Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
- (i)
- The slurries were prepared by dispersing the ceramic or porcelain powder into distilled water together with a poly-vinylic binder. The porcelain powder morphology is characterized by a certain granulometric distribution, as presented in Section 3.2. The porcelain ceramic slurry was prepared by adding solid porcelain powder 70 wt% to distilled water. The milling process was carried out using a Fritzch Pulverisette planetary mill using porcelain 10 mm balls. As binder, 1.5 wt% Polyvinylalcohol PVA (Sigma Aldrich, Darmstadt, Germany) was added after the milling procedure. This amount is necessary to preserve the slurry stability and to assure the ability of the solid particles to coat the sponge. The ceramic slurries were mixed under magnetic stirring.
- (ii)
- The polymeric sponges were coated with the prepared slurry and then cut to the necessary shape. These sponges were manually immersed in the slurry, and unlike in the traditional replication method, they were not squeezed but kept fully loaded with slurry. The sponges were immediately dried with a multidirectional air flux at 120 °C for 30 min.
- (iii)
- All of the sponges were burned out during a heat treatment which, at the same time, sinters the ceramic powders. Thus, this method has the advantage of obtaining a mass containing an excess of ceramic material; this has a favorable effect on the mechanical resistance of the final product. In consequence, the main aspect which must be discussed—as it may become critical—is related to the porosity of the obtained materials. This process was performed in two steps under atmospheric conditions using an electric furnace (Nabertherm L15/12/P330 Muffle Furnace). The first step which was conducted at 250 °C for 30 min was necessary for burning the sacrificial polyurethane scaffold. In the second step, the ceramic body was sintered at two different temperatures, depending on the precursors that were used: 600 °C for the D and E ceramics, and 900 °C for the novel porcelain C, respectively. The holding time at the sintered temperature was one hour for all of the investigated ceramics, followed by cooling with 100 °C/h until room temperature was reached in order to avoid thermally-induced cracks. Thus, the sponge acted as a template for the porous scaffold.
- (iv)
