New Materials Based on Polyvinylpyrrolidone-Containing Copolymers with Ferromagnetic Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Technique of pHEMA-gr-PVP Copolymers and FMF-Filled Composites Based on Them
2.3. Measurements and Characterization
2.3.1. Investigation of Polymerization Kinetics of HEMA/PVP Compositions
2.3.2. Standard Methods of Instrumental Research
2.3.3. PVP Grafting
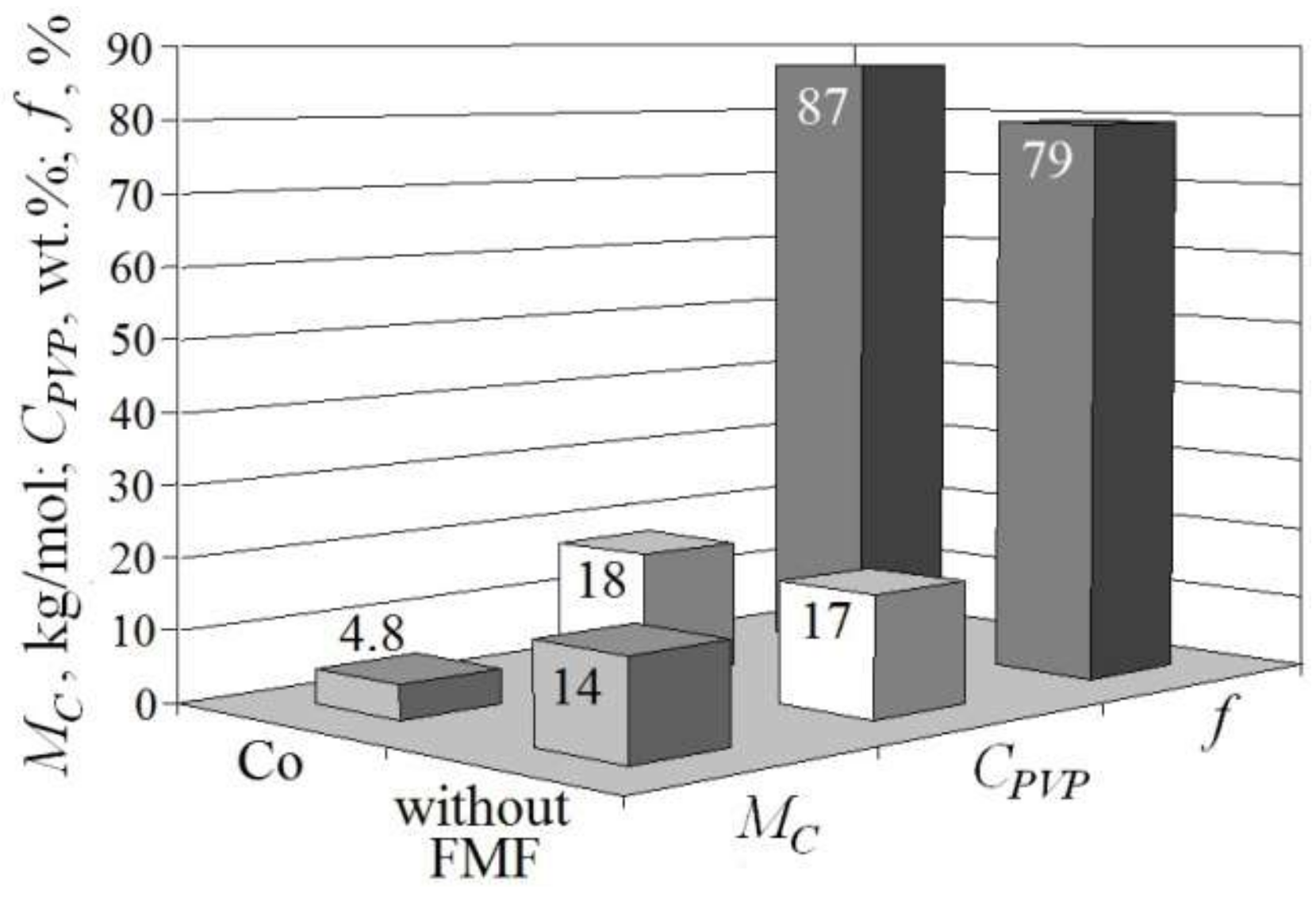
2.3.4. The Molecular Weight between Crosslinks in Polymer Network
2.3.5. Physico-Mechanical Characteristics of FMF/pHEMA-gr-PVP Copolymers
2.3.6. Thermophysical Properties
2.3.7. Conductivity
2.3.8. Magnetic Properties
