Photocatalytic Activity of Silicon Nanowires Decorated with PbS Nanoparticles Deposited by Pulsed Laser Deposition for Efficient Wastewater Treatment
Abstract
:1. Introduction
2. Elaboration of Silicon Nanowires Decorated with PbS Nanoparticles
2.1. Silicon Cleaning
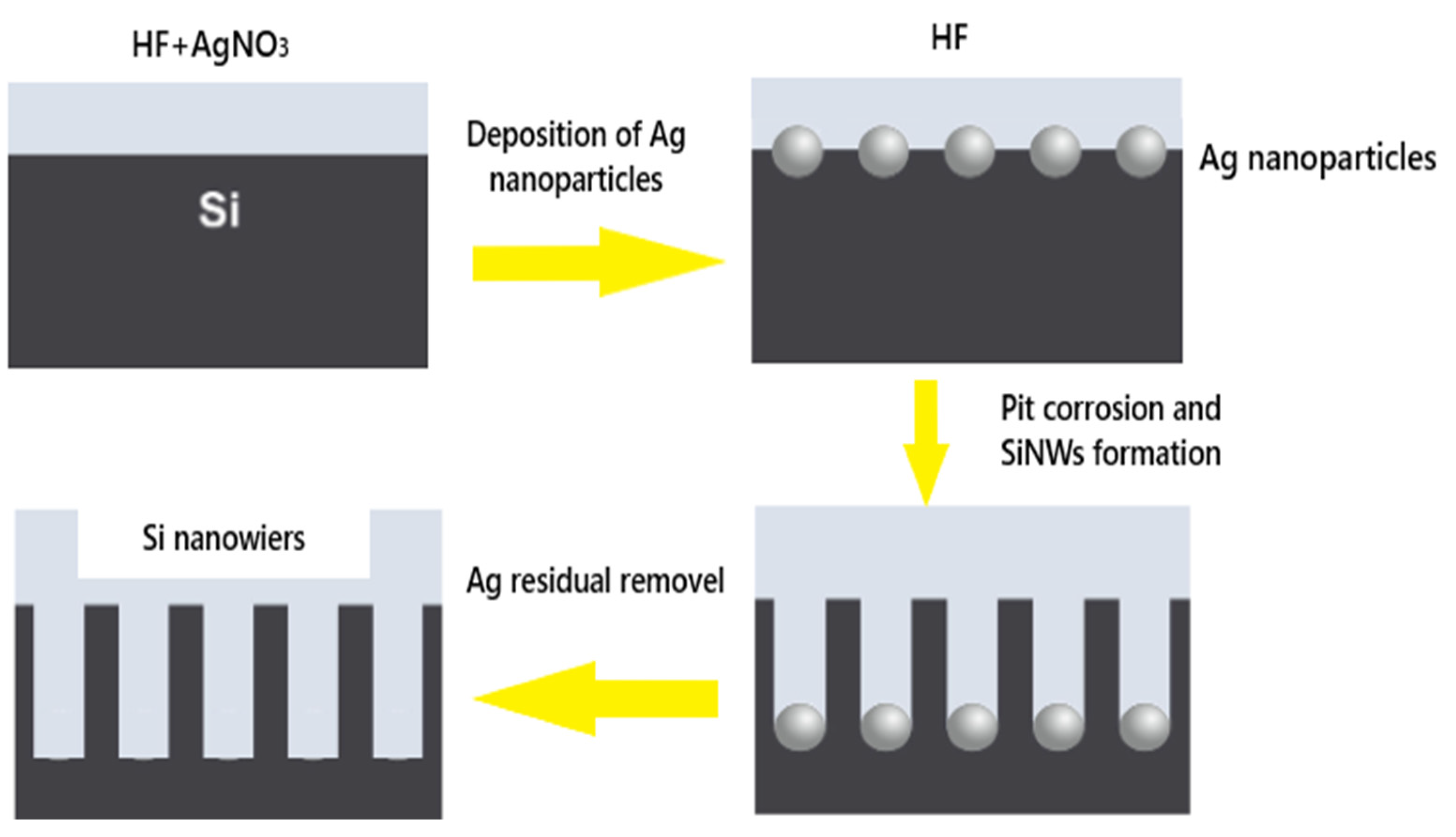
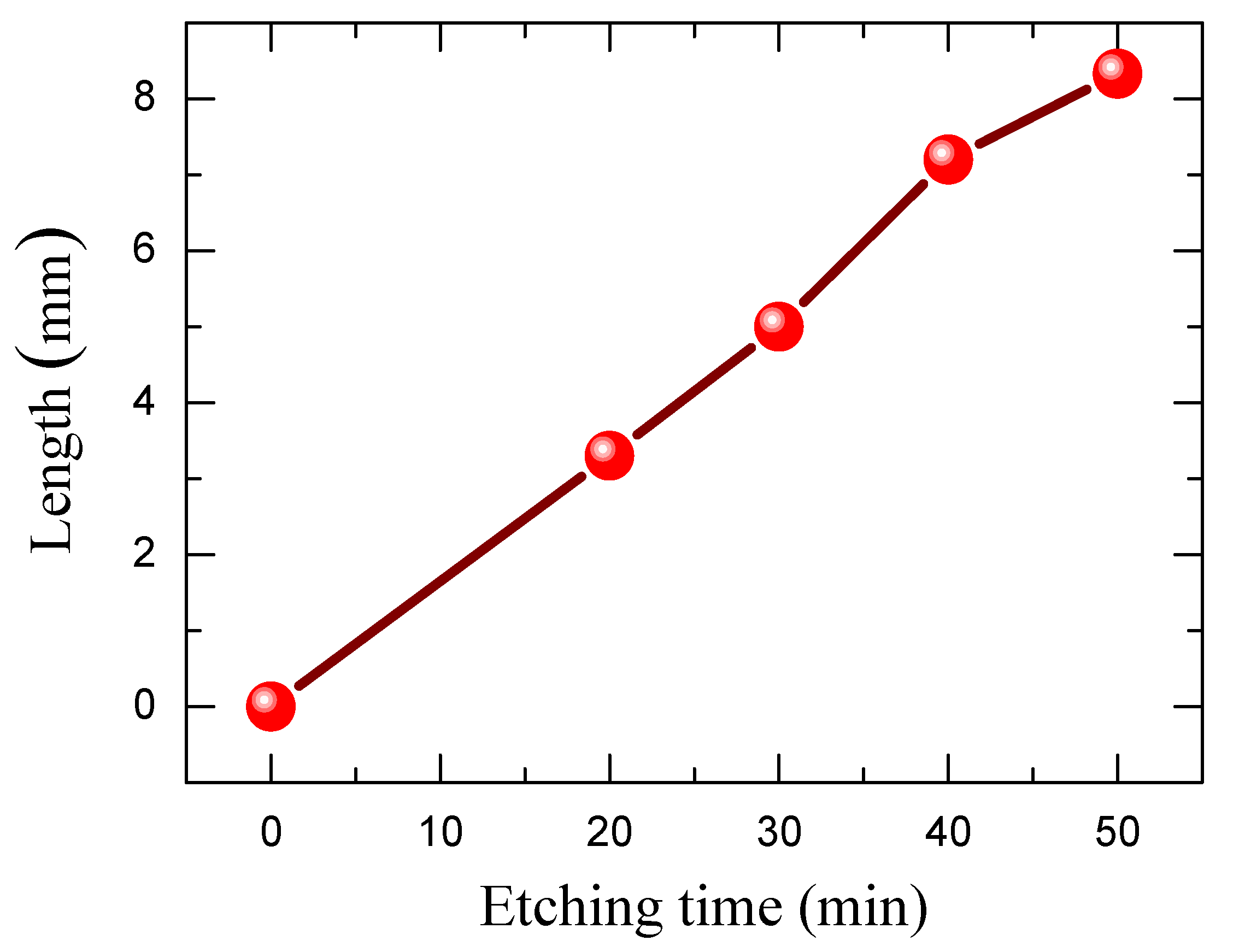
2.2. Elaboration of Silicon Nanowires
2.3. Silicon Nanowires Decorated with PbS Nanoparticles Deposited by PLD
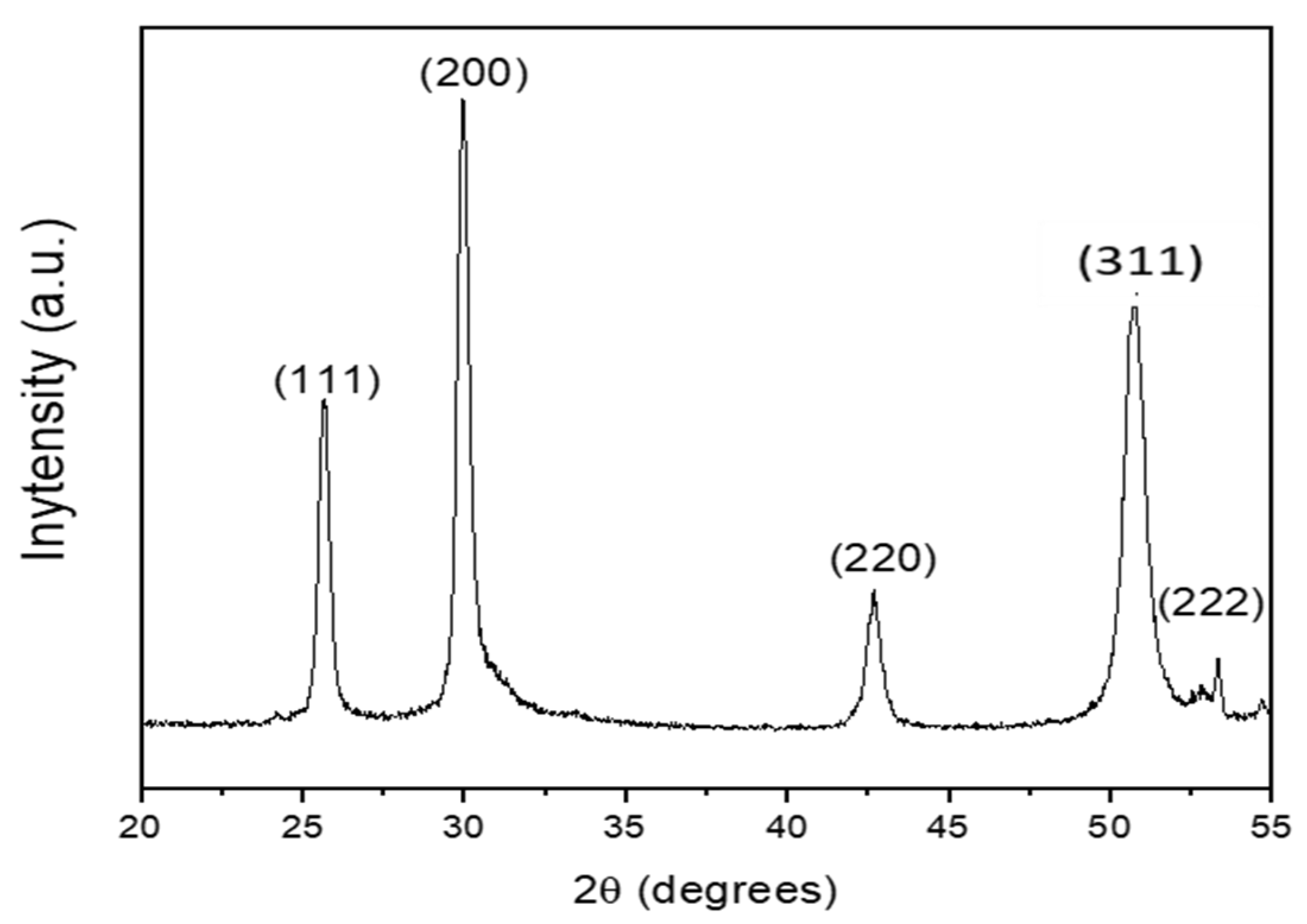
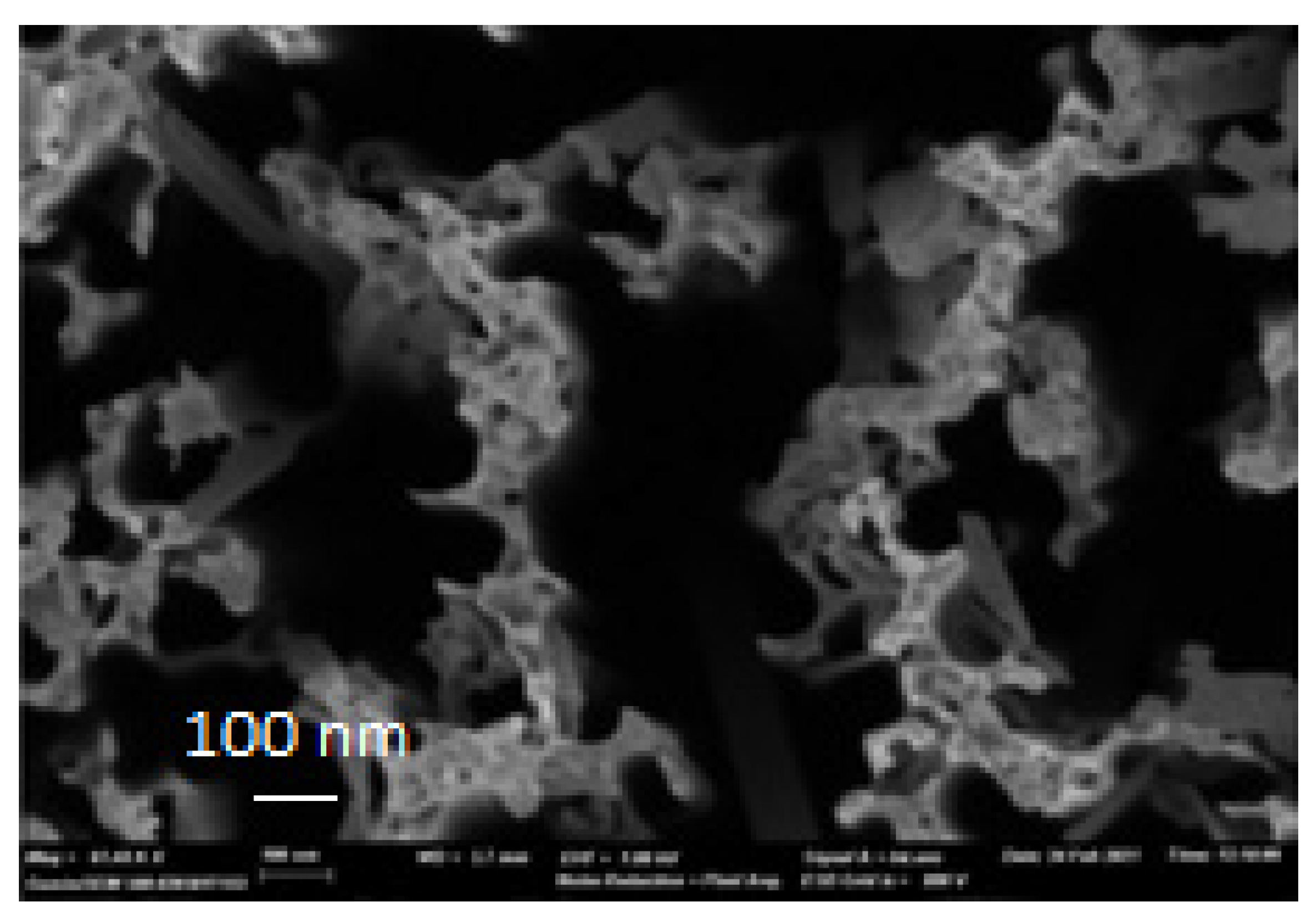
2.4. Microstructure and Morphology of PbS/SiNWs Nanohybrids
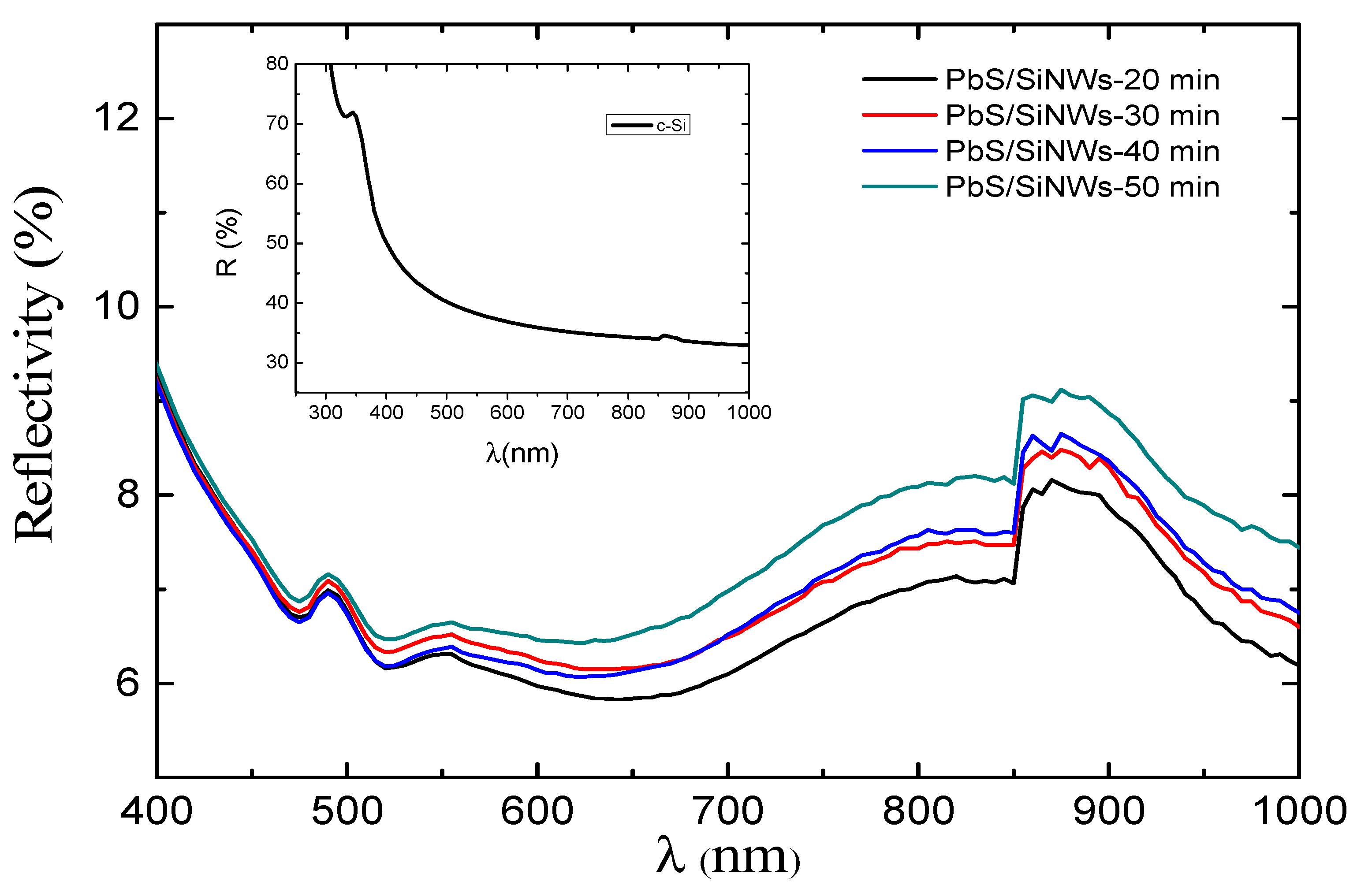
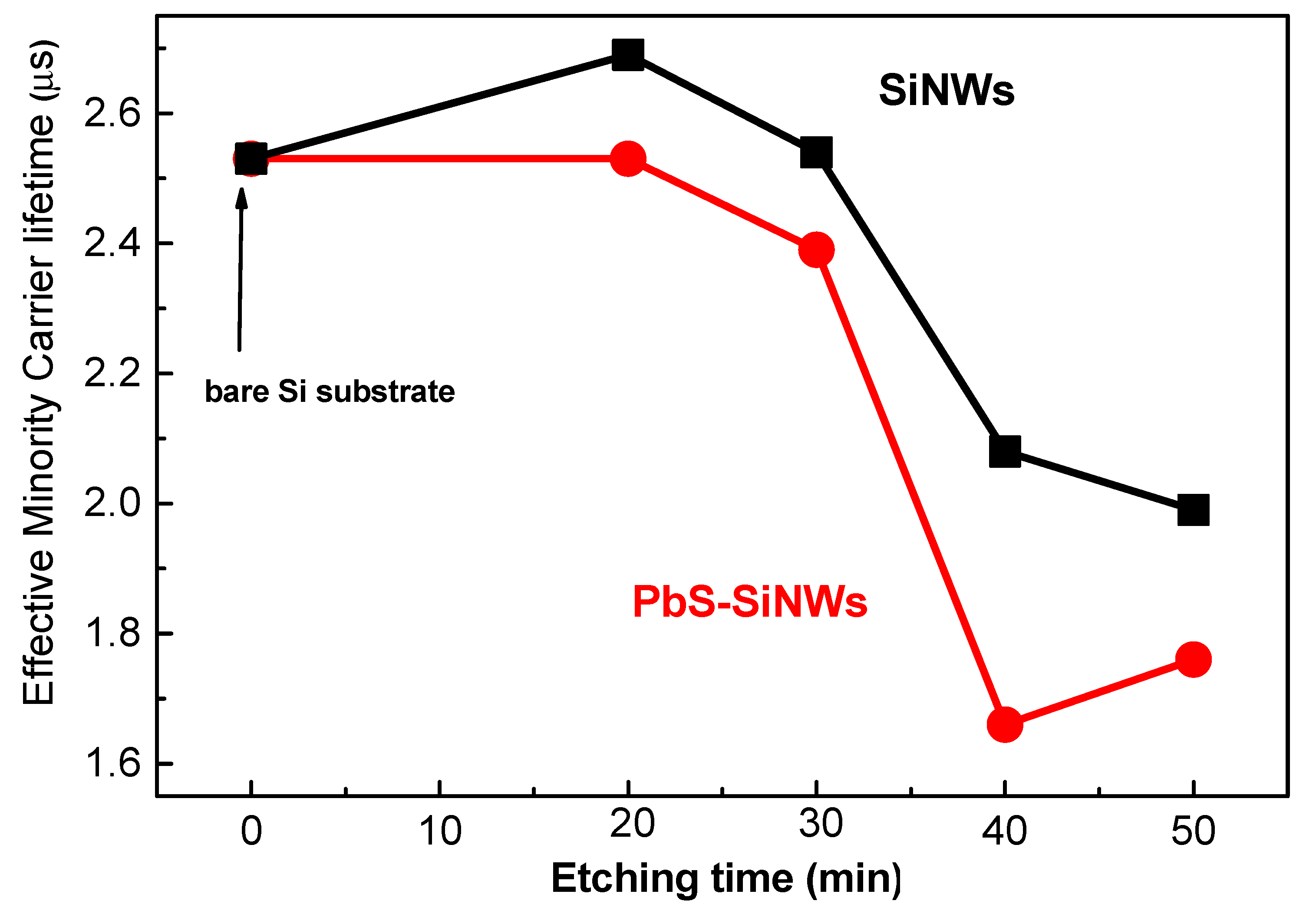
2.5. Optoelectronic and Microstructural Properties of PbS/SiNWs Nanohybrids
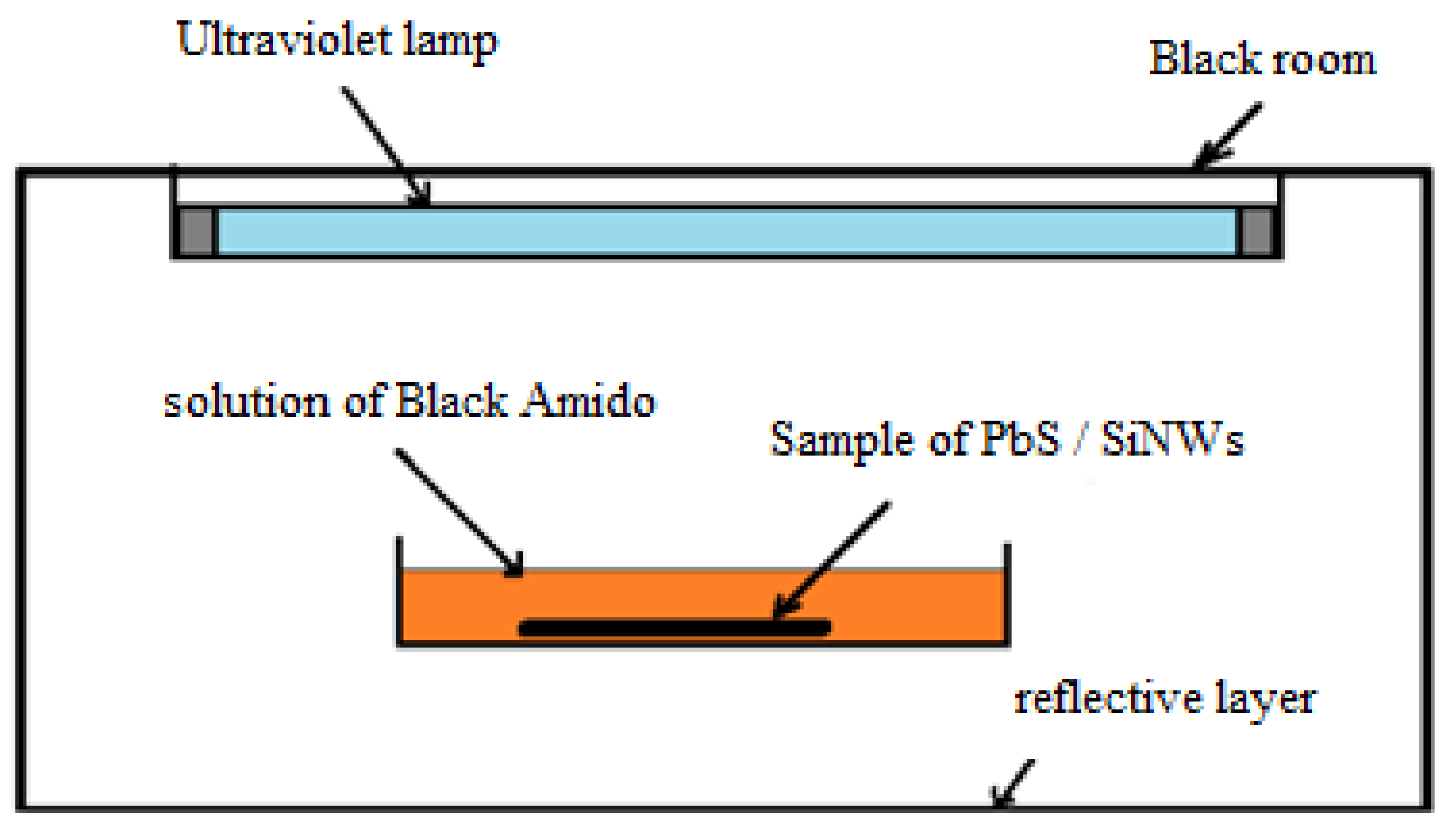
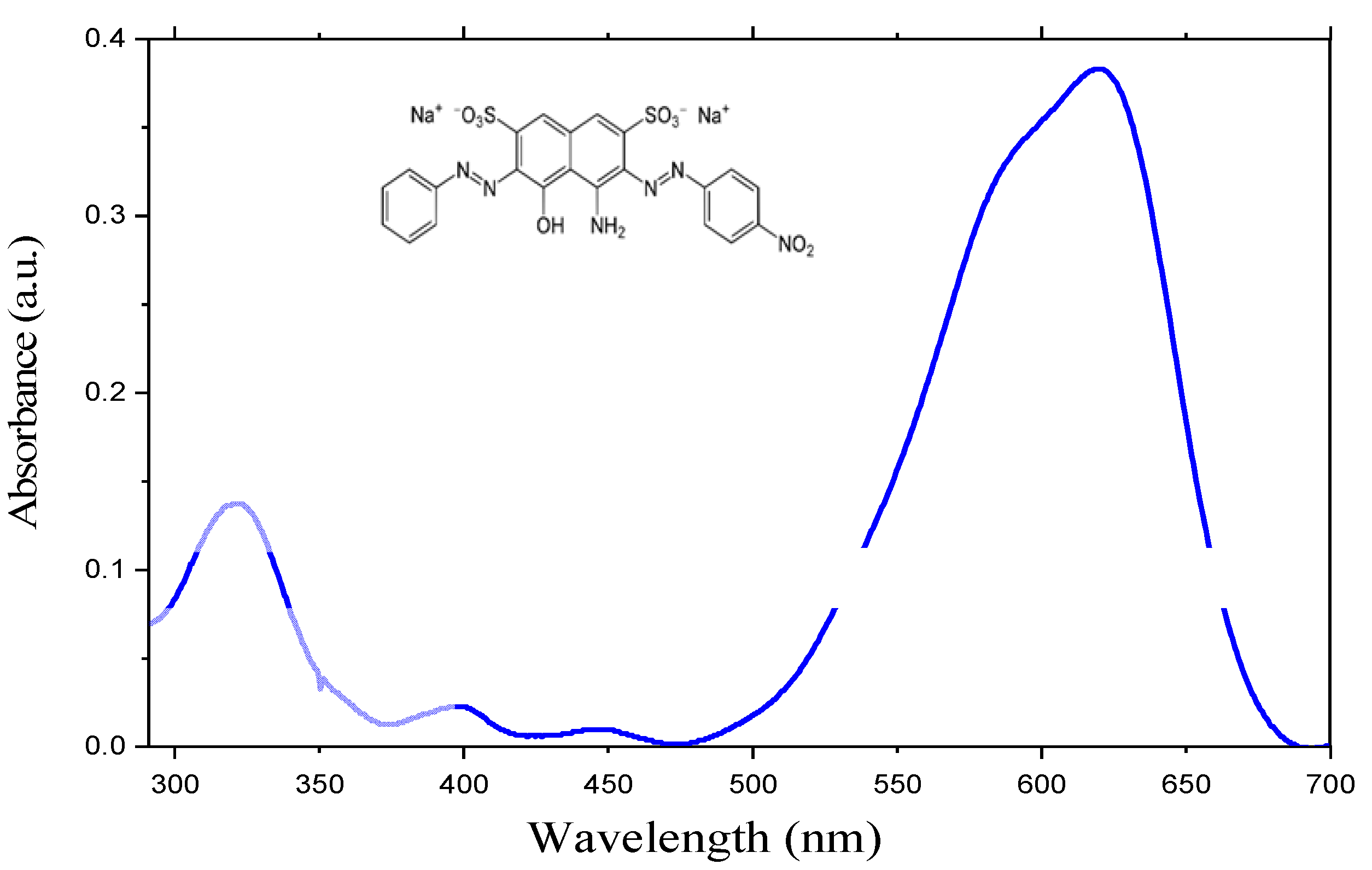
3. Photocatalytic Application of the PbS/SiNWs Nanohybrids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Synthesis of carbon nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Ghosh, R.; Giri, P.K. Silicon nanowire heterostructures for advanced energy and environmental applications: A review. Nanotechnology 2017, 28, 012001. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, F.; Wang, T.; Shao, M. Silicon nanowires: Applications in catalysis with distinctive surface property. J. Mater. Sci. Mater. Electron. 2015, 26, 4722–4729. [Google Scholar] [CrossRef]
- Picca, R.A.; Calvano, C.D.; Lo Faro, M.J.; Fazio, B.; Trusso, S.; Ossi, P.M.; Neri, F.; D’Andrea, C.; Irrera, A.; Cioffi, N. Functionalization of silicon nanowire arrays by silver nanoparticles for the laser desorption ionization mass spectrometry analysis of vegetable oils. J. Mass Spectrom. 2016, 51, 849–856. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzo, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2017, 5, 471–479. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Da, P.; Xu, M.; Wu, H.; Zheng, G. Silicon Nanowires for Biosensing, Energy Storage, and Conversion. Adv. Mater. 2013, 25, 5177–5195. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Gösele, U. Growth, Thermodynamics, and Electrical Properties of Silicon Nanowires. Chem. Rev. 2010, 110, 361–388. [Google Scholar] [CrossRef] [Green Version]
- Nafie, N.; Lachiheb, M.A.; Ben Rabha, M.; Dimassi, W.; Bouaïcha, M. Effect of the doping concentration on the properties of silicon nanowires. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 427–430. [Google Scholar] [CrossRef]
- Gaidi, M.; Daoudi, K.; Columbus, S.; Hajjaji, A.; El Khakani, M.A.; Bessais, B. Enhanced photocatalytic activities of silicon nanowires/graphene oxide nanocomposite: Effect of etching parameters. J. Environ. Sci. 2020, 101, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ben Rabha, M.; Khezami, L.; Jemai, A.B.; Alhathlool, R.; Ajbar, A. Surface passivation of silicon nanowires based metal nano-particle assisted chemical etching for photovoltaic applications. J. Cryst. Growth 2017, 462, 35–40. [Google Scholar] [CrossRef]
- Rebhi, A.; Hajjaji, A.; Leblanc-Lavoie, J.; Aouida, S.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films. Nanomaterials 2021, 11, 1254. [Google Scholar] [CrossRef]
