Binary Promoter Improving the Moderate-Temperature Adhesion of Addition-Cured Liquid Silicone Rubber for Thermally Conductive Potting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
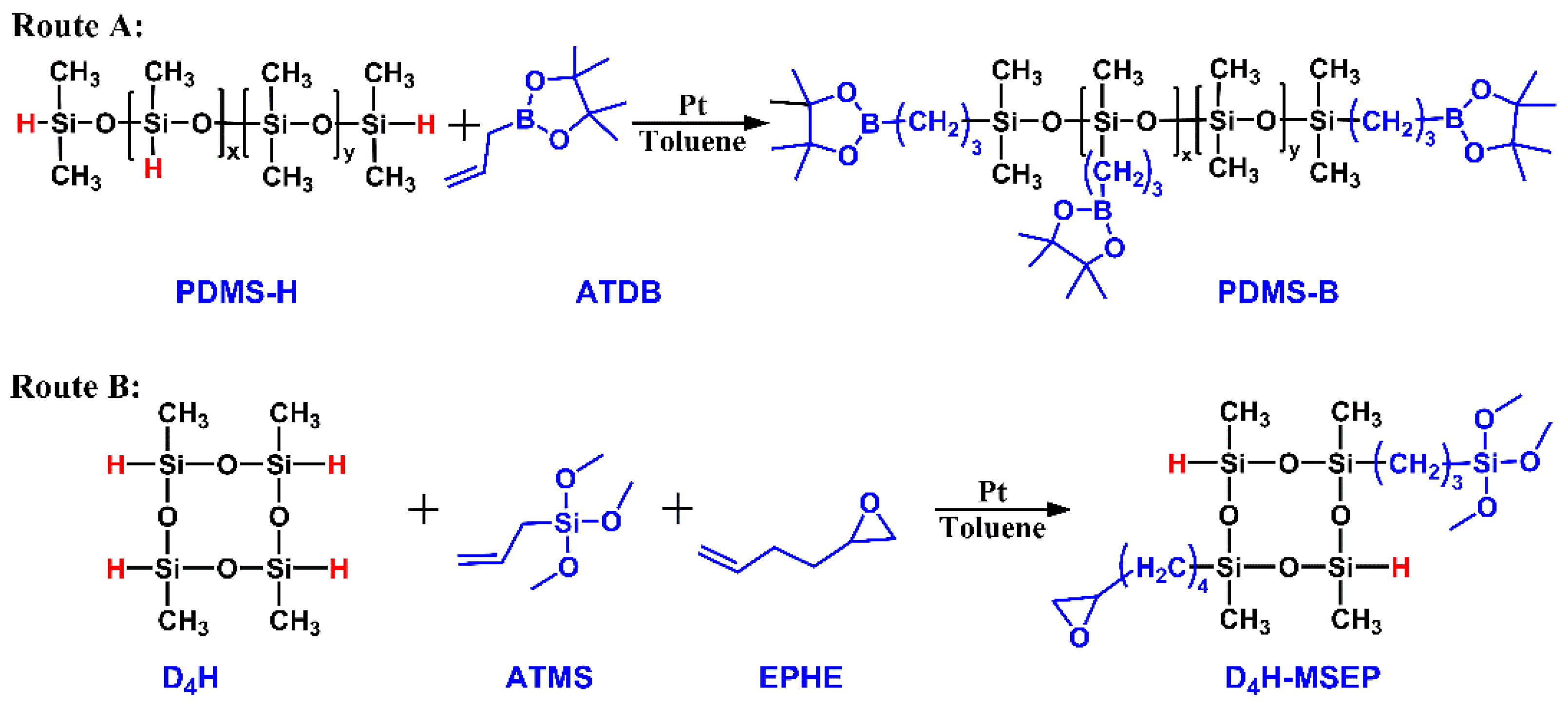
2.2. Synthesis of Boron-Modified PDMS
2.3. Synthesis of Epoxy and Alkoxy Groups-Bifunctionalized D4H
2.4. Preparation of Self-Adhesive Two-Component Addition-Curing LSR
2.5. Characterization
2.5.1. Fourier Transform Infrared (FTIR) Analysis
2.5.2. Viscosity Measurement
2.5.3. Mechanical Properties Test
2.5.4. Thermal Conductivities Test
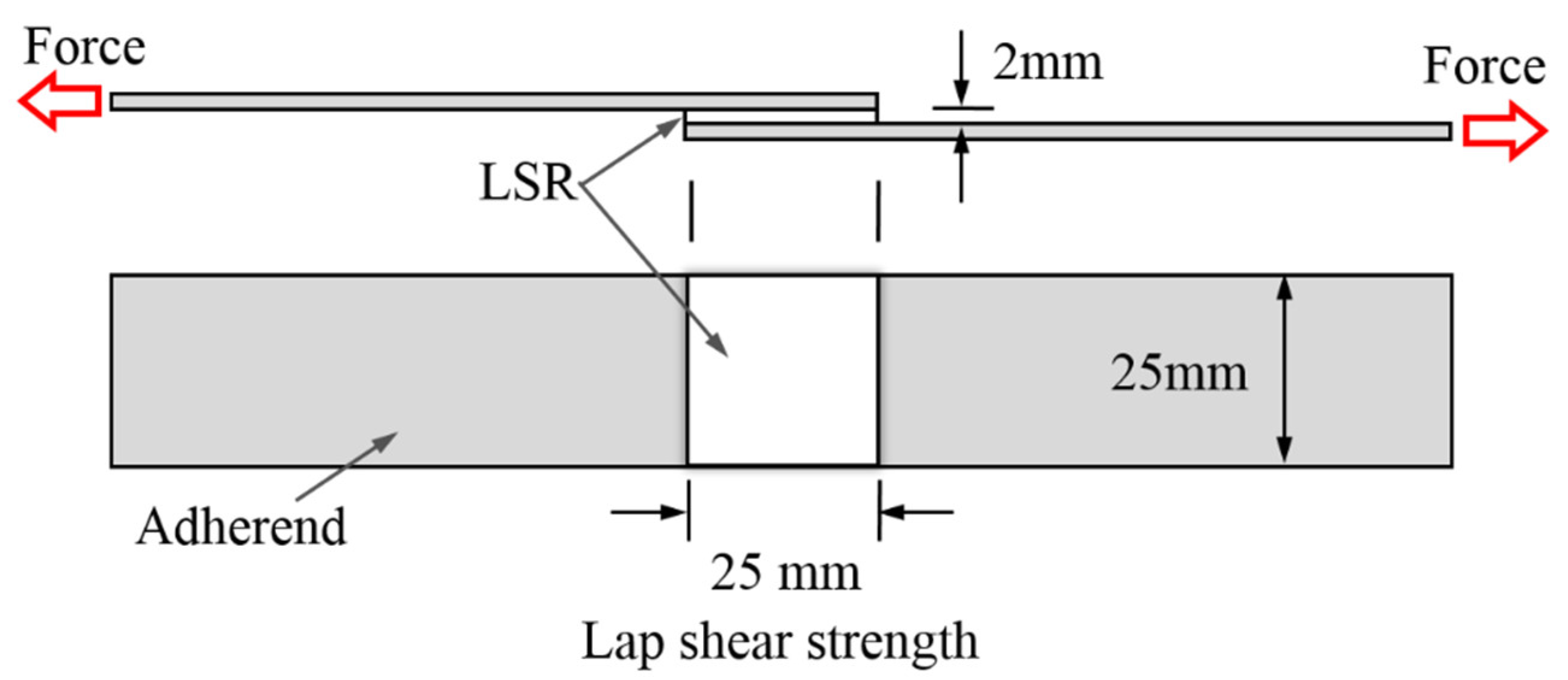
2.5.5. Adhesion Performance
3. Results
3.1. Structural Characterization of PDMS-B and D4H-MSEP
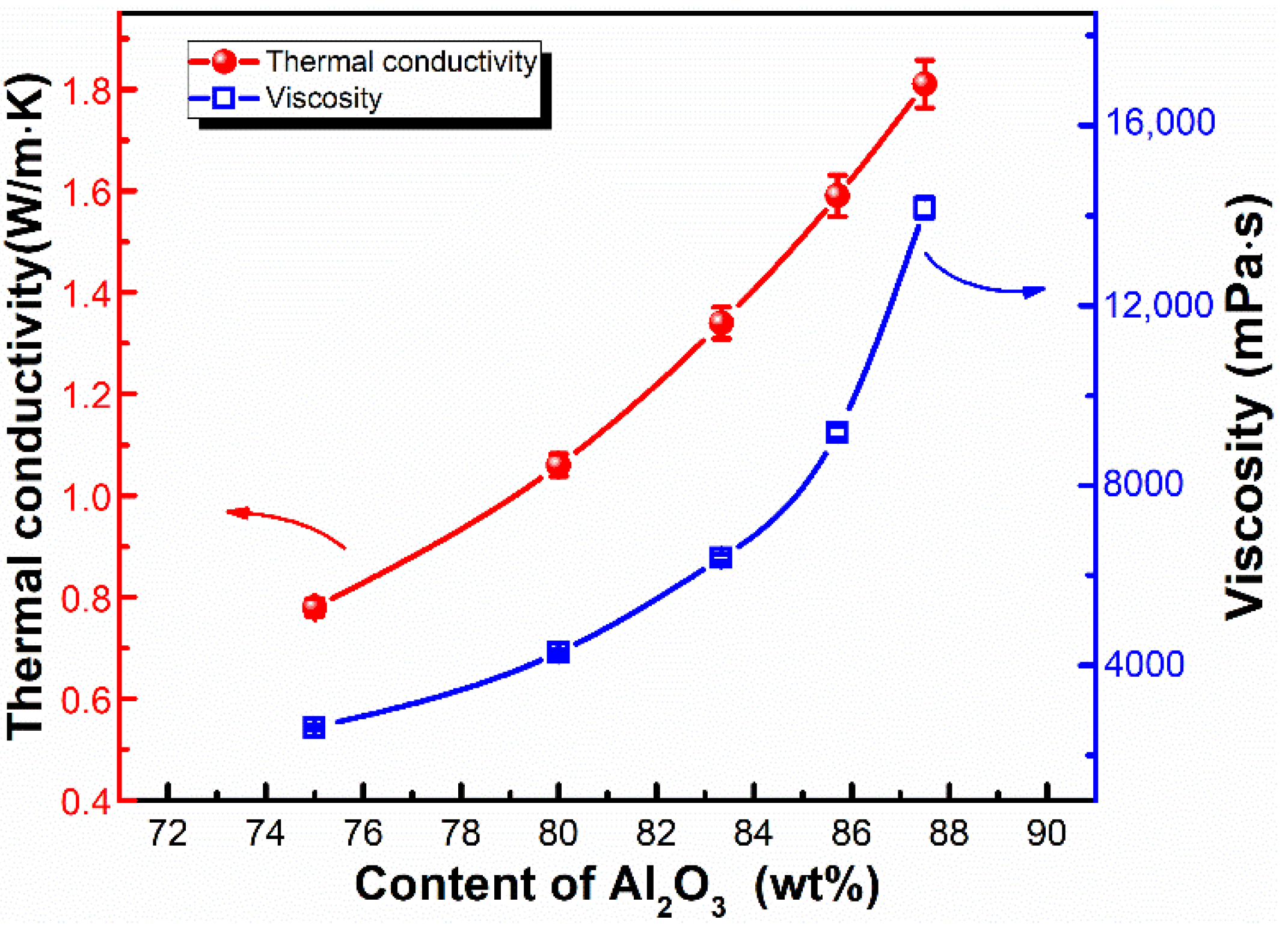
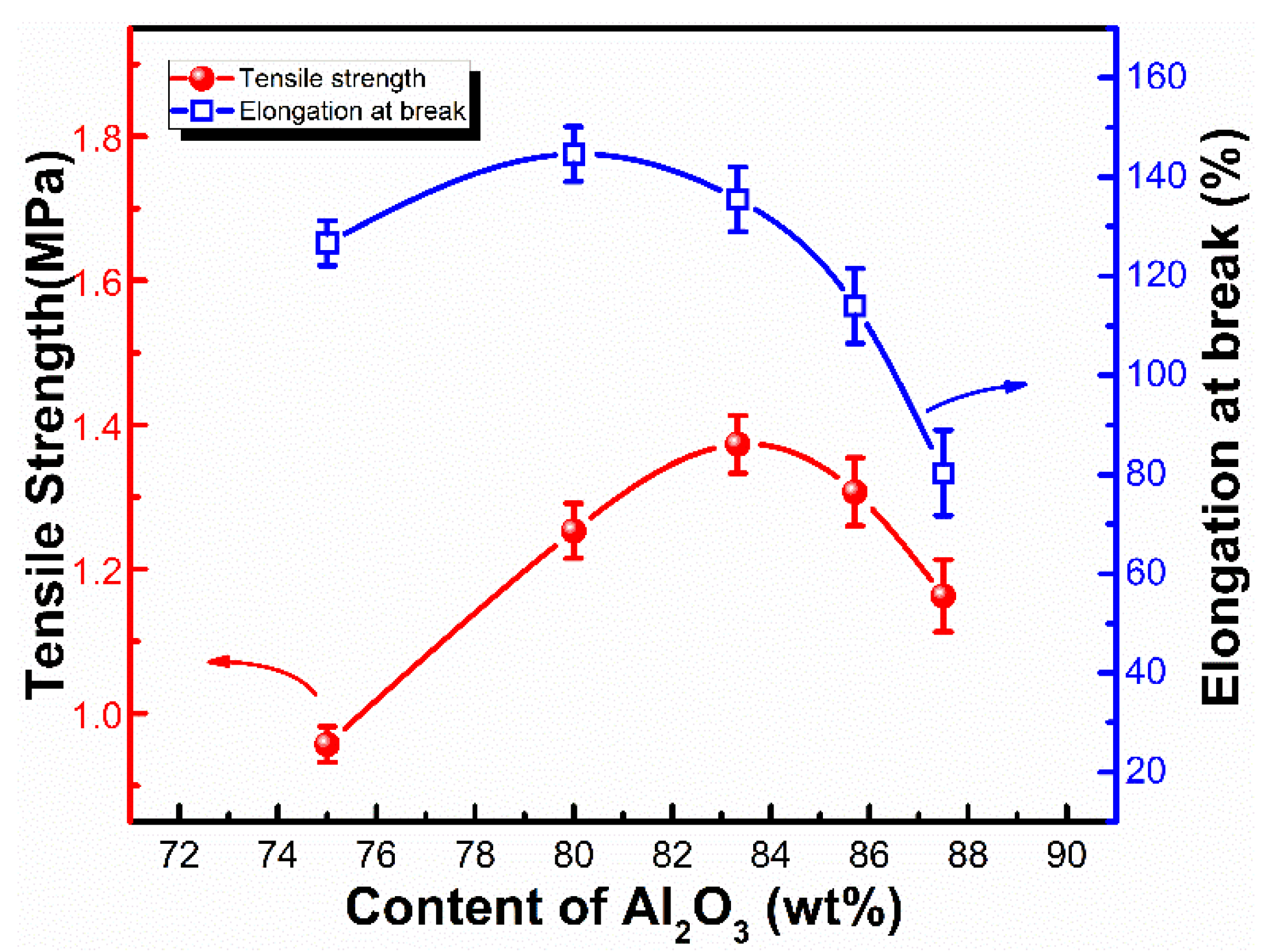
3.2. Thermal Conductivity and Mechanical Properties of LSR