3. Results and Discussion
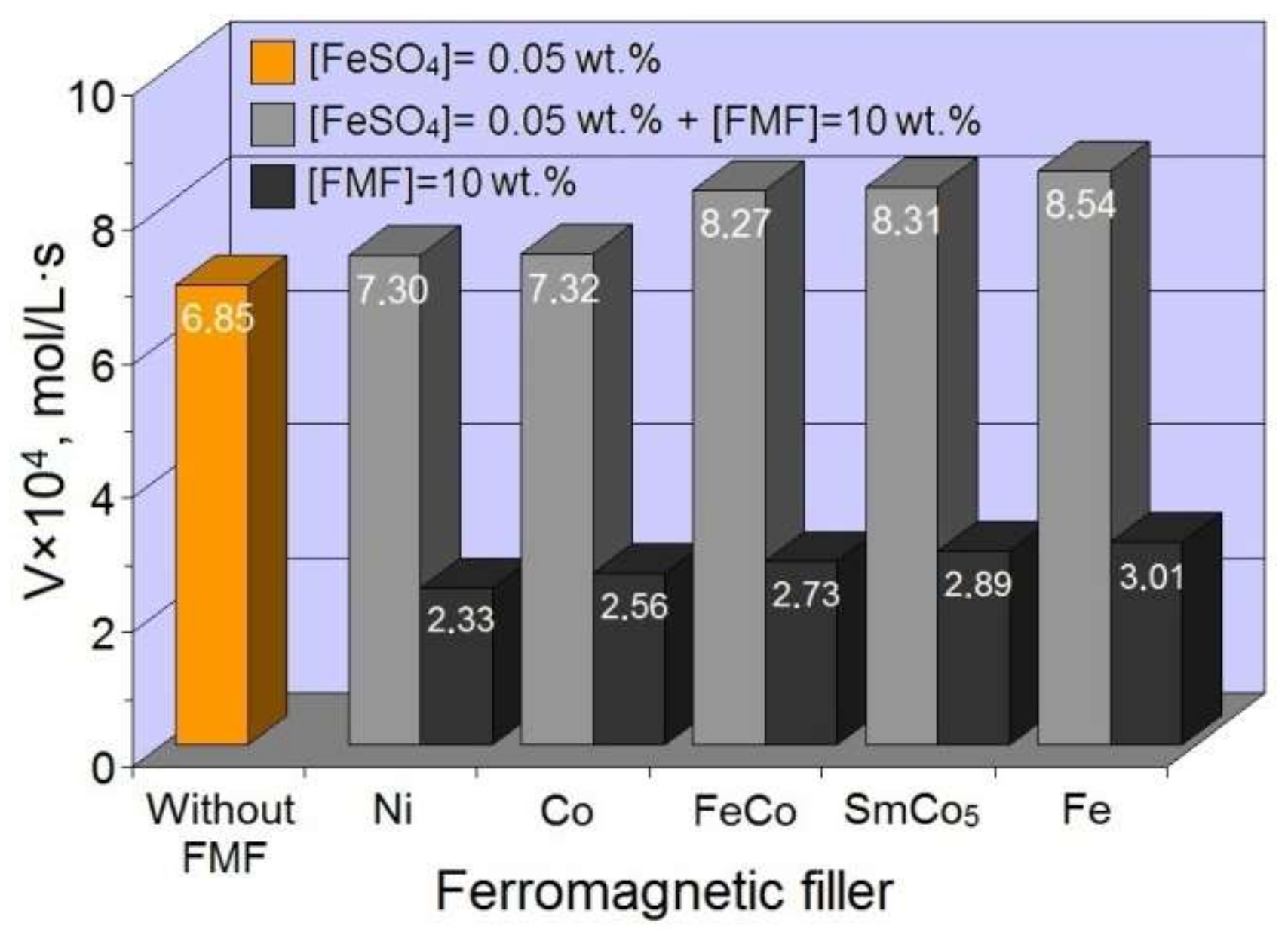
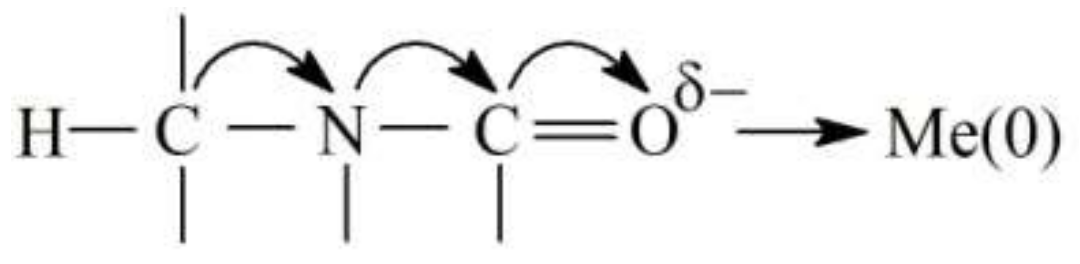
3.1. Features of FMF Effect on the Formation of the Structure of HEMA-gr-PVP Copolymers
3.2. Properties of FMF/HEMA-gr-PVP Composites
3.2.1. Sorption Capacity of FMF-Filled pHEMA-gr-PVP Copolymers
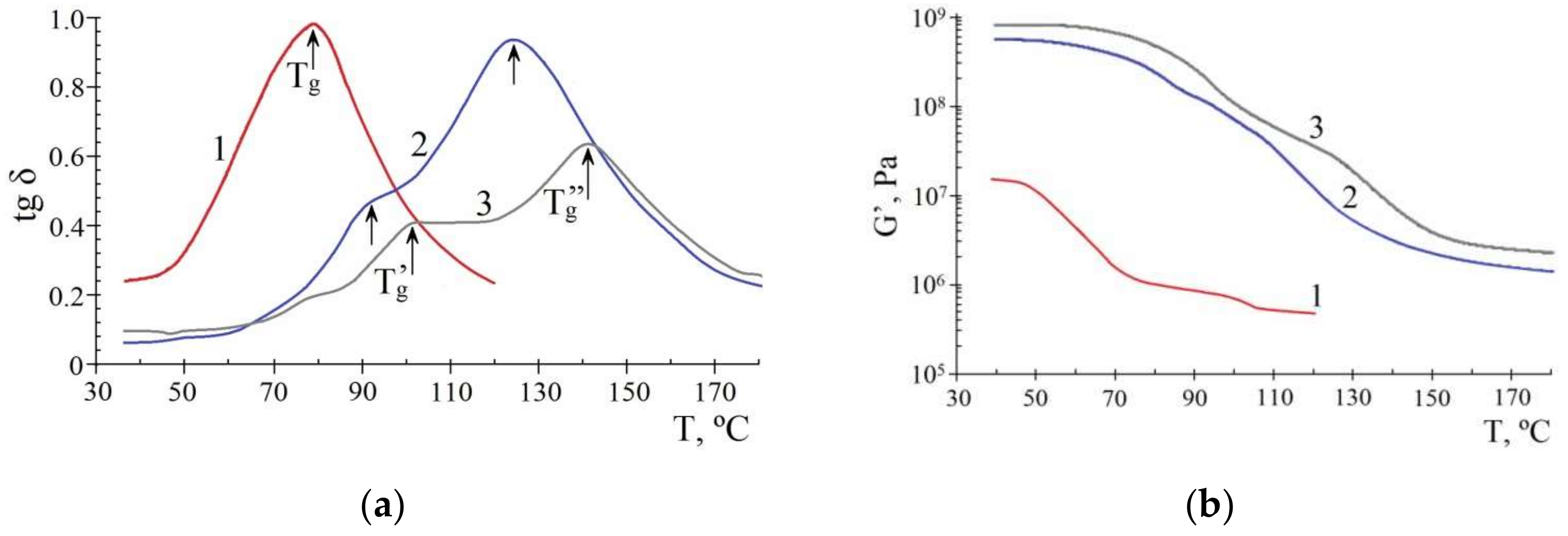
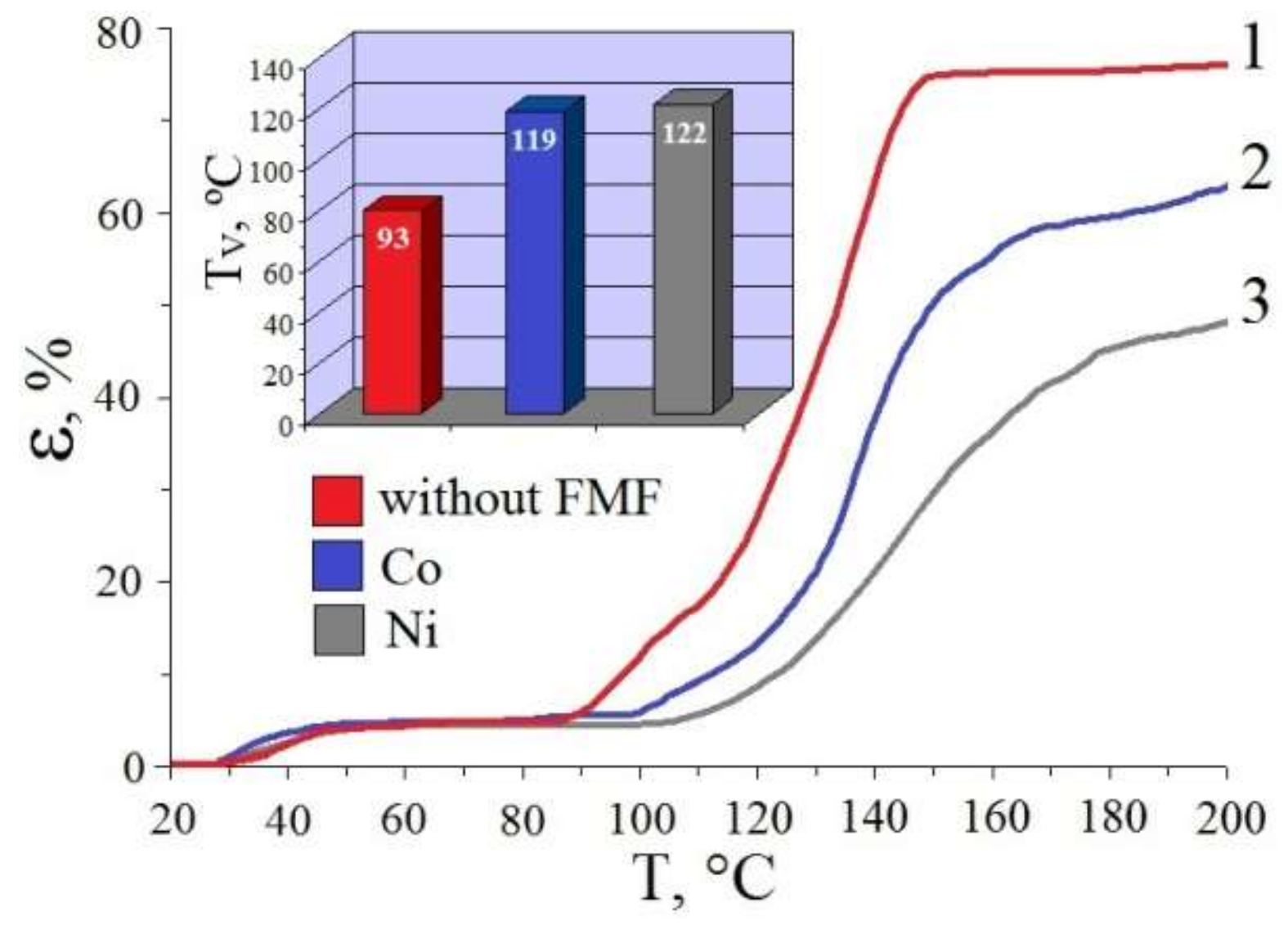
3.2.2. Influence of Metallic Filler on Thermophysical Properties of pHEMA-gr-PVP Copolymers
3.2.3. Magnetic Properties of FMF/pHEMA-gr-PVP Composites
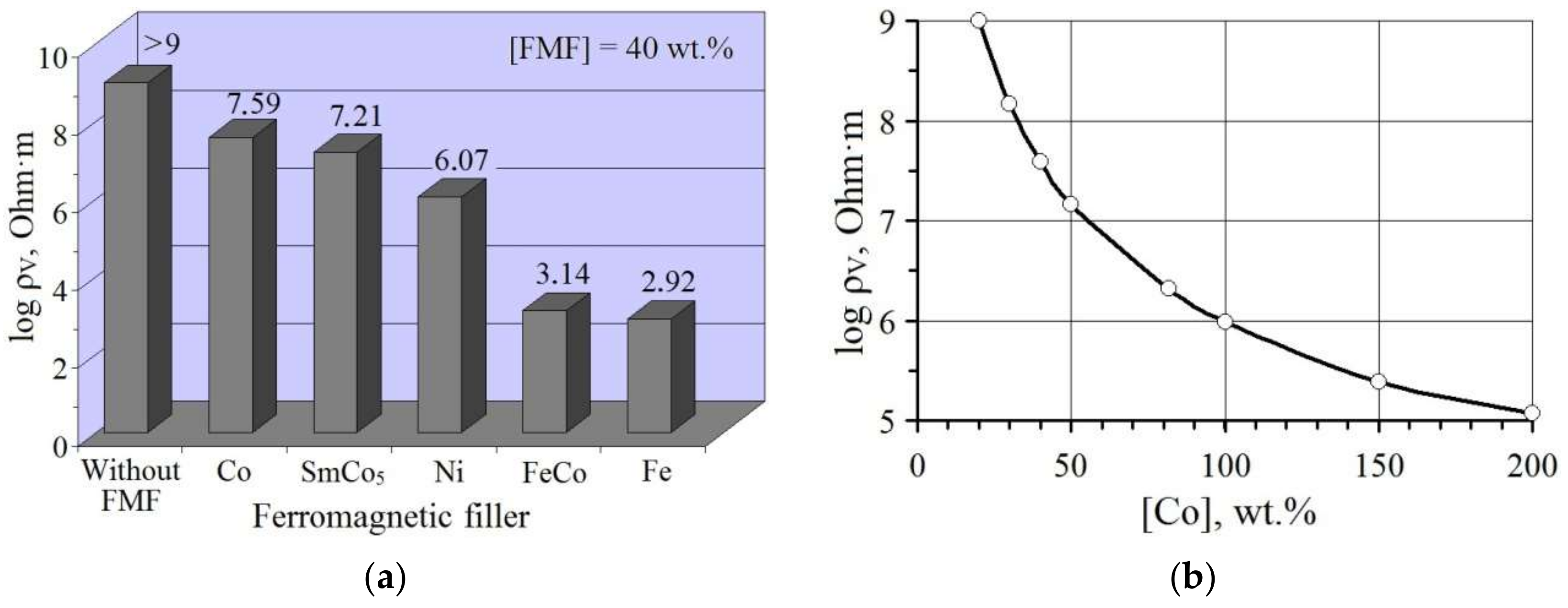
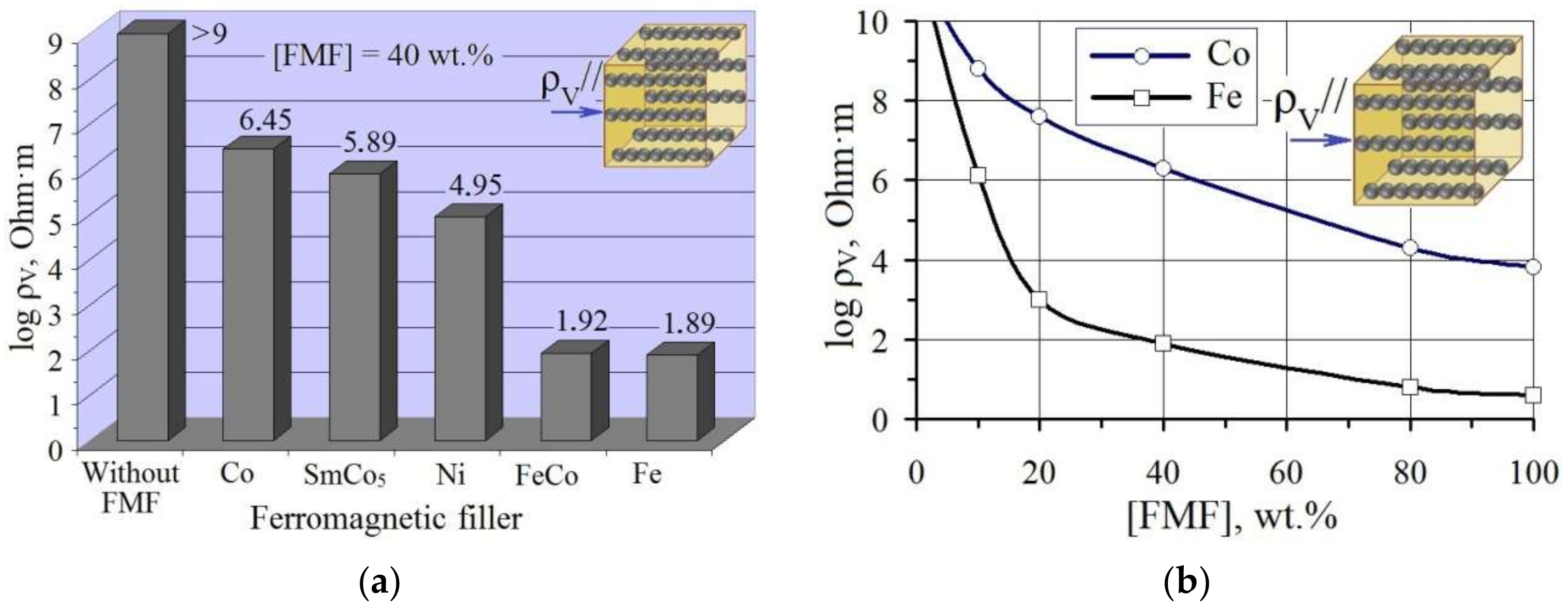
