Antibacterial Activity of Antibiotic-Releasing Polydopamine-Coated Nephrite Composites for Application in Drug-Eluting Contact Lens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
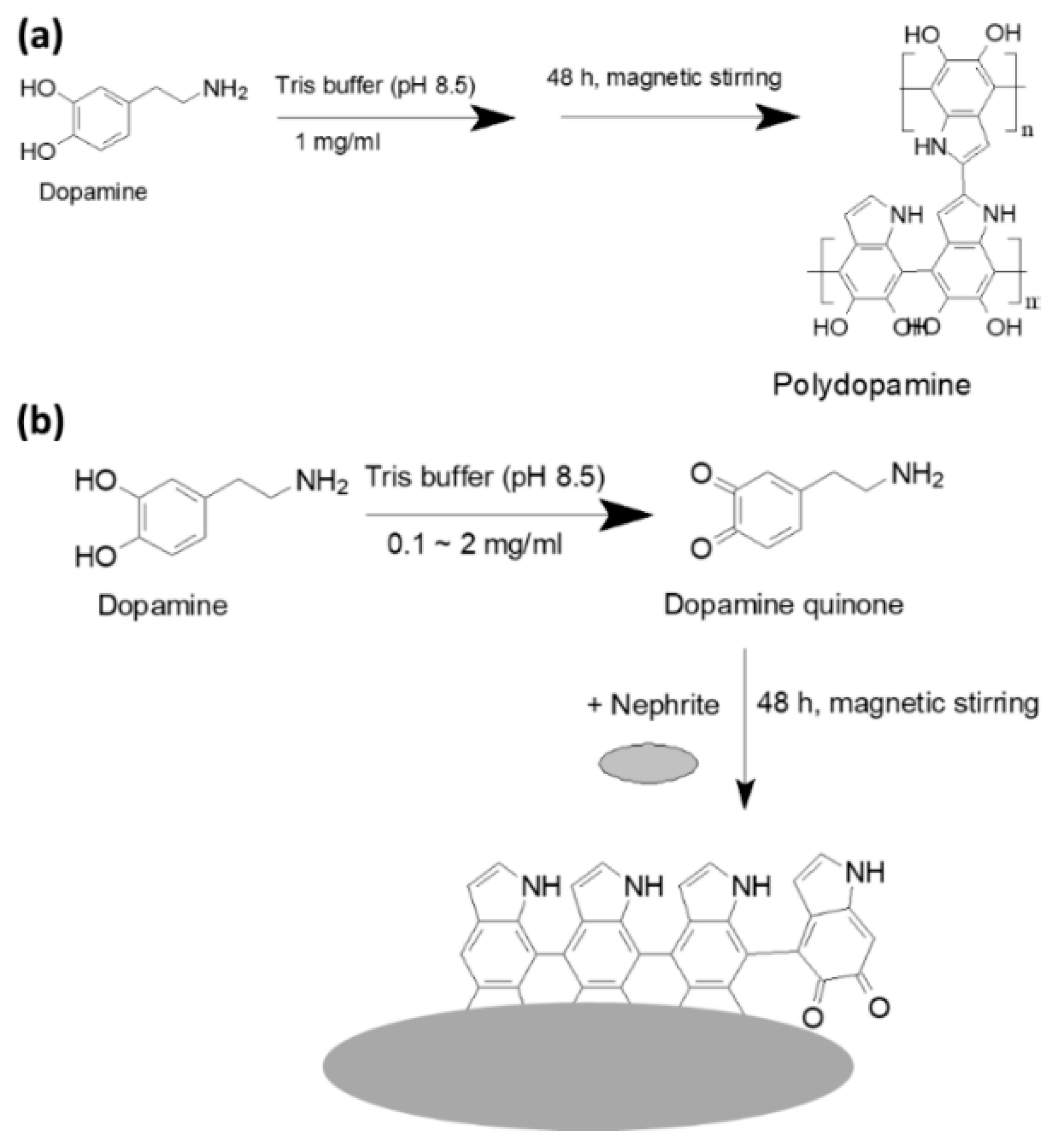
2.2. Fabrication of PDA-Coated Nephrite and Antibiotic Incorporation
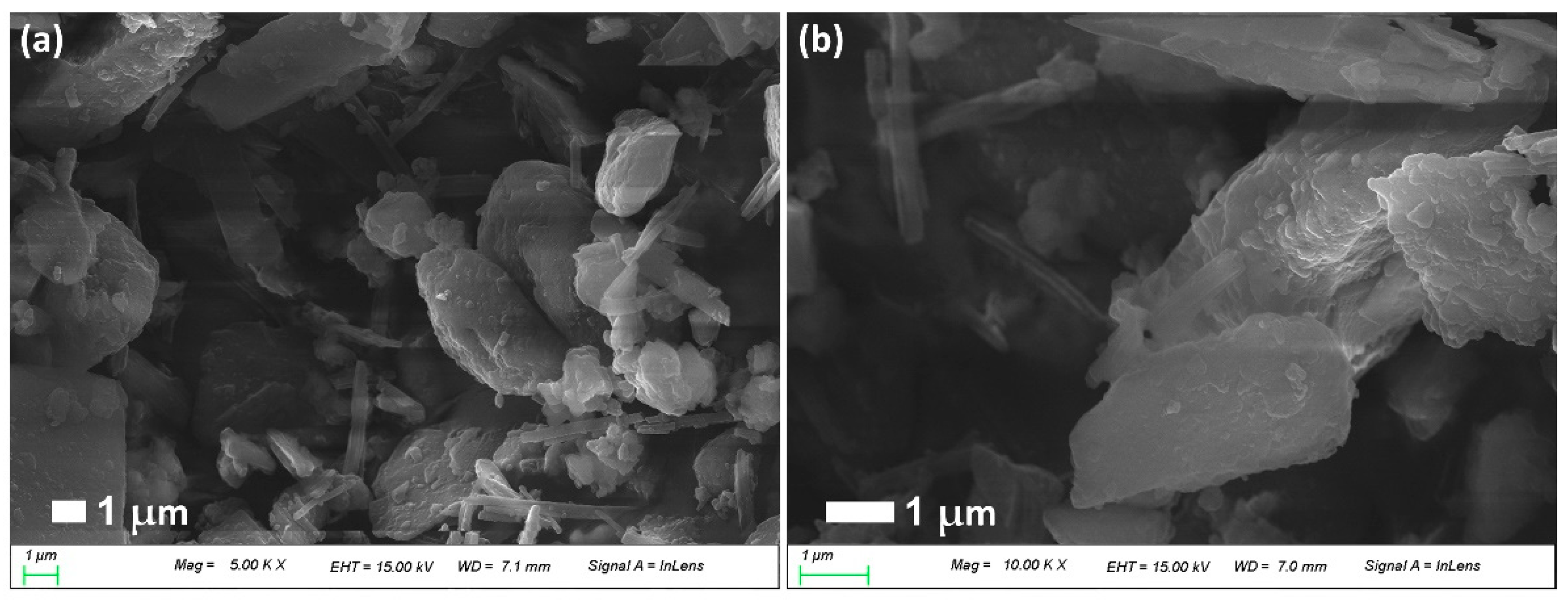
2.3. Morphology of CIP (or LEVO)-Incorporated/PDA-Coated Nephrite Composites
2.4. Crystalline Properties of CIP-Incorporated/PDA-Coated Nephrite Composites
- Data type = binary; goniometer = 1; attachment = 1; scan mode = continuous.
- Mode 2 (R/T) = reflection; scan axis = 2θ/θ.
- Start angle = 10.000; stop angle = 80.000; scan speed = 5.000; sampling interval = 0.050; θ angle = 5.000; 2θ angle = 10.000; fixed time = 0.01; full scale = 1000; counting unit = CPS; target= Cu.
- Wavelength Ka1 = 1.540510; wavelength Ka2 = 1.544330; wavelength Ka = 1.541780; wavelength Kb = 1.392170; 40.0 kV; 20.0 mA.