- Once this polymer sacrificial template was removed, the samples were sintered to the desired structure using an optimized heat-treatment schedule, in two phases: the first one at 250 °C for burning the organic phase; the second one at 600 °C for the D and E ceramics, and at 900 °C for the novel porcelain C, for one hour.
2.2. Characterization of the Samples
- (1)
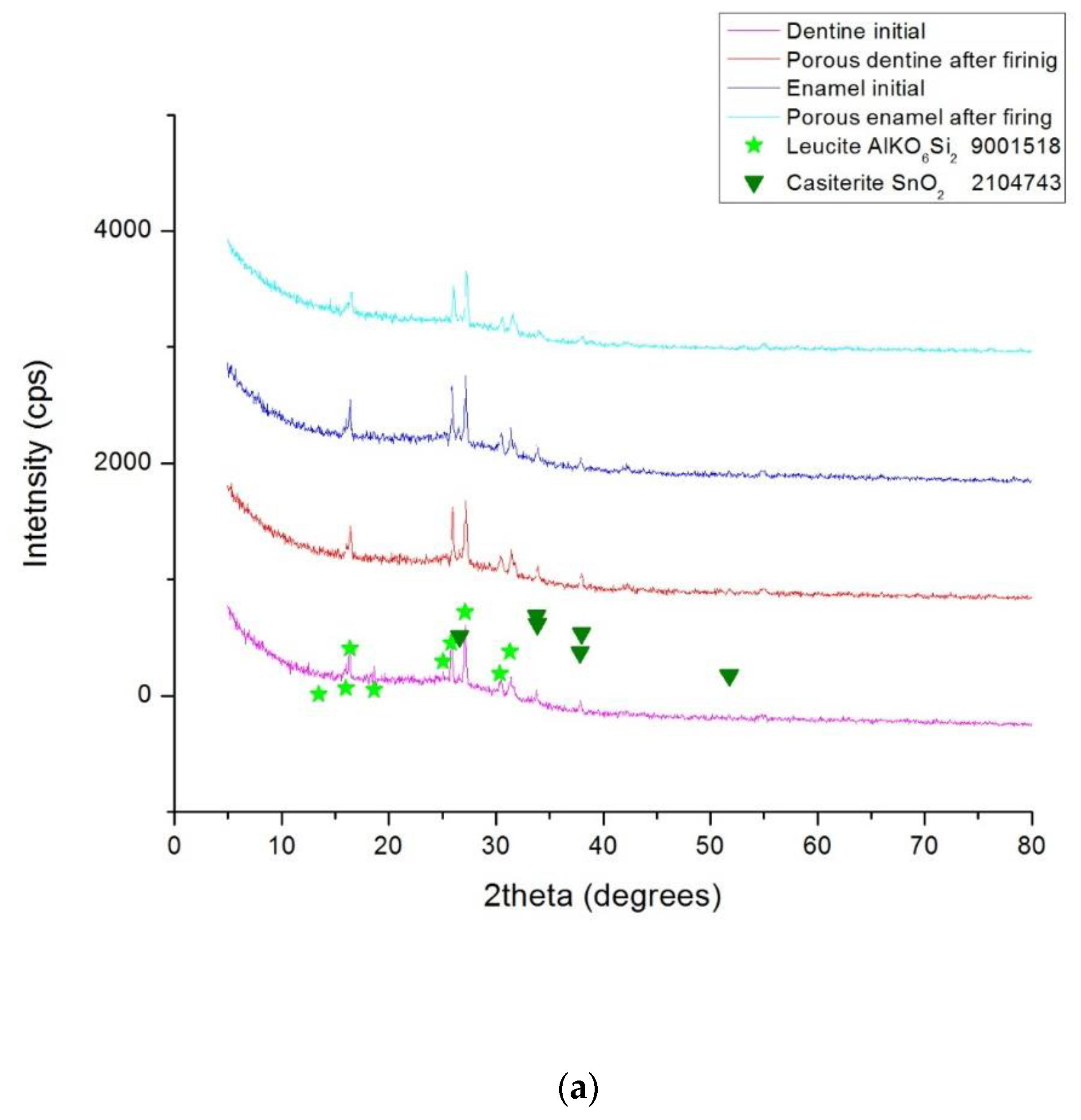
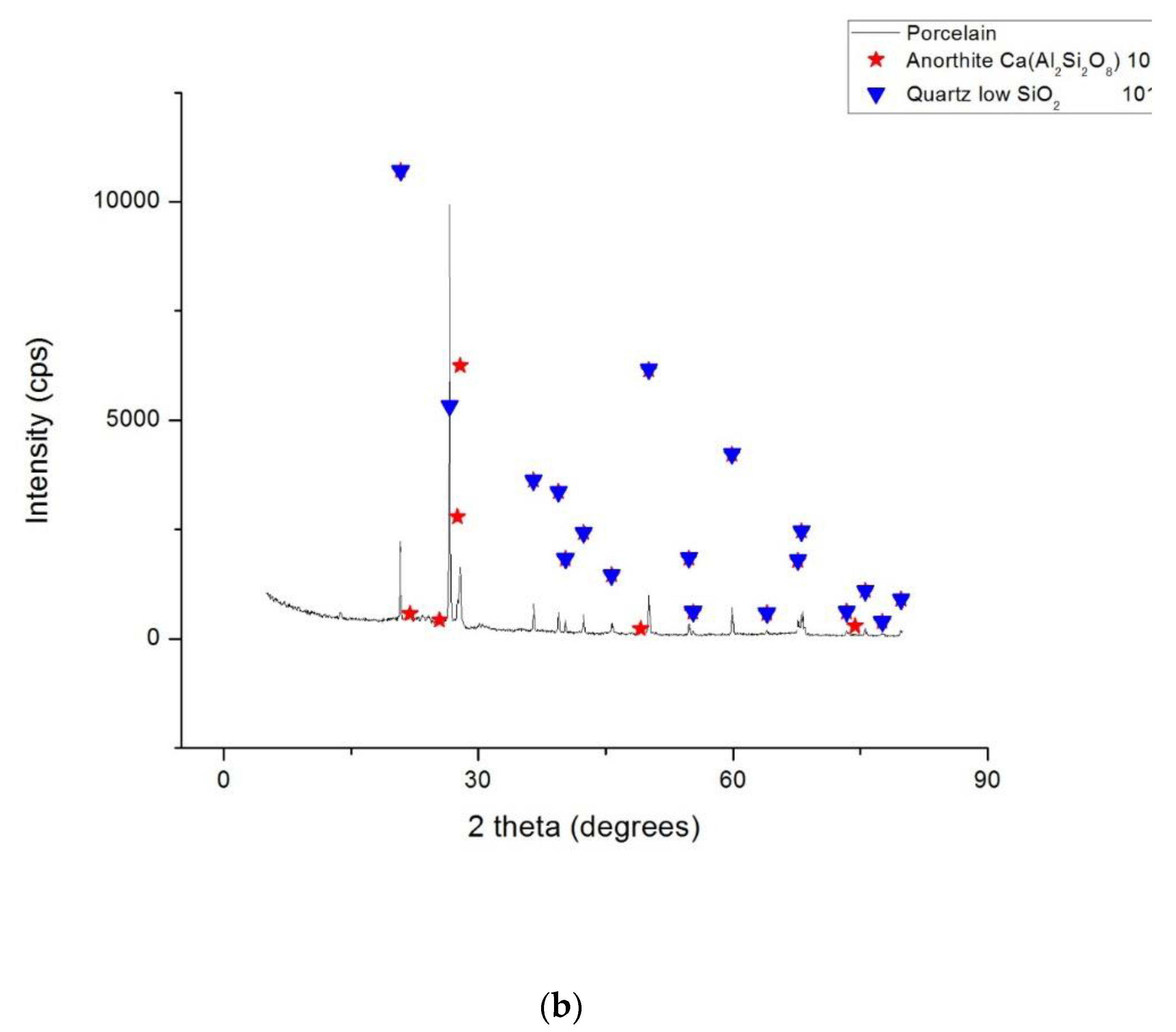
- X-ray diffraction (XRD) analysis. The phase compositions of the studied ceramic samples were determined with a Rigaku Ultima 4 diffractometer (Rigaku Corp., Tokyo, Japan) using a monochromatic Cu-Kα radiation. The XRD measurements were performed using powdered scaffolds that were obtained after the heat treatment, and dentine and enamel commercial powders for the precursors. All of the samples were scanned over an angular range of 5 to 80° and measurements were recorded using a scanning speed of 20°/min at every 0.05° interval.
- (2)
- Ascertainment of the apparent porosity, pap, is represented by the ratio between the open pore volume and the apparent volume of the material. It is expressed in % of the latter. In the case of the obtained glass ceramics, the apparent porosity was measured using the liquid saturation method under vacuum, with water as the working liquid. The apparent density [kg/m3] is:where m [kg] represents the mass of the specimen in its dry state; [m3] is the apparent volume of the specimens, where m1 is the mass of the sample when it is saturated with liquid, m2 is the mass of liquid-weighted specimen, and [kg/m3] is the density of the utilized liquid. Therefore, according to the above definition, the apparent porosity is:where a is the absorption capacity of the studied material.
- (3)
- Scanning electronic microscopy (SEM) was used to analyze the macro- and micro-porous structure of the obtained ceramics with a Quanta FEG 250 microscope (Thermo Fisher Scientific, Hillsboro, OR, USA) using a low vacuum mode at 3.0 kV.
- (4)
- Confocal microscopy (CM) was used to analyze the obtained samples with a LEXT 3D Measuring Laser Microscope OLS4000 (Olympus Europa SE & Co. KG., Hamburg, Germany), which works with a laser centered at a 405 nm wavelength. This scanning microscope is equipped with a confocal system that captures the in-focus image while simultaneously eliminating the flare.