- Hajjaji, A.; Jemai, S.; Trabelsi, K.; Kouki, A.; Assaker, I.B.; Ka, I.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Study of TiO2 Nanotubes Decorated with PbS Nanoparticles elaborated by Pulsed-Laser-Deposition: Microstructural, Optoelectronic and Photoelectrochemical Properties. J. Mater. Sci. Mater. Electron. 2019, 24, 20935–20946. [Google Scholar] [CrossRef]
- Hajjaji, A.; Rebhi, A.; Ka, I.; Trabelsi, K.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Pulsedlaser deposited lead sulfide nanoparticles based decoration of porous silicon layer as an effective passivation treatment for multicrystalline silicon. J. Appl. Surf. Sci. 2020, 505, 144590. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Kashyap, J. Enhancement of photocatalytic properties of transitional metal oxides using conducting polymers: A mini review. Mater. Res. Bull. 2015, 71, 75–90. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Kashyap, J. Role of Conducting Polymers in Enhancing TiO2-based Photocatalytic Dye Degradation: A Short Review. Polym.-Plast. Technol. Eng. 2015, 54, 1850–1870. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.P.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef]
- Khezami, L.; Lounissi, I.; Hajjaji, A.; Guesmi, A.; Assadi, A.A.; Bessais, B. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds. Materials 2021, 14, 7341. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Elfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) with Ag Silver Nanoparticles (Ag-NPs): Photocatalytic Performance for Wastewater Treatment under Visible Light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, W.; Zhou, Y.; Chen, X.; Xiao, Y.; Ma, M.; Zhu, W.; Wei, F. Fabrication of porous silicon nanowires by MACE method in HF/H2O2/AgNO3 system at room temperature. Nanoscale Res. Lett. 2014, 9, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.Q.; Hu, J.J.; Yan, Y.J.; Wu, Y.; Fang, H.; Xu, Y.; Lee, S.; Zhu, J. Fabrication of Single-Crystalline Silicon Nanowires by Scratching a Silicon Surface with Catalytic Metal Particles. Adv. Funct. Mater. 2006, 16, 387–394. [Google Scholar] [CrossRef]
