Low-Temperature Rapid Sintering of Dense Cubic Phase ZrW2−xMoxO8 Ceramics by Spark Plasma Sintering and Evaluation of Its Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZrW2−xMoxO7(OH)2·2H2O Precursors
2.2. Fabrication of Cubic-ZrW2−xMoxO8 Ceramics
2.3. Evaluation Method
3. Results and Discussion
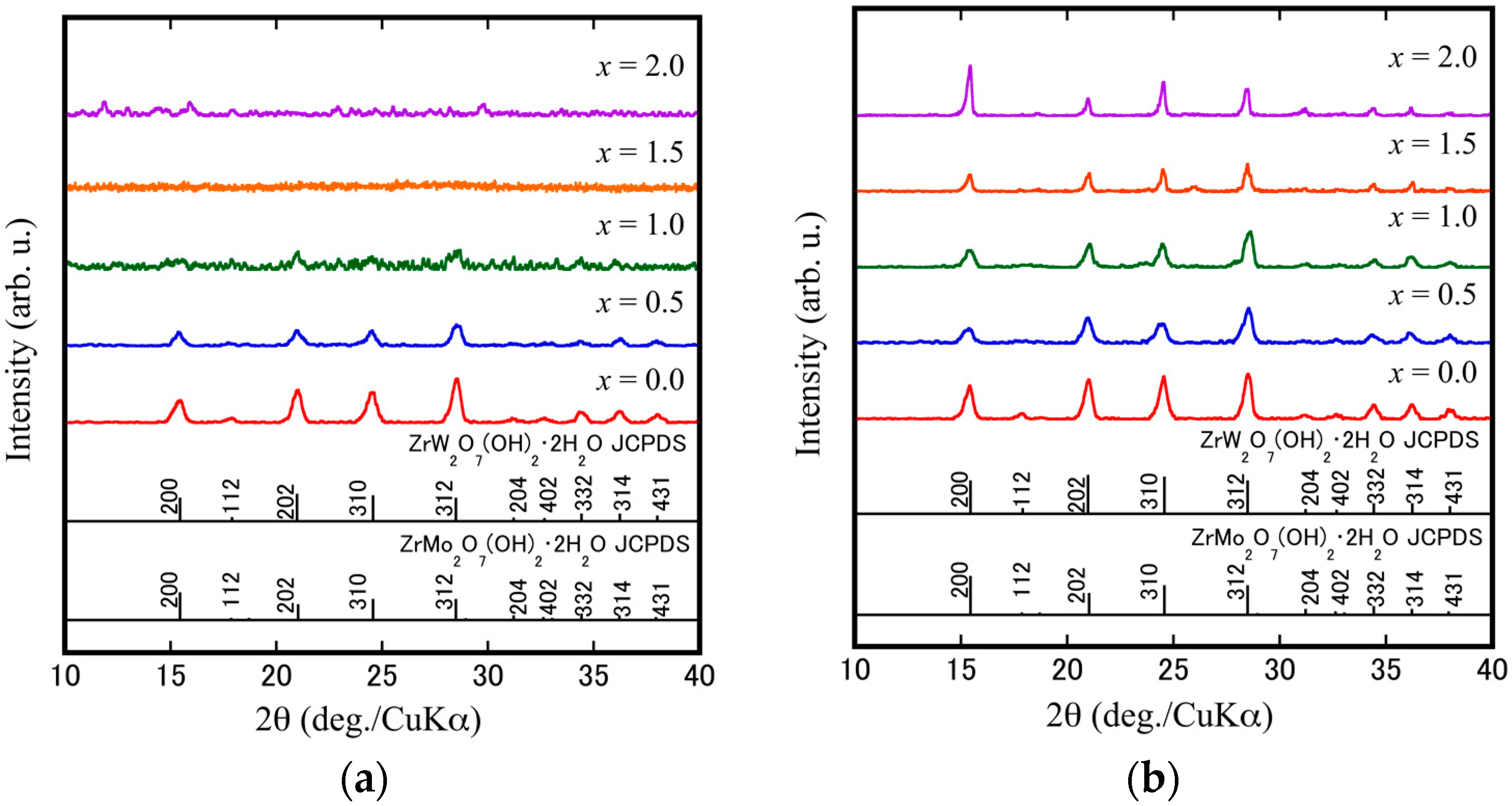