3.2.4. Electrically Conductive Properties of FMF/pHEMA-gr-PVP Composites
3.2.5. Physico-Mechanical Properties
4. Conclusions
- It was found that the polymerization of FMF-filled compositions occurred without additional catalysts, which is the evidence of the participation of the FMF surface in initiating the polymerization process of HEMA/PVP compositions. The formation of grafted spatially crosslinked copolymer of pHEMA-gr-PVP was confirmed. The introduction of FMF particles into the original composition formulation caused the formation of copolymers with a greater degree of crosslinking, which indicates the appearance of additional spatial network due to the formation of physical nodes, the elements of which may be metal particles. It was established that in the interfacial layer, on the filler surface and in the volume, a copolymer formed with a different structure.
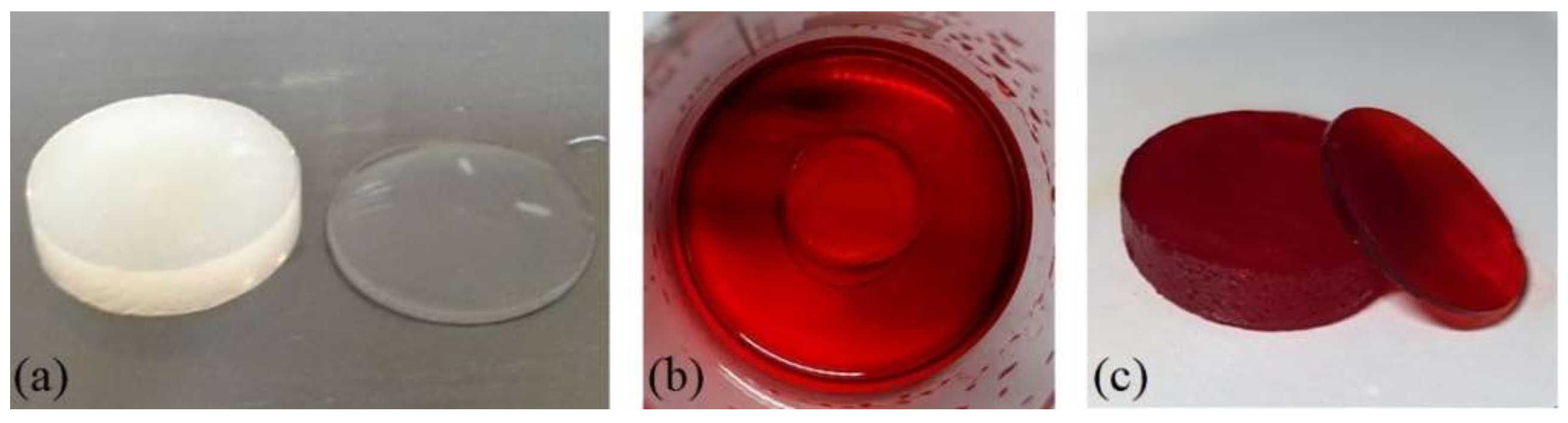
- Using the example of water and ethyl alcohol, it was shown that the obtained composites are able to swell in solvents. It was established that the introduction of FMF particles into the structure of pHEMA-gr-PVP copolymers in any case led to a deterioration of the sorption capacity. At the same time, the water content decreased by 16–18% and lay within 42–43% by mass, but remained relatively high. The sorption possibility of pHEMA-gr-PVP copolymers of synthetic dyes of organic origin was shown on the example of methyl red.
- The filling of pHEMA-gr-PVP copolymers with micro-sized FMF powders increase their heat resistance (Vicat softening temperature is 39–42 °C higher compared to the unfilled material), as well as strength characteristics (in both dry and swollen states). The surface hardness of dry samples with a FMF content of only 10 wt.% increased by 20–25%, and the hardness number of samples in a swollen state is doubled. At the same time, resilience slightly deteriorated, and so plasticity increased.
