The Effect of Antimony (III) Oxide on the Necessary Amount of Precursors Used in Laser-Activated Coatings Intended for Electroless Metallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Polinitrate (V) complex of [(2-amino-5-guanidine-pentane) (mi-O,O′-nitrate(V)) (2,2′-dipyridile) copper (II)]{[Cu(μ-O,O′-NO3)(l-arg)(2,2′-bpy)]·NO3}n abbreviated as compound A;
- Copper (II) acetylacetonate Cu(acac)2 (Sigma Aldrich, Saint Louis, MO, USA) abbreviated as compound B;
- Antimony (III) oxide Sb2O3 (particle size < 250 nm) (Sigma Aldrich, Saint Louis, MO, USA);
- Polyurethane resin B4060 (Haering, Bubsheim, Germany);
- Polycarbonate (PC) Xantar 19 UR (DSM Engineering Plastics, Holand);
- Autocatalytic metallization bath M-Copper-85 (MacDermid, Łysomice, Poland);
- Formaldehyde HCHO, 36% (POCH, Gliwice, Poland), molecular weight 30.03 g mol−1;
- Adhesive Araldite 2011 (Huntsman, Basel, Switzerland).
2.2. Laser Irradiation and Metallization
2.3. Methodology
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.T.; Xu, L.; Chen, J.H.; Zhao, B.; Fu, X.Z.; Sun, R.; Chen, Q.; Wong, C.P. Electroless Deposition Metals on Poly(dimethylsiloxane) with Strong Adhesion as Flexible and Stretchable Conductive Materials. ACS Appl. Mater. Interfaces 2018, 10, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, P.; Rytlewski, P.; Moraczewski, K.; Mazurkiewicz, A. A review on the direct electroplating of polymeric materials. J. Mater. Sci. 2021, 56, 14881–14899. [Google Scholar] [CrossRef]
