Elastic Properties and Hardness of Mixed Alkaline Earth Silicate Oxynitride Glasses
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Effective Cation Field Strength, Bridging Oxygen, and Network Connectivity
3.2. Density, Molar Volume, and Glass Packing Density
3.3. Hardness
3.4. Elastic Properties
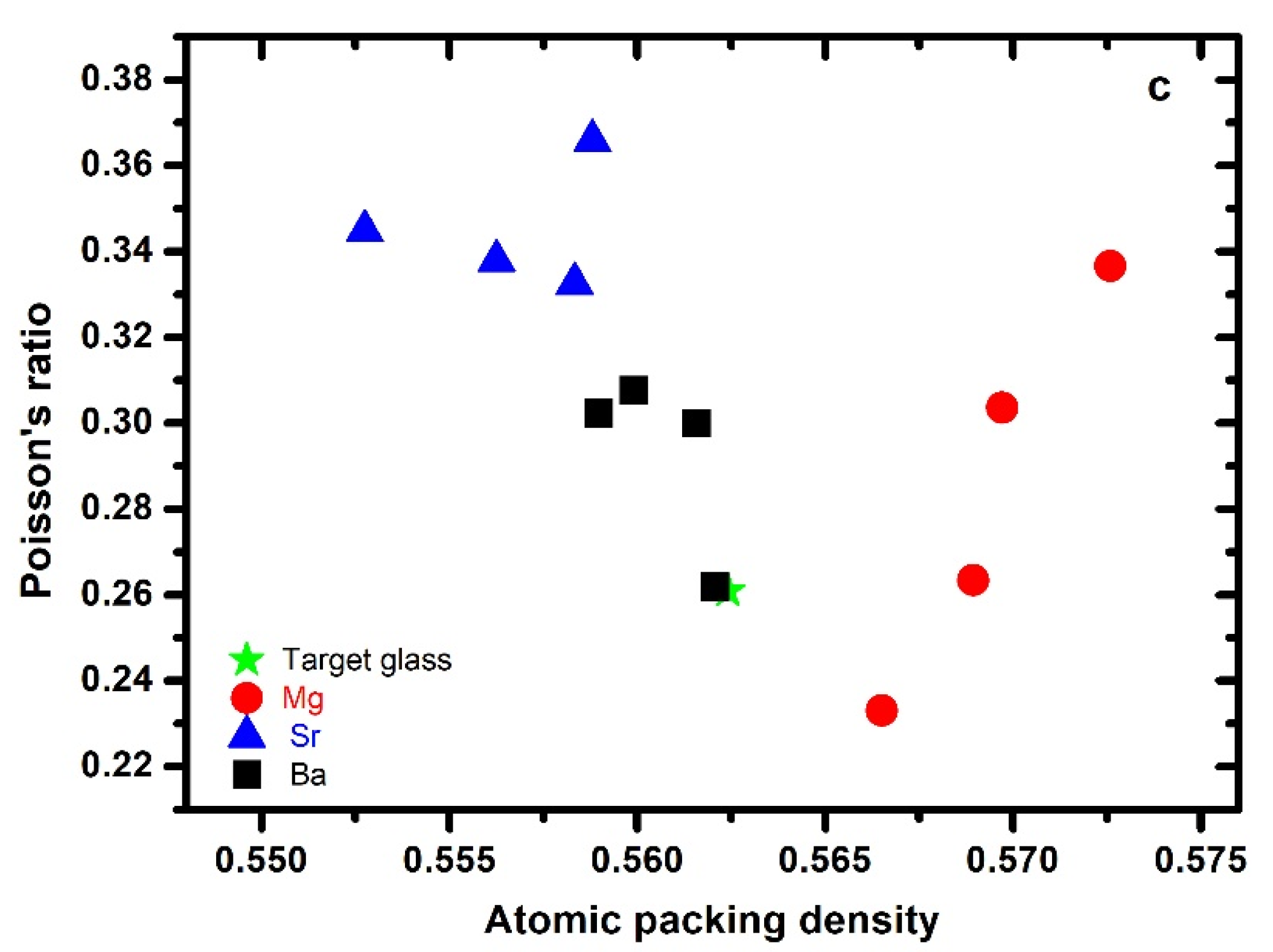
3.5. Poisson’s Ratio
3.6. Absence of Mixed Modifier Effects
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loehman, R.E. Preparation and Properties of Oxynitride Glasses. J. Non-Cryst. Solids 1983, 56, 123–134. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K.; Yoko, T. Preparation and Properties of Ca-Al-Si-O-N Oxynitride Glasses. J. Non-Cryst. Solids 1983, 56, 147–152. [Google Scholar] [CrossRef]
- Day, D.E.; Frischat, G.H.; Pastuszak, R.; Verdier, P. Preparation, Properties, and Structure of Special Glasses M-S-A-O-N glasses (M = Mg, Ca, Ba, Mn, Nd), existence range and comparative study of some properties. J. Non-Cryst. Solids 1983, 56, 141–146. [Google Scholar] [CrossRef]
- Tredway, W.K.; Risbud, S.H. Melt Processing and Properties of Barium-Sialon Glasses. J. Am. Ceram. Soc. 1983, 66, 324–327. [Google Scholar] [CrossRef]
- Homeny, J.; Mcgarry, D.L. Preparation and Mechanical-Properties of Mg-Al-Si-O-N Glasses. J. Am. Ceram. Soc. 1984, 67, C225–C227. [Google Scholar] [CrossRef]
- Lemercier, H.; Rouxel, T.; Fargeot, D.; Besson, J.L.; Piriou, B. Yttrium SiAlON glasses: Structure and mechanical properties—Elasticity and viscosity. J. Non-Cryst. Solids 1996, 201, 128–145. [Google Scholar] [CrossRef]
- Sun, E.Y.; Becher, P.F.; Hwang, S.L.; Waters, S.B.; Pharr, G.M.; Tsui, T.Y. Properties of silicon-aluminum-yttrium oxynitride glasses. J. Non-Cryst. Solids 1996, 208, 162–169. [Google Scholar] [CrossRef]
