Nonconventional 1,8-Diazafluoren-9-One Aggregates for Green Light Enhancement in Hybrid Biocompatible Media
Abstract
1. Introduction
2. Materials and Methods
- 1,8-diazafluoren-9-one (Sigma-Aldrich, Munich, Germany), and it was spectroscopically pure (dye content 99%).
- Titanium(IV) tetra(2-propanolate)-99.000% trace metals basis, poly(ethylene glycol) p-(1,1,3,3-tetramethylbutyl)-phenyl ether (Triton X-100), hydrochloric acid, propan-2-ol, and pentane-2,4-dione (Sigma-Aldrich, Munich, Germany).
- Ethanol (POCH Company, Gliwice, Poland). DFO/TiO2 thin films were obtained using the sol–gel method.
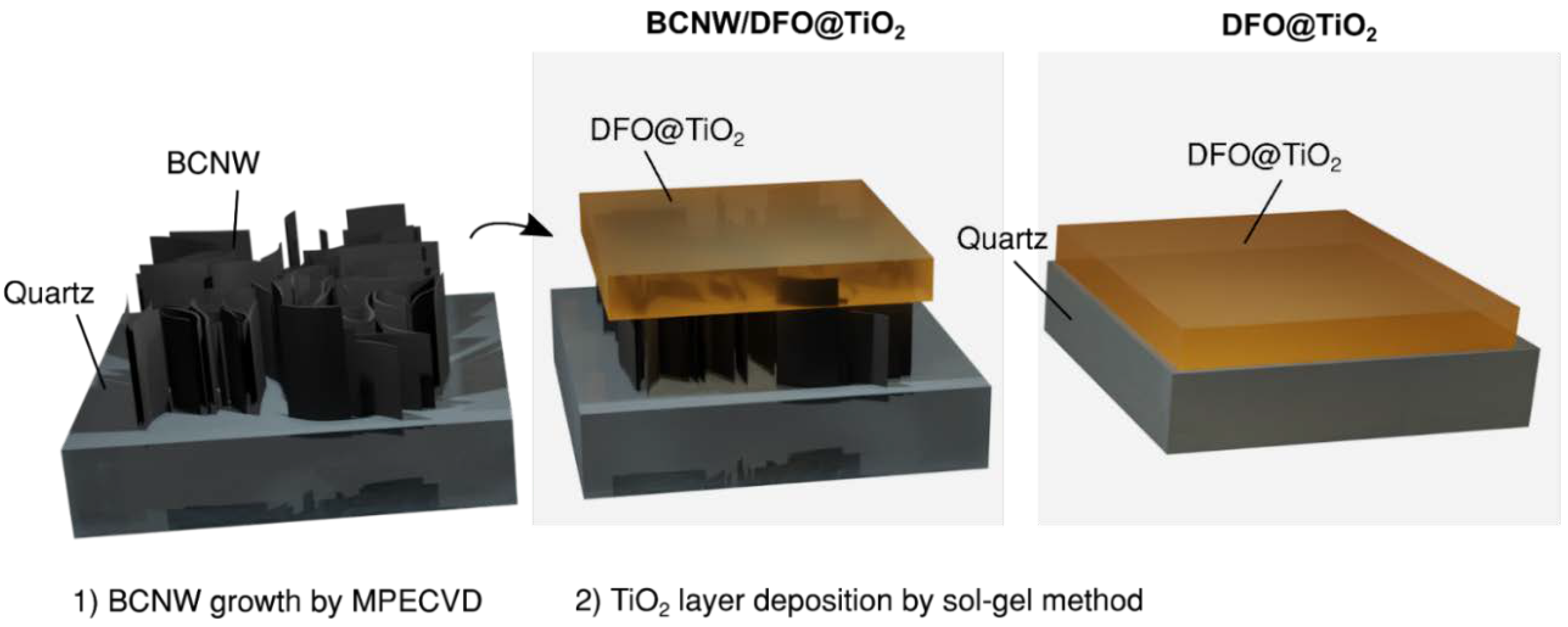
2.1. Growth of BCNW
2.2. DFO/TiO2 Sol–Gel Synthesis
2.3. Apparatus
2.4. Quantum Chemistry Calculations Details
3. Results
3.1. The Structural Profile of Thin Films
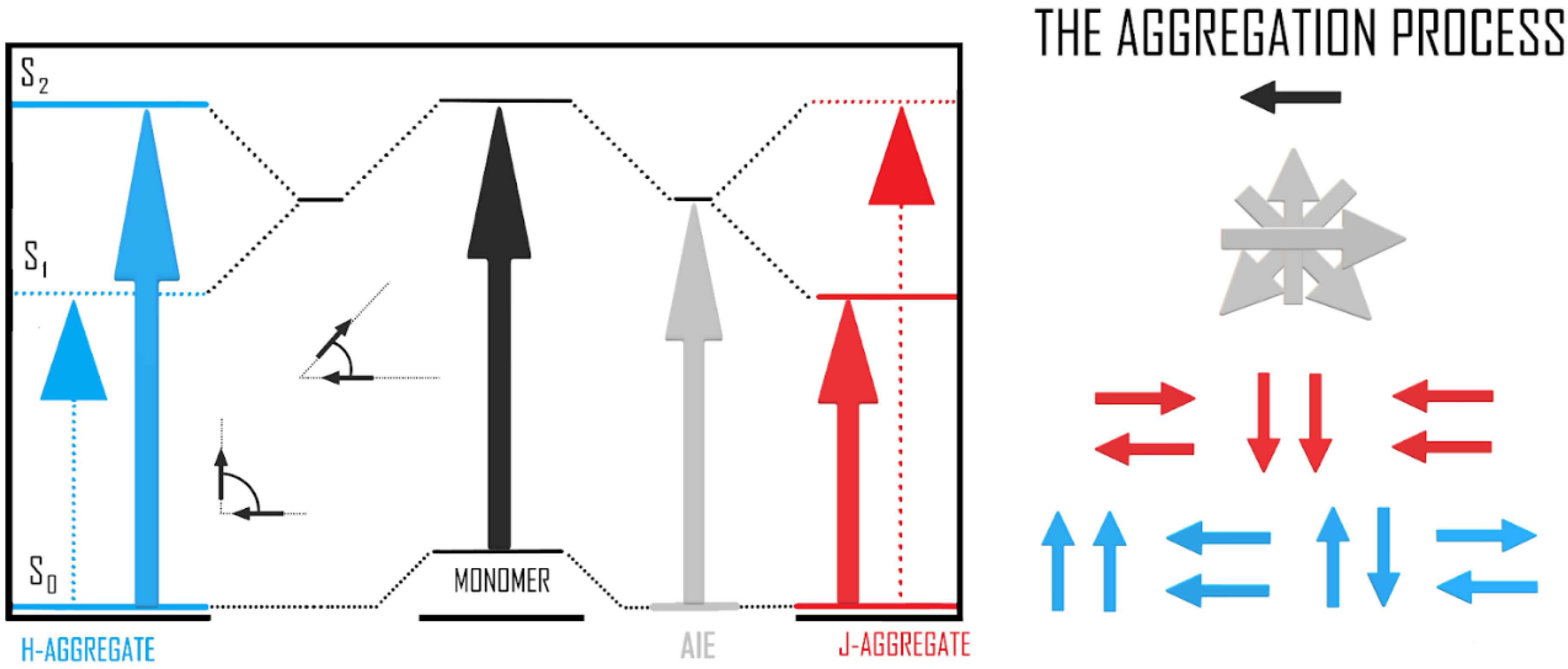
3.2. The Spectroscopic Nature of Thin Films
3.3. The Surface Energy Properties—Liquid/Solid Wettability Energetics
3.4. Quantum Chemistry Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, M.M.; Imai, H.; Hirashima, H. Preparation of porous titania film by modified sol-gel method and its application to photocatalyst. J. Sol-Gel Sci. Technol. 2002, 25, 65–74. [Google Scholar] [CrossRef]
- Xagas, A.P.; Androulaki, E.; Hiskia, A.; Falaras, P. Preparation, fractal surface morphology and photocatalytic properties of TiO2 films. Thin Solid Films 1999, 357, 173–178. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Cheng, B.; Zhao, X.; Zheng, Z.; Li, A.S.K. Atomic force microscopic studies of porous TiO2 thin films prepared by the sol-gel method. J. Sol-Gel Sci. Technol. 2002, 24, 229–240. [Google Scholar] [CrossRef]