- (5)
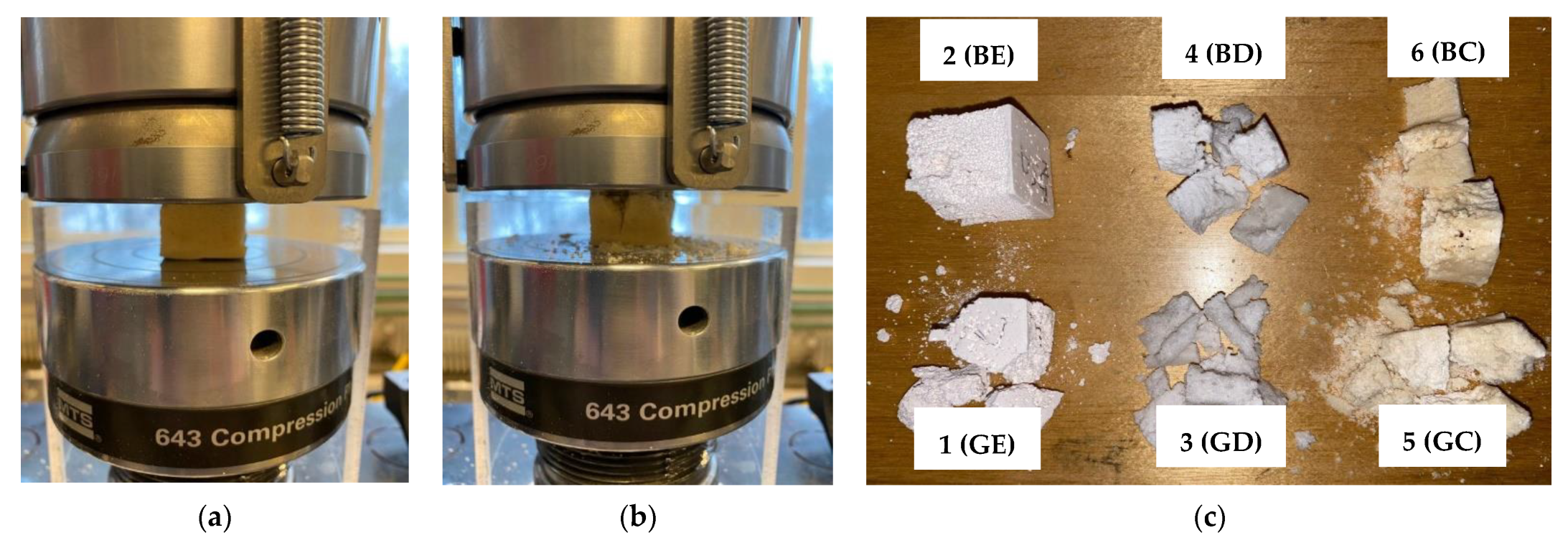
- Mechanical testing was carried out for each study group using an MTS 643 Compression Plateus (MTS System, Eden Prairie, MN, USA), as shown in the examples that are presented in Section 3. Thus, the compressive strength was determined to assess the capability of the scaffolds to be handled, as well as to perform their functions. Although the standard (i.e., ASTM C1424-15(2019), Standard Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature) recommends cylindrical shape specimens with the length/diameter range from 1.5 to 2.5, we chose cubic specimens for the following technological reasons: (i) the sacrificial polyurethane foam is easier and more precisely cut in this shape compared to the cylindrical shape; (ii) the final ceramic scaffold is easy to be shaped in the desired form starting from cubic form using regular abrasive tools. It should be mentioned that similar specimens were successfully used for compression tests of highly porous alumina-based foam [28].
- (6)
- Biocompatibility or cytotoxicity can be verified using two different methods: (i) in vivo tests using human materials, which are too complex and require ethical approvals; (ii) in vitro testing on human or animal cell lines, which is the most common method and it was applied in the present study for the new material C. Biocompatibility was demonstrated in our previous study [25] for the two commercially available materials E and D.
3. Results
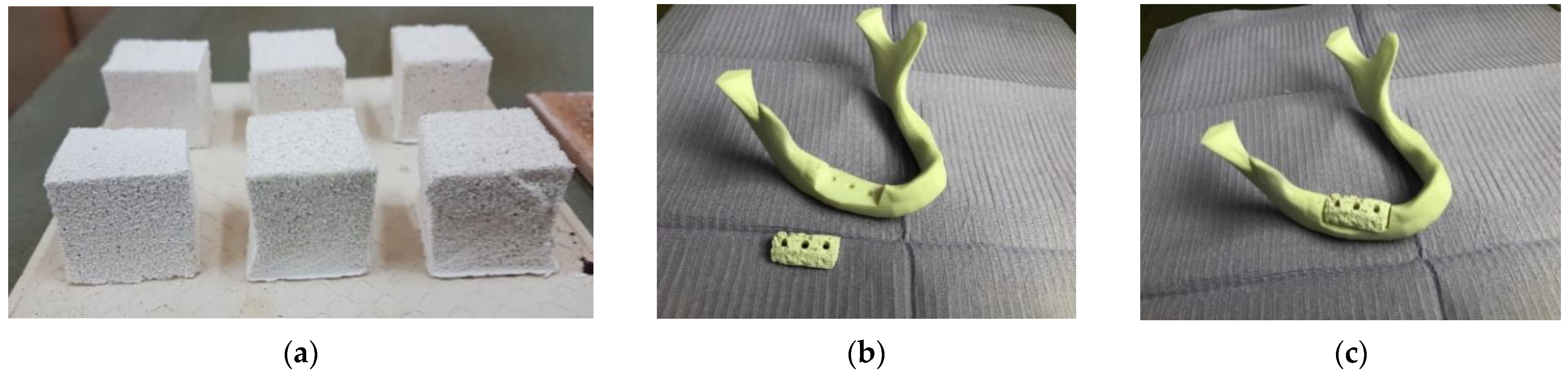
3.1. Sponges Characterization
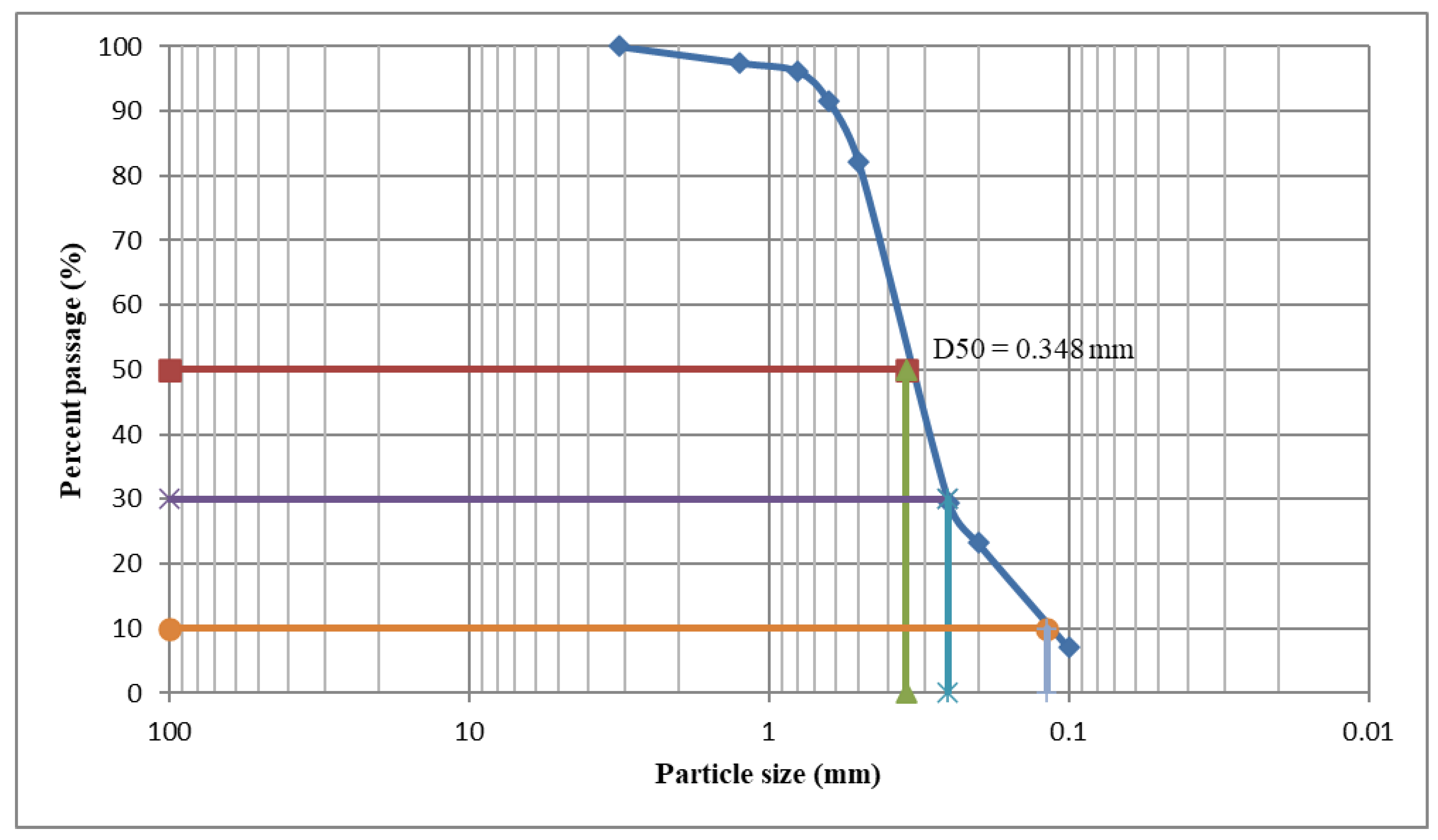
3.2. Powder Characterization
3.3. Phase Composition