- Ramesh, R.; Nestor, E.; Pomeroy, M.J.; Hampshire, S. Formation of Ln-Si-Al-O-N glasses and their properties. J. Eur. Ceram. Soc. 1997, 17, 1933–1939. [Google Scholar] [CrossRef]
- Lofaj, F.; Deriano, S.; LeFloch, M.; Rouxel, T.; Hoffmann, M.J. Structure and rheological properties of the RE-Si-Mg-O-N (RE = Sc, Y, La, Nd, Sm, Gd, Yb and Lu) glasses. J. Non-Cryst. Solids 2004, 344, 8–16. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Grins, J.; Esmaeilzadeh, S. La-Si-O-N glasses—Part I. Extension of the glass forming region. J. Eur. Ceram. Soc. 2007, 27, 4773–4781. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Grins, J.; Esmaeilzadeh, S. La-Si-O-N glasses—Part II: Vickers hardness and refractive index. J. Eur. Ceram. Soc. 2007, 27, 4783–4787. [Google Scholar] [CrossRef]
- Leonova, E.; Hakeem, A.S.; Jansson, K.; Stevensson, B.; Shen, Z.; Grins, J.; Esmaeilzadeh, S.; Edén, M. Nitrogen-rich La-Si-Al-O-N oxynitride glass structures probed by solid state NMR. J. Non-Cryst. Solids 2008, 354, 49–60. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Glass-forming region in the Ca–Si–O–N system using CaH2 as Ca source. J. Eur. Ceram. Soc. 2008, 28, 2659–2664. [Google Scholar] [CrossRef]
- Sharafat, A. Preparation, Characterization and Properties of Nitrogen Rich Glasses in Alkaline Earth-Si-O-N Systems. Ph.D Thesis, Institutionen för Fysikalisk Kemi, Oorganisk Kemi och Strukturkemi, Stockholm University, Stockholm, Sweden, 2009; p. 116. [Google Scholar]
- Sharafat, A.; Forslund, B.; Grins, J.; Esmaeilzadeh, S. Formation and properties of nitrogen-rich strontium silicon oxynitride glasses. J. Mater. Sci. 2009, 44, 664–670. [Google Scholar] [CrossRef]
- Sharafat, A.; Bo, J. Preparation of oxynitride glasses from woody biofuel ashes. J. Non-Cryst. Solids 2010, 356, 2774–2777. [Google Scholar]