- Fabes, B.D.; Birnie, D.P., III; Zelinski, B.J.J. Porosity and composition effects in sol-gel derived interference filters. Thin Solid Films 1995, 254, 175–180. [Google Scholar] [CrossRef]
- Carbonaro, C.M. Tuning the formation of aggregates in silica–Rhodamine 6G hybrids by thermal treatment. J. Photochem. Photobiol. A Chem. 2011, 222, 56–63. [Google Scholar] [CrossRef]
- Vogel, R.; Meredith, P.; Kartini, I.; Harvey, M.; Riches, J.D.; Bishop, A.; Heckenberg, N.; Trau, M.; Rubinsztein-Dunlop, H. Absorption and fluorescence spectroscopy of rhodamine 6G in titanium dioxide nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 245–249. [Google Scholar] [CrossRef]
- Vogel, R.; Meredith, P.; Kartini, I.; Harvey, M.; Riches, J.D.; Bishop, A.; Heckenberg, N.; Trau, M.; Rubinsztein-Dunlop, H. Mesostructured Dye-Doped Titanium Dioxide for Micro-Optoelectronic Applications. ChemPhysChem 2003, 4, 595–603. [Google Scholar] [CrossRef]
- Jelley, E.E. Spectral absorption and fluorescence of dyes in the molecular state. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Bojarski, P.; Matczuk, A.; Bojarski, C.; Kawski, A.; Kukliński, B.; Zurkowska, G.; Diehl, H. Fluorescent dimers of rhodamine 6G in concentrated ethylene glycol solution. Chem. Phys. 1996, 210, 485–499. [Google Scholar] [CrossRef]
