Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
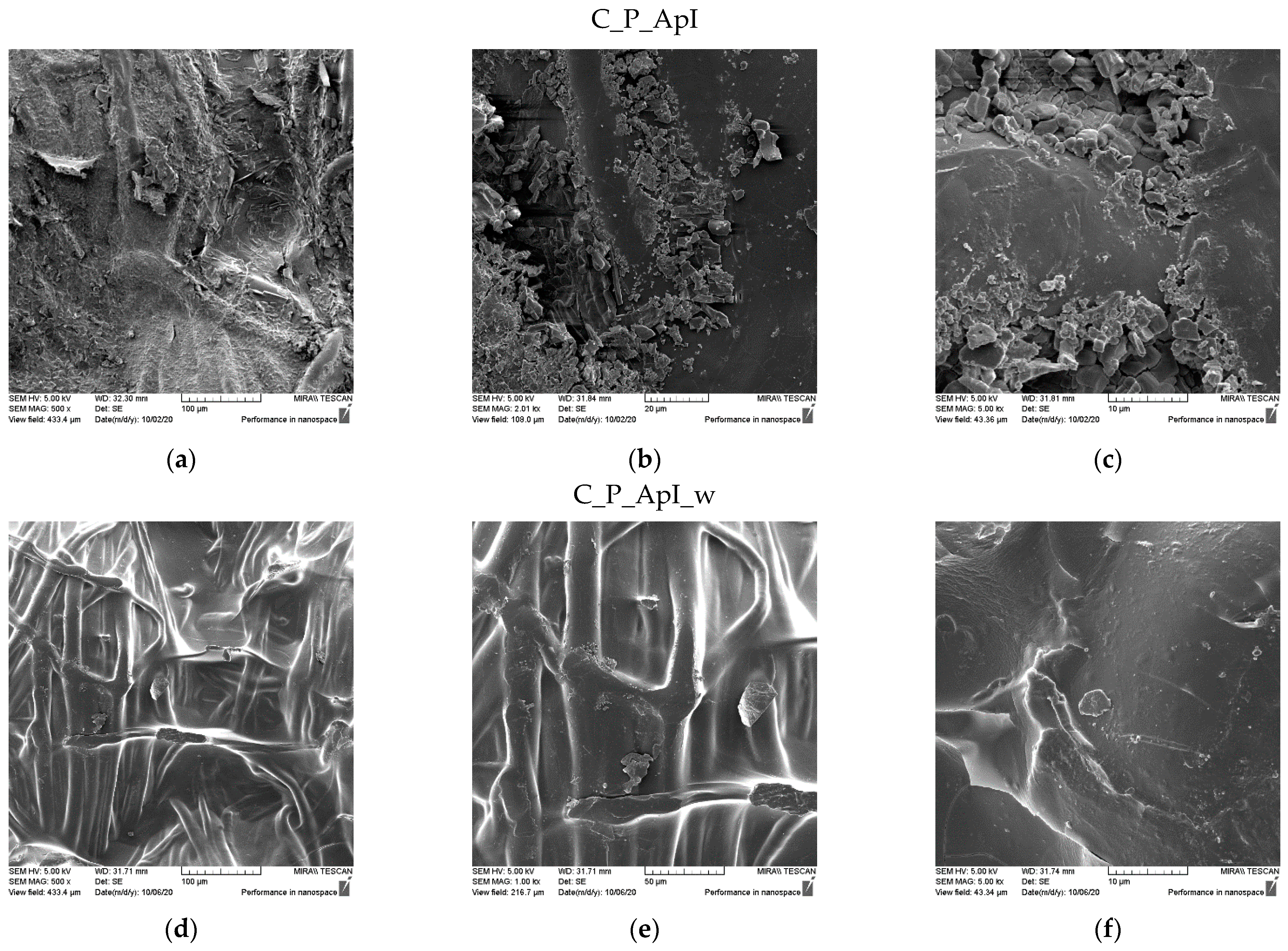
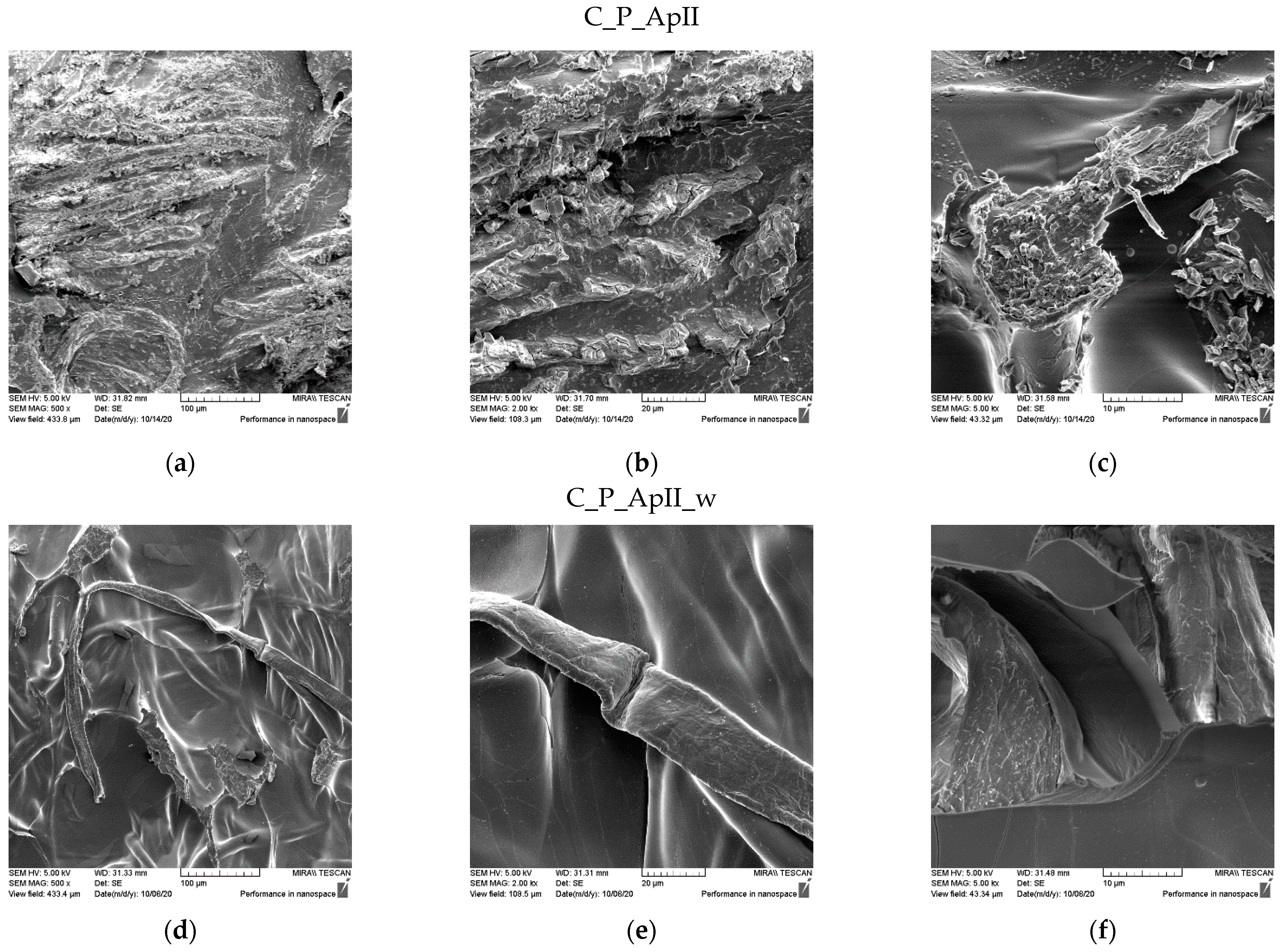
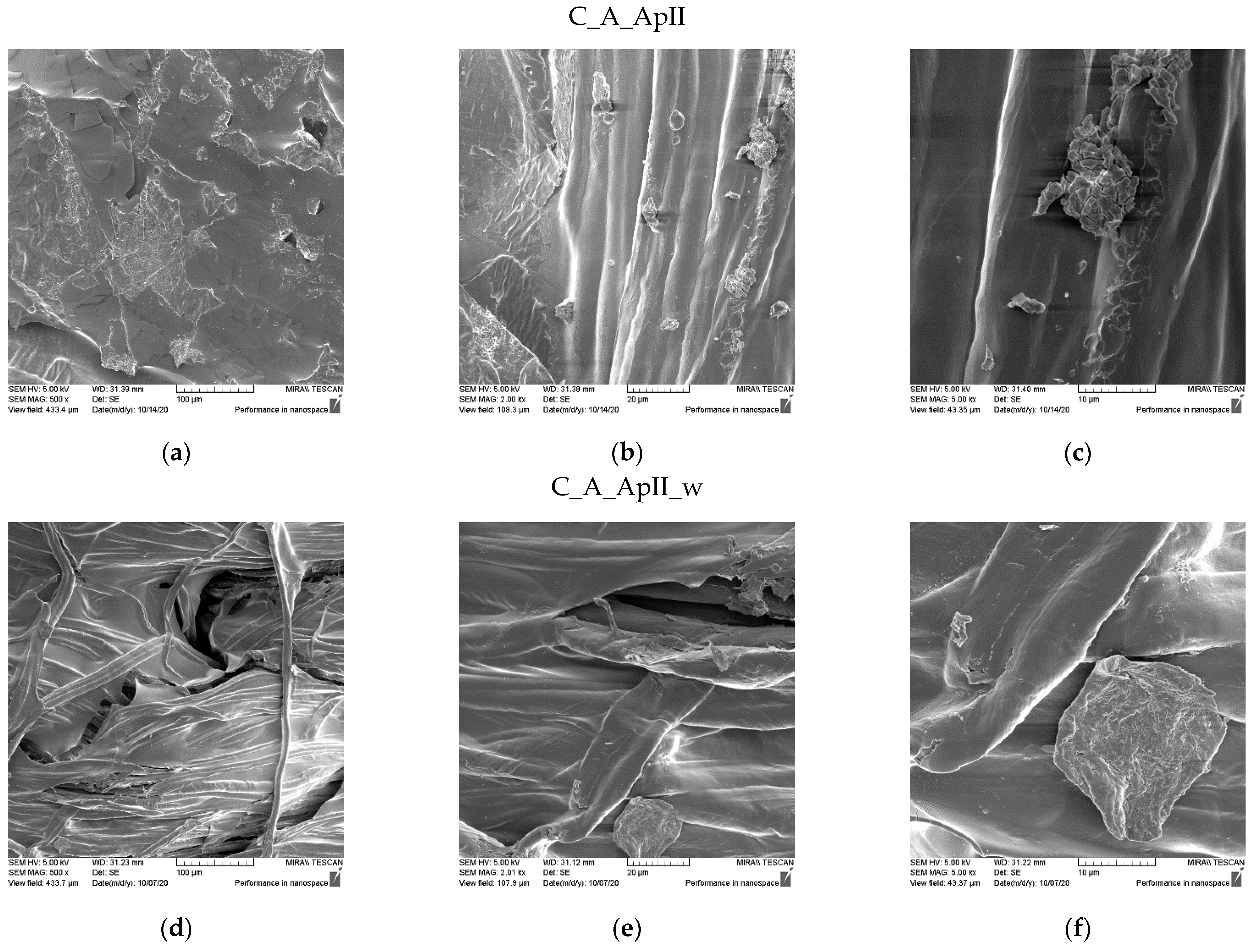
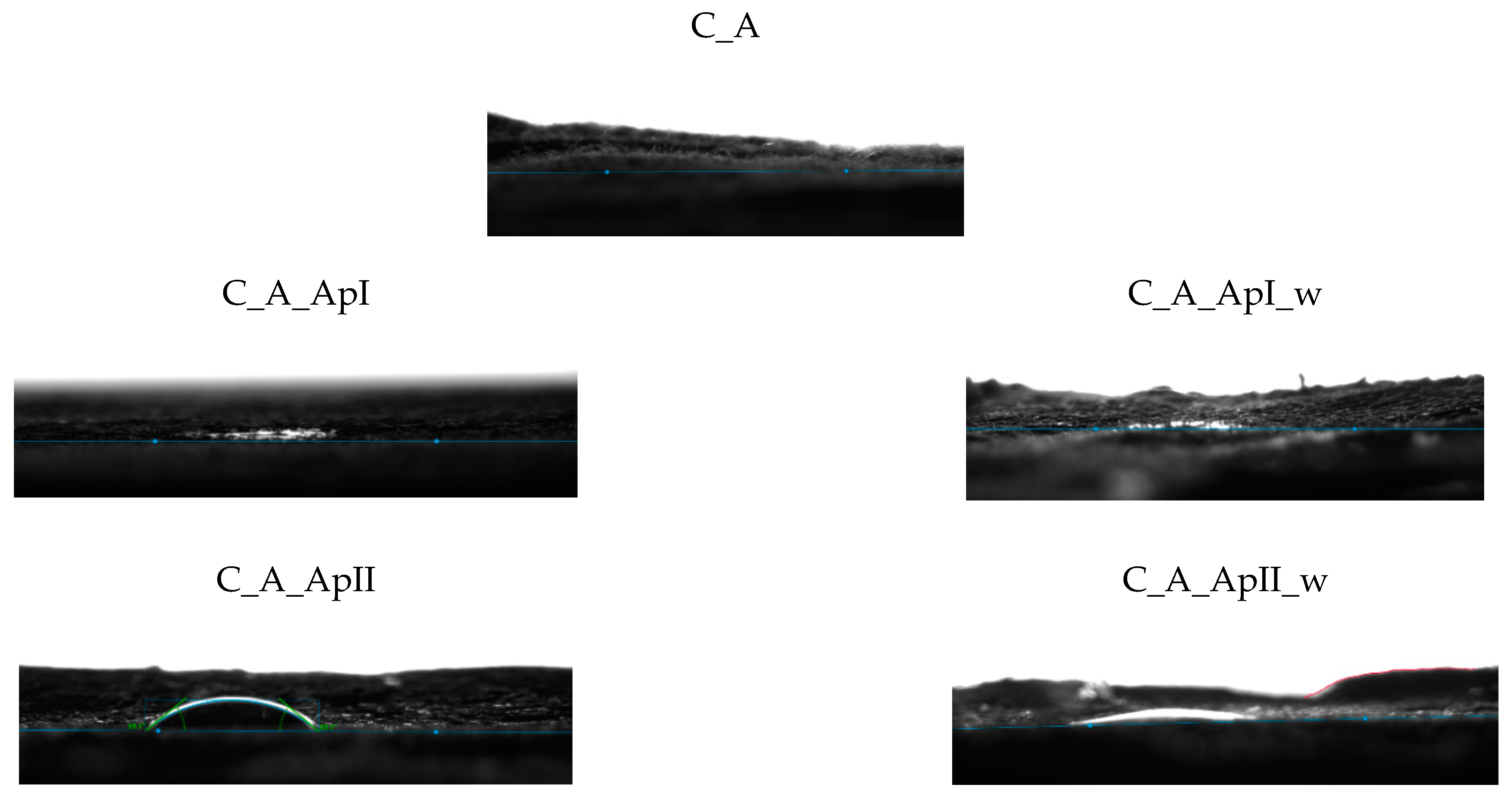
3.1. Morphological Characterization of the Sample Surfaces
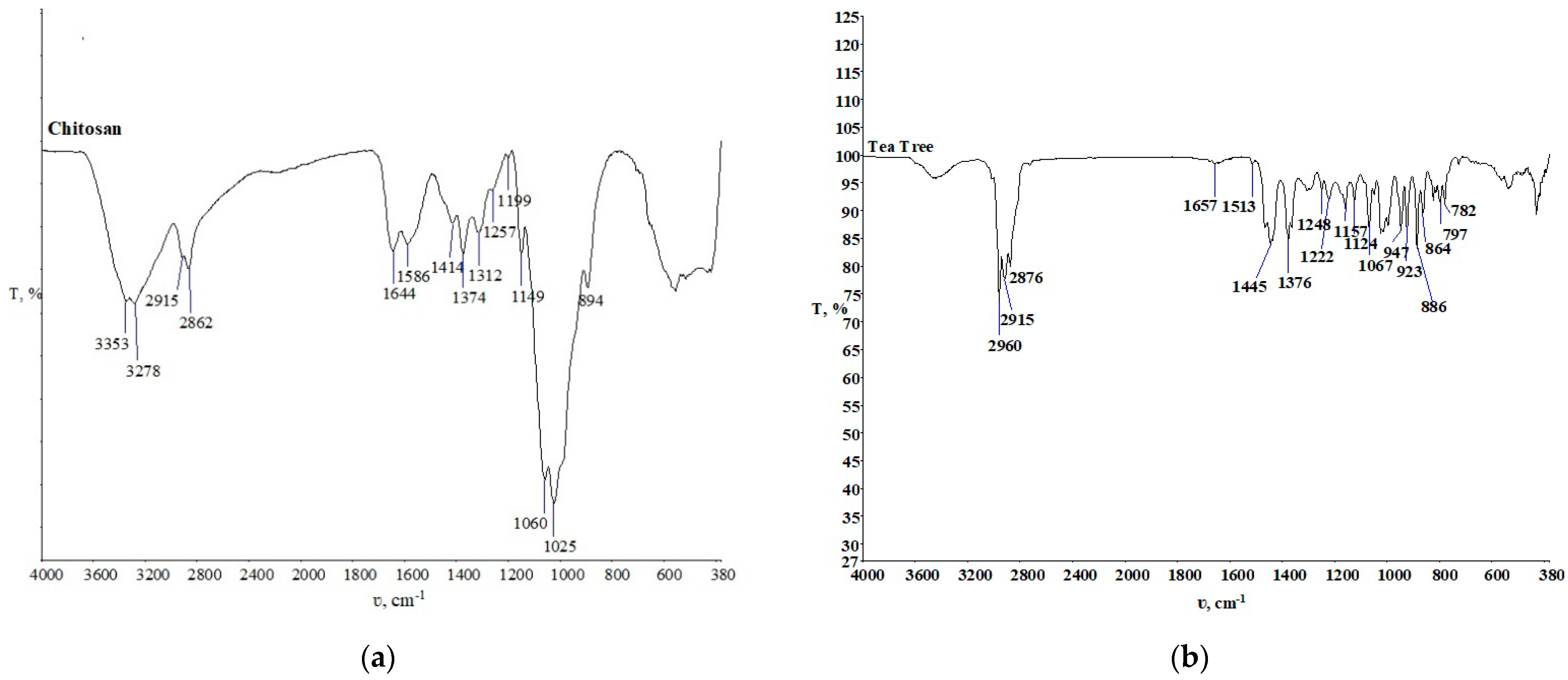