- Ali, S.; Jonson, B. Glasses in the Ba-Si-O-N System. J. Am. Ceram. Soc. 2011, 94, 2912–2917. [Google Scholar] [CrossRef]
- Lofaj, F.; Satet, R.; Hoffmann, M.J.; de Arellano Lopez, A.R. Thermal expansion and glass transition temperature of the rare-earth doped oxynitride glasses. J. Eur. Ceram. Soc. 2004, 24, 3377–3385. [Google Scholar] [CrossRef]
- Lofaj, F.; Hvizdos, P.; Dorcakova, F.; Satet, R.; Hoffmann, M.J.; de Arellano-Lopez, A.R. Indentation moduli and microhardness of RE-Si-Mg-O-N glasses (RE = Sc, Y, La, Sm, Yb and Lu) with different nitrogen content. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Processing 2003, 357, 181–187. [Google Scholar] [CrossRef]
- Pomeroy, M.J.; Mulcahy, C.; Hampshire, S. Independent effects of nitrogen substitution for oxygen and yttrium substitution for magnesium on the properties of Mg-Y-Si-Al-O-N glasses. J. Am. Ceram. Soc. 2003, 86, 458–464. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Dauce, R.; Leonova, E.; Edén, M.; Shen, Z.J.; Grins, J.; Esmaeilzadeh, S. Silicate glasses with unprecedented high nitrogen and electropositive metal contents obtained by using metals as precursors. Adv. Mater. 2005, 17, 2214–2216. [Google Scholar] [CrossRef]
- Ali, S.; Jonson, B.; Pomeroy, M.J.; Hampshire, S. Issues associated with the development of transparent oxynitride glasses. Ceram. Int. 2015, 41, 3345–3354. [Google Scholar] [CrossRef]
- Ali, S.; Paul, B.; Magnusson, R.; Ekström, E.; Pallier, C.; Jonson, B.; Eklund, P.; Birch, J. Optical and mechanical properties of amorphous Mg-Si-O-N thin films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2019, 372, 9–15. [Google Scholar] [CrossRef]
- Ali, S.; Paul, B.; Magnusson, R.; Broitman, E.; Jonson, B.; Eklund, P.; Birch, J. Synthesis and characterization of the mechanical and optical properties of Ca-Si-O-N thin films deposited by RF magnetron sputtering. Surf. Coat. Technol. 2017, 315, 88–94. [Google Scholar] [CrossRef]