- Gruszecki, W.I. Structural characterization of the aggregated forms of violaxanthin. J. Biol. Phys. 1991, 18, 99–109. [Google Scholar] [CrossRef]
- Inglot, K.; Martyński, T.; Bauman, D. Molecular organization and aggregation in Langmuir and Langmuir-Blodgett films of azo dye/liquid crystal mixtures. Opto-Electron. Rev. 2009, 17, 120–128. [Google Scholar] [CrossRef]
- McRae, E.G.; Kasha, M. Enhancement of phosphorescence ability upon aggregation of dye molecules. J. Chem. Phys. 1958, 28, 721–722. [Google Scholar] [CrossRef]
- Rohatgi, K.K. Absorption spectra of the dimers of ionic dyes. J. Mol. Spectrosc. 1968, 27, 545–548. [Google Scholar] [CrossRef]
- Monahan, A.R.; Blossey, D.F. Aggregation of arylazonaphthols. I. Dimerization of Bonadur Red in aqueous and methanolic systems. J. Phys. Chem. 1970, 74, 4014–4021. [Google Scholar] [CrossRef]
- Somsen, O.J.; van Grondelle, R.; van Amerongen, H. Spectral broadening of interacting pigments: Polarized absorption by photosynthetic proteins. Biophys. J. 1996, 71, 1934–1951. [Google Scholar] [CrossRef][Green Version]
- Kunzler, J.; Samha, L.; Zhang, R.; Samha, H. Investigation of the effect of concentration on the molecular aggregation of cyanine dyes in aqueous solution. Am. J. Undergrad. Res. 2011, 9, 1–4. [Google Scholar] [CrossRef]
- Antonov, L.; Gergov, G.; Petrov, V.; Kubista, M.; Nygren, J. UV–Vis spectroscopic and chemometric study on the aggregation of ionic dyes in water. Talanta 1999, 49, 99–106. [Google Scholar] [CrossRef]