- FMF-filled pHEMA-gr-PVP copolymers, after band magnetization with profile inductors, possess magnetic properties. It was established that the obtained composites in dry state are characterized by a coercive force of 200 kA × m−1 and induction of a magnetic field at the poles of 4–5 mT and 10–15 mT, respectively.
- The introduction of fine FMF particles into pHEMA-gr-PVP copolymers (which are dielectrics in the dry state) provides them with electrical conductivity. Depending on the nature and content of FMF, the resistance of filled materials can be regulated within 103–106 Ohm·m. The structuring of filler particles into chains using their orientation during polymerization in a constant magnetic field provided a 10-fold increase in the electrical conductivity of composites with relatively small amounts of FMF.
- Depending on the need and the task in hand, the research results showed, while choosing the nature of the metal-filler, it is possible to obtain hydrogel composite materials with unique predictable properties, having electrically conductive, magnetic, or sorption activities and combinations of the same. The obtained composites were not contaminated with other substances (by-products from the obtaining reaction of metal-filler), which also allowed the avoidance of an additional longtime stage of purification of the composite, washing. The high reactive capacity of the polymer-monomer composition, based on HEMA and PVP in the presence of FeSO4, provides the ability to obtain hydrogel materials filled with FMF at a high rate using simple technology, at room temperature, in air, without the usage of sophisticated equipment and facilities. Preliminary results indicate the prospects for the use of the obtained materials, for example, as magnetic sorbents for various applications, as well as electrically conductive materials that may respond to changes in conductivity from various factors (e.g., moisture content, differences in pressure, temperature).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, V.; Namdeo, M.; Murali Mohan, Y.; Bajpai, S.K.; Bajpai, M. Review on polymer, hydrogel and microgel metal nanocomposites: A facile nanotechnological approach. J. Macromol. Sci. 2007, 45, 107–119. [Google Scholar] [CrossRef]
- Kucherenko, A.; Moravskyi, V.; Kuznetsova, M.; Grytsenko, O.; Masyuk, A.; Dulebova, L. Regularities of obtaining metal-filled polymer composites. In Nanomaterials in Biomedical Application and Biosensors (NAP-2019); Pogrebnjak, A., Pogorielov, M., Viter, R., Eds.; Springer Proceedings in Physics; Springer: Singapore, 2020; Volume 244, pp. 59–66. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Li, H.; Yang, P.; Pageni, P.; Tang, C. Recent advances in metal-containing polymer hydrogels. Macromol. Rapid Commun. 2017, 38, 1700109. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. App. Polym. Sci. 2021, 138, e50376. [Google Scholar] [CrossRef]
- Nosova, N.G.; Samaryk, V.J.; Varvarenko, S.M.; Ferens, M.V.; Voronovska, A.V.; Nagornyak, M.I.; Khomyak, S.V.; Nadashkevych, Z.J.; Voronov, S.A. Porous polyacrylamide hydrogels: Preparation and properties. Vopr. Khimii Khimicheskoi Tekhnologii. 2016, 5–6, 78–86. [Google Scholar]
- Jumadilov, T.; Abilov, Z.; Grazulevicius, J.; Zhunusbekova, N.; Kondaurov, R.; Agibayeva, L.; Akimov, A. Mutual activation and sorption ability of rare cross-linked networks in intergel system based on polymethacrylic acid and poly-4-vinylpyridine hydrogels in relation to lanthanum ions. Chem. Chem. Technol. 2017, 11, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Tarnavchyk, I.; Voronov, A.; Kohut, A.; Nosova, N.; Varvarenko, S.; Samaryk, V.; Voronov, S. Reactive hydrogel networks for the fabrication of metal-polymer nanocomposites. Macromol. Rapid Commun. 2009, 30, 1564–1569. [Google Scholar] [CrossRef]
- Burkeev, M.Z.; Shibayeva, S.R.; Khamitova, T.O.; Plocek, J.; Tazhbayev, Y.M.; Davrenbekov, S.Z.; Nurmaganbetova, M.T.; Kazhmuratova, A.T.; Zhumagalieva, T.S.; Kezdikbayeva, A.T. Synthesis and catalytic properties of new polymeric monometallic composites based on copolymers of polypropylene glycol maleate phthalate with acrylic acid. Polymers 2021, 13, 4369. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Q.; Du, J. Recent advances in magnetic hydrogels. Polym. Int. 2016, 65, 1365–1372. [Google Scholar] [CrossRef]
- Shah, L.A.; Ambreen, J.; Bibi, I.; Sayed, M.; Siddiq, M. Silver nanoparticles fabricated hybrid microgels for optical and catalytic study. J. Chem. Soc. Pak. 2016, 38, 850–858. [Google Scholar]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Electrically conducting silver/guar gum/poly(acrylic acid) nanocomposite. Int. J. Biol. Macromol. 2014, 69, 456–463. [Google Scholar] [CrossRef]