3.4. Apparent Porosities of the Samples
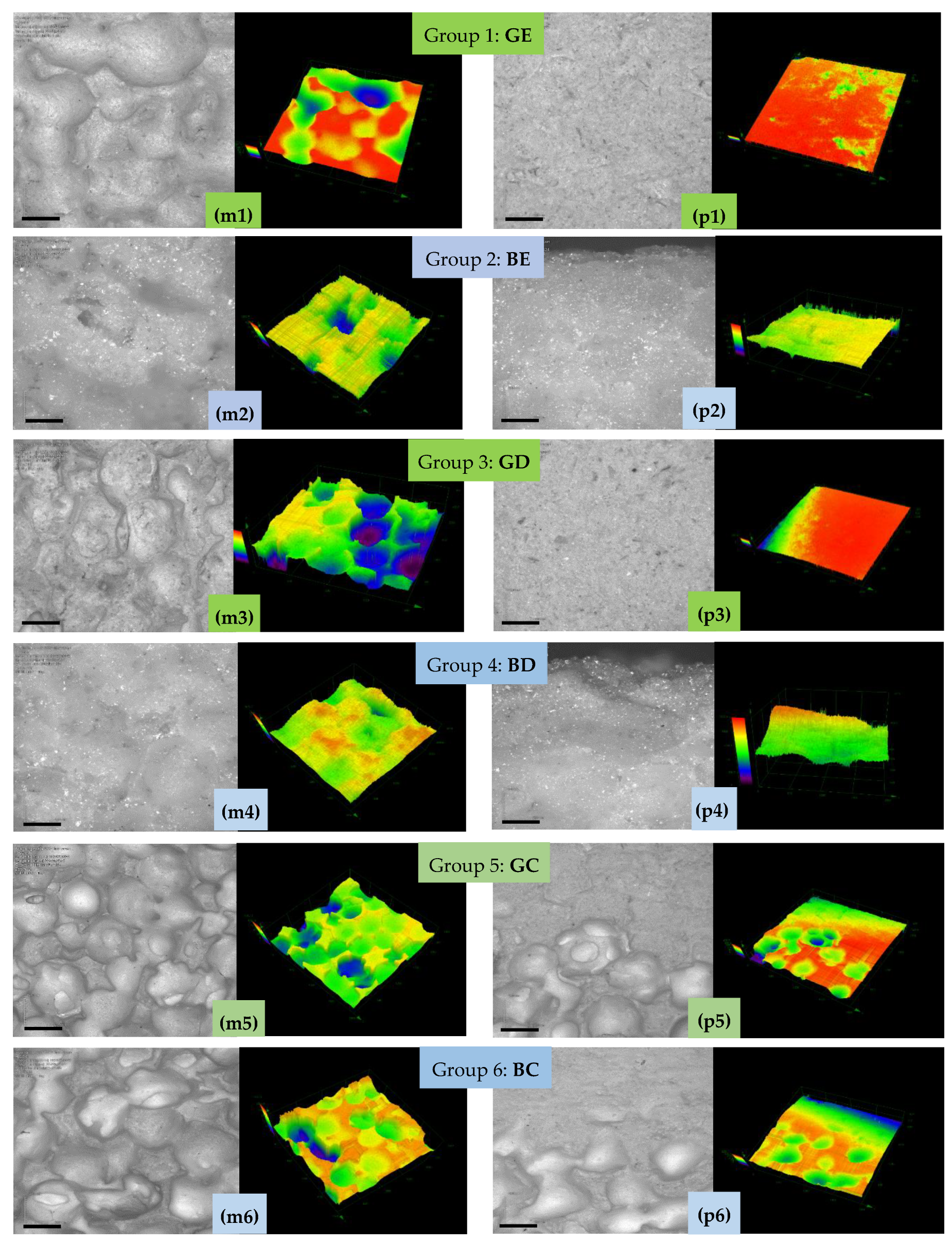
3.5. Pores Morphology—SEM Investigations
3.6. Confocal Microscopy (CM)
3.7. Mechanical Testing Results
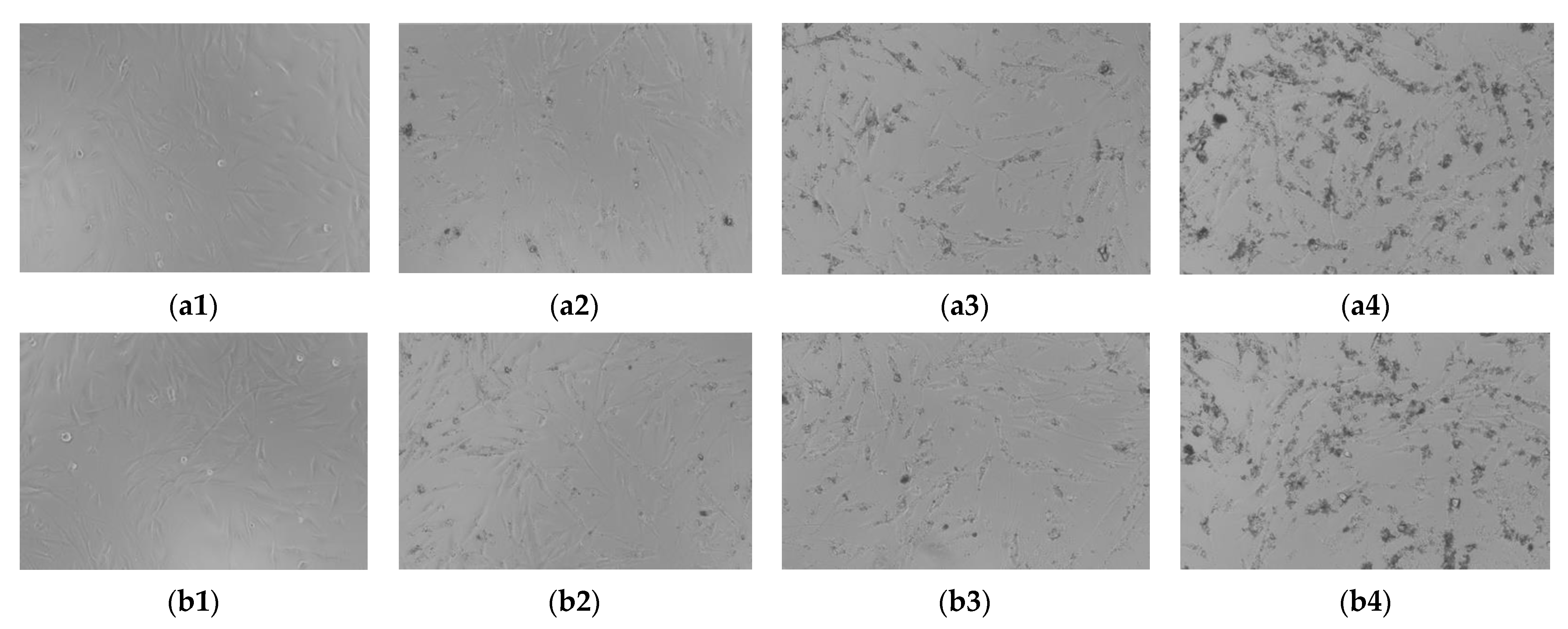
3.8. Biocompatibility
4. Discussion
4.1. Manufacturing Methods
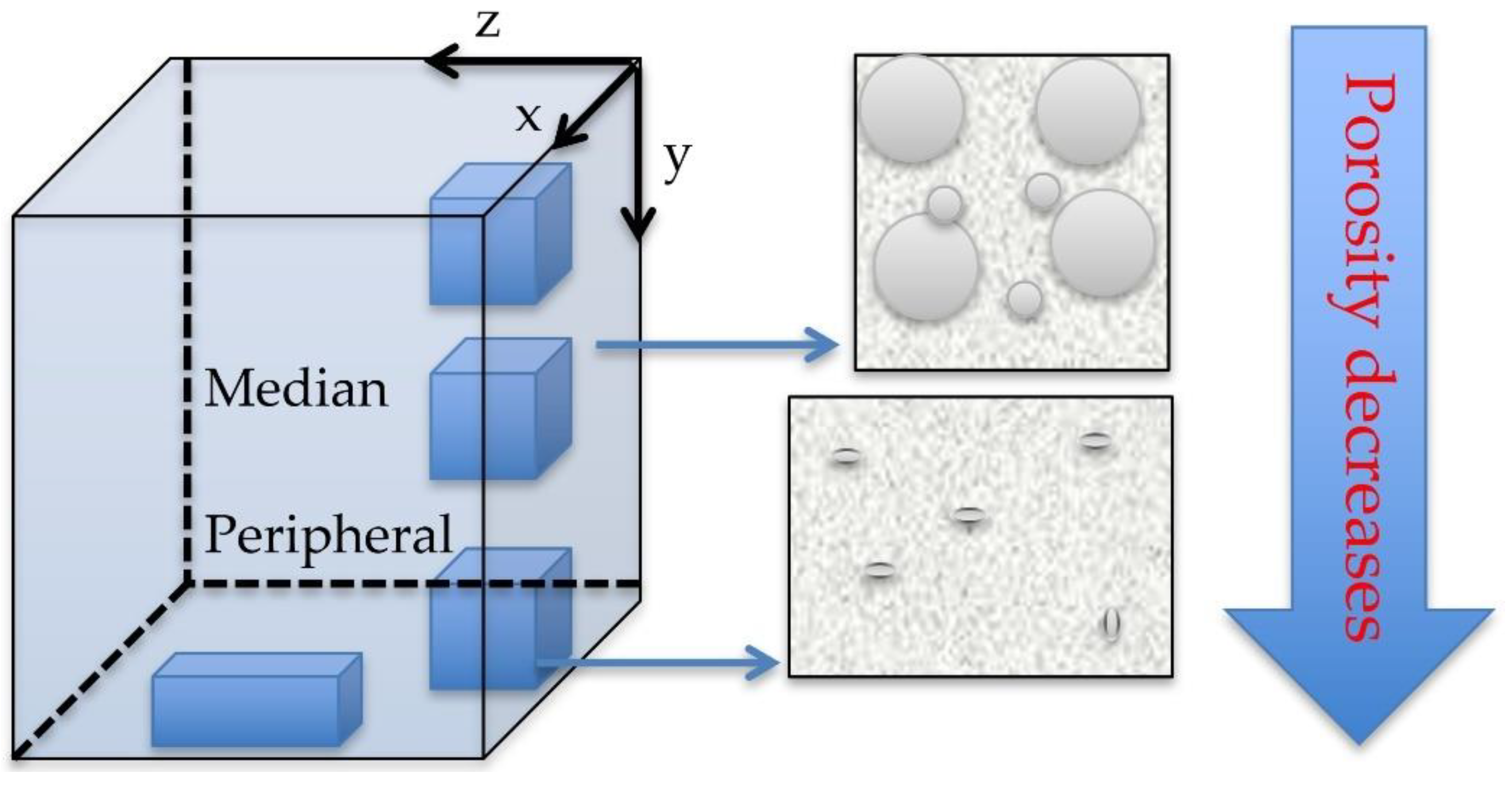
4.2. Porosity Versus SEM and CM
4.3. Mechanical Properties
4.4. Biocompatibility
4.5. On the Potential of Other Imaging Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascalho, M.; Platt, J.L. The future of organ replacement: Needs, potential applications, and obstacles to application. Transplant. Proc. 2006, 38, 362–364. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, D.; Cannillo, V.; Sola, A. Shell Scaffolds: A new approach towards high strength bioceramic scaffolds for bone regeneration. Mater. Lett. 2010, 64, 203–206. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, T.; Omata, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Alveolar bone tissue engineering using composite scaffolds for drug delivery. Jpn. Dent. Sci. Rev. 2010, 46, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Francesca, G.; Alessandro, S.; Giuseppe, M.P. The biomaterialist’s task: Scaffold Biomaterials and Fabrication Technologies. Joints 2013, 1, 130–137. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Gerecht-Nir, S.; Radisic, M.; Park, H.; Cannizzaro, C.; Boublik, J.; Langer, R.; Vunjak-Novakovic, G. Biophysical regulation during cardiac development and application to tissue engineering. Int. J. Dev. Biol. 2006, 50, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Luca, R.; Todea, C.D.; Duma, V.