- Rytlewski, P.; Jagodziński, B.; Moraczewski, K. Laser-assisted Electroless Metallization of Polymer Materials: A critical review. Rev. Adhes. Adhes. 2016, 4, 334–366. [Google Scholar] [CrossRef]
- Teixeira, L.A.C.; Santini, M.C. Surface conditioning of ABS for metallization without the use of chromium baths. J. Mater. Process. Technol. 2005, 170, 37–41. [Google Scholar] [CrossRef]
- Mai, T.T.; Schultze, J.W.G.; Staikov, G. Relation between surface preconditioning and metal deposition in direct galvanic metallization of insulating surfaces. J. Solid State Electrochem. 2004, 8, 201–208. [Google Scholar] [CrossRef]
- Aronson, C.L.; Beholz, L.G.; Beloskur, D.; Burland, B.; Perez, J. New pathways for modifying the surface of high density polyethylene: Chemically benign adhesion promotion and subsequent reactivity. Polym. Bull. 2005, 53, 401–412. [Google Scholar] [CrossRef]
- Ratautas, K.; Andrulevicius, M.; Jagminienė, A.; Stankevičienė, I.; Norkus, E.; Raciukaitis, G. Laser-assisted selective copper deposition on commercial PA6 by catalytic electroless plating—Process and activation mechanism. Appl. Surf. Sci. 2019, 470, 405–410. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, J.; Jia, L.; Zhang, G.; Sun, S.; Zhou, T. Laser-Induced Selective Metallization on Polymer Substrates Using Organocopper for Portable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 13714–13723. [Google Scholar] [CrossRef]
- Bachya, B.; Franke, J. Experimental investigation and optimization for the effective parameters in the laser direct structuring process. J. Laser Micro Nanoeng. 2015, 10, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Balzereita, S.; Proesb, F.; Altstädta, V.; Emmelmann, C. Properties of copper modified polyamide 12-powders and their potential for the use as laser direct structurable electronic circuit carriers. Addit. Manuf. 2018, 23, 347–354. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Moraczewski, K.; Rytlewski, P.; Stepczyńska, M.; Jagodziński, B. Metalizowaniebezprądowetworzywpolimerowych. Polimery 2017, 62, 163–169. [Google Scholar] [CrossRef]
- Rytlewski, P.; Żenkiewicz, M.; Tracz, A.; Moraczewski, K.; Mróz, W. Surface morphology studies of laser irradiated and chemically metalized polyamide composites. Surf. Coat. Technol. 2011, 205, 5248–5253. [Google Scholar] [CrossRef]
- Rytlewski, P. Laser induced electroactivity of polyamide composites. Electrochim. Acta 2012, 61, 191–197. [Google Scholar] [CrossRef]