- Grytsenko, O.; Pukach, P.; Suberlyak, O.; Shakhovska, N.; Karovič, V. Usage of mathematical modeling and optimization in development of hydrogel medical dressings production. Electronics 2021, 10, 620. [Google Scholar] [CrossRef]
- Chen, T.; Hou, K.; Ren, Q.; Chen, G.; Wei, P.; Zhu, M. Nanoparticle-polymer synergies in nanocomposite hydrogels: From design to application. Macromol. Rapid Commun. 2018, 39, 1800337. [Google Scholar] [CrossRef]
- Filipcsei, G.; Csetneki, I.; Szilágyi, A.; Zrínyi, M. Magnetic field-responsive smart polymer composites. In Oligomers-Polymer Composites-Molecular Imprinting. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2007; Volume 206, pp. 137–189. [Google Scholar] [CrossRef]
- Yin, C.S.; Cheung, P.Y.; Kohler, A.C.; Compton, J.T.; Hsu, L.L.; Lau, K.M.; Kim, S.; Lee, B.W.; Lee, F.Y.; Sia, S.K. Additive manufacturing of hydrogel-based materials for next-generation implantable medical devices. Sci. Robot. 2017, 2, eaah6451. [Google Scholar] [CrossRef]
- Grytsenko, O.; Gajdos, I.; Spišák, E.; Krasinskyi, V.; Suberlyak, O. Novel Ni/pHEMA-gr-PVP composites obtained by polymerization with simultaneous metal deposition: Structure and properties. Materials 2019, 12, 1956. [Google Scholar] [CrossRef] [Green Version]
- Ansari, T.M.; Ajmal, M.; Saeed, S.; Naeem, H.; Ahmad, H.B.; Mahmood, K.; Farooqi, Z.H. Synthesis and characterization of magnetic poly(acrylic acid) hydrogel fabricated with cobalt nanoparticles for adsorption and catalytic applications. J. Iran. Chem. Soc. 2019, 16, 2765–2776. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Rasika Dias, H.V.; Kharisov, B.I. Magnetic adsorbents based on micro- and nano-structured materials. RSC Adv. 2015, 5, 6695–6719. [Google Scholar] [CrossRef]
- Zulfiqar; Afzal, S.; Khan, R.; Zeb, T.; Rahman, M.; Burhanullah; Ali, S.; Khan, G.; Rahman, Z.; Hussain, A. Structural, optical, dielectric and magnetic properties of PVP coated magnetite (Fe3O4) nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 20040–20050. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Zasońska, B.; Brož, A.; Šlouf, M.; Hodan, J.; Petrovský, E.; Hlídková, H.; Horák, D. Magnetic superporous poly(2-hydroxyethyl methacrylate) hydrogel scaffolds for bone tissue engineering. Polymers 2021, 13, 1871. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar] [CrossRef]
- Mun, S.J.; Ko, D.; Kim, H.U.; Han, Y.; Roh, Y.H.; Kim, B.-G.; Na, H.B.; Bong, K.W. Photopolymerization-based synthesis of uniform magnetic hydrogels and colorimetric glucose detection. Materials 2020, 13, 4401. [Google Scholar] [CrossRef]
- Cai, H.; Lu, P.; Dong, J. Robust nickel–polymer nanocomposite particles for hydrogen generation from sodium borohydride. Fuel 2016, 166, 297–301. [Google Scholar] [CrossRef]
- Ajmal, M.; Aftab, F.; Bibi, I.; Iqbal, M.; Ambreen, J.; Ahmad, H.B.; Akhtar, N.; Haleem, A.; Siddiq, M. Facile synthesis of porous anionic hydrogel embedded with nickel nanoparticles and evaluation of its catalytic performance for the rapid reduction of 4-nitrophenol. J. Porous Mater. 2019, 26, 281–290. [Google Scholar] [CrossRef]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Grytsenko, O.M.; Naumenko, O.P.; Suberlyak, O.V.; Dulebova, L.; Berezhnyy, B.V. Optimization of the technological parameters of the graft copolymerization of 2-hydroxyethyl methacrylate with polyvinylpyrrolidone for nickel deposition from salts. Vopr. Khimii Khimicheskoi Tekhnologii 2020, 1, 25–32. [Google Scholar] [CrossRef]
- Yuan, Q.; Williams, R. Large scale manufacture of magnetic polymer particles using membranes and microfluidic devices. China Particuology 2007, 5, 26–42. [Google Scholar] [CrossRef]
- Jean, I.; Baek, J. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.; Lo, I.; Yin, K.; Tang, S. Removal of natural organic matter by cationic hydrogel with magnetic properties. J. Environ. Manag. 2011, 92, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.K.; Labena, A.; Fakhry, S.H.; Safwat, G.; Diab, A.; Atta, A.M. Antimicrobial activity of hybrids terpolymers based on magnetite hydrogel nanocomposites. Materials 2019, 12, 3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magnetothermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Suberlyak, O.; Grytsenko, O.; Hischak, K.; Hnatchuk, N. Research of influence the nature of metal on mechanism of synthesis polyvinilpyrrolidone metal copolymers. Chem. Chem. Technol. 2013, 7, 289–294. [Google Scholar] [CrossRef]
- Suberlyak, O.; Grytsenko, O.; Kochubei, V. The role of FeSO4 in the obtaining of polyvinylpirolidone copolymers. Chem. Chem. Technol. 2015, 9, 429–434. [Google Scholar] [CrossRef]