- Bojarski, P. Concentration quenching and depolarization of rhodamine 6G in the presence of fluorescent dimers in polyvinyl alcohol films. Chem. Phys. Lett. 1997, 278, 225–232. [Google Scholar] [CrossRef]
- Tyurin, O.V.; Bercov, Y.M.; Zhukov, S.O.; Levitskaya, T.F.; Gevelyuk, S.A.; Doycho, I.K.; Rysiakiewicz-Pasek, E. Aggregation of dyes in the porous glass. Opt. Appl. 2010, 40, 311–321. [Google Scholar]
- Lewkowicz, A.; Bogdanowicz, R.; Bojarski, P.; Pierpaoli, M.; Gryczyński, I.; Synak, A.; Mońka, M.; Karczewski, J.; Struck-Lewicka, W.; Wawrzyniak, R.; et al. The luminescence of 1,8-diazafluoren-9-one/titanium dioxide composite thin films for optical application. Materials 2020, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Zygadło, P.; Lewkowicz, A. Structural-spectroscopic analysis of DFO/PVA films as potential materials used in revealing fingerprints on non-porous surfaces. Issue Forensic Sci. 2021, 312, 32–40. [Google Scholar] [CrossRef]
- Lewkowicz, A.; Kantor, M.; Zalewski, W.; Bojarski, P.; Mońka, M. Spectroscopic evidence of fluorescence by 1,8-diazafluoren-9-one aggregates—A prospective new ultrasensitive method for fingerprint trace detection. J. Forensic Sci. 2022, 67, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Strobel, M.; Lyons, C.S. An essay on contact angle measurements. Plasma Processes Polym. 2011, 8, 8–13. [Google Scholar] [CrossRef]
- Chibowski, E. Surface free energy of a solid from contact angle hysteresis. Adv. Colloid Interface Sci. 2003, 103, 149–172. [Google Scholar] [CrossRef]