- Rytlewski, P.; Jagodziński, B.; Malinowski, R.; Budner, B.; Moraczewski, K.; Wojciechowska, A.; Augustyn, P. Laser-induced surface activation and electroless metallization of polyurethane coating containing copper(II) L-tyrosine. Appl. Surf. Sci. 2020, 505, 144429. [Google Scholar] [CrossRef]
- Jagodziński, B.; Rytlewski, P.; Moraczewski, K. Comparative Evaluation of Cu(acac)2 and {[Cu(μ-O,O′-NO3) (L-arg) (2,2′-bpy)]·NO3}n as potential precursors of electroless metallization of laser-activated polymer materials. Materials 2021, 14, 978. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Goepfert, Y.; Leonard, D.; Bessuueille, F.; Bouadi, M. Palladium (+2) reduction: A key step for the electroless Ni metallization of insulating substrates by a tin-free process. Thin Solid Film. 2006, 515, 1623–1633. [Google Scholar] [CrossRef]
- Rytlewski, P.; Jagodziński, B.; Malinowski, R.; Budner, B.; Moraczewski, K.; Wojciechowska, A.; Augustyn, P. Laser Activated and Electroless Metalized Polyurethane Coatings Containing Copper(II) L-Tyrosine and Glass Microspheres. Molecules 2021, 26, 5571. [Google Scholar] [CrossRef]