- Ali, S.; Paul, B.; Magnusson, R.; Greczynski, G.; Broitman, E.; Jonson, B.; Eklund, P.; Birch, J. Novel transparent MgSiON thin films with high hardness and refractive index. Vacuum 2016, 131, 1–4. [Google Scholar] [CrossRef][Green Version]
- Hänninen, T.; Schmidt, S.; Jensen, J.; Hultman, L.; Högberg, H. Silicon oxynitride films deposited by reactive high power impulse magnetron sputtering using nitrous oxide as a single-source precursor. J. Vac. Sci. Technol. A 2015, 33, 05E121. [Google Scholar] [CrossRef]
- Liu, G.H.; Zhou, Z.Z.; Wei, Q.H.; Fei, F.; Yang, H.; Liu, Q. Preparation and tunable optical properties of ion beam sputtered SiAlON thin films. Vacuum 2014, 101, 1–5. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kato, K.; Omoto, H.; Tomioka, T.; Takamatsu, A. Stable deposition of silicon oxynitride thin films with intermediate refractive indices by reactive sputtering. Thin Solid Films 2012, 520, 3862–3864. [Google Scholar] [CrossRef]
- Chen, J.; Wei, P.; Huang, Y. Formation and properties of La-Y-Si-O-N oxynitride glasses. J. Mater. Sci. Lett. 1997, 16, 1486–1488. [Google Scholar] [CrossRef]
- Rocherulle, J.; Matecki, M.; Delugeard, Y. Heat capacity measurements of Mg-Y-Si-Al-O-N glasses. J. Non-Cryst. Solids 1998, 238, 51–56. [Google Scholar] [CrossRef]
- Pomeroy, M.J.; Nestor, E.; Ramesh, R.; Hampshire, S. Properties and crystallization of rare-earth Si-Al-O-N glasses containing mixed trivalent modifiers. J. Am. Ceram. Soc. 2005, 88, 875–881. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Ali, S.; Jonson, B. Preparation and properties of mixed La–Pr silicate oxynitride glasses. J. Non-Cryst. Solids 2013, 368, 93–97. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Properties of high nitrogen content mixed alkali earth oxynitride glasses (AE(x)Ca(1-x))(1.2(1))SiO1.9(1)N0.86(6), AE = Mg, Sr, Ba. J. Non-Cryst. Solids 2009, 355, 1259–1263. [Google Scholar] [CrossRef]