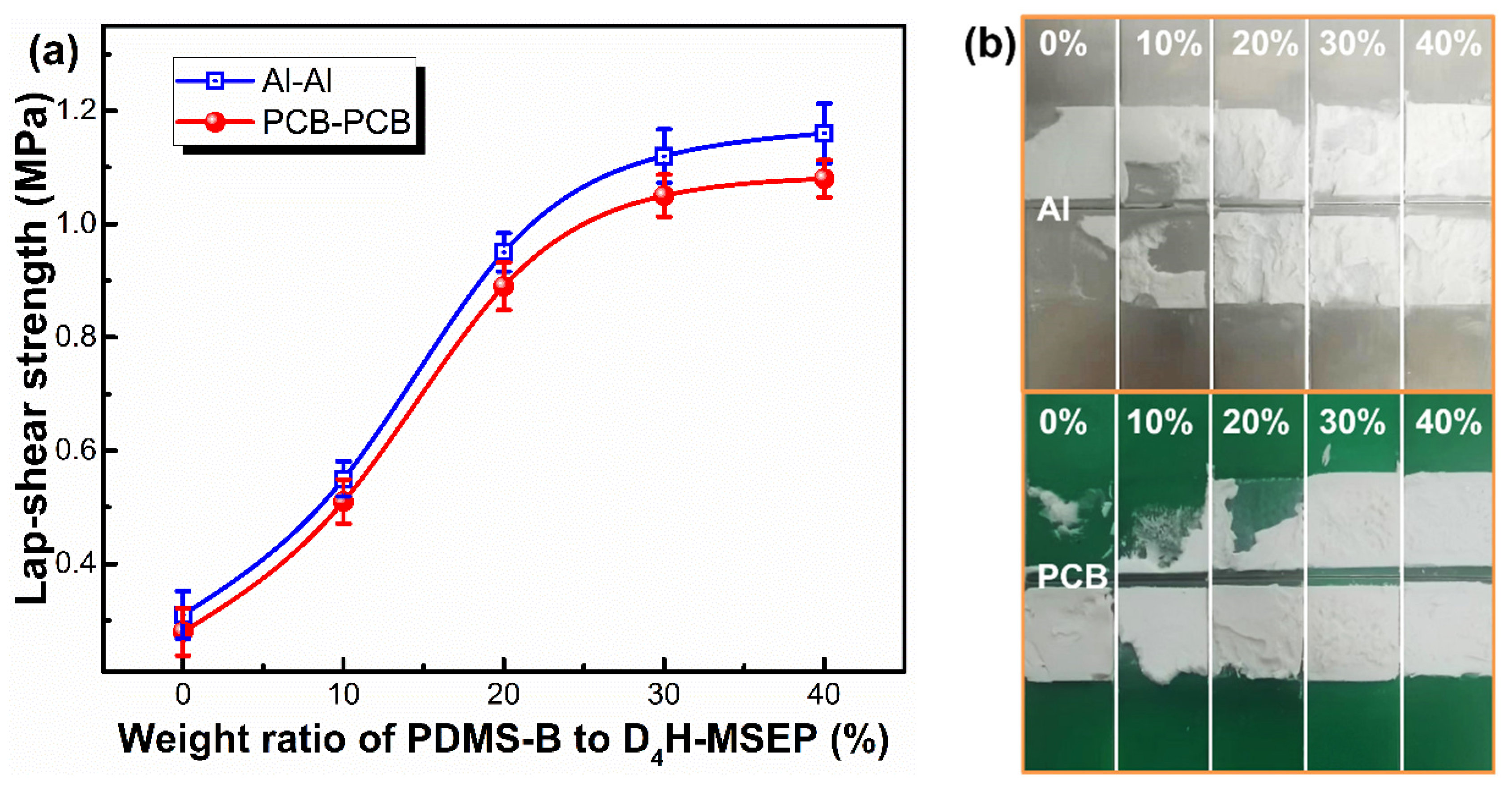
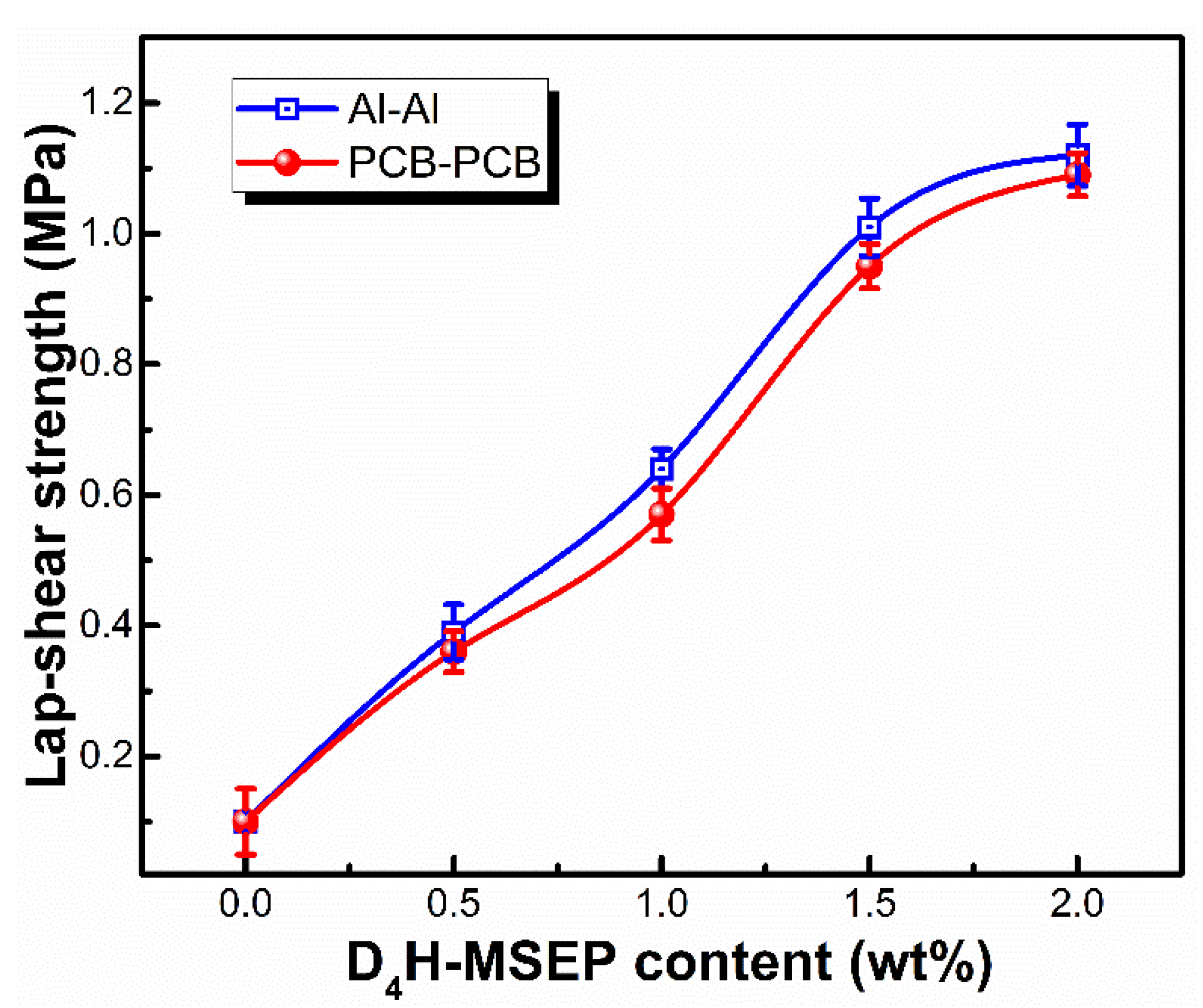
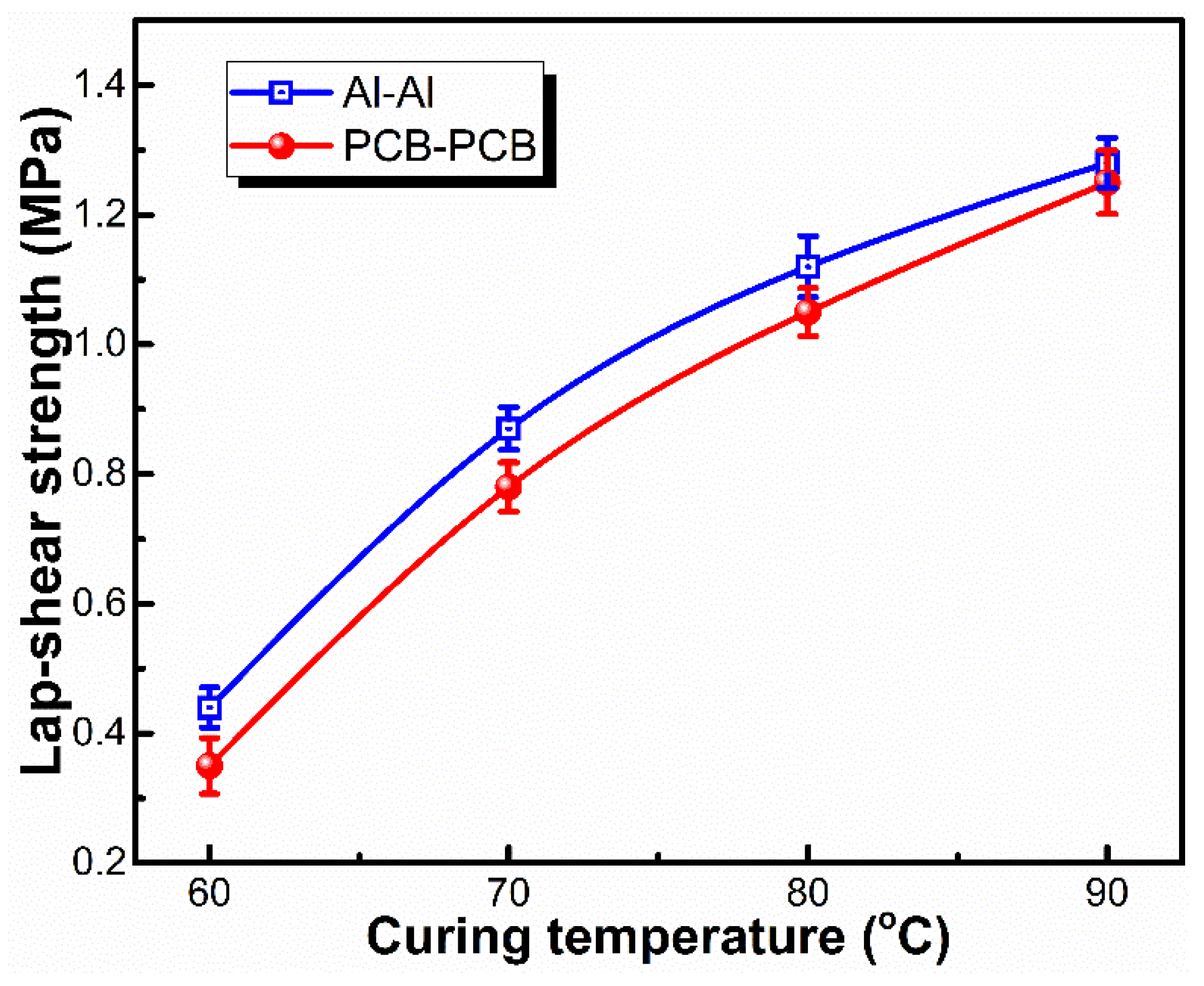
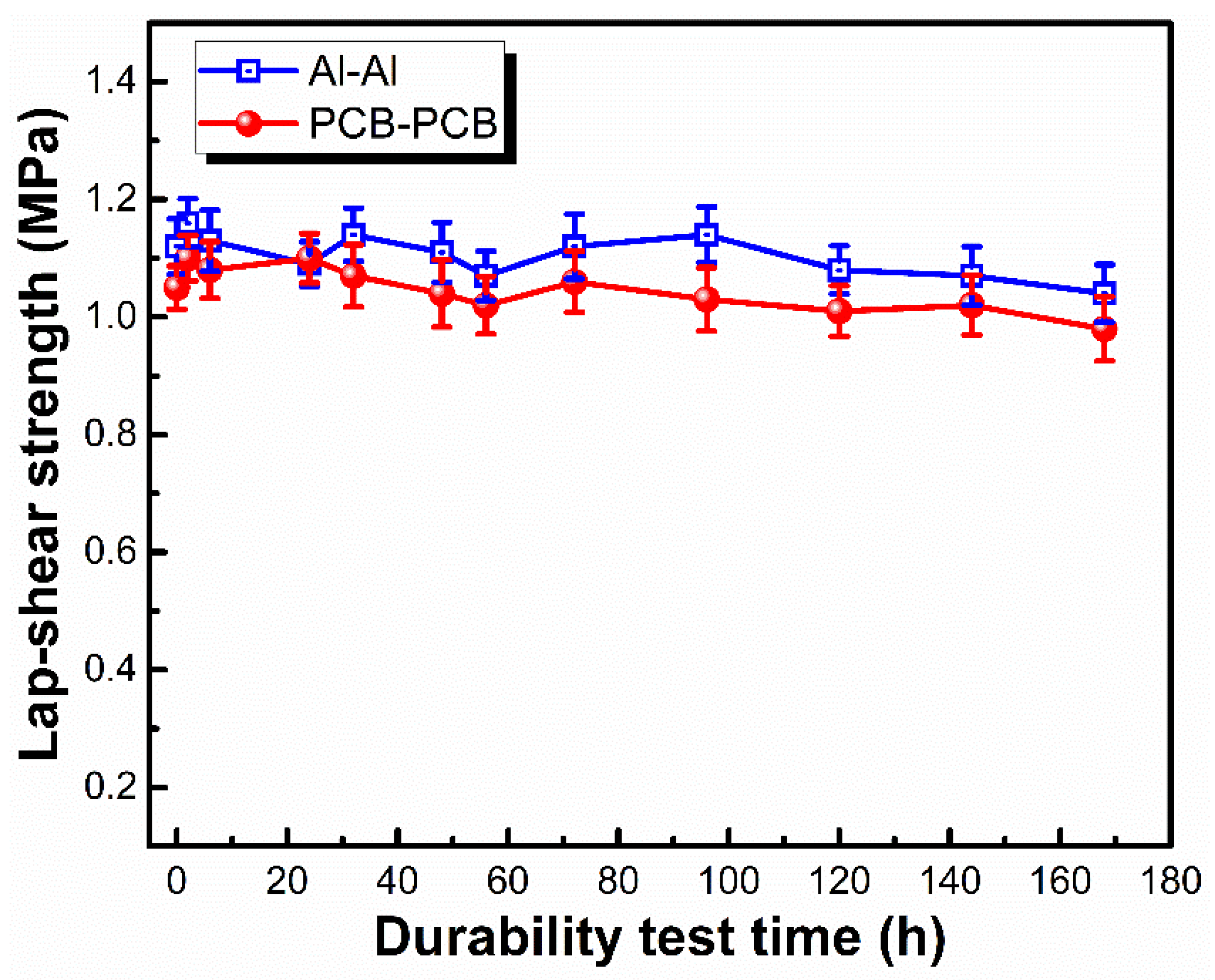
3.3. Adhesion Performance of LSR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziar, H.; Manganiello, P.; Isabella, O.; Zeman, M. Photovoltatronics: Intelligent PV-based devices for energy and information applications. Energ. Environ. Sci. 2021, 14, 106–126. [Google Scholar] [CrossRef]
- Watanabe, A.O.; Ali, M.; Sayeed, S.Y.B.; Tummala, R.R.; Pulugurtha, M.R. A review of 5G Front-End systems package integration. IEEE Trans. Compon. Packag. Manufact. Technol. 2021, 11, 118–133. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Malhouitre, S.; Gradkowski, K.; Morrissey, P.E.; O’Brien, P.; Caillaud, C.; Vaissiere, N.; Decobert, J.; Lei, S.; Enright, R.; et al. III-V-on-Silicon integration: From hybrid devices to heterogeneous photonic integrated circuits. IEEE J. Sel. Top. Quant. 