- Cuevas, A.; Donald, D.M. Measuring and interpreting the lifetime of silicon wafers. Sol. Energy 2004, 76, 255–262. [Google Scholar] [CrossRef]
- Tang, J.; Shi, J.; Zhou, L.; Ma, Z. Fabrication and Optical Properties of Silicon Nanowires Arrays by Electroless Ag-catalyzed Etching. Nano-Micro Lett. 2011, 3, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Rabha, M.B.; Salem, M.; ElKhakani, M.A.; Bessais, B.; Gaidi, M. Monocrystalline Silicon surface passivation by Al2O3/porous silicon combined treatment. Mater. Sci. Eng. B 2013, 178, 695–697. [Google Scholar] [CrossRef]
- Joice, J.A.I.; Sivakumar, T.; Ramakrishnan, R.; Ramya, G.; Prasad, K.P.S.; Selvan, D.A. Visible active metal decorated titania catalysts for the photocatalytic degradation of Amidoblack-10B. Chem. Eng. J. 2012, 210, 385–397. [Google Scholar] [CrossRef]
- Qamar, M.; Saquib, M.; Muneer, M. Photocatalytic degradation of two selected dye derivatives, chromotrope 2B and amido black 10B, in aqueous suspensions of titanium dioxide. Dyes Pigments 2005, 65, 1–9. [Google Scholar] [CrossRef]