- Ecolivet, C.; Verdier, P. Elastic Properties and Refraction Indexes of Nitrided Glasses. Mater. Res. Bull. 1984, 19, 227–231. [Google Scholar] [CrossRef]
- Weigel, C.; Le Losq, C.; Vialla, R.; Dupas, C.; Clément, S.; Neuville, D.R.; Rufflé, B. Elastic moduli of XAlSiO4 aluminosilicate glasses: Effects of charge-balancing cations. J. Non-Cryst. Solids 2016, 447, 267–272. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic-Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Rocherulle, J.; Guyader, J.; Verdier, P.; Laurent, Y. Li-Si-Al-O-N and Li-Si-O-N Oxynitride Glasses Study and Characterization. J. Mater. Sci. 1989, 24, 4525–4530. [Google Scholar] [CrossRef]
- Hampshire, S.; Hanifi, A.R.; Genson, A.; Pomeroy, M.J. Ca-Si-Al-O-N glasses: Effects of fluorine on glass formation and properties. Key Eng. Mater. 2007, 352, 165–172. [Google Scholar]
- Sharafat, A.; Bo, J. Compositional effects on the properties of high nitrogen content alkaline-earth silicon oxynitride glasses, AE = Mg, Ca, Sr, Ba. J. Eur. Ceram. Soc. 2011, 31, 611–618. [Google Scholar] [CrossRef]
- Paraschiv, G.L.; Gomez, S.; Mauro, J.C.; Wondraczek, L.; Yue, Y.; Smedskjaer, M.M. Hardness of Oxynitride Glasses: Topological Origin. J. Phys. Chem. B 2015, 119, 4109–4115. [Google Scholar] [CrossRef]
- García-Bellés, Á.R.; Monzó, M.; Barba, A.; Clausell, C.; Pomeroy, M.J.; Hanifi, A.R.; Hampshire, S. Factors Controlling Properties of Ca-Mg, Ca-Er, Ca-Nd, or Ca-Y-Modified Aluminosilicate Glasses Containing Nitrogen and Fluorine. J. Am. Ceram. Soc. 2013, 96, 2839–2845. [Google Scholar] [CrossRef]
