Microwave Treatment of Calcium Phosphate/Titanium Dioxide Composite to Improve Protein Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 Nanomaterials
2.2. Preparation of Calcium Phosphate Coatings and Microwave Treatment
2.3. Characterization of CAP/TiNF Coatings
2.4. Protein Adsorption
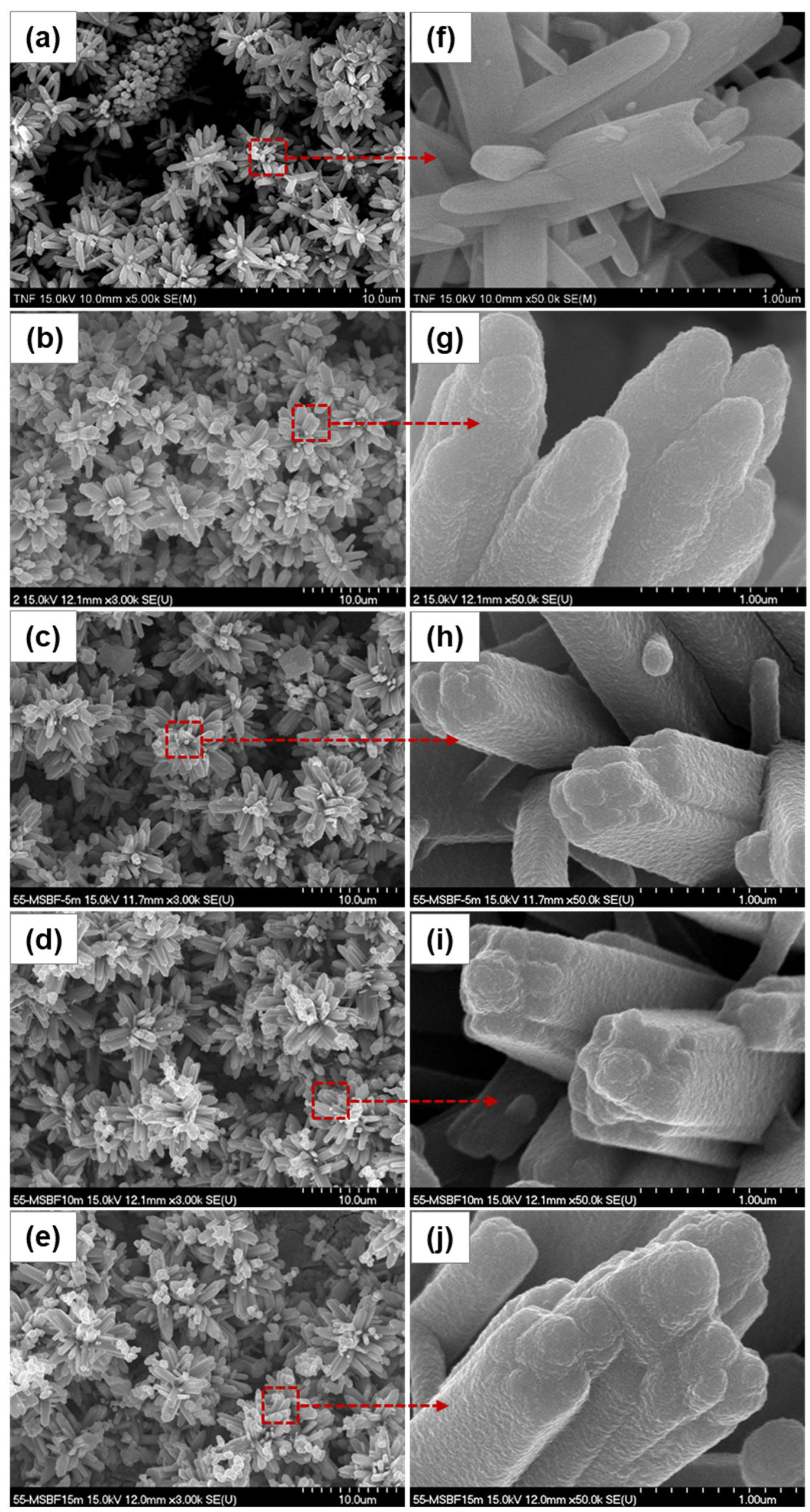
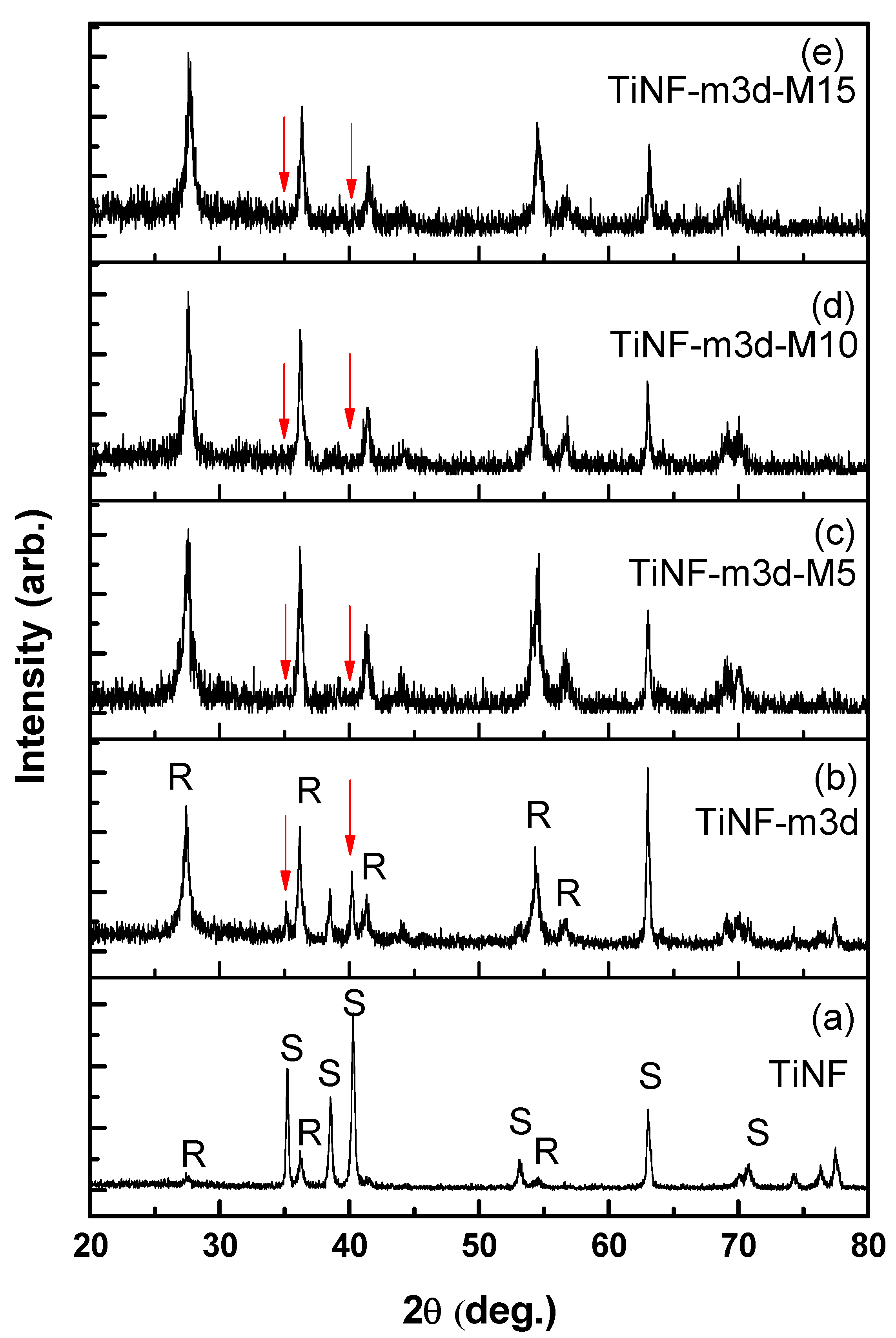
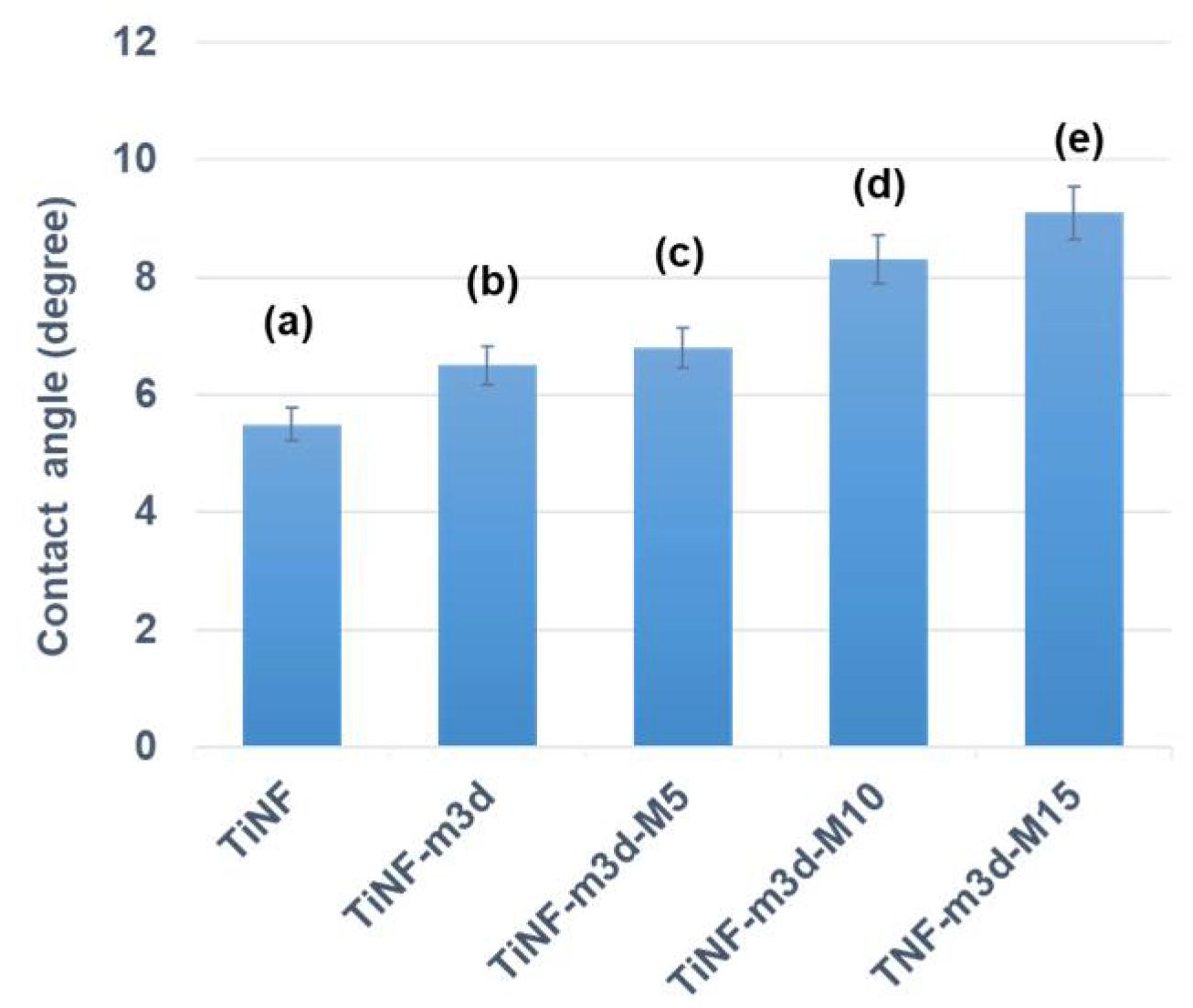
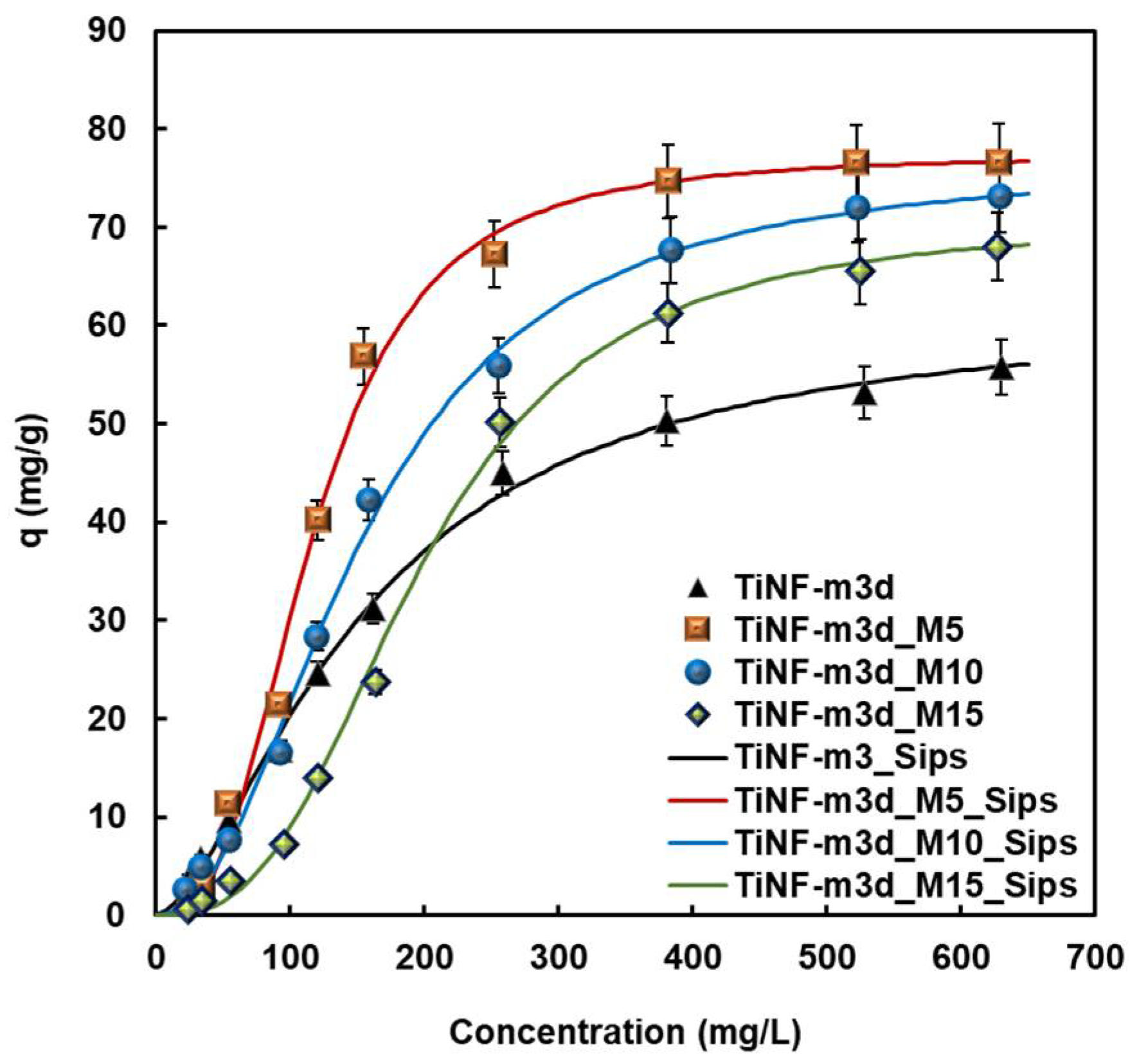
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate–TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Mangkonsu, C.; Kunio, I.; Bunhan, L.; Otman, R.; Noor, A.-F.M. The effect of microwave sintering on the microstructure and properties of calcium phosphate ceramic. Procedia Chem. 2016, 19, 498–504. [Google Scholar] [CrossRef][Green Version]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef] [PubMed]
- Stipniece, L.; Kondratjeva, A.; Salma-Ancane, K. Influence of precursor characteristics on properties of porous calcium phosphate-titanium dioxide composite bioceramics. Ceram. Int. 2020, 46, 243–250. [Google Scholar] [CrossRef]
- Fang, Y.; Agrawal, D.K.; Roy, D.M.; Roy, R. Microwave sintering of hydroxyapatite ceramics. J. Mater. Res. 1994, 9, 180–187. [Google Scholar] [CrossRef]