2020, 26, 6100213. [Google Scholar] [CrossRef]
- Chen, C.; Luo, F.; Kang, Y. A review of SiC power module packaging: Layout, material system and integration. CPSS Trans. Power Electron. Appl. 2017, 2, 170–186. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhang, C.; Liang, H.; Li, J.; Du, B. Non-Linear conductivity Epoxy/SiC composites for emerging power module packaging: Fabrication, characterization and application. Materials 2020, 13, 3278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Zhu, Z.X.; Zhang, H.; Wang, F.; Jiang, W.J.; Lin, S.; Yang, Y. Polyurethane sponge-derived nitrogen-doped carbon-encapsulation composite for enhanced lithium-ion battery performances. Appl. Surf. Sci. 2020, 534, 147631. [Google Scholar] [CrossRef]
- Otsuka, K.; Shirai, Y.; Okutani, K. A new silicone gel sealing mechanism for high reliability encapsulation. IEEE Trans. Comp. Hybrids Manufact. Technol. 1984, 3, 249–256. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Fink, J.K. Liquid Silicone Rubber: Chemistry, Materials, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Bahrain, S.H.K.; Masdek, N.R.N.; Mahmud, J.; Mohammed, M.N.; Sapuan, S.M.; Ilyas, R.A.; Mohamed, A.; Shamseldin, M.A.; Abdelrahman, A.; Asyraf, M.R.M. Morphological, physical, and mechanical properties of Sugar-Palm (Arenga pinnata (Wurmb) merr.)-Reinforced silicone rubber biocomposites. Materials 2022, 15, 4062. [Google Scholar] [CrossRef]
- Bahrain, S.H.K.; Rahim, N.N.C.A.; Mahmud, J.; Mohammed, M.N.; Sapuan, S.M.; Ilyas, R.A.; Alkhatib, S.E.; Asyraf, M.R.M. Hyperelastic properties of bamboo cellulosic fibre–reinforced silicone rubber biocomposites via compression test. Int. J. Mol. Sci. 2022, 23, 6338. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.K.; Zhang, D.L.; Zha, J.W.; Yin, L.J.; Dang, Z.M. Thermal, electrical, and mechanical properties of addition-type liquid silicone rubber co-filled with Al2O3 particles and BN sheets. J. Appl. Polym. Sci. 2020, 137, 49399. [Google Scholar] [CrossRef]
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M.J. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef] [Green Version]
- Ou, Z.Z.; Gao, F.; Zhao, H.J.; Dang, S.M.; Zhu, L.J. Research on the thermal conductivity and dielectric properties of AlN and BN co-filled addition-cure liquid silicone rubber composites. RSC Adv. 2019, 9, 28851–28856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.W.; Wang, F.Z.; Dai, J.; Huang, Z.X. Effect of functionalization of graphene nanoplatelets on the mechanical and thermal properties of silicone rubber composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.Y.; Ren, Y.J.; Guo, H.C.; Małycha, K.; Orzechowski, K.; Bai, S.L. Recent progress on thermally conductive and electrical insulating rubber composites: Design, processing and applications. Compos. Commun. 2020, 22, 100430. [Google Scholar] [CrossRef]
- Nguyen, N.; Dinh, V.; Nguyen-Duc, T.; Ta, Q.; Dao, X.; Pham, T.; Nguyen-Duc, T. Effect of potting materials on LED bulb’s driver temperature. Microelectron. Reliab. 2018, 86, 77–81. [Google Scholar] [CrossRef]
- Liquid Silicone Rubbers for Electrical & Electronic Applications. Available online: http://www.shinetsusilicone-global.com/catalog/pdf/LiquidSiliconeRubbers-ele_E.pdf (accessed on 2 May 2022).