-F.; Bradu, A.; Podoleanu, A. Quantitative assessment of rat bone regeneration using complex master–slave optical coherence tomography. Quant. Imaging Med. Surg. 2019, 9, 782–798. [Google Scholar] [CrossRef]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, B.; Nath, S. Fundamentals of Biomaterials and Biocompatibility. In Advanced Biomaterials; Basu, B., Katti, D.S., Kumar, A., Eds.; John Willey & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 3–18. [Google Scholar]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef]
- Lanza, R.P.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering, 2nd ed.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Das Lala, S. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. A Revised Replication Method for Bioceramic Scaffolds. Bioceram. Dev. Appl. 2011, 1, D110401. [Google Scholar] [CrossRef]
- Shahgholia, M.; Olivierod, S.; Bainob, F.; Vitale-Brovaroneb, C.; Gastaldia, D.; Venaa, P. Mechanical characterization of glass-ceramic scaffolds at multiple characteristic lengths through nanoindentation. J. Eur. Ceram. Soc. 2016, 36, 2403–2409. [Google Scholar] [CrossRef]
- Vargas, G.E.; Mesones, R.V.; Bretcanu, O.; Porto Lopez, J.M.; Boccaccini, A.R.; Gorustovich, A. Biocompatibility and bone mineralization potential of 45S5 Bioglass-derived glass–ceramic scaffolds in chick embryos. Acta Biomater. 2009, 5, 374–380. [Google Scholar] [CrossRef]
- Ren, X.; Tuo, Q.; Tian, K.; Huang, G.; Lid, J.; Xu, T.; Lv, X.; Wu, J.; Chen, Z.; Weng, J.; et al. Enhancement of osteogenesis using a novel porous hydroxyapatite scaffold T in vivo and vitro. Ceram. Int. 2018, 44, 21656–21665. [Google Scholar]