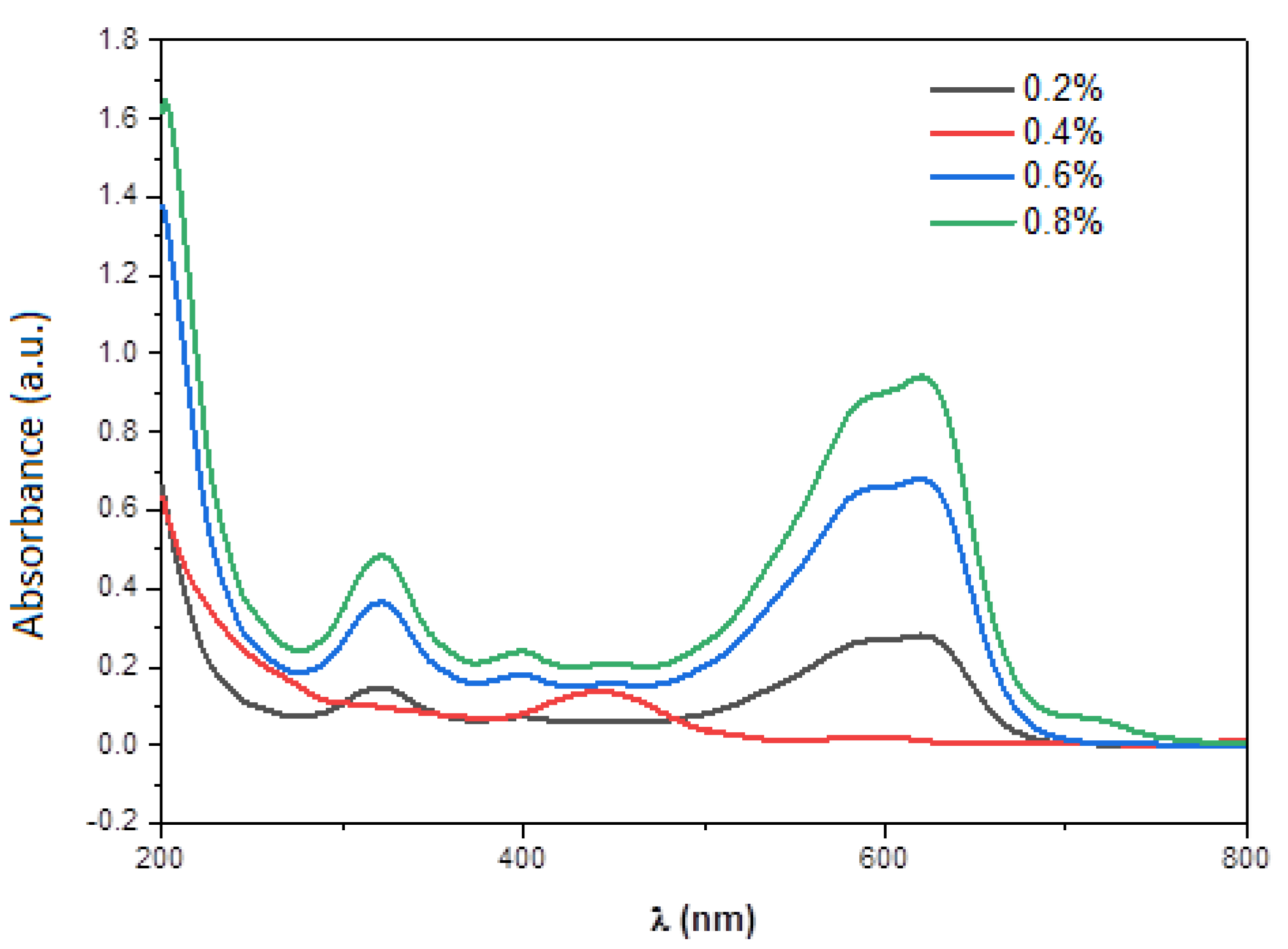
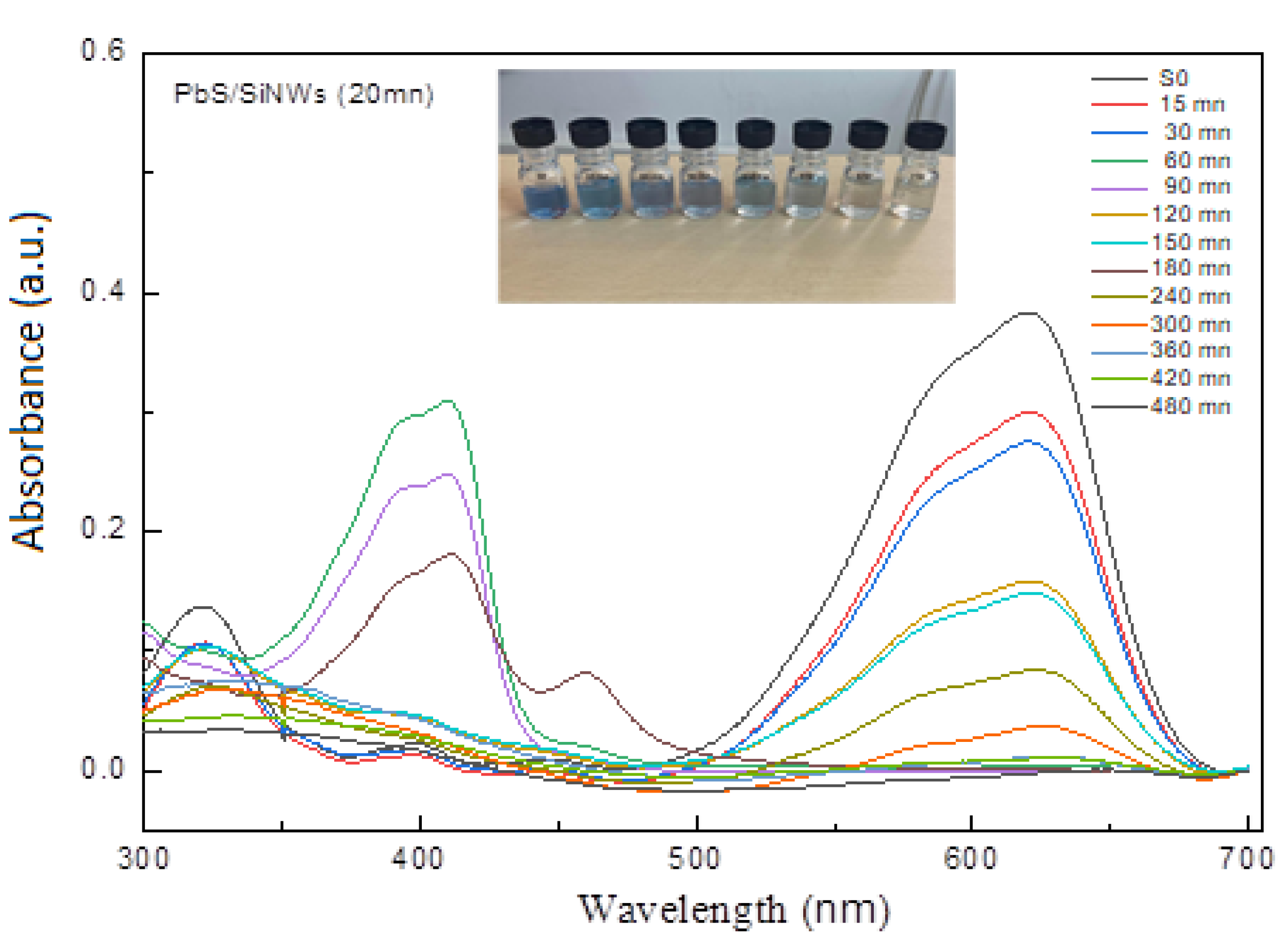
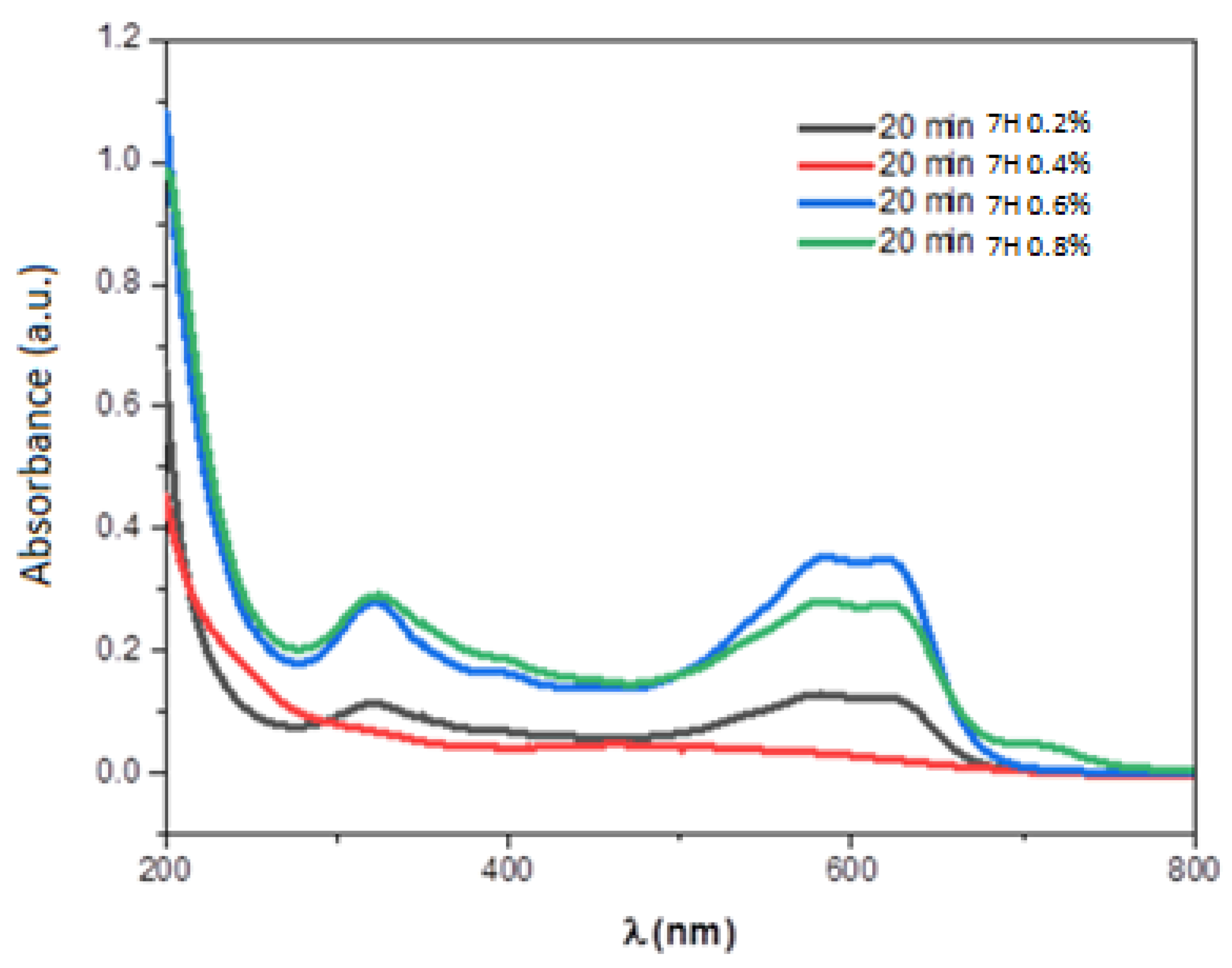
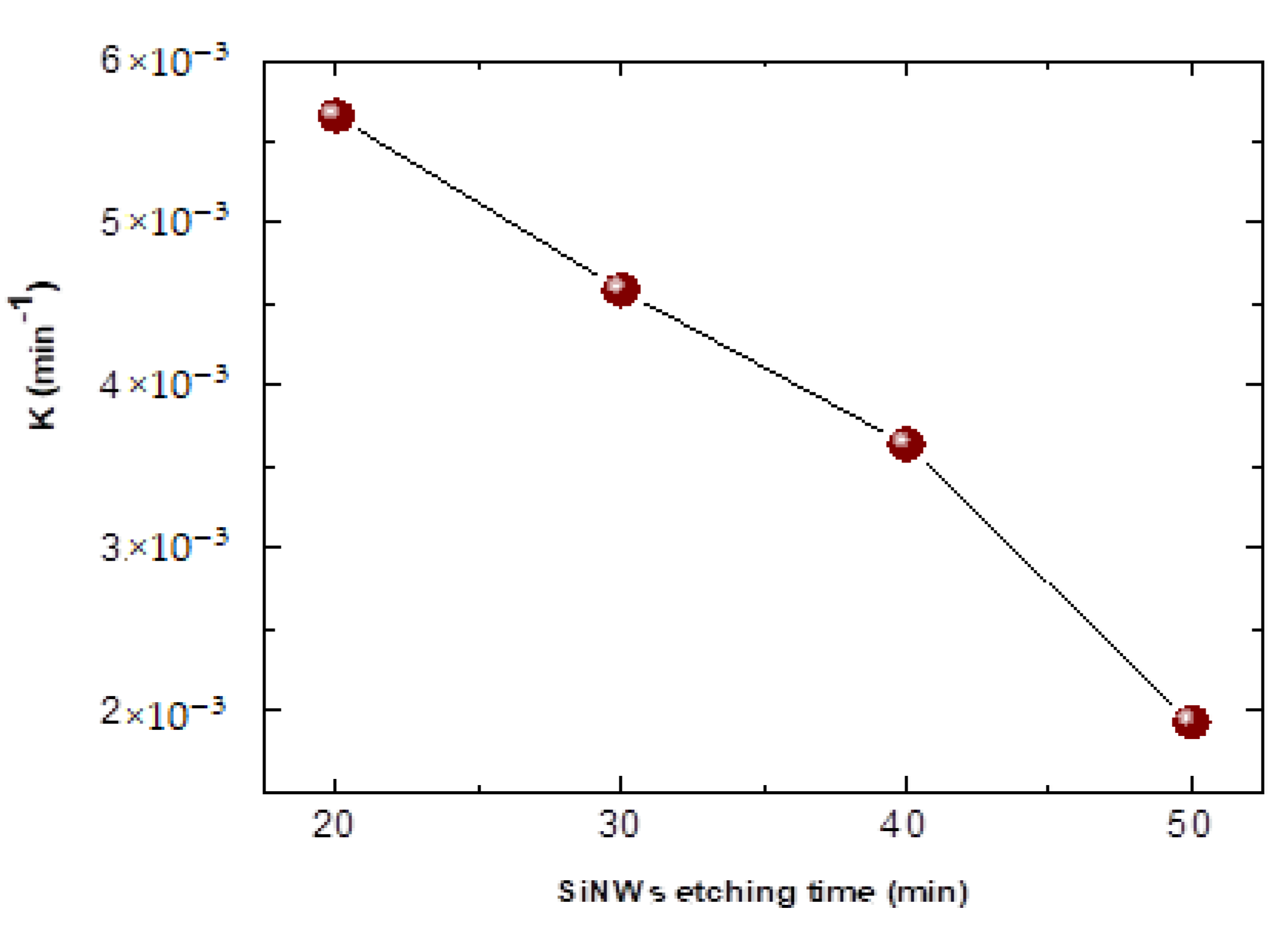
| Element | Line Type | Apparent Concentration | k Ratio | wt% | wt% Sigma | Atomic % |
|---|---|---|---|---|---|---|
| C | K series | 5.71 | 0.05708 | 10.83 | 0.14 | 24.15 |
| O | K series | 7.19 | 0.06297 | 6.46 | 0.05 | 10.81 |
| Si | K series | 112.41 | 1.03086 | 61.64 | 0.25 | 58.77 |
| S | K series | 3.46 | 0.03667 | 2.39 | 0.07 | 2 |
| Ag | L series | 17.76 | 0.17758 | 15.61 | 0.21 | 3.88 |
| Pb | M series | 3.09 | 0.02972 | 3.07 | 0.26 | 0.4 |
| Total | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algethami, F.K.; Trabelsi, K.; Hajjaji, A.; Rabha, M.B.; Khezami, L.; Elamin, M.R.; Bessais, B.; El Khakani, M.A. Photocatalytic Activity of Silicon Nanowires Decorated with PbS Nanoparticles Deposited by Pulsed Laser Deposition for Efficient Wastewater Treatment. Materials 2022, 15, 4970. https://doi.org/10.3390/ma15144970