- Rytlewski, P. Application of Nd:YAG laser in electroless metallization of polymer composites. Mater. Manuf. Proc. 2014, 29, 1111–1116. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Wojkiewicz, A.; Gągor, A.; Późniak, B. Krystaliczna Forma Kompleksu Azotan (V) [((S)-2-Amino-5-guanidynopentan-N,O) (μ-O,O′-Azotano(V)) (2,2′-Dipirydyl) Miedzi(II)] i Sposób jej Wytwarzania, Polska. Patent nr 227112, 31 October 2017. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rytlewski, P.; Żenkiewicz, M. Laserowe modyfikowanie materiałów polimerowych. Cz. III, Ablacja laserowa i zmiany struktury geometrycznej powierzchni. Polimery 2007, 52, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, R.J.; Afonso, C.N.; Bator, M.; Lippert, T. Laser ablation of ceramic Al2O3 at 193 nm and 248 nm: The importance of sin-gle-photon ionization processes. J. Appl. Phys. 2013, 113, 223301. [Google Scholar] [CrossRef] [Green Version]
- Żenkiewicz, M. Analiza głównych uwarunkowań metody van Ossa-Chaunhury’ego-Gooda w badaniach warstwy wierzchniej materiałów polimerowych. Polimery 2006, 51, 169–176. [Google Scholar]
- Żenkiewicz, M. Analiza głównych metod badania swobodnej energii powierzchniowej materiałów polimerowych. Polimery 2007, 52, 760–767. [Google Scholar]
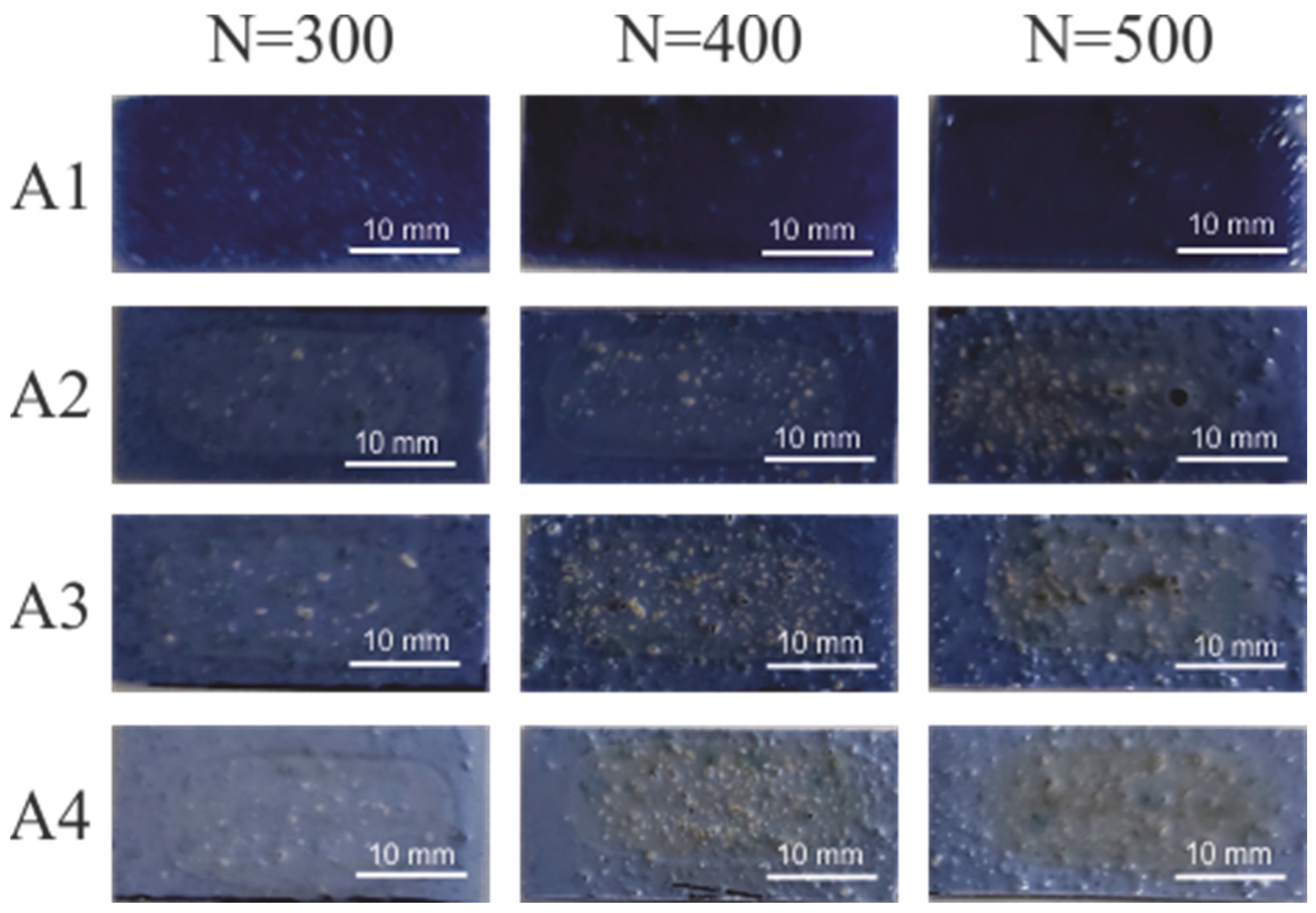
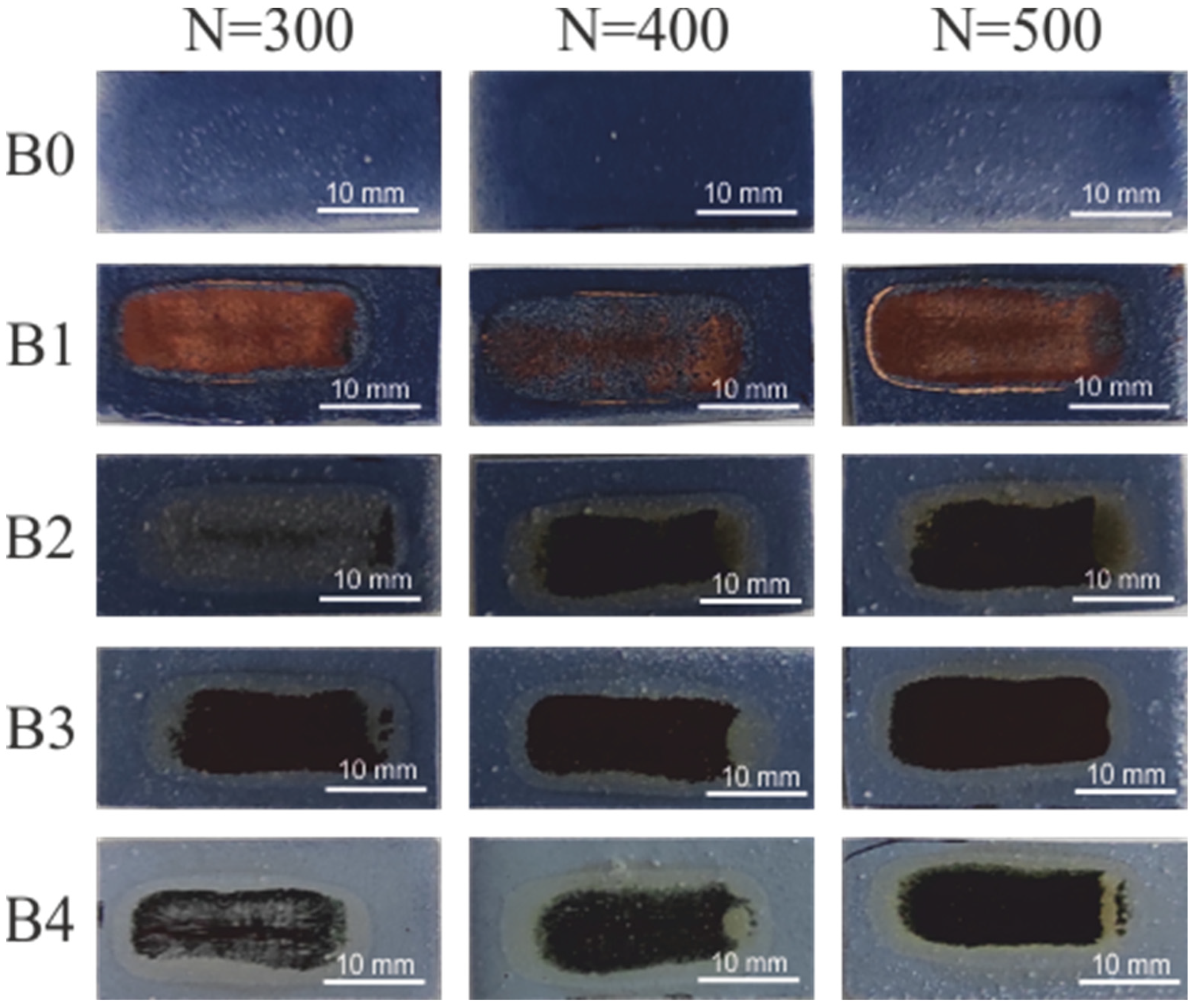

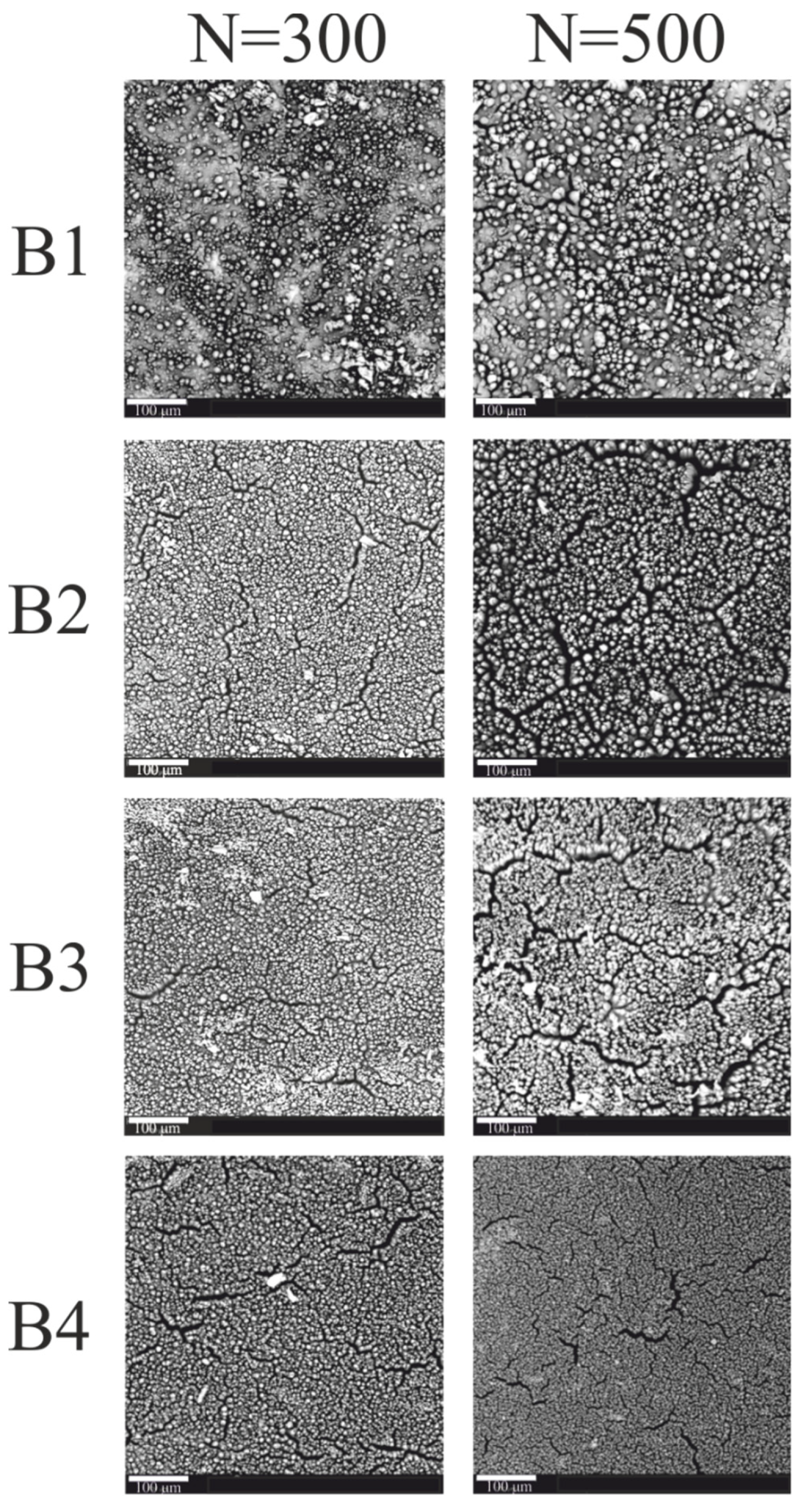
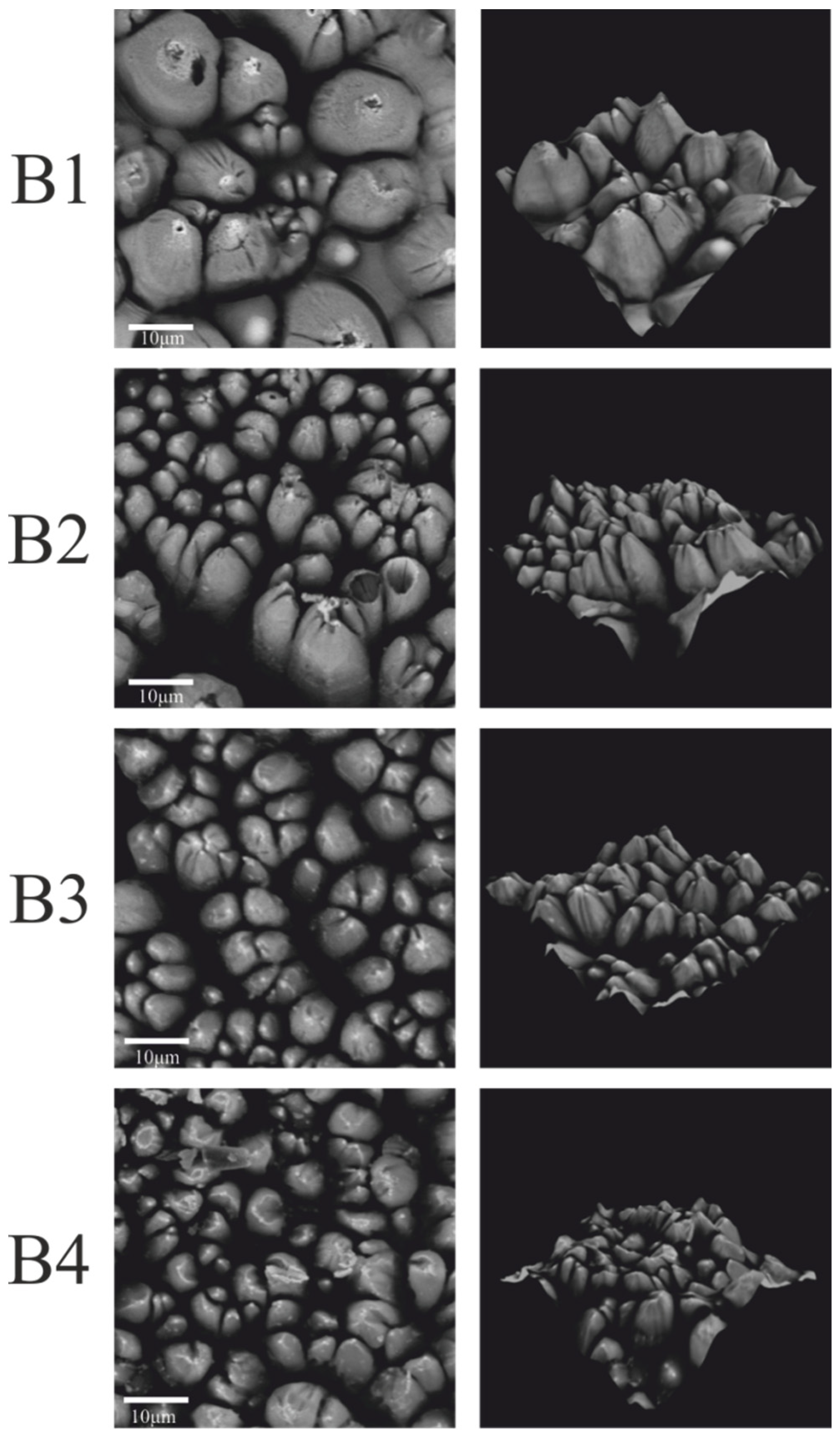