- Engin, N.O.; Tas, A.C. Manufacture of Macroporous Calcium Hydroxyapatite Bioceramics. J. Eur. Ceram. Soc. 1999, 19, 2569–2572. [Google Scholar] [CrossRef]
- Appel, A.A.; Anastasio, M.A.; Larson, J.C.; Brey, E.M. Imaging challenges in biomaterials and tissue engineering. Biomaterials 2013, 34, 6615–6630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
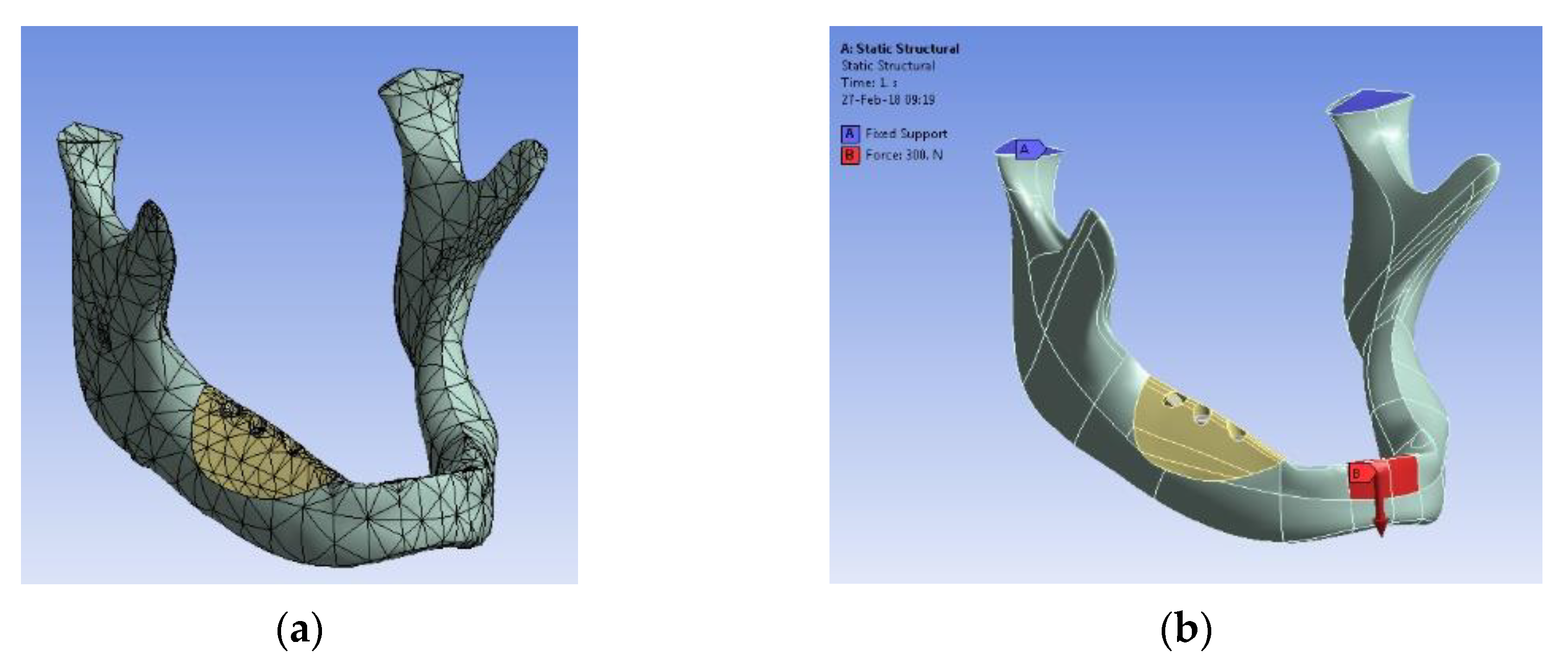
- Gabor, A.; Zaharia, C.; Todericiu, V.; Szuhanek, C.; Cojocariu, A.C.; Duma, V.-F.; Sticlaru, C.; Negrutiu, M.L.; Antoniac, I.V.; Sinescu, C. Adhesion of scaffolds with implants to the mandibular bone with a defect: A Finite Element Analysis. Mater. Plast. 2018, 55, 394–397. [Google Scholar] [CrossRef]
- Fabricky, M.M.C.; Gabor, A.-G.; Milutinovici, R.A.; Watz, C.G.; Avram, Ș.; Drăghici, G.; Mihali, C.V.; Moacă, E.-A.; Dehelean, C.A.; Galuscan, A.; et al. Scaffold-Type Structure Dental Ceramics with Different Compositions Evaluated through Physicochemical Characteristics and Biosecurity Profiles. Materials 2021, 14, 2266. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.dentsplysirona.com/en (accessed on 1 September 2021).
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Buncianu, D.; Tessier-Doyen, N.; Absi, J.; Negru, R.; Şerban, D.A.; Marşavina, L. Multi-Scale Mechanical Behaviour of a Highly Porous Alumina Based Foam. Met. Mater. Int. 2020, 26, 1524–1532. [Google Scholar] [CrossRef]
- Renders, G.A.; Mulder, L.; van Ruijven, L.J.; van Eijden, T.M. Porosity of human mandibular condylar bone. J. Anat. 2007, 210, 239–248. [Google Scholar] [CrossRef]