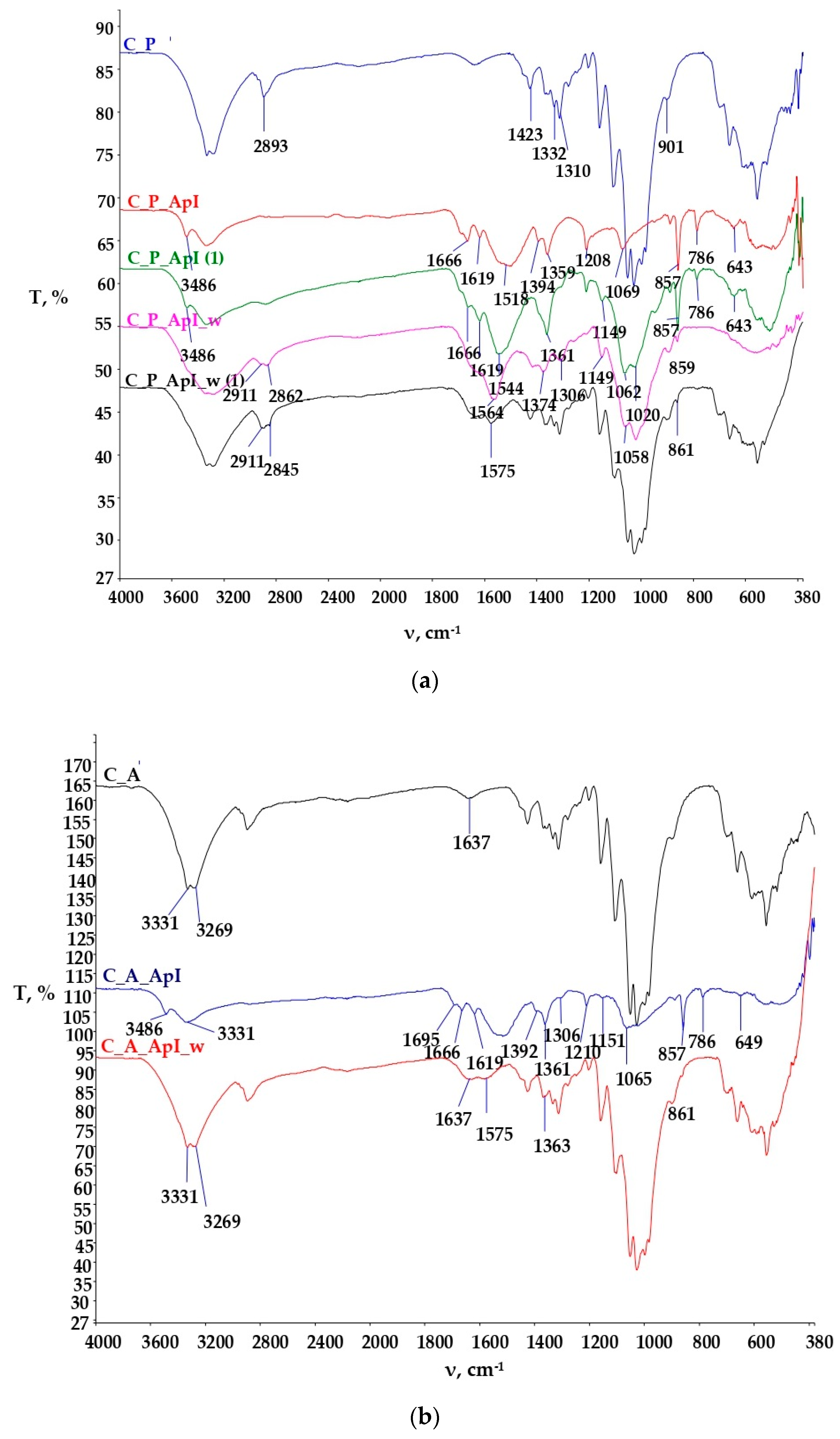
3.2. Physico-Chemical Characterization of the Samples Established by Spectroscopy with Fourier Transform of the Infrared Spectrum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sang-Hoon, L.; Hudson, S.M. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Prog. Coloration Relat. Top. 2004, 3, 108–113. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Ul-Islam, S.; Butola, B.S. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Tamanadar, E.; Hajabdollahi, N.; Vatankhah, M.; Thakur, V.K.; Skorik, Y.A. Chitosan microencapsulation of rhizobacteria for biological control of plant pests and diseases: Recent advances and applications. Rhizosphere 2022, 23, 100565. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, A.; Mohamad Ibrahim, M.N.; Rashid, M. Chitosan-based nanocomposites for gene delivery: Application and future perspectives. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering, 1st ed.; Bhawani, S.A., Karim, Z., Jawaid, M., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 245–262. [Google Scholar]
- Tiwari, A. Handbook of Antimicrobial Coatings, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2017; Available online: https://www.elsevier.com/books/handbook-of-antimicrobial-coatings/tiwari/978-0-12-811982-2 (accessed on 20 November 2021).