- García-Bellés, Á.R.; Clausell, C.; Barba, A.; Pomeroy, M.J.; Hampshire, S. Effect of fluorine and nitrogen content on the properties of Ca-Mg-Si-Al-O-(N)-(F) glasses. Ceram. Int. 2017, 43, 4197–4204. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Hardness and refractive index of Ca-Si-O-N glasses. J. Non-Cryst. Solids 2009, 355, 301–304. [Google Scholar] [CrossRef]
- Hampshire, S. Oxynitride glasses, their properties and crystallization—A review. J. Non-Cryst. Solids 2003, 316, 64–73. [Google Scholar] [CrossRef]
- Becher, P.F.; Waters, S.B.; Westmoreland, C.G.; Riester, L. Compositional Effects on the Properties of Si-Al-RE-Based Oxynitride Glasses (RE = La, Nd, Gd, Y, or Lu). J. Am. Ceram. Soc. 2002, 85, 897–902. [Google Scholar] [CrossRef]
- Saddeek, Y.B. Study of elastic moduli of lithium borobismuthate glasses using ultrasonic technique. J. Non-Cryst. Solids 2011, 357, 2920–2925. [Google Scholar] [CrossRef]
- Coon, D.N.; Weidner, J.R. Elastic-Moduli of Y-Al-Si-O-N Glasses. J. Non-Cryst. Solids 1990, 116, 201–205. [Google Scholar] [CrossRef]
- Rouxel, T. Elastic properties and short-to medium-range order in glasses. J. Am. Ceram. Soc. 2007, 90, 3019–3039. [Google Scholar] [CrossRef]
- Sellappan, P.; Sharafat, A.; Keryvin, V.; Houizot, P.; Rouxel, T.; Grins, J.; Esmaeilzadeh, S. Elastic properties and surface damage resistance of nitrogen-rich (Ca,Sr)-Si-O-N glasses. J. Non-Cryst. Solids 2010, 356, 2120–2126. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Rzoska, S.J.; Bockowski, M.; Mauro, J.C. Mixed alkaline earth effect in the compressibility of aluminosilicate glasses. J. Chem. Phys. 2014, 140, 054511. [Google Scholar] [CrossRef]
- Grund Bäck, L.; Ali, S.; Karlsson, S.; Möncke, D.; Kamitsos, E.I.; Jonson, B. Mixed alkali/alkaline earth-silicate glasses: Physical properties and structure by vibrational spectroscopy. Int. J. Appl. Glass Sci. 2019, 10, 349–362. [Google Scholar] [CrossRef]