| Coating | Content of Compounds: [wt.%] | ||
|---|---|---|---|
| Complex A | Complex B | Sb2O3 | |
| A1 | 20 | - | - |
| A2 | 16.67 | - | 3.33 |
| A3 | 15 | - | 5 |
| A4 | 10 | - | 10 |
| B0 | - | 10 | - |
| B1 | - | 20 | - |
| B2 | - | 16.67 | 3.33 |
| B3 | - | 15 | 5 |
| B4 | - | 10 | 10 |
| Nazwa Próbki | N = 300 | N = 500 | ||||||
|---|---|---|---|---|---|---|---|---|
| Cu at. [%] | O at. [%] | C at. [%] | Sb at. [%] | Cu at. [%] | O at. [%] | C at. [%] | Sb at. [%] | |
| A1 | - | 30.45 | 69.55 | - | 0.41 | 29.84 | 69.45 | - |
| A2 | 0.36 | 27.90 | 64.75 | - | 2.38 | 24.69 | 67.85 | 5.07 |
| A3 | 0.46 | 29.13 | 70.41 | - | 1.46 | 21.33 | 68.05 | 9.16 |
| A4 | 0.19 | 29.83 | 69.97 | - | 1.19 | 25.14 | 63.93 | 9.74 |
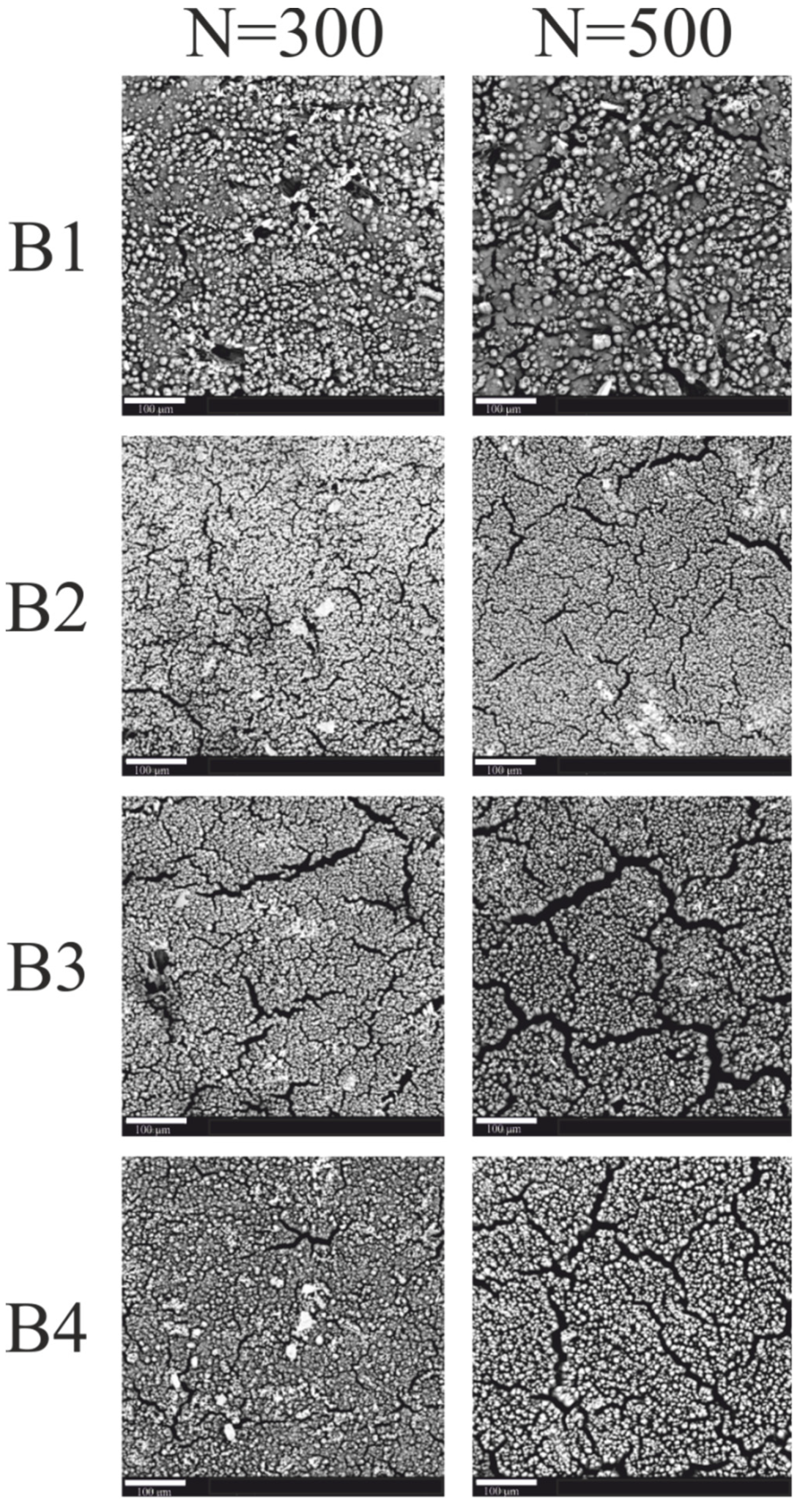
| B1 | 7.37 | 21.18 | 71.45 | - | 9.40 | 21.48 | 69.12 | - |
| B2 | 4.94 | 20.91 | 74.14 | - | 7.10 | 23.66 | 67.54 | 1.70 |
| B3 | 7.92 | 20.83 | 67.62 | 3.62 | 7.64 | 22.28 | 64.05 | 6.02 |
| B4 | 4.03 | 19.75 | 59.08 | 17.14 | 4.86 | 25.11 | 55.65 | 14.38 |
| Coating | Surface Energy [mJ/m2] | ||
|---|---|---|---|
| Unmodified | Modified N = 300 | Modified N = 500 | |
| A1 | 42.7 | 45.3 | 42.9 |
| A2 | 38.3 | 55.2 | 61.2 |
| A3 | 47 | 50.8 | 59.1 |
| A4 | 42.1 | 51.5 | 68 |
| B1 | 42.1 | 82.6 | 74.8 |
| B2 | 38.6 | 86.4 | 75 |
| B3 | 37.3 | 67.5 | 75 |