- Niedziałkowski, P.; Ossowski, T.; Zięba, P.; Cirocka, A.; Rochowski, P.; Pogorzelski, S.J.; Ryl, J.; Sobaszek, M.; Bogdanowicz, R. Poly-l-lysine-modified boron-doped diamond electrodes for the amperometric detection of nucleic acid bases. J. Electroanal. Chem. 2015, 756, 84–93. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Ficek, M.; Jakóbczyk, P.; Karczewski, J.; Bogdanowicz, R. Self-assembly of vertically orientated graphene nanostructures: Multivariate characterization by Minkowski functionals and fractal geometry. Acta Mater. 2021, 214, 116989. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Ficek, M.; Rycewicz, M.; Sawczak, M.; Karczewski, J.; Ruello, M.L.; Bogdanowicz, R. Tailoring electro/optical properties of transparent boron-doped carbon nanowalls grown on quartz. Materials 2019, 12, 547. [Google Scholar] [CrossRef]
- Kubicki, A.A.; Bojarski, P.; Grinberg, M.; Sadownik, M.; Kukliński, B. Time-resolved streak camera system with solid-state laser and optical parametric generator in different spectroscopic applications. Opt. Commun. 2006, 263, 275–280. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Berezowski, Z.; Rochowski, P.; Szurkowski, S. A novel methodology based on contact angle hysteresis approach for surface changes monitoring in model PMMA-Corega Tabs system. Appl. Surf. Sci. 2012, 258, 3652–3658. [Google Scholar] [CrossRef]