2.5. Drug Content Measurement and Drug Release Study
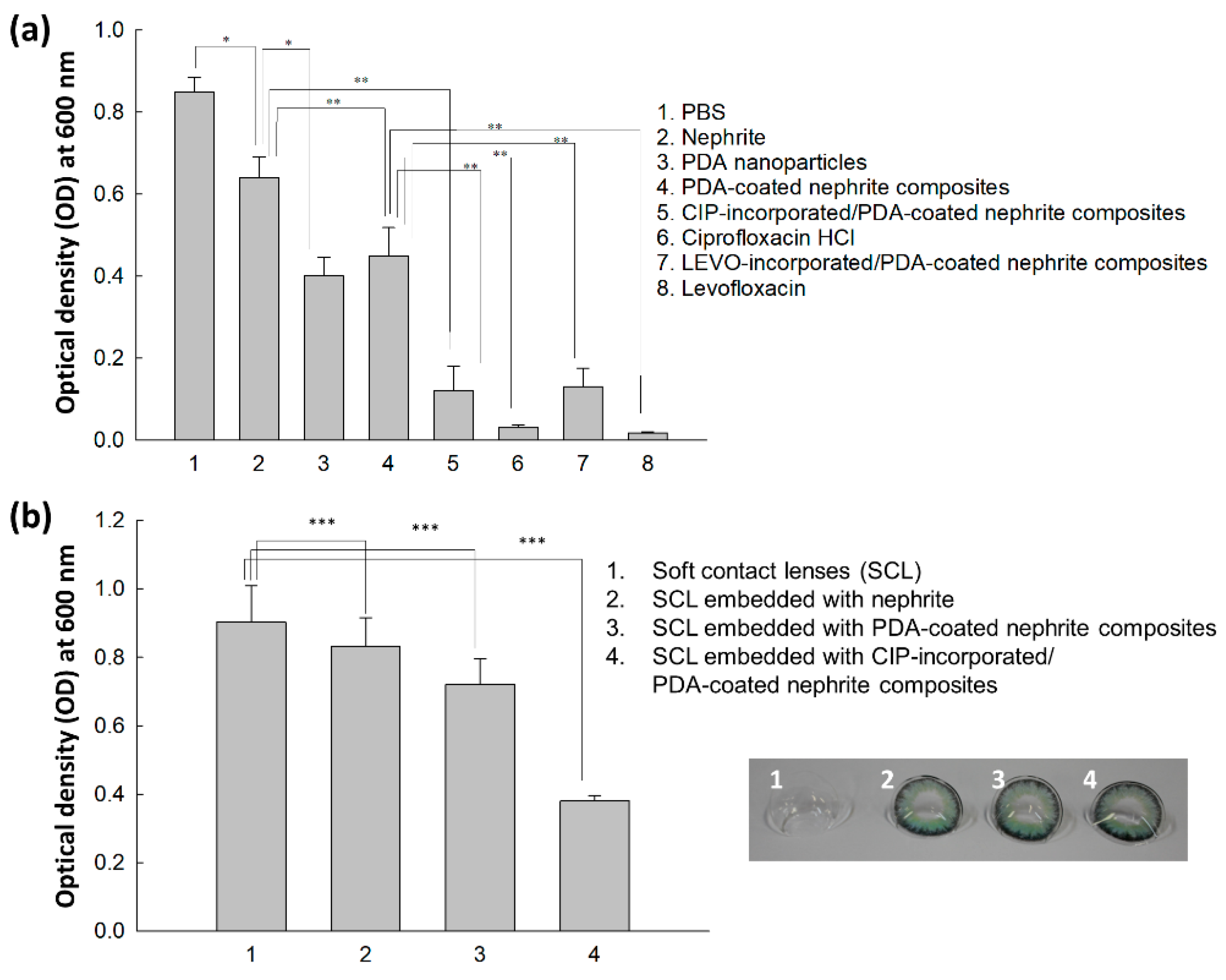
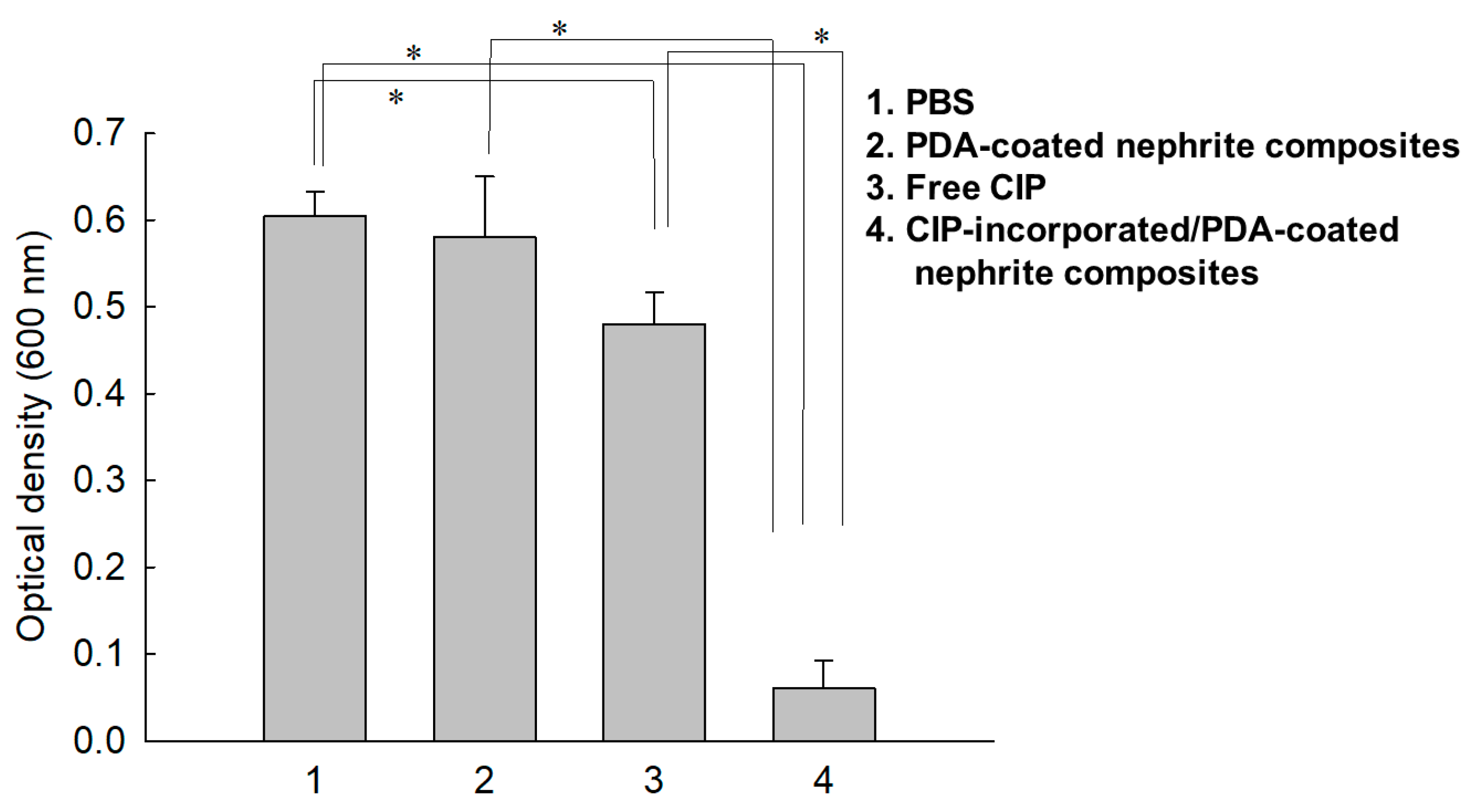
2.6. Antibacterial Activity In Vitro
2.7. Cell Cytotoxicity
2.8. Statistical Analysis
3. Results
3.1. CIP-Incorporated and PDA-Coated Nephrite Composites
3.2. Drug Release Study
3.3. Antibacterial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poggio, E.C.; Glynn, R.J.; Schein, O.D.; Seddon, J.M.; Shannon, M.J.; Scardino, V.A.; Kenyon, K.R. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 1989, 321, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Buehler, P.O.; Schein, O.D.; Stamler, J.F.; Verdier, D.D.; Katz, J. The increased risk of ulcerative keratitis among disposable soft contact lens users. Arch. Ophthalmol. 1992, 110, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Buehler, P.O.; Stamler, J.F.; Verdier, D.D.; Katz, J. The impact of overnight wear on the risk of contact lens-associated ulcerative keratitis. Arch. Ophthalmol. 1994, 112, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.B.; Nixon, A.D.; Rueff, E.M. Contact lens associated microbial keratitis: Practical considerations for the optometrist. Clin Optom. 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Hammersmith, K. Contact lens-related microbial keratitis: Recent outbreaks. Curr. Opin. Ophthalmol. 2008, 19, 302–306. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Lalitha, P.; Prajna, N.V.; Srinivasan, M.; Das, M.; D’Silva, S.S.; Oldenburg, C.E.; Borkar, D.S.; Esterberg, E.J.; Lietman, T.M.; et al. Acanthamoeba, fungal, and bacterial keratitis: A comparison of risk factors and clinical features. Am. J. Ophthalmol. 2014, 157, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Ung, L.; Chodosh, J. Foundational concepts in the biology of bacterial keratitis. Exp. Eye Res. 2021, 209, 108647. [Google Scholar] [CrossRef]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis antimicrobial resistance surveillance program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the management of infectious keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Kowalski, R.P.; Gordon, Y.J. Emerging fluoroquinolone resistance in bacterial keratitis: A 5-year review. Ophthalmology 1999, 106, 1313–1318. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, S.; Garg, P.; Rao, G.N. Clinical resistance of Staphylococcus keratitis to ciprofloxacin monotherapy. Indian J. Ophthalmol. 2004, 52, 287–292. [Google Scholar] [PubMed]
- Park, S.H.; Lee, S.U.; Kim, Y.K.; Yu, H.S.; Park, S.H.; Ahn, J.H.; Kim, S.J.; Shin, J.H.; Lee, J.E. Anti-staphylococcal Effect of a Nephrite-containing Contact Lens Storage Case. J. Korean Ophthalmol. Soc. 2020, 61, 868–875. [Google Scholar] [CrossRef]
- Lee, S.M.; Jung, J.W.; Lee, D.H.; Park, S.H.; Lee, J.H.; Yu, H.S.; Kim, Y.K.; Lee, J.E. Anti-pseudomonal Effect of a Nephrite-containing Contact Lens Storage Case. J. Korean Ophthalmol. Soc. 2018, 59, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Lee, J.H.; Park, S.H.; Yu, H.S.; Kim, Y.K.; Lee, J.E. Amoebicidal Effect of Nephrite-containing Contact Lens Storage Case. J. Korean Ophthalmol. Soc. 2017, 58, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Bär, F.W.; van der Veen, F.H.; Benzina, A.; Habets, J.; Koole, L.H. New biocompatible polymer surface coating for stents results in a low neointimal response. J. Biomed. Mater. Res. 2000, 52, 193–198. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Zhao, H.F.; Shen, J.C.; Fan, D.Z.; Sun, Y.F. A novel crosslinkable polymer as drug-loaded coating for biomedical device. J. Mater. Sci. Mater. Med. 2004, 15, 137–143. [Google Scholar] [CrossRef]
- McManamon, C.; de Silva, J.P.; Delaney, P.; Morris, M.A.; Cross, G.L.W. Characteristics, interactions and coating adherence of heterogeneous polymer/drug coatings for biomedical devices. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 102–108. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine antioxidant hydrogels for wound healing applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent advances in a polydopamine-mediated antimicrobial adhesion system. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, R.; Zou, Y.; Xing, D.; Zhong, K. Light-driven self-healing polyurethane based on PDA@Ag nanoparticles with improved mechanical and antibacterial properties. J. Mater. Chem. B 2022, 10, 1085–1093. [Google Scholar] [CrossRef]