- Bose, S.; Dasgupta, S.; Tarafder, S.; Bandyopadhyay, A. Microwave-processed nanocrystalline hydroxyapatite: Simultaneous enhancement of mechanical and biological properties. Acta Biomater. 2010, 6, 3782–3790. [Google Scholar] [CrossRef]
- Khorami, M.; Hesaraki, S.; Ebadzadeh, T.; Farhangdoust, S.; Zamanian, A. In The effect of microwave irradiation on structural and mechanical properties of nano-structured bone-like carbonated hydroxyapatite. Key Eng. Mater. 2012, 493–494, 231–235. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Titanium as a reconstruction and implant material in dentistry: Advantages and pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M.; Arslan, O. Surface properties of bovine serum albumin–adsorbed oxides: Adsorption, adsorption kinetics and electrokinetic properties. Microporous Mesoporous Mater. 2006, 96, 331–340. [Google Scholar] [CrossRef]
- Qaid, T.H.; Ramesh, S.; Yusof, F.; Basirun, W.J.; Ching, Y.C.; Chandran, H.; Ramesh, S.; Krishnasamy, S. Micro-arc oxidation of bioceramic coatings containing eggshell-derived hydroxyapatite on titanium substrate. Ceram. Int. 2019, 45, 18371–18381. [Google Scholar] [CrossRef]