- Extrand, C.W.; Kumagai, Y. Liquid Drops on an Inclined Plane: The Relation between Contact Angles, Drop Shape, and Retentive Force. J. Colloid Interface Sci. 1995, 170, 515–521. [Google Scholar] [CrossRef]
- Mazurek, A.Z.; Pogorzelski, S.J.; Boniewicz-Szmyt, K. Adsorption of natural surfactants present in sea waters at surfaces of minerals: Contact angle measurements. Oceanologia 2009, 51, 377–403. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Boniewicz-Szmyt, K.; Grzegorczyk, M.; Rochowski, P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials 2022, 15, 1830. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2020, 132, 154104. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Lewkowicz, A.; Rycewicz, M.; Szczodrowski, K.; Ruello, M.L.; Bogdanowicz, R. Enhanced photocatalytic activity of transparent carbon nanowall/TiO2 heterostructures. Mater. Lett. 2020, 262, 127155. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in the biomedical field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- Sebastian, E.; Philip, A.M.; Benny, A.; Hariharan, M. Inside Cover: Null Exciton Splitting in Chromophoric Greek Cross (+) Aggregate (Angew. Chem. Int. Ed. 48/2018). Angew. Chem. Int. Ed. 2018, 57, 15696–15701. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Gast, A.P.; Adamson, A.W. Physical Chemistry of Surfaces, 6th ed.; Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Rodrıguez-Valverde, M.A.; Cabrerizo-Vılchez, M.A.; Rosales-Lopez, P.; Paez-Duenas, A.; Hidalgo-Alvarez, R. Contact angle measurements on two (wood and stone) non-ideal surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 485–495. [Google Scholar] [CrossRef]