| B4 | 72.8 | 78.8 | 77.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagodziński, B.; Rytlewski, P.; Moraczewski, K.; Trafarski, A.; Karasiewicz, T. The Effect of Antimony (III) Oxide on the Necessary Amount of Precursors Used in Laser-Activated Coatings Intended for Electroless Metallization. Materials 2022, 15, 5155. https://doi.org/10.3390/ma15155155
Jagodziński B, Rytlewski P, Moraczewski K, Trafarski A, Karasiewicz T. The Effect of Antimony (III) Oxide on the Necessary Amount of Precursors Used in Laser-Activated Coatings Intended for Electroless Metallization. Materials. 2022; 15(15):5155. https://doi.org/10.3390/ma15155155
Chicago/Turabian StyleJagodziński, Bartłomiej, Piotr Rytlewski, Krzysztof Moraczewski, Andrzej Trafarski, and Tomasz Karasiewicz. 2022. "The Effect of Antimony (III) Oxide on the Necessary Amount of Precursors Used in Laser-Activated Coatings Intended for Electroless Metallization" Materials 15, no. 15: 5155. https://doi.org/10.3390/ma15155155
APA StyleJagodziński, B., Rytlewski, P., Moraczewski, K., Trafarski, A., & Karasiewicz, T. (2022). The Effect of Antimony (III) Oxide on the Necessary Amount of Precursors Used in Laser-Activated Coatings Intended for Electroless Metallization. Materials, 15(15), 5155. https://doi.org/10.3390/ma15155155