- Santos Morais, D.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Rejane, C.; Goy Sinara, T.B.; Morais Odilio, B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Braz. J. Pharmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A. Biochemical significance of exogenous chitins and chitosans in animals and patients. Carbohydr. Polym. 1993, 20, 7–16. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Janjić, S.; Kostić, M.; Vučinić, V.; Dimitrijević, S.; Popović, K.; Ristić, M.; Skundrić, P. Biologically active fibres based on chitosan-coated lyocell fibers. Carbohydr. Polym. 2009, 78, 747–759. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. The Good, the Bad and the Ugly of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Flinčec Grgac, S.; Tarbuk, A.; Dekanić, T.; Sujka, W.; Draczyński, Z. The Chitosan Implementation into Cotton and Polyester/Cotton Blend Fabrics. Materials 2020, 13, 1616. [Google Scholar] [CrossRef] [Green Version]
- Flinčec Grgac, S.; Biruš, T.-D.; Tarbuk, A.; Malinar, R.; Draczyński, Z. The influence of maleic acid concentration on the binding of chitosan with cotton cellulose. In Proceedings of the 13th International Scientific-Professional Symposium Textile Science and Economy, Chinese–Croatian Forum, Innovation, Design and Digitalization in the Textile and Leather Sector, Zagreb, Croatia, 18 September 2020. [Google Scholar]
- Wasko, J.; Fraczyk, J.; Becht, A.; Kaminski, Z.J.; Flinčec Grgac, S.; Tarbuk, A.; Kaminska, M.; Dudek, M.; Gliscinska, E.; Draczynski, Z.; et al. Conjugates of Chitosan and Calcium Alginate with Oligoproline and Oligohydroxyproline Derivatives for Potential Use in Regenerative Medicine. Materials 2020, 13, 3079. [Google Scholar] [CrossRef]
- Flinčec Grgac, S.; Dekanić, T.; Kiepiela, P.; Sujka, W.; Tarbuk, A.; Draczyński, Z. The chitosan implementation into cotton/polyester blended fabric. In Proceedings of the 10th Central European Conference Fibre Grade Polymers, Chemical Fibres and Special Textiles, Lodz, Poland, 22–23 October 2019. [Google Scholar]
- Rytwo, G.; Zakai, R.; Wicklein, B. The Use of ATR-FTIR Spectroscopy for Quantification of Adsorbed Compounds. J. Spectrosc. 2015, 1, 727595. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. Int. J. Biol. Macromol. 2012, 50, 1195–1200. [Google Scholar] [CrossRef]
- Saini, S.; Gupta, A.; Singh, N.; Sheikh, J. Functioalization of linen fabric using layer by layer treatment with chitosan and green tea extract. J. Ind. Eng. Chem. 2019, 82, 6233. [Google Scholar]
- Benltoufa, S.; Miled, W.; Trad, M.; Slama, R.B.; Fayala, F. Chitosan hydrogel-coated cellulosic fabric for medical end-use: Antibacterial properties, basic mechanical and comfort properties. Carbohydr. Polym. 2020, 227, 115352. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J.; Bramhecha, I. Multifunctional modification of linen fabric using chitosan-based formulations. Int. J. Biol. Macromol. 2018, 118, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, L.; Ji, J.; Huang, Y.; Chen, D. Antibacterial Properties of Cotton Fabrics Treated with Chitosan. Text. Res. J. 2003, 73, 1103–1106. [Google Scholar] [CrossRef]
- Gatahi, D.; Wanyika, H.; Kihurani, A.; Gatebe, E. Effect of Biological Control Antagonists Adsorbed on Chitosan Immobilized Silica Nanocomposite on Ralstonia solanacearum and Growth of Tomato Seedlings. Adv. Res. 2016, 6, 1–23. [Google Scholar] [CrossRef]
- Lin, G.; Chen, H.; Zhou, H.; Zhou, X.; Xu, H. Preparation of Tea Tree Oil/Poly (styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties. Materials 2018, 11, 710. [Google Scholar] [CrossRef] [Green Version]
- Anicuta, S.G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier Transform Infrared (FTIR) Spectroscopy for Characterization of Antimicrobial Films Containing Chitosan. 2010. Available online: http://protmed.uoradea.ro/facultate/anale/ecotox_zooteh_ind_alim/2010/ipa/120%20Stoica.pdf (accessed on 18 October 2021).