- Cooper, D.M.L.; Kawalilak, C.E.; Harrison, K.; Johnston, B.D.; Johnston, J.D. Cortical Bone Porosity: What Is It, Why Is It Important, and How Can We Detect It? Curr. Osteoporos. Rep. 2016, 14, 187–198. [Google Scholar] [CrossRef]
- Ryshkewitch, E. Compression strength of porous sintered alumina and zirconia. J. Am. Ceram. Soc. 1953, 36, 65–68. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; De Groot, K.; Weiqun, C.; Yubao, L.; Xingdong, Z. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials 1994, 15, 31–34. [Google Scholar] [CrossRef]
- Yubao, L.; Klein, C.P.A.T.; Xingdong, Z.; De Groot, K. Formation of a bone apatite-like layer on the surface of porous HA ceramics. Biomaterials 1994, 15, 835–841. [Google Scholar] [CrossRef]
- Biggemann, J.; Pezoldt, M.; Stumpf, M.; Greil, P.; Fey, T. Modular ceramic scaffolds for individual implants. Acta Biomater. 2018, 80, 390–400. [Google Scholar] [PubMed]
- Zhang, H.; Li, X.; Wen, J.; Zhao, C. Preparation and characterisation of HA/TCP biphasic porous ceramic scaffolds with pore-oriented structure. Ceram. Int. 2017, 43, 11780–11785. [Google Scholar] [CrossRef]
- Melli, V.; Lefebvre, L.-P.; Lenci, M.; Mondon, M.; Sao-Joao, S.; Cigada, A.; Delafosse, D.; De Nardo, L. Resorbability of a Bioglass®-based glass-ceramic scaffold produced via a powder metallurgy approach. Ceram. Int. 2017, 43, 8625–8635. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Vila, M.; Diaz, A.; Acosta, C.; Barton, I.; Escobar, A.; Vallet-Regí, M.; Synthesis of bioceramic foams from natural products. Mater. Sci. 2017. Available online: https://eprints.ucm.es/id/eprint/41103/1/ (accessed on 11 July 2022).