- Xiong, F.; Wei, S.; Sheng, H.; Han, X.; Jiang, W.; Zhang, Z.; Li, B.; Xuan, H.; Xue, Y.; Yuan, H. In situ polydopamine functionalized poly-L-lactic acid nanofibers with near-infrared-triggered antibacterial and reactive oxygen species scavenging capability. Int. J. Biol. Macromol. 2022, 201, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Murari, G.; Bock, N.; Zhou, H.; Yang, L.; Liew, T.; Fox, K.; Tran, P.A. Effects of polydopamine coatings on nucleation modes of surface mineralization from simulated body fluid. Sci. Rep. 2020, 10, 14982. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Peng, Y.Y.; Cheng, Q.; Ishihara, K.; Liu, Y.; Narain, R. Dopamine assisted self-cleaning, antifouling, and antibacterial coating via dynamic covalent interactions. ACS Appl. Mater. Interfaces 2022, 14, 9557–9569. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Na, H.S.; Seo, D.H.; Kim, D.G.; Lee, H.C.; Jang, M.K.; Na, S.K.; Roh, S.H.; Kim, S.I.; Nah, J.W. Ciprofloxacin-encapsulated poly(DL-lactide-co-glycolide) nanoparticles and its antibacterial activity. Int. J. Pharm. 2008, 352, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.W.; Jia, H.R.; Wu, F.G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-generation contact lenses: Towards bioresponsive drug delivery and smart technologies in ocular therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Phan, C.M.; Concheiro, A.; Jones, L.; Alvarez-Lorenzo, C. Testing drug release from medicated contact lenses: The missing link to predict in vivo performance. J. Control. Release 2022, 343, 672–702. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.K.; Nirmal, J.; Garg, P.; Venuganti, V.V.K. Corneal delivery of moxifloxacin and dexamethasone combination using drug-eluting mucoadhesive contact lens to treat ocular infections. Int. J. Pharm. 2020, 591, 120023. [Google Scholar] [CrossRef] [PubMed]
