymer With Potential for CO2 Adsorption, Antibacterial Activity, and Selective Sensing of Fe3+ /Cr3+ Ions and TNP. Front. Chem. 2021, 9, 678993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mandal, S.K. A robust and water-stable two-fold interpenetrated metal-organic framework containing both rigid tetrapodal carboxylate and rigid bifunctional nitrogen linkers exhibiting selective CO2 capture. Dalton Trans. 2019, 48, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Cheedarala, R.K.; Gaikwad, R.; Kim, S.; Han, S. Controllable Synthesis of 1, 3, 5-tris (1H-benzo[d]imidazole-2-yl) Benzene-Based MOFs. Appl. Sci. 2021, 11, 9856. [Google Scholar] [CrossRef]
- He, T.J.; Xiao, Y.H.; Zhao, Q.D.; Zhou, M.X.; He, G.H. Ultramicroporous Metal-Organic Framework Qc-5-Cu for Highly Selective Adsorption of CO2 from C2H4 Stream. Ind. Eng. Chem. Res. 2020, 59, 3153–3161. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Huo, J.; Brightwell, M.; Hankari, S.E.; Garai, A.; Bradshaw, D. A versatile, industrially relevant, aqueous room temperature synthesis of HKUST-1 with high space-time yield. J. Mater. Chem. A 2013, 1, 15220–15223. [Google Scholar] [CrossRef]
- Duan, C.X.; Zhang, H.; Li, F.E.; Xiao, J.; Luo, S.J.; Xi, H.X. Hierarchically porous metal-organic frameworks: Rapid synthesis and enhanced gas storage. Soft Matter 2018, 14, 9589–9598. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.W.; Zeng, H.C. An alternative synthetic approach for macro-meso-microporous metal-organic frameworks via a “domain growth” mechanism. Chem. Commun. 2016, 52, 8432–8435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, H.V.; Sartbaeva, A.; Eioi, J.-C.; Davis, S.A.; Ting, V.P. Defective hierarchical porous copper-based metal-organic frameworks synthesised via facile acid etching strategy. Sci. Rep. 2019, 9, 10887. [Google Scholar] [CrossRef]
| Materials | SBET (m2 g−1) | CO2 Uptake | Ref. |
|---|---|---|---|
| CuBTC-Water | 1697 | 94.147 | This work |
| UiO-66-DAM | 860 | 54.1 | [29] |
| Zn porous MOF | 447.92 | 59.2 | [30] |
| NJFU-5 | 473 | 37.0 | [31] |
| Co3(L)2 (2,2′-bipy)2](DMF)3(H2O)3 | 658 | 50 | [32] |
| {[Co2(4,4′-bpy)(L)]·H2O·0.5(DMF)}n | 224 | 36.4 | [33] |
| TIBM-Cu MOF | 1073.5 | 88.2 | [34] |
| Qc-5-Cu | 211.3 | 60.76 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Gao, S.; Fu, Y.; Xu, D.; Kong, D. Template-Mediated Synthesis of Hierarchically Porous Metal–Organic Frameworks for Efficient CO2/N2 Separation. Materials 2022, 15, 5292. https://doi.org/10.3390/ma15155292
Qiu T, Gao S, Fu Y, Xu D, Kong D. Template-Mediated Synthesis of Hierarchically Porous Metal–Organic Frameworks for Efficient CO2/N2 Separation. Materials. 2022; 15(15):5292. https://doi.org/10.3390/ma15155292
Chicago/Turabian StyleQiu, Tianjie, Song Gao, Yanchun Fu, Dong Xu, and Dekai Kong. 2022. "Template-Mediated Synthesis of Hierarchically Porous Metal–Organic Frameworks for Efficient CO2/N2 Separation" Materials 15, no. 15: 5292. https://doi.org/10.3390/ma15155292
APA StyleQiu, T., Gao, S., Fu, Y., Xu, D., & Kong, D. (2022). Template-Mediated Synthesis of Hierarchically Porous Metal–Organic Frameworks for Efficient CO2/N2 Separation. Materials, 15(15), 5292. https://doi.org/10.3390/ma15155292