- Park, K.H.; Song, H.-J.; Park, Y.-J. Fabrication and Electrochemical Properties of Hierarchical Titanium Dioxide Nanoflower-Calcium Phosphate Composites. Int. J. Electrochem. Sci. 2021, 16, 210412. [Google Scholar] [CrossRef]
- García, A.J. Get a grip: Integrins in cell–biomaterial interactions. Biomaterials 2005, 26, 7525–7529. [Google Scholar] [CrossRef] [PubMed]
- Jenney, C.R.; Anderson, J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. Part B 2000, 49, 435–447. [Google Scholar] [CrossRef]
- He, H.; Li, G.; Li, B.; Chen, Z. Effects of surface microstructure of hydroxyapatite on protein adsorption and biological performance of osteoblasts. Appl. Surf. Sci. 2008, 255, 565–567. [Google Scholar] [CrossRef]
- Sahu, D.P.; Jammalamadaka, S.N. Detection of bovine serum albumin using hybrid TiO2 + graphene oxide based Bio-resistive random access memory device. Sci. Rep. 2019, 9, 16141. [Google Scholar] [CrossRef]
- Chen, F.L.; Luh, H.T.; Hsiao, Y.C. Label-free, color-indicating, polarizer-free dye-doped liquid crystal microfluidic polydimethylsiloxane biosensing chips for detecting albumin. Polymers 2021, 13, 2587. [Google Scholar] [CrossRef]
- Chen, F.L.; Fan, Y.J.; Lin, J.D.; Hsiao, Y.C. Label-free, color-indicating, and sensitive biosensors of cholesteric liquid crystals on a single vertically aligned substrate. Biomed. Opt. Express 2019, 10, 4636–4642. [Google Scholar] [CrossRef]