- Bengani, L.C.; Kobashi, H.; Ross, A.E.; Zhai, H.; Salvador-Culla, B.; Tulsan, R.; Kolovou, P.E.; Mittal, S.K.; Chauhan, S.K.; Kohane, D.S.; et al. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020, 116, 149–161. [Google Scholar] [CrossRef] [PubMed]




| Composition | HEMA Soft Contact Lenses (%, w/w) |
|---|---|
| 2-HEMA | 98.9 1.0 0.1 |
| EGDMA AIBN |
| Composition Ratio (g) Antibiotics/Dopamine/Nephrite | Drug Contents (%, w/w) | |
|---|---|---|
| Theoretical a | Experimental b | |
| PDA-coated nephrite composites * | ||
| 0/1.0/1.0 | - | - |
| CIP-incorporated/ PDA-coated | ||
| nephrite composites | ||
| 0.2/0.1/1.0 | 15.4 | |
| 0.2/0.5/1.0 | 11.8 | 6.8 |
| 0.2/1.0/1.0 | 9.1 | 7.4 |
| 0.2/2.0/1.0 | 6.3 | 3.1 |
| 0.1/1.0/1.0 | 4.8 | 3.7 |
| LEVOP-incorporated/ PDA-coated | ||
| nephrite composites | ||
| 0.2/1.0/1.0 | 9.1 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.-S.; Moon, K.-J.; Lee, J.-E.; Jeong, Y.-I. Antibacterial Activity of Antibiotic-Releasing Polydopamine-Coated Nephrite Composites for Application in Drug-Eluting Contact Lens. Materials 2022, 15, 4823. https://doi.org/10.3390/ma15144823
Kang M-S, Moon K-J, Lee J-E, Jeong Y-I. Antibacterial Activity of Antibiotic-Releasing Polydopamine-Coated Nephrite Composites for Application in Drug-Eluting Contact Lens. Materials. 2022; 15(14):4823. https://doi.org/10.3390/ma15144823
Chicago/Turabian StyleKang, Min-Seung, Kyung-Jin Moon, Ji-Eun Lee, and Young-IL Jeong. 2022. "Antibacterial Activity of Antibiotic-Releasing Polydopamine-Coated Nephrite Composites for Application in Drug-Eluting Contact Lens" Materials 15, no. 14: 4823. https://doi.org/10.3390/ma15144823
APA StyleKang, M.-S., Moon, K.-J., Lee, J.-E., & Jeong, Y.-I. (2022). Antibacterial Activity of Antibiotic-Releasing Polydopamine-Coated Nephrite Composites for Application in Drug-Eluting Contact Lens. Materials, 15(14), 4823. https://doi.org/10.3390/ma15144823