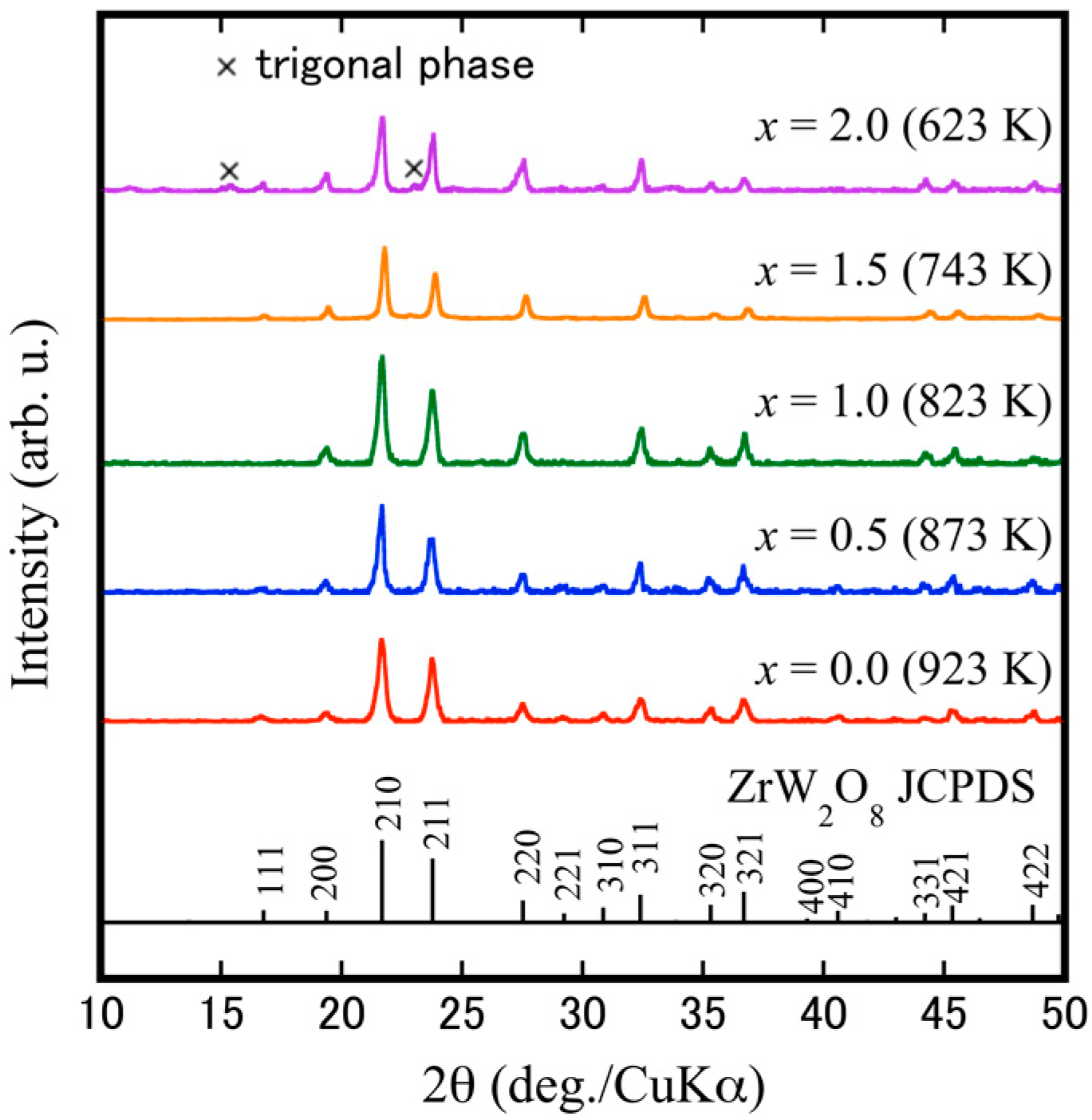
3.1. Preparation of ZrW2−xMoxO7(OH)2·2H2O Precursors