- Gupta, D.; Bhaumik, S.; Fabrics, S.; Nagar, M. Antimicrobial treatment for textiles. Indian J. Fibre Text. Res. 2007, 32. Available online: http://nopr.niscair.res.in/handle/123456789/419 (accessed on 18 October 2021).
- Naebe, M.; Li, Q.; Onur, A.; Denning, R. Investigation of chitosan adsorption onto cotton fabric with atmospheric helium/oxygen plasma pre-treatment. Cellulose 2016, 23, 2129–2142. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of Hydrophilic Plasticizers on Mechanical, Thermal, and Surface Properties of Chitosan Films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef]
- Nechita, P. Applications of Chitosan in Wastewater Treatment, Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; IntechOpen: Rijeka, Croatia, 2017; Available online: https://www.intechopen.com/chapters/52359 (accessed on 18 October 2021).
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.; Min, K. Antimicrobial finishing of polypropylene nonwoven fabric by treatment with chitosan oligomer. J. Appl. Polym. Sci. 1999, 74, 2911–2916. [Google Scholar] [CrossRef]






| Ingredients | Bath I (ApI) | Bath II (ApII) |
|---|---|---|
| Maleic acid (Sigma Aldrich, Spain) | 10 g/L | |
| Sodium hypophosphite monohydrate (Sigma Aldrich, Stockholm, Sweden) | 6 g/L | |
| Chitosan (Tricomed SA, Łódź, Poland) | 50% by weight of material | |
| Tea Tree Oil (Sigma Aldrich, Stockholm, Sweden) | 40% tea tree oil by weight of chitosan | - |
| C_P | cotton sample in plain weave |
| C_P_w | cotton sample in plain weave, and washed once |
| C_A | cotton sample in satin weave |
| C_A_w | cotton sample in satin weave and washed once |
| C_P_API | cotton sample in plain weave, treated with Bath I in an autoclave at 80 °C, 24 h |
| C_P_API_w | cotton sample in plain weave, treated with Bath I in an autoclave at 80 °C, 24 h, and washed once |
| C_A _API | cotton sample in satin weave, treated with Bath I in an autoclave at 80 °C, 24 h |
| C_A_API_w | cotton sample in satin weave, treated with Bath I in an autoclave at 80 °C, 24 h, and washed once |
| C_P_APII | cotton sample in plain weave, treated with Bath II in an autoclave at 80 °C, 24 h |
| C_P_APII_w | cotton sample in plain weave, treated with Bath II in an autoclave at 80 °C, 24 h, and washed once |
| C_A_APII | cotton sample in satin weave, treated with Bath II in an autoclave at 80 °C, 24 h |
| C_A_APII_w | cotton sample in satin weave, treated with Bath II in an autoclave at 80 °C, 24 h, and washed once |
| Samples | CA (m) | CA (l) | CA (r) | Temp | Mean Three-Phase Point (l)/(mm) | Mean Three-Phase Point (r)/(mm) | Mean Diameter (mm) | Mean Volume (µL) |
|---|---|---|---|---|---|---|---|---|
| C_P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_P_ApI | 19.0 ± 4.5 | 19.0 ± 4.5 | 19.0 ± 4.5 | 20 | 12 ± 0.2 | 30.8 ± 0.7 | 18.8 ± 0.7 | 228.3 ± 78.5 |
| C_P_ApI (1) | 24.0 ± 6.1 | 24.0 ± 6.1 | 24.0 ± 6.1 | 20 | 13.1 ± 1.1 | 33 ± 0.2 | 19.8 ± 1.1 | 324.4 ± 64.1 |