Algethami FK, Trabelsi K, Hajjaji A, Rabha MB, Khezami L, Elamin MR, Bessais B, El Khakani MA. Photocatalytic Activity of Silicon Nanowires Decorated with PbS Nanoparticles Deposited by Pulsed Laser Deposition for Efficient Wastewater Treatment. Materials. 2022; 15(14):4970. https://doi.org/10.3390/ma15144970
Chicago/Turabian StyleAlgethami, Faisal K., Khaled Trabelsi, Anouar Hajjaji, Mohamed B. Rabha, Lotfi Khezami, Mohamed R. Elamin, Brahim Bessais, and My Ali El Khakani. 2022. "Photocatalytic Activity of Silicon Nanowires Decorated with PbS Nanoparticles Deposited by Pulsed Laser Deposition for Efficient Wastewater Treatment" Materials 15, no. 14: 4970. https://doi.org/10.3390/ma15144970
APA StyleAlgethami, F. K., Trabelsi, K., Hajjaji, A., Rabha, M. B., Khezami, L., Elamin, M. R., Bessais, B., & El Khakani, M. A. (2022). Photocatalytic Activity of Silicon Nanowires Decorated with PbS Nanoparticles Deposited by Pulsed Laser Deposition for Efficient Wastewater Treatment. Materials, 15(14), 4970. https://doi.org/10.3390/ma15144970