- Lv, P.; Wang, C.; Stevensson, B.; Yu, Y.; Wang, T.; Edén, M. Impact of the cation field strength on physical properties and structures of alkali and alkaline-earth borosilicate glasses. Ceram. Int. 2022, 48, 18094–18107. [Google Scholar] [CrossRef]
- Wang, H.; Hou, X.; Zhang, Y.; Zhao, D.; Li, S.; Huang, W.; Zhou, Y. The influence of the mixed alkaline earth effect on the structure and properties of (Ca, Mg)–Si–Al–O–N glasses. Ceram. Int. 2021, 47, 12276–12283. [Google Scholar] [CrossRef]
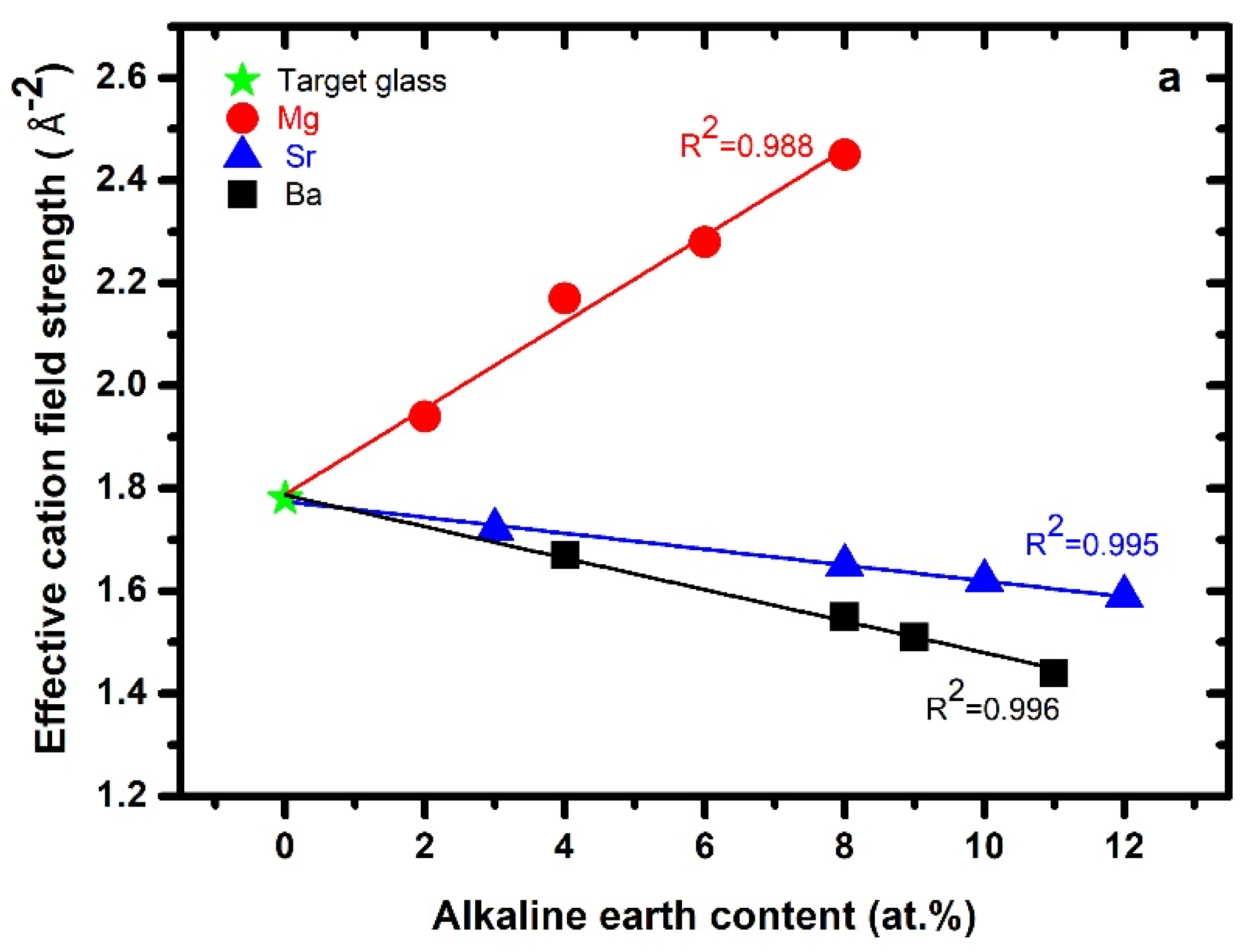
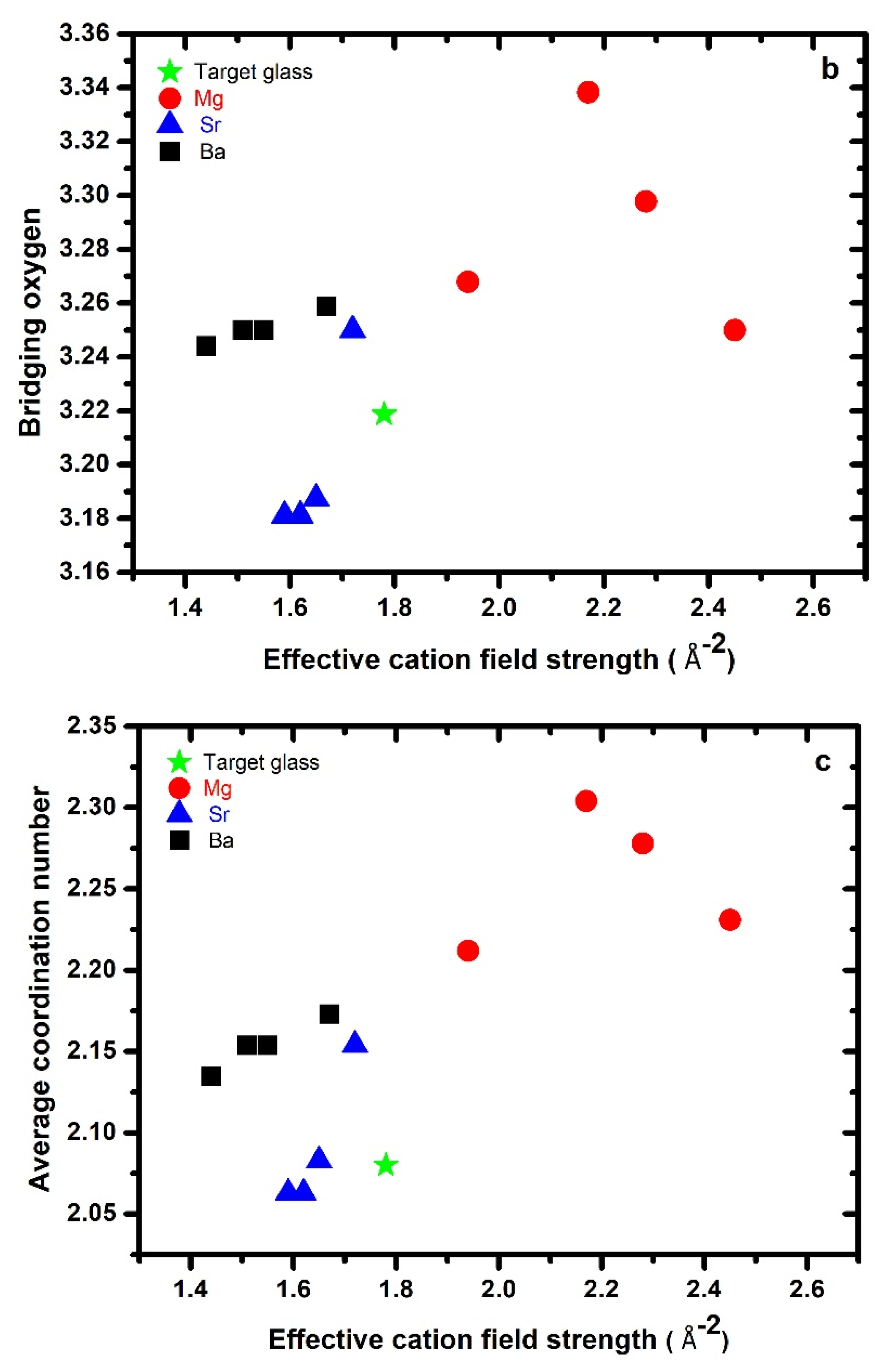
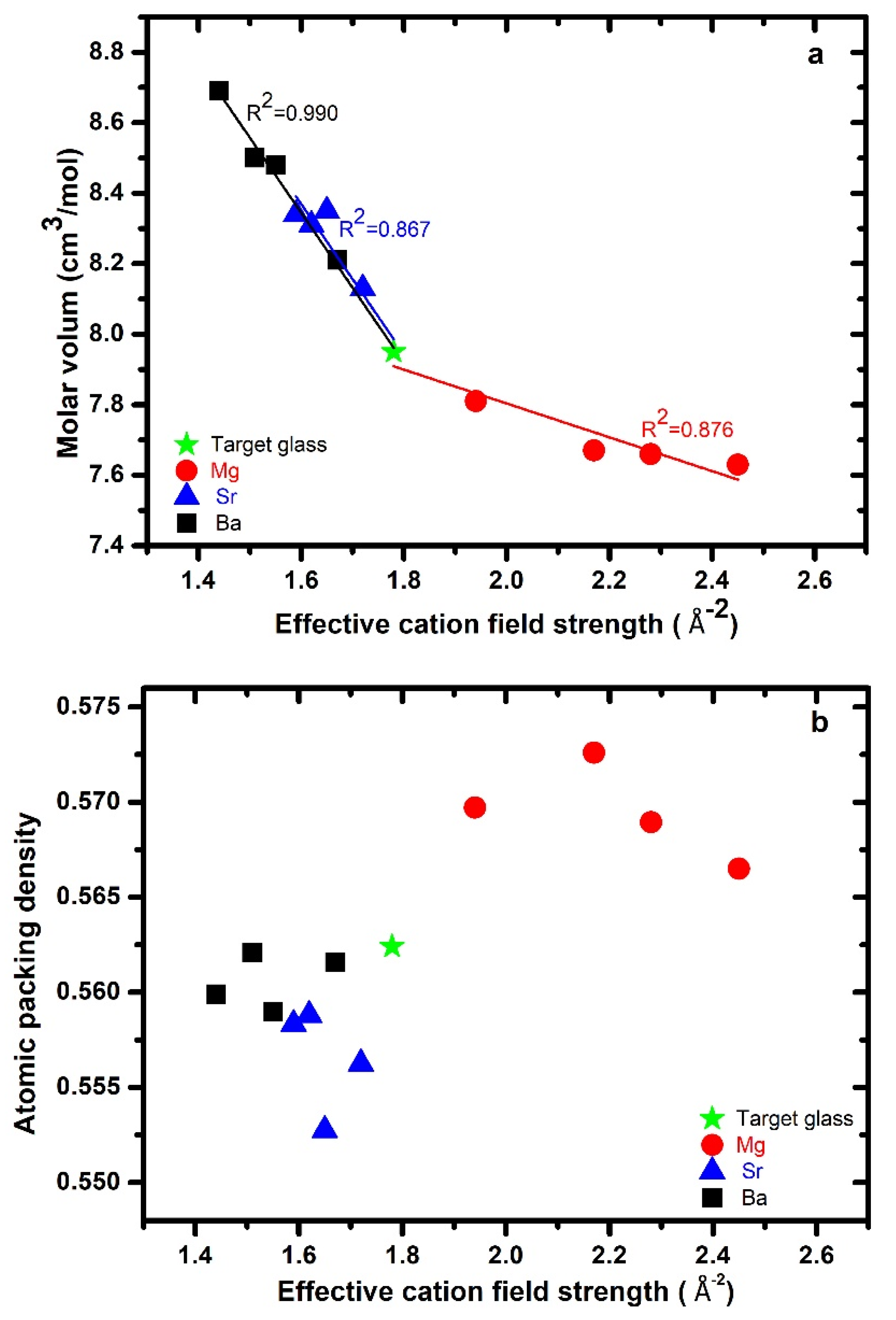
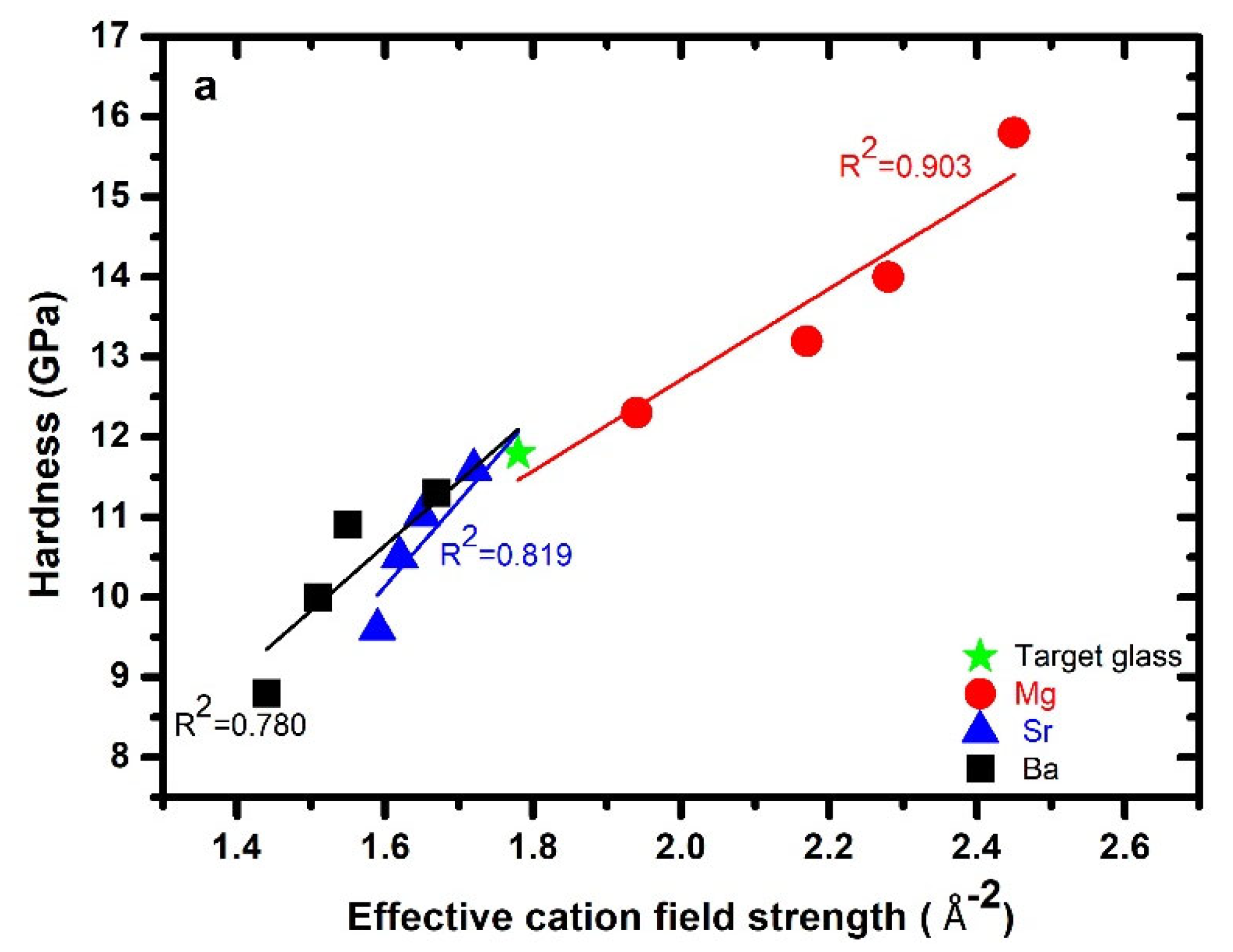



| ID | * Glass Composition (atomic %) | ECFS Å−2 | # ρ g/cm3 | # Mv cm3/mol | Cg | nBO | |
|---|---|---|---|---|---|---|---|
| Target glass | Ca25Si19O40N16 | 1.78 | 3.02 | 7.95 | 0.562 | 3.22 | 2.080 |
| Series-Mg, Mgx–Ca(25−x)–Si19 O40N16 | |||||||
| Mg-1 | Mg2Ca22Si20O37N19 | 1.94 | 3.02 | 7.81 | 0.570 | 3.27 | 2.212 |
| Mg-2 | Mg4Ca18Si21O38N19 | 2.17 | 3.00 | 7.67 | 0.573 | 3.34 | 2.304 |
| Mg-3 | Mg6Ca17Si21O37N19 | 2.28 | 2.98 | 7.66 | 0.569 | 3.30 | 2.278 |
| Mg-4 | Mg8Ca16Si21O37N18 | 2.45 | 2.96 | 7.63 | 0.567 | 3.25 | 2.231 |
| Series-Sr, Srx–Ca(25−x)–Si19 O40N16 | |||||||
| Sr-1 | Sr3Ca21Si20O40N16 | 1.72 | 3.14 | 8.13 | 0.556 | 3.25 | 2.154 |
| Sr-2 | Sr8Ca18Si19O37N18 | 1.65 | 3.35 | 8.35 | 0.553 | 3.19 | 2.083 |
| Sr-3 | Sr10Ca16Si19O38N17 | 1.62 | 3.49 | 8.31 | 0.559 | 3.18 | 2.063 |
| Sr-4 | Sr12Ca14Si19O38N17 | 1.59 | 3.55 | 8.34 | 0.559 | 3.18 | 2.063 |
| Series-Ba, Bax–Ca(25−x)–Si19 O40N16 | |||||||
| Ba-1 | Ba4Ca20Si20O39N17 | 1.67 | 3.35 | 8.21 | 0.562 | 3.26 | 2.173 |
| Ba-2 | Ba8Ca16Si20O40N16 | 1.55 | 3.67 | 8.48 | 0.559 | 3.25 | 2.154 |