- Grundke, K.; Michel, S.; Knispel, G.; Grundler, A. Wettability of silicone and polyether impression materials: Characterization by surface tension and contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 598–609. [Google Scholar] [CrossRef]
- Vudayagiri, S.; Junker, M.D.; Skov, A.L. Factors affecting the surface and release properties of thin polydimethylsiloxane films. Polym. J. 2013, 45, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.W.; Wan, Z.M.; Chen, X.; Wan, J.H.; Luo, L.; Zhang, H.N.; Shu, S.M.; Tu, Z.K. Temperature and humidity effect on aging of silicone rubbers as sealing materials for proton exchange membrane fuel cell applications. Appl. Therm. Eng. 2016, 104, 472–478. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, G.; Boroyevich, D.; Ngo, K.D.T. Survey of High-Temperature polymeric encapsulants for power electronics packaging. IEEE Trans. Comp. Pack. Man. 2015, 5, 168–181. [Google Scholar]
- Chan, S.I.; Hong, W.S.; Kim, K.T.; Yoon, Y.G.; Han, J.H.; Jang, J.S. Accelerated life test of high power white light emitting diodes based on package failure mechanisms. Microelectron. Reliab. 2011, 51, 1806–1809. [Google Scholar] [CrossRef]
- Pan, B.; Wang, Y.; Jin, J.M.; Wang, L.; Ma, M.D. Mechanical shock ability of different potting materials and packaging processes for electronic components. Appl. Mech. Mater. 2012, 271–272, 50–54. [Google Scholar] [CrossRef]
- Bloomfield, L.A. Primer system for bonding conventional adhesives and coatings to silicone rubber. Int. J. Adhes. Adhes. 2016, 68, 239–247. [Google Scholar] [CrossRef]
- Grard, A.; Belec, L.; Perrin, F.X. Effect of surface morphology on the adhesion of silicone elastomers on AA6061 aluminum alloy. Int. J. Adhes. Adhes. 2020, 102, 102656. [Google Scholar] [CrossRef]
- Roth, J.; Albrecht, V.; Nitschke, M.; Bellmann, C.; Simon, F.; Zschoche, S.; Michel, S.; Luhmann, C.; Grundke, K.; Voit, B. Surface functionalization of silicone rubber for permanent adhesion improvement. Langmuir 2008, 24, 12603–12611. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Mirzadeh, H.; Katbab, A. Modification of polysiloxane polymers forbiomedical applications a review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, Y.; Li, Y.; Li, G.; Wang, Y.; Han, Y.; Sun, X.; Tan, X. Fabrication of siloxane hybrid material with high adhesion and high refractive index for light emitting diodes (LEDs) encapsulation. J. Macromol. Sci. A 2014, 51, 653–658. [Google Scholar] [CrossRef]
- Pan, Z.Q.; Huang, B.S.; Zhu, L.Q.; Zeng, K.L. Synthesis of siloxane oligomers containing boron and epoxy groups for promoting the adhesion of addition-curable silicone rubber to PPA and copper plate. J. Adhes. Sci. Technol. 2021, 35, 626–640. [Google Scholar] [CrossRef]
- Wu, J.K.; Zheng, K.W.; Nie, X.C.; Ge, H.R.; Wang, Q.Y.; Xu, J.T. Promoters for improved adhesion strength between Addition-Cured liquid silicone rubber and Low-Melting-Point thermoplastic polyurethanes. Materials 2022, 15, 991. [Google Scholar] [CrossRef]
- Pan, K.X.; Zeng, X.R.; Li, H.Q.; Lai, X.J. Synthesis of silane oligomers containing vinyl and epoxy group for improving the adhesion of addition-cure silicone encapsulant. J. Adhes. Sci. Technol. 2016, 30, 1131–1142. [Google Scholar] [CrossRef]
- Pan, K.X.; Zeng, X.R.; Li, H.Q.; Lai, X.J.; Chen, Z.H.; Liang, G.Q. Synthesis of phenyl silicone resin with epoxy and acrylate group and its adhesion enhancement for addition-cure silicone encapsulant with high refractive index. J. Adhes. Sci. Technol. 2016, 30, 2699–2709. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Zhou, S.T.; Chen, Y.; Liang, M.; Zou, H.W. Preparation of high-performance epoxy-containing silicone rubber via hydrosilylation reaction. J. Appl. Polym. Sci. 2019, 137, 48397. [Google Scholar] [CrossRef]
- Wang, W.J.; Perng, L.H.; Hsiue, G.H.; Chang, F.C. Characterization and properties of new silicone-containing epoxy resin. Polymer 2000, 41, 6113–6122. [Google Scholar] [CrossRef]
- Singh, G.P.; Kaur, P.; Kaur, S.; Arora, D.; Singh, P.; Singh, D.P. Density and FTIR studies of multiple transition metal doped Borate Glass. Mater. Phys. Mech. 2012, 14, 31–36. [Google Scholar]