- Lee, M.J.; Duan, F.F.; Wu, P.C.; Lee, W. Liquid crystal–photopolymer composite films for label-free single-substrate protein quantitation and immunoassay. Biomed. Opt. Express 2020, 11, 4915–4927. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Huang, T.; Long, M.; Huo, B. Competitive Binding to Cuprous Ions of Protein and BCA in the Bicinchoninic Acid Protein Assay. Open Biomed. Eng. 2010, 4, 271–278. [Google Scholar] [CrossRef]
- Kopac, T.; Bozgeyik, K.; Yener, J. Effect of pH and temperature on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 19–28. [Google Scholar] [CrossRef]
- Zhou, H.; Nabiyouni, M.; Bhaduri, S.B. Microwave assisted apatite coating deposition on Ti6Al4V implants. Mater. Sci. Eng. C 2013, 33, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Ren, F.; Bhaduri, S.B. Microwave processing of calcium phosphate and magnesium phosphate based orthopedic bioceramics: A state-of-the-art review. Acta Biomater. 2020, 111, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915–5927. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite-Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Saito, S.; Hamai, R.; Shiwaku, Y.; Hasegawa, T.; Sakai, S.; Tsuchiya, K.; Sai, Y.; Iwama, R.; Amizuka, N.; Takahashi, T.; et al. Involvement of distant octacalcium phosphate scaffolds in enhancing early differentiation of osteocytes during bone regeneration. Acta Biomater. 2021, 129, 309–322. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and Qualitative Evaluation of Adsorption/Desorption of Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces. Langmuir 2009, 25, 11614–11620. [Google Scholar] [CrossRef]
- Wassell, D.T.H.; Hall, R.C.; Embery, G. Adsorption of bovine serum albumin onto hydroxyapatite. Biomaterials 1995, 16, 697–702. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and protein adsorption: An overview of mechanisms and effects of surface features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]


| Reagent | SBF | m-SBF | ||
|---|---|---|---|---|
| mM | g/L | mM | g/L | |
| NaCl | 141.0 | 8.240 | 141.0 | 8.240 |
| KCl | 4.0 | 0.300 | 4.0 | 0.300 |
| MgSO4 | 0.5 | 0.060 | 0.5 | 0.060 |
| MgCl2 | 1.0 | 0.950 | 1.0 | 0.950 |
| NaHCO3 | 4.2 | 0.353 | 4.2 | 0.353 |
| CaCl2·2H2O | 2.5 | 0.368 | 5.0 | 0.736 |
| KH2PO4 | 1.0 | 0.136 | 2.0 | 0.272 |
| Tri-HCl | 3.940 | 3.940 | ||