| Ba-3 | Ba9Ca15Si20O40N16 | 1.51 | 3.84 | 8.50 | 0.562 | 3.25 | 2.154 |
| Ba-4 | Ba11Ca13Si20O41N15 | 1.44 | 3.99 | 8.69 | 0.560 | 3.24 | 2.135 |
| ID | # Hv GPa | H GPa | Er GPa | E GPa | G GPa | K GPa | υ |
|---|---|---|---|---|---|---|---|
| Target glass | 9.41 | 11.8 | 120 | 119 | 44.5 | 86 | 0.337 |
| Series-Mg, Mgx–Ca(25−x)–Si19 O40N16 | |||||||
| Mg-1 | 10.48 | 12.3 | 130 | 127 | 48.7 | 94 | 0.304 |
| Mg-2 | 11.33 | 13.2 | 141 | 135 | 52.5 | 98 | 0.286 |
| Mg-3 | 12.01 | 14 | 145 | 141 | 55.8 | 110 | 0.263 |
| Mg-4 | 12.16 | 15.8 | 153 | 146 | 59.2 | 117 | 0.233 |
| Series-Sr, Srx–Ca(25−x)–Si19 O40N16 | |||||||
| Sr-1 | 9.42 | 11.6 | 120 | 118 | 44.1 | 82 | 0.338 |
| Sr-2 | 9.47 | 11.02 | 119 | 117 | 43.5 | 81 | 0.345 |
| Sr-3 | 9.45 | 10.5 | 117 | 112 | 41.0 | 77 | 0.366 |
| Sr-4 | 9.30 | 9.6 | 114 | 109 | 40.9 | 82 | 0.332 |
| Series-Ba, Bax–Ca(25−x)–Si19 O40N16 | |||||||
| Ba-1 | 9.47 | 11.3 | 118 | 117 | 45.0 | 86 | 0.300 |
| Ba-2 | 9.31 | 10.9 | 115 | 112 | 43.0 | 81 | 0.302 |
| Ba-3 | 8.92 | 10.0 | 108 | 106 | 42.0 | 79 | 0.261 |
| Ba-4 | 8.29 | 8.8 | 106 | 102 | 39.0 | 73 | 0.308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S. Elastic Properties and Hardness of Mixed Alkaline Earth Silicate Oxynitride Glasses. Materials 2022, 15, 5022. https://doi.org/10.3390/ma15145022
Ali S. Elastic Properties and Hardness of Mixed Alkaline Earth Silicate Oxynitride Glasses. Materials. 2022; 15(14):5022. https://doi.org/10.3390/ma15145022
Chicago/Turabian StyleAli, Sharafat. 2022. "Elastic Properties and Hardness of Mixed Alkaline Earth Silicate Oxynitride Glasses" Materials 15, no. 14: 5022. https://doi.org/10.3390/ma15145022
APA StyleAli, S. (2022). Elastic Properties and Hardness of Mixed Alkaline Earth Silicate Oxynitride Glasses. Materials, 15(14), 5022. https://doi.org/10.3390/ma15145022