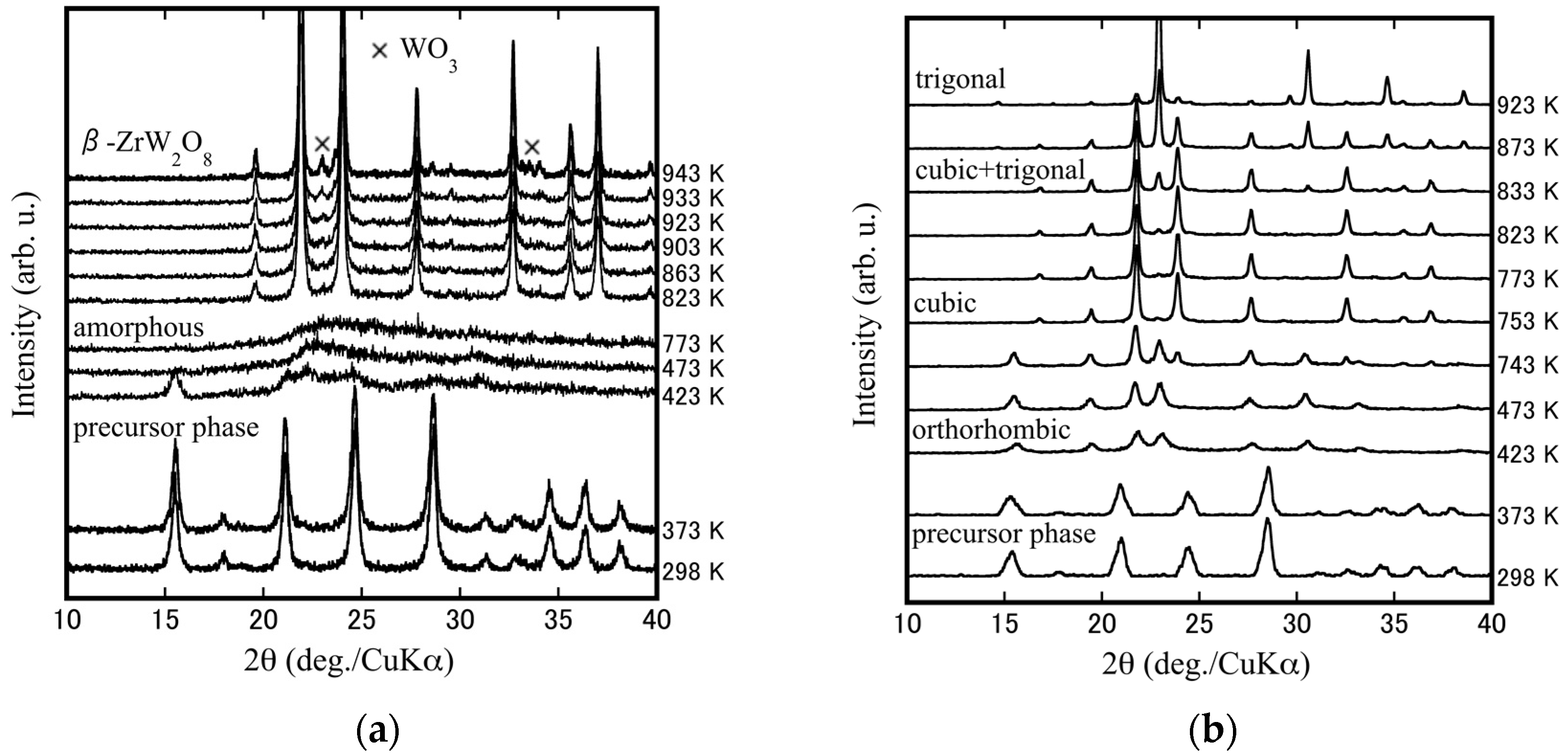
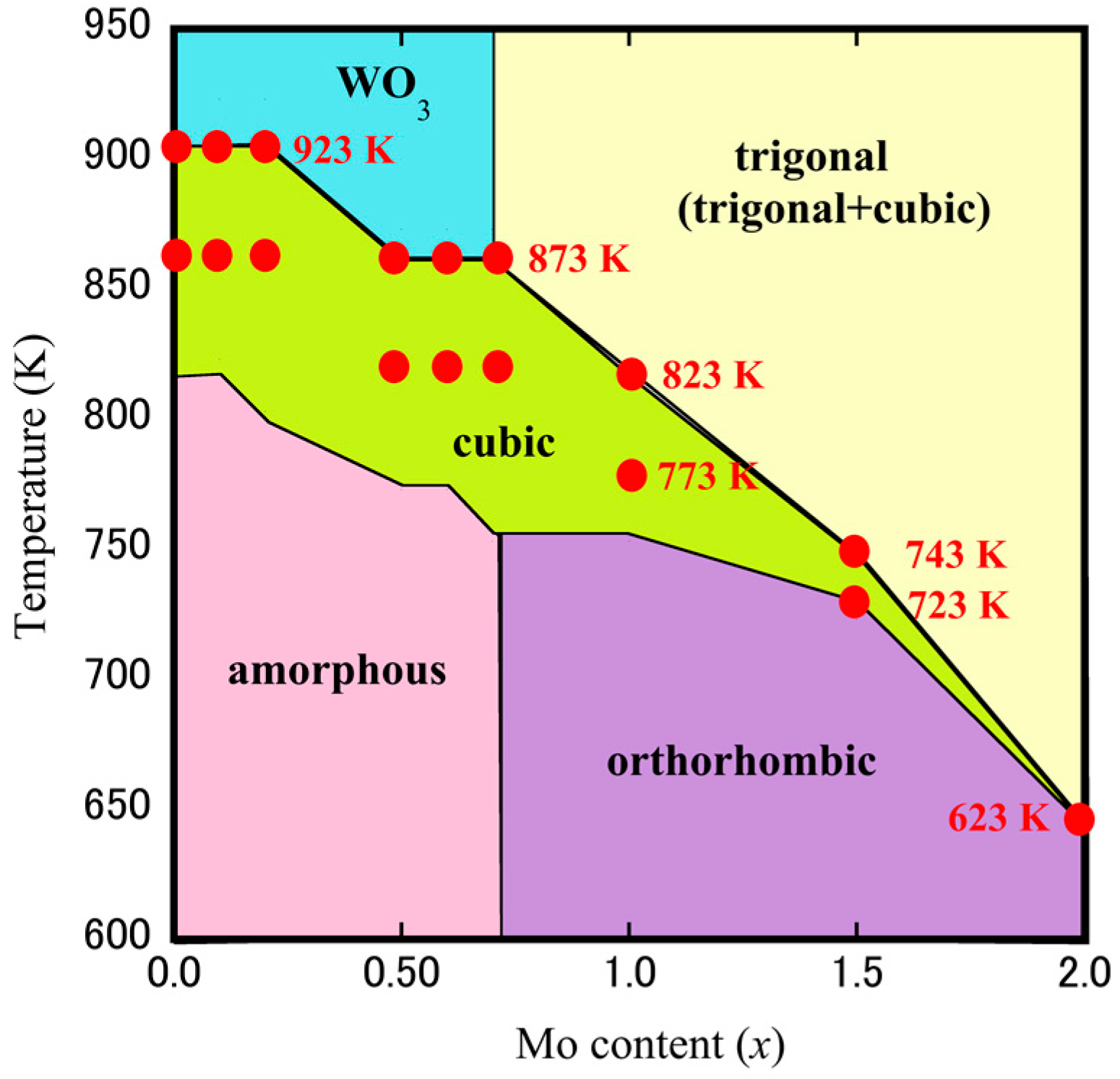
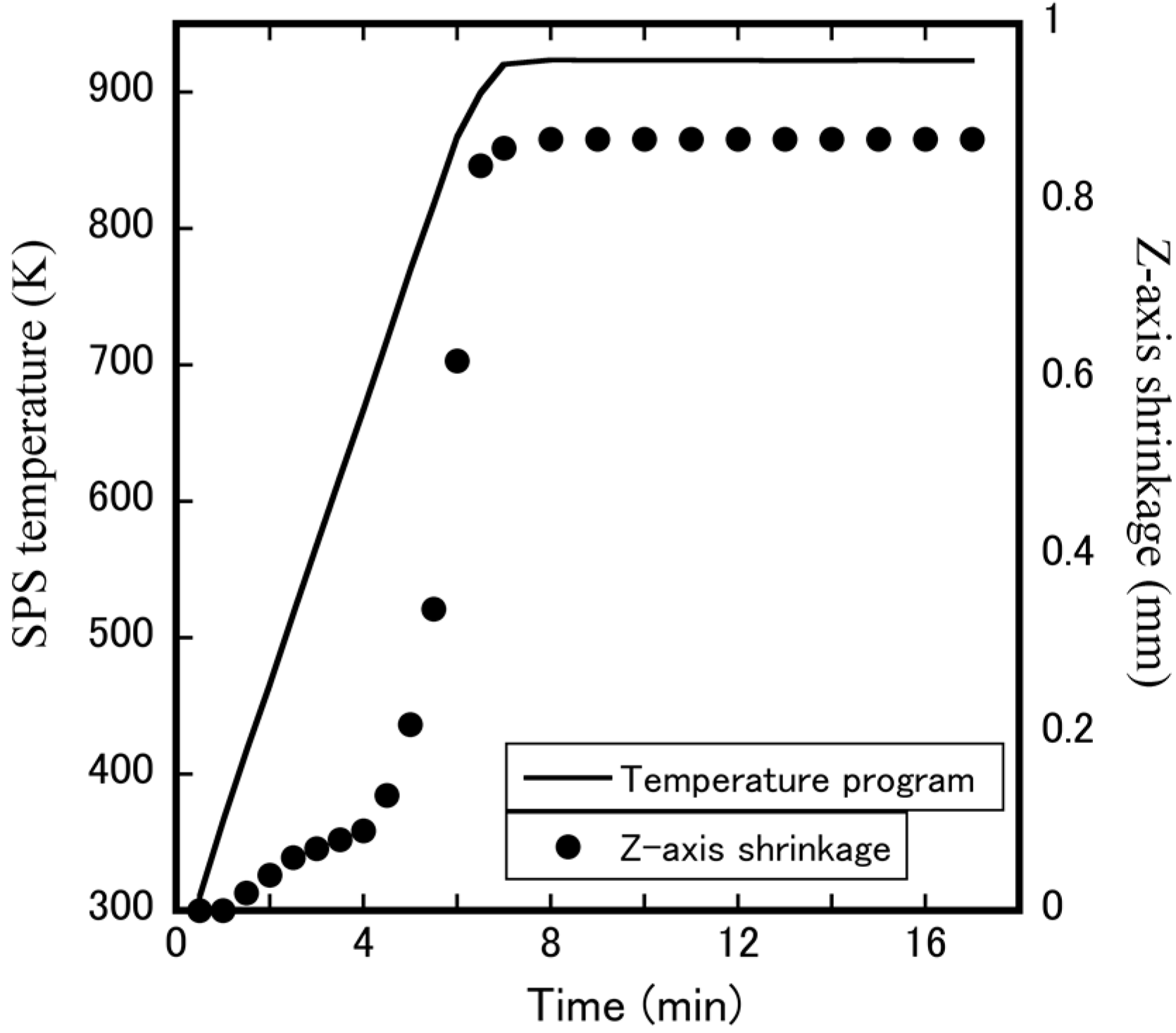