| Samples | Simulation Model | qm (mg g−1) | b (L mg−1) | m(-) | RMSE 1 |
|---|---|---|---|---|---|
| TiNF-m3d | Langmuir Sips | 91.97 | 2.93 × 10−3 | 0.973 | |
| 61.63 | 2.94 × 10−3 | 1.612 | 0.994 | ||
| TiNF-m3d-M5 | Sips | 77.30 | 1.49 × 10−6 | 2.818 | 0.991 |
| TiNF-m3d-M10 | Sips | 76.56 | 1.76 × 10−5 | 2.175 | 0.995 |
| TiNF-m3d-M15 | Sips | 70.49 | 2.93 × 10−7 | 2.849 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Song, H.-J.; Park, Y.-J. Microwave Treatment of Calcium Phosphate/Titanium Dioxide Composite to Improve Protein Adsorption. Materials 2022, 15, 4773. https://doi.org/10.3390/ma15144773
Park KH, Song H-J, Park Y-J. Microwave Treatment of Calcium Phosphate/Titanium Dioxide Composite to Improve Protein Adsorption. Materials. 2022; 15(14):4773. https://doi.org/10.3390/ma15144773
Chicago/Turabian StylePark, Kyung Hee, Ho-Jun Song, and Yeong-Joon Park. 2022. "Microwave Treatment of Calcium Phosphate/Titanium Dioxide Composite to Improve Protein Adsorption" Materials 15, no. 14: 4773. https://doi.org/10.3390/ma15144773
APA StylePark, K. H., Song, H.-J., & Park, Y.-J. (2022). Microwave Treatment of Calcium Phosphate/Titanium Dioxide Composite to Improve Protein Adsorption. Materials, 15(14), 4773. https://doi.org/10.3390/ma15144773