- Suberlyak, O.; Skorokhoda, V. Hydrogels based on polyvinylpyrrolidone copolymers. In Hydrogels; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2018; pp. 136–214. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Khromiak, U.; Levytskyi, V.; Stepova, K.; Tarnawsky, A. Synthesis and properties of adhesive polymer-methylmethacrylate materials. Int. J. Polym. Sci. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Suberlyak, O.V.; Levitskij, V.E.; Skorokhoda, V.Y.; Godij, A.B. Physical-chemical fenomena on phase boundary vinyl monomer-water solution of polyvinylpyrrolidone. Ukr. Khimicheskij Zhurnal 1998, 64, 122–125. [Google Scholar]
- Grytsenko, O.; Dulebova, L.; Suberlyak, O.; Skorokhoda, V.; Spišák, E.; Gajdos, I. Features of structure and properties of pHEMA-gr-PVP block copolymers, obtained in the presence of Fe2+. Materials 2020, 13, 4580. [Google Scholar] [CrossRef]
- Krasinskyi, V.; Suberlyak, O.; Dulebová, L.; Antoniuk, V. Nanocomposites on the basis of thermoplastics and montmorillonite modified by polyvinylpyrrolidone. Key Eng. Mater. 2017, 756, 3–10. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, L.; Shen, H.; Wu, H.; Zhao, J.; Wang, S.; Hu, L. Biodegradable MoSe2-polyvinylpyrrolidone nanoparticles with multi-enzyme activity for ameliorating acute pancreatitis. J. Nanobiotechnol. 2022, 20, 113. [Google Scholar] [CrossRef]
- Levytskyi, V.Y.; Masyuk, A.S.; Suberlyak, O.V. Preparation and properties of polymer-silicate composites based on hydrophilic polymers. Vopr. Khimii Khimicheskoi Tekhnologii 2017, 6, 68–74. [Google Scholar]
- Krasinskyi, V.; Suberlyak, O.; Zemke, V.; Klym, Y.; Gaidos, I. The role of polyvinylpyrrolidone in the formation of nanocomposites based on acompatible polycaproamide and polypropylene. Chem. Chem. Technol. 2019, 13, 59–63. [Google Scholar] [CrossRef]
- Grytsenko, O.; Pukach, P.; Suberlyak, O.; Moravskyi, V.; Kovalchuk, R.; Berezhnyy, B. The Scheffe’s method in the study of mathematical model of the polymeric hydrogels composite structures optimization. Math. Modeling Comput. 2019, 6, 258–267. [Google Scholar] [CrossRef]
- Ciardelli, G.; Cristallini, C.; Barbani, N.; Benedetti, G.; Crociani, A.; Travison, L.; Giusti, P. Bioartificial polymeric materials: -amylase, poly(2-hydroxyethyl methacrylate), poly(N-vinylpyrrolidone) system. Macromol. Chem. Phys. 2002, 203, 1666–1673. [Google Scholar] [CrossRef]
- Muller, K. Identification and determination of polyvinylpyrrolidone (PVP) as well as determination of active substances in PVP-containing drug preparations. Pharm. Acta Helv. 1968, 43, 107–122. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.; Hu, X.; Chu, L. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [Green Version]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–254. [Google Scholar] [CrossRef]
- Krasinskyi, V.; Suberlyak, O.; Sikora, J.; Zemke, V. Nanocomposites based on polyamide-6 and montmorillonite intercalated with polyvinylpyrrolidone. Polym.-Plast. Technol. Mater. 2021, 60, 1641–1655. [Google Scholar] [CrossRef]
- Suberlyak, O.; Melnyk, J.; Skorokhoda, V. Formation and properties of hydrogel membranes based on cross-linked copolymers of methacrylates and water-soluble polymers. Eng. Biomater. 2009, 12, 5–8. [Google Scholar]
- Xu, J.; Zhao, P.; Zhang, Y. Iodine-responsive poly(HEMA-PVP) hydrogel for self-regulating burst-free extended release. J. Biomater. Sci. Polym. Ed. 2017, 28, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Mamakhel, M.; Christensen, M. High coercivity SmCo5 synthesized with assistance of colloidal SiO2. Sci. Rep. 2021, 11, 4682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, R.; Yue, M.; Liu, W. Microstructure and magnetic properties of SmCo5 sintered magnets. Rare Met. 2020, 39, 1295–1299. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef]
- Yaman, K. Fractal characterization of electrical conductivity and mechanical properties of copper particulate polyester matrix composites using image processing. Polym. Bull. 2022, 79, 3309–3332. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2003, 257, 24–40. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grytsenko, O.; Dulebova, L.; Spišák, E.; Berezhnyy, B. New Materials Based on Polyvinylpyrrolidone-Containing Copolymers with Ferromagnetic Fillers. Materials 2022, 15, 5183. https://doi.org/10.3390/ma15155183
Grytsenko O, Dulebova L, Spišák E, Berezhnyy B. New Materials Based on Polyvinylpyrrolidone-Containing Copolymers with Ferromagnetic Fillers. Materials. 2022; 15(15):5183. https://doi.org/10.3390/ma15155183
Chicago/Turabian StyleGrytsenko, Oleksandr, Ludmila Dulebova, Emil Spišák, and Bohdan Berezhnyy. 2022. "New Materials Based on Polyvinylpyrrolidone-Containing Copolymers with Ferromagnetic Fillers" Materials 15, no. 15: 5183. https://doi.org/10.3390/ma15155183
APA StyleGrytsenko, O., Dulebova, L., Spišák, E., & Berezhnyy, B. (2022). New Materials Based on Polyvinylpyrrolidone-Containing Copolymers with Ferromagnetic Fillers. Materials, 15(15), 5183. https://doi.org/10.3390/ma15155183