| C_P_ApI_w | 27.3 ± 4.4 | 27.3 ± 4.4 | 27.3 ± 4.4 | 20 | 14.1 ± 0.9 | 30.4 ± 0.3 | 16.3 ± 1.2 | 219.6 ± 83.3 |
| C_P_ApI_w (1) | 36.8 ± 5.3 | 36.8 ± 5.3 | 36.8 ± 5.3 | 20 | 14.2 ± 0.2 | 29.8 ± 0.1 | 15.6 ± 0.2 | 260.9 ± 49.2 |
| C_P_ApII | 30.5 ± 14.0 | 30.5 ± 14.0 | 30.5 ± 14.0 | 20 | 11 ± 2.2 | 30.4 ± 0.7 | 19.5 ± 2.9 | 360.1 ± 57.4 |
| C_P_ApII_w | 22.3 ± 6.0 | 22.3 ± 6.0 | 22.3 ± 6.0 | 20 | 12.8 ± 0.2 | 29.2 ± 0.1 | 16.5 ± 0.3 | 177.4 ± 56.3 |
| Samples | CA (m) | CA (l) | CA (r) | Temp | Mean Three-Phase Point (l)/(mm) | Mean Three-Phase Point (r)/(mm) | Mean Diameter (mm) | Mean Volume (µL) |
|---|---|---|---|---|---|---|---|---|
| C_A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_A_ApI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_A_ApI_w | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_A_ApII | 27.7 ± 7.7 | 27.7 ± 7.7 | 27.7 ± 7.7 | 20 | 11.8 ± 0.1 | 27.8 ± 0.2 | 16.0 ± 0.2 | 205.4 ± 69.9 |
| C_A_ApII_w | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Samples | Mass per Unit Area, g/m2 * | STDEV (σ), g/m2 | Weight Difference, g | Weight Change, % |
|---|---|---|---|---|
| C_P | 158.3 ± 0.4 | 0.4 | ||
| C_P_w | 159.0 ± 0.7 | 0.7 | 0.74 | 0.47 |
| C_P_API | 206.0 ± 2.0 | 2.0 | 47.65 | 30.11 |
| C_P_API_w | 185.9 ± 0.6 | 0.6 | 26.91 | 16.93 |
| C_P_APII | 204.5 ± 0.6 | 0.6 | 45.51 | 28.62 |
| C_P_APII_w | 198.1 ± 0.7 | 0.7 | 39.8 | 25.15 |
| C_A | 178.5 ± 1.9 | 1.9 | ||
| C_A_w | 196.5 ± 2.2 | 2.2 | 18 | 10.09 |
| C_A_API | 239.4 ± 1.8 | 1.8 | 60.95 | 34.16 |
| C_A_API_w | 216.1 ± 1.7 | 1.7 | 19.65 | 10.00 |
| C_A_APII | 211.1 ± 2.6 | 2.6 | 32.65 | 18.30 |
| C_A_APII_w | 203.9 ± 0.2 | 0.2 | 7.45 | 3.79 |
| Samples | Staphylococcus aureus | Escherichia coli | Candida albicans |
|---|---|---|---|
| C_P | − | − | − |
| C_A | − | − | − |
| C_P_ApI | +/− | +/− | +/− |
| C_P_ApI_w | +/− | +/− | +/− |
| C_A_ApI | +/− | +/− | + |
| C_A_ApI_w | +/− | +/− | + |
| C_P_ApII | +/− | +/− | +/− |
| C_P_ApII_w | +/− | +/− | +/− |
| C_A_ApII | +/− | +/− | +/− |
| C_A_ApII_w | +/− | +/− | +/− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flinčec Grgac, S.; Tesla, T.; Čorak, I.; Žuvela Bošnjak, F. Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics. Materials 2022, 15, 5034. https://doi.org/10.3390/ma15145034
Flinčec Grgac S, Tesla T, Čorak I, Žuvela Bošnjak F. Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics. Materials. 2022; 15(14):5034. https://doi.org/10.3390/ma15145034
Chicago/Turabian StyleFlinčec Grgac, Sandra, Tea Tesla, Ivana Čorak, and Franka Žuvela Bošnjak. 2022. "Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics" Materials 15, no. 14: 5034. https://doi.org/10.3390/ma15145034
APA StyleFlinčec Grgac, S., Tesla, T., Čorak, I., & Žuvela Bošnjak, F. (2022). Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics. Materials, 15(14), 5034. https://doi.org/10.3390/ma15145034