- Clary, J.W.; Rettenmaier, T.J.; Snelling, R.; Bryks, W.; Banwell, J.; Wipke, W.T.; Singaram, B. Hydride as a leaving group in the reaction of pinacolborane with halides under ambient grignard and barbier conditions. One-Pot synthesis of alkyl, aryl, heteroaryl, vinyl, and allyl pinacolboronic esters. J. Org. Chem. 2011, 76, 9602–9610. [Google Scholar] [CrossRef]
- Zhai, Q.Q.; Zhao, S.G.; Zhou, C.J.; Li, W.J.; Peng, C. Determination of the Si-H content of hydrogen silicone oil by a combination of the fourier transform near infrared, attenuated total reflectance-fourier transform infrared, and partial least squares regression models. J. Appl. Polym. Sci. 2014, 131, 40694. [Google Scholar] [CrossRef]
- Hastings, D.L.; Schoenitz, M.; Ryan, K.M.; Dreizin, E.L.; Krumpfer, J.W. Stability and ignition of a siloxane-coated magnesium powder. Prop. Explos. Pyrotech. 2020, 45, 621–627. [Google Scholar] [CrossRef]
- Gupta, S.; Ramamurthy, P.C.; Madras, G. Covalent Grafting of Polydimethylsiloxane over Surface-Modified Alumina Nanoparticles. Ind. Eng. Chem. Res. 2011, 50, 6585–6593. [Google Scholar] [CrossRef]
- Yahya, S.N.; Lin, C.K.; Ramli, M.R.; Jaafar, M.; Ahmad, Z. Effect of cross-link density on optoelectronic properties of thermally cured 1,2-epoxy-5-hexene incorporated polysiloxane. Mater. Des. 2013, 47, 416–423. [Google Scholar] [CrossRef]
- Langer, S.H.; Elbling, I.N.; Finestone, A.B.; Thomas, W.R. The chemistry of new latent curing systems for epoxy resins. J. Appl. Polym. Sci. 1961, 5, 370–374. [Google Scholar] [CrossRef]
- DOWSIL™ SE 1816 CV Kit. Available online: https://www.dow.com/documents/en-us/productdatasheet/11/11-32/11-3268-dowsil-se-1816-cv-kit.pdf (accessed on 15 July 2022).
- Shimpi, T.M.; Moffett, C.; Sampath, W.S.; Barth, K.L. Materials selection investigation for thin film photovoltaic module encapsulation. Sol. Energy 2019, 187, 226–232. [Google Scholar] [CrossRef]
- Hacke, P.; Spataru, S.; Terwilliger, K.; Perrin, G.; Glick, S.; Kurtz, S.; Wohlgemuth, J. Accelerated testing and modeling of Potential-induced degradation as a function of temperature and relative humidity. IEEE J. Photovolt. 2015, 5, 1549–1553. [Google Scholar] [CrossRef]

| Compositions | Content (g) | |
|---|---|---|
| Component A | Component B | |
| PDMS-Vi | 75.0 | 66.0 |
| PDMS-mVi | 25.0 | 22.0 |
| PHMS-H | / | 12.0 |
| Spherical α-Al2O3 1 | 300–700 | 300–700 |
| PDMS-B | 0–9.6 | / |
| D4H-MSEP | / | 0–32.0 |
| Platinum catalyst | 0.8 | / |
| 3-methyl-1-pentyn-3-ol | / | 1.0 |
| Adhesion Promoter | Substrates | Curing Tempureture | Shear Strength | Ref. |
|---|---|---|---|---|
| Siloxane oligomers-containing boron and epoxy groups | Cu/Cu | 150 °C | 3.16 MPa | [30] |
| Vinyl and epoxy groups-modified silane oligomer | Al/Al | 130 °C | 1.06 MPa | [32] |
| Phenyl silicone resin with epoxy and acrylate groups | Al/Al | 150 °C | 4.43 MPa | [33] |
| Epoxy, alkoxy and acrylate groups-modified prepolymer | Al/Al | 100 °C | 0.83 MPa | [34] |
| KE-1285-A/B 1 | Al/Al | 120 °C | 1.50 MPa | [18] |
| Dow Corning® SE 1816 CV Kit 2 | Al/Al | 150 °C | 1.80 MPa | [43] |
| D4H-MSEP/PDMS-B | Al/Al | 80 °C | 1.12 MPa | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-K.; Zheng, K.-W.; Wang, Q.-Y.; Nie, X.-C.; Wang, R.; Xu, J.-T. Binary Promoter Improving the Moderate-Temperature Adhesion of Addition-Cured Liquid Silicone Rubber for Thermally Conductive Potting. Materials 2022, 15, 5211. https://doi.org/10.3390/ma15155211
Wu J-K, Zheng K-W, Wang Q-Y, Nie X-C, Wang R, Xu J-T. Binary Promoter Improving the Moderate-Temperature Adhesion of Addition-Cured Liquid Silicone Rubber for Thermally Conductive Potting. Materials. 2022; 15(15):5211. https://doi.org/10.3390/ma15155211
Chicago/Turabian StyleWu, Jia-Kai, Kai-Wen Zheng, Qiong-Yan Wang, Xin-Cheng Nie, Rui Wang, and Jun-Ting Xu. 2022. "Binary Promoter Improving the Moderate-Temperature Adhesion of Addition-Cured Liquid Silicone Rubber for Thermally Conductive Potting" Materials 15, no. 15: 5211. https://doi.org/10.3390/ma15155211
APA StyleWu, J.-K., Zheng, K.-W., Wang, Q.-Y., Nie, X.-C., Wang, R., & Xu, J.-T. (2022). Binary Promoter Improving the Moderate-Temperature Adhesion of Addition-Cured Liquid Silicone Rubber for Thermally Conductive Potting. Materials, 15(15), 5211. https://doi.org/10.3390/ma15155211