- Chibowski, E. On some relations between advancing, receding and Young’0027s contact angles. Adv. Colloid Interface Sci. 2007, 133, 51–59. [Google Scholar] [CrossRef] [PubMed]

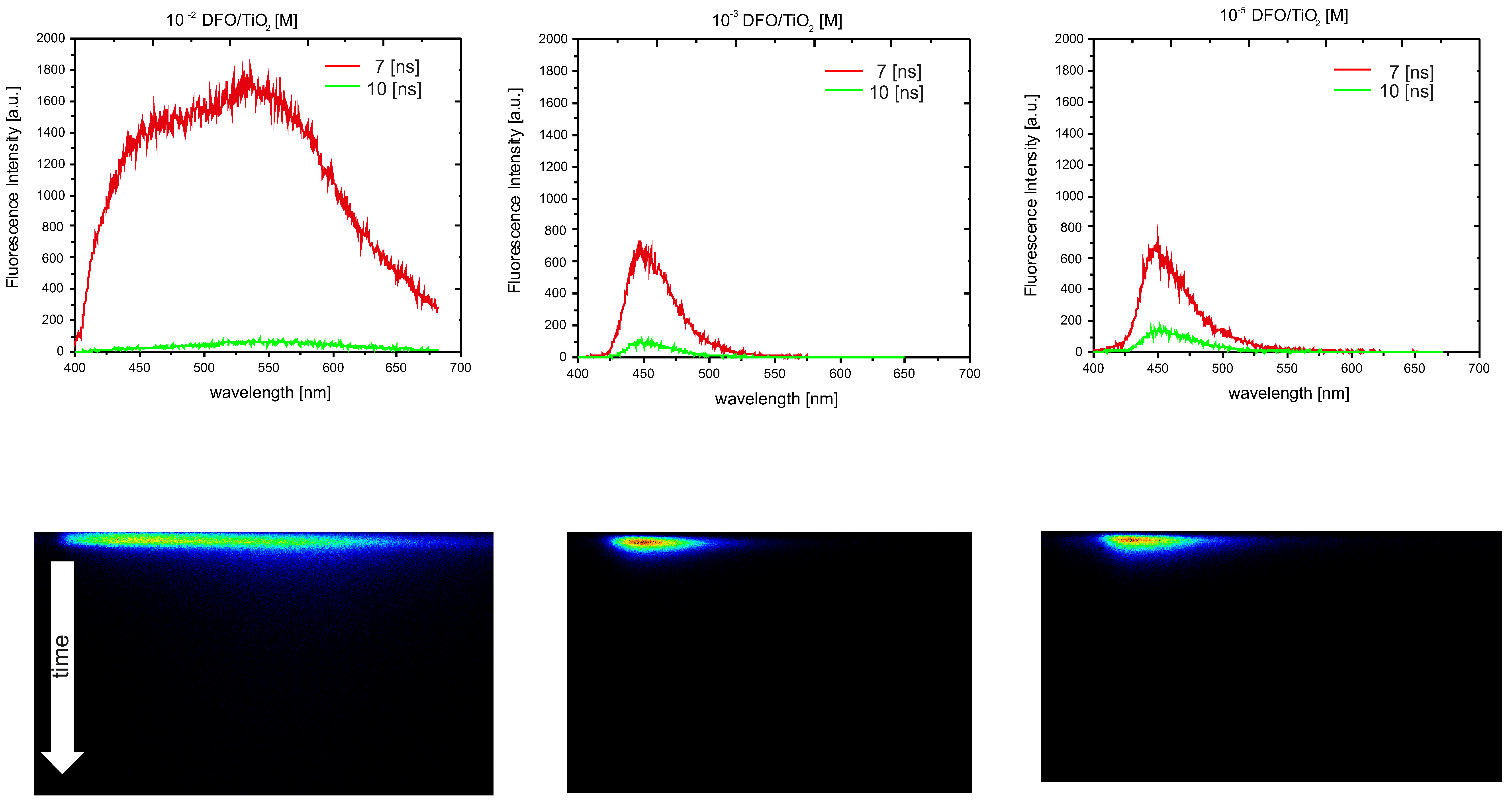
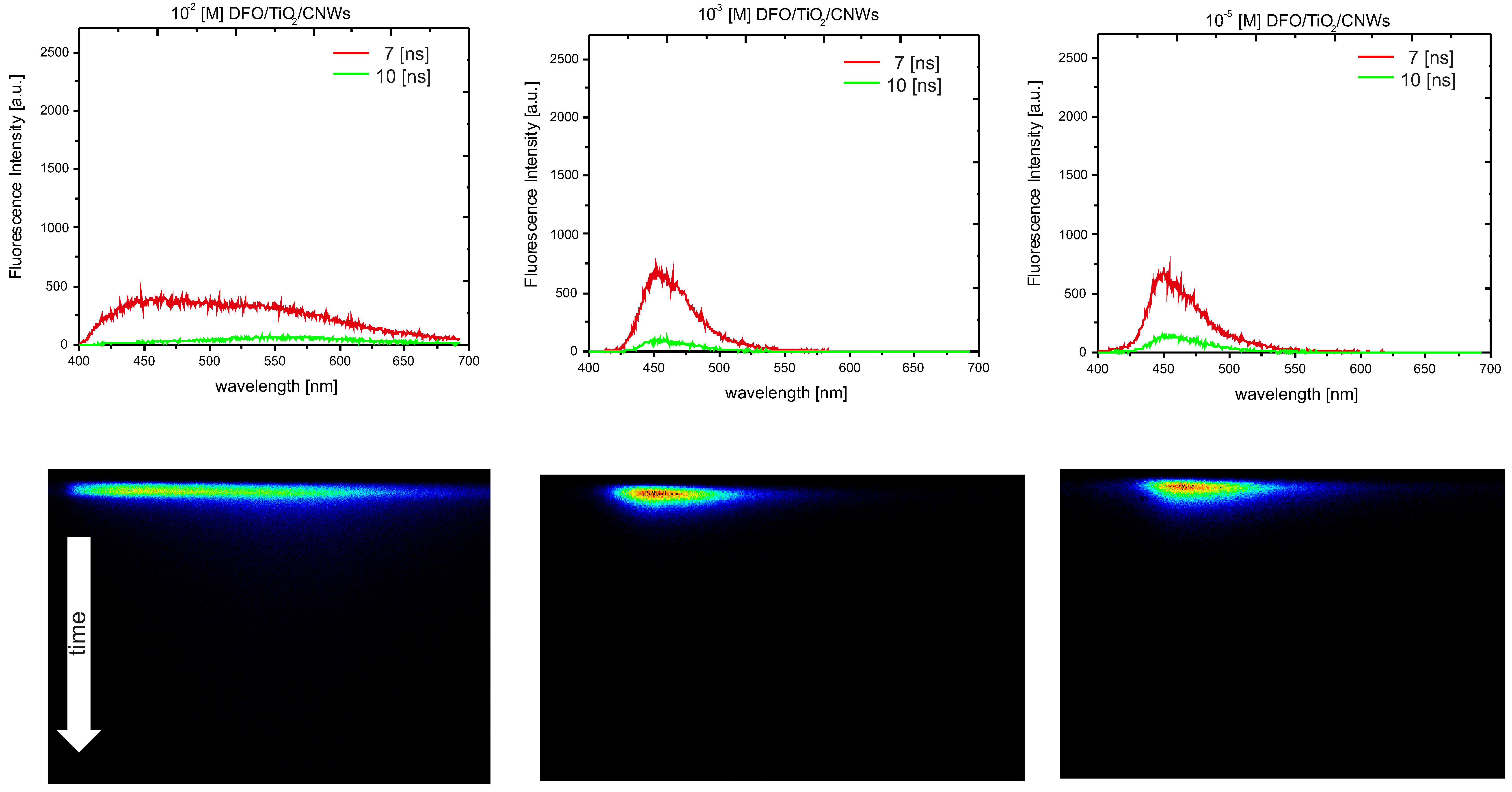



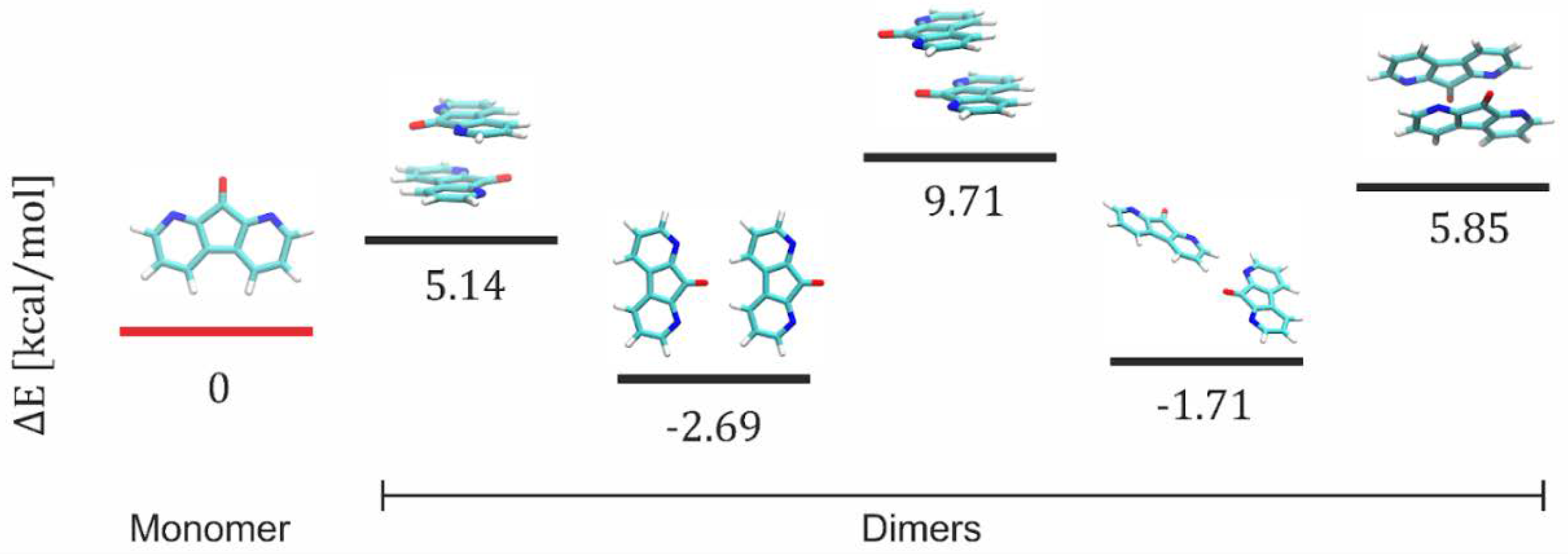
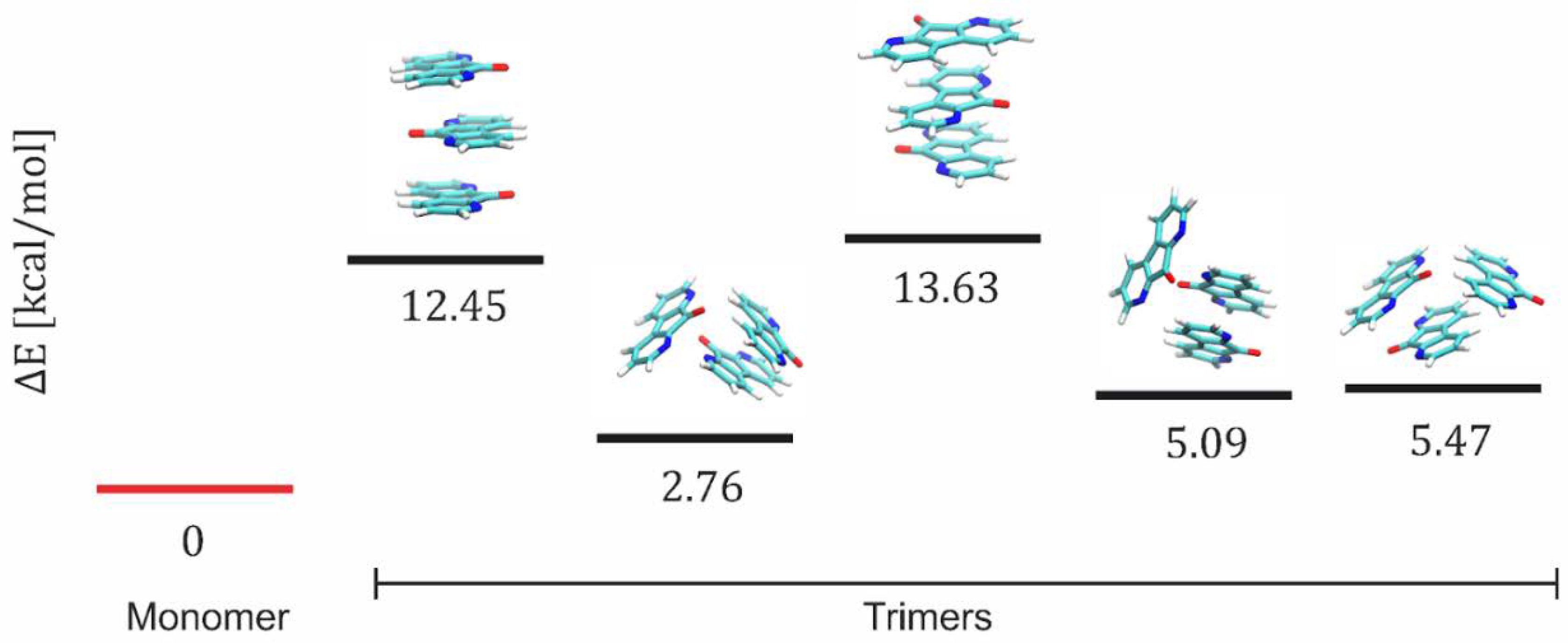
| c [M] DFO/TiO2 or TiO2/CNWs | n (TiO2) | d (TiO2) [nm] | n (DFO/TiO2/CNW) | d (DFO/TiO2/CNW) [nm] |
|---|---|---|---|---|
| 0 [M] | 1.514 | 300 | 2.32/1.76 | 135/533 |
| 10−5 [M] | 1.592 | 386 | - */1.77 | 30/520 |
| 10−2 [M] | 1.624 | 356 | 2.58/1.75 | 90/530 |
| CDFO [mol/dm3] | θϒ [deg.] | θA [deg.] | θR [deg.] | CAH [deg.] | Π [mN/m] | ϒSV [mJ/m2] | WA [mJ/m2] | ϒSVd [mJ/m2] | ϒd/ϒSV [a.u.] |
|---|---|---|---|---|---|---|---|---|---|
| TiO2 | 37.67 | 51.89 | 14.79 | 37.10 | 25.4 | 53.10 | 117.7 | 47.58 | 0.896 |
| 10−5 DFO/TiO2 | 13.04 | 39.25 | 12.06 | 27.19 | 14.86 | 61.06 | 129.1 | 57.28 | 0.938 |
| 10−3 DFO/TiO2 | 21.01 | 28.07 | 13.49 | 14.58 | 6.6 | 66.89 | 137.0 | 64.46 | 0.964 |
| 10−2 DFO/TiO2 | 10.49 | 25.16 | 8.36 | 16.8 | 6.1 | 67.82 | 138.6 | 66.03 | 0.974 |
| TiO2/CNWs | 24.45 | 40.5 | 14.13 | 26.37 | 15.2 | 60.46 | 128.1 | 56.38 | 0.933 |
| 10−5 DFO/TiO2/CNWs | 29.31 | 46.76 | 12.92 | 33.84 | 21.1 | 56.46 | 122.6 | 51.66 | 0.915 |
| 10−3 DFO/TiO2/CNWs | 15.43 | 34.71 | 11.99 | 22.72 | 11.4 | 63.57 | 132.6 | 60.40 | 0.950 |