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Haugh, M.; O’Brien, F. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.-C.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [Green Version]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Dental Diagnosis and Treatment Assessments: Between X-rays Radiography and Optical Coherence Tomography. Materials 2020, 13, 4825. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe for distortion-free images in Gabor-Domain Optical Coherence Microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef] [PubMed]
- Hutiu, G.; Duma, V.-F.; Demian, D.; Bradu, A.; Podoleanu, A.G. Surface imaging of metallic material fractures using optical coherence tomography. Appl. Opt. 2014, 53, 5912–5916. [Google Scholar] [CrossRef]
- Hutiu, G.; Duma, V.-F.; Demian, D.; Bradu, A.; Podoleanu, A.G. Assessment of Ductile, Brittle, and Fatigue Fractures of Metals Using Optical Coherence Tomography. Metals 2018, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Sughanthy, A.P.; Ansari, M.N.M. A Review on Bone Scaffold Fabrication Methods. Int Res. J. Eng. Technol. 2015, 2, 1232–1238. [Google Scholar]
- Monroy, G.L.; Won, J.; Spillman, D.R.; Dsouza, R.; Boppart, S.A. Clinical translation of handheld optical coherence tomography: Practical considerations and recent advancements. J. Biomed. Opt. 2017, 22, 121715. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.D.; Kraus, M.F.; Potsaid, B.; Liu, J.J.; Choi, W.; Jayaraman, V.; Cable, A.E.; Hornegger, J.; Duke, J.S.; Fujimoto, J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed. Opt. Express 2014, 5, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Demian, D.; Duma, V.-F.; Sinescu, C.; Negrutiu, M.L.; Cernat, R.; Topala, F.I.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Design and testing of prototype handheld scanning probes for optical coherence tomography. J. Eng. Med. 2014, 228, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duma, V.-F.; Dobre, G.; Demian, D.; Cernat, R.; Sinescu, C.; Topala, F.I.; Negrutiu, M.L.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Handheld scanning probes for optical coherence tomography. Rom. Rep. Phys. 2015, 67, 1346–1358. [Google Scholar]



| Group | N | Mean [%] | Standard Deviation [%] | Standard Error | 95% Confidence Interval for the Mean | Min. | Max. | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| 1 (GE) | 10 | 29.040 | 0.391 | 0.124 | 28.760 | 29.319 | 28.62 | 29.75 |
| 2 (BE) | 10 | 45.350 | 0.573 | 0.181 | 44.940 | 45.760 | 44.33 | 45.92 |
| 3 (GD) | 10 | 32.230 | 0.589 | 0.187 | 31.808 | 32.652 | 31.39 | 32.98 |
| 4 (BD) | 10 | 51.260 | 0.462 | 0.146 | 50.929 | 51.591 | 50.53 | 51.86 |
| 5 (GC) | 10 | 51.679 | 0.449 | 0.142 | 51.358 | 52.000 | 50.90 | 52.30 |
| 6 (BC) | 10 | 65.850 | 0.621 | 0.197 | 65.406 | 66.295 | 64.60 | 66.90 |
| Group | Maximum Dimension of the Pores (μm)—SEM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Mean | Standard Deviation | ||||||||
| m | p | m | p | m | p | m | p | m | p | m | p | m | p | |
| 1 (GE) | 713.5 | 67.3 | 710 | 82.3 | 695.5 | 77.3 | 684.9 | 103.1 | 709.3 | 102.7 | 702.6 | 86.5 | 10.79 | 14.21 |
| 2 (BE) | 665.5 | 313.7 | 255.2 | 364.1 | 576.2 | 317.2 | 679.4 | 343.1 | 635.3 | 307.3 | 562.3 | 329.1 | 157.61 | 21.33 |
| 3 (GD) | 724.4 | 97.6 | 592.1 | 113.2 | 671.2 | 98.3 | 689.6 | 107.9 | 591.6 | 122.3 | 653.8 | 107.9 | 53.38 | 9.31 |
| 4 (BD) | 671.3 | 131.9 | 673 | 317.7 | 602.8 | 144.6 | 592.3 | 272.3 | 617.7 | 178.5 | 631.4 | 209 | 34.23 | 73,27 |
| 5 (GC) | 621.4 | 316.3 | 623 | 274.8 | 615.7 | 272.3 | 624.1 | 302.9 | 607.3 | 283.1 | 618.3 | 289.9 | 6.23 | 17,03 |
| 6 (BC) | 754.9 | 521.3 | 733.4 | 366.6 | 711.3 | 472.2 | 729.2 | 476.7 | 716.2 | 423.7 | 729 | 452.1 | 15.26 | 52.75 |
| Group | Dimension of the Pores (μm)—CM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Mean | Standard Deviation | ||||||||
| m | p | m | p | m | p | m | p | m | p | m | p | m | p | |
| 1 (GE) | 527.4 | 54.2 | 773.4 | 62.4 | 721.4 | 60.1 | 697.4 | 82.3 | 659.3 | 89.8 | 675.78 | 69.76 | 82.9 | 13.78 |
| 2 (BE) | 634.6 | 318.2 | 752.4 | 275.4 | 629.6 | 224.1 | 702.6 | 307.2 | 654.1 | 296.4 | 674.66 | 284.26 | 46.65 | 42.57 |
| 3 (GD) | 621.2 | 83.4 | 774.5 | 97.5 | 754.4 | 126.4 | 724.2 | 92.8 | 696.4 | 112.3 | 714.14 | 102.48 | 53.51 | 15.2 |
| 4 (BD) | 470.4 | 125.4 | 642.5 | 276.5 | 539.9 | 208.3 | 597.3 | 176.9 | 608.9 | 109.5 | 571.8 | 179.32 | 60.54 | 60.12 |
| 5 (GC) | 603.7 | 406.8 | 637.5 | 213.2 | 592.3 | 471.2 | 616.2 | 204.7 | 597.6 | 262.8 | 609.46 | 311.74 | 15.96 | 107.73 |
| 6 (BC) | 641.4 | 341.2 | 723.1 | 310.5 | 670.5 | 518.4 | 694.4 | 273.2 | 707.3 | 406.8 | 687.34 | 370.02 | 29.48 | 86.16 |
| Group | 1 (GE) | 2 (BE) | 3 (GD) | 4 (BD) | 5 (GC) | 6 (BC) | |
|---|---|---|---|---|---|---|---|
| Compressive strength σ (MPa) | Mean | 1.50 | 0.94 | 1.57 | 3.71 | 1.02 | 2.09 |
| SD | 0.30 | 0.24 | 0.16 | 0.96 | 0.15 | 0.36 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabor, A.G.; Duma, V.-F.; Fabricky, M.M.C.; Marsavina, L.; Tudor, A.; Vancea, C.; Negrea, P.; Sinescu, C. Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy. Materials 2022, 15, 4899. https://doi.org/10.3390/ma15144899
Gabor AG, Duma V-F, Fabricky MMC, Marsavina L, Tudor A, Vancea C, Negrea P, Sinescu C. Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy. Materials. 2022; 15(14):4899. https://doi.org/10.3390/ma15144899
Chicago/Turabian StyleGabor, Alin Gabriel, Virgil-Florin Duma, Mihai M. C. Fabricky, Liviu Marsavina, Anca Tudor, Cosmin Vancea, Petru Negrea, and Cosmin Sinescu. 2022. "Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy" Materials 15, no. 14: 4899. https://doi.org/10.3390/ma15144899
APA StyleGabor, A. G., Duma, V.-F., Fabricky, M. M. C., Marsavina, L., Tudor, A., Vancea, C., Negrea, P., & Sinescu, C. (2022). Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy. Materials, 15(14), 4899. https://doi.org/10.3390/ma15144899