| 10−2 DFO/TiO2/CNWs | 21.72 | 38.95 | 10.20 | 28.75 | 15.0 | 61.13 | 129.4 | 57.49 | 0.940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewkowicz, A.; Pierpaoli, M.; Walczewska-Szewc, K.; Czarnomska, M.; Bojarski, P.; Bogdanowicz, R.; Pogorzelski, S.; Kułak, L.; Karczewski, J. Nonconventional 1,8-Diazafluoren-9-One Aggregates for Green Light Enhancement in Hybrid Biocompatible Media. Materials 2022, 15, 5012. https://doi.org/10.3390/ma15145012
Lewkowicz A, Pierpaoli M, Walczewska-Szewc K, Czarnomska M, Bojarski P, Bogdanowicz R, Pogorzelski S, Kułak L, Karczewski J. Nonconventional 1,8-Diazafluoren-9-One Aggregates for Green Light Enhancement in Hybrid Biocompatible Media. Materials. 2022; 15(14):5012. https://doi.org/10.3390/ma15145012
Chicago/Turabian StyleLewkowicz, Aneta, Mattia Pierpaoli, Katarzyna Walczewska-Szewc, Martyna Czarnomska, Piotr Bojarski, Robert Bogdanowicz, Stanisław Pogorzelski, Leszek Kułak, and Jakub Karczewski. 2022. "Nonconventional 1,8-Diazafluoren-9-One Aggregates for Green Light Enhancement in Hybrid Biocompatible Media" Materials 15, no. 14: 5012. https://doi.org/10.3390/ma15145012
APA StyleLewkowicz, A., Pierpaoli, M., Walczewska-Szewc, K., Czarnomska, M., Bojarski, P., Bogdanowicz, R., Pogorzelski, S., Kułak, L., & Karczewski, J. (2022). Nonconventional 1,8-Diazafluoren-9-One Aggregates for Green Light Enhancement in Hybrid Biocompatible Media. Materials, 15(14), 5012. https://doi.org/10.3390/ma15145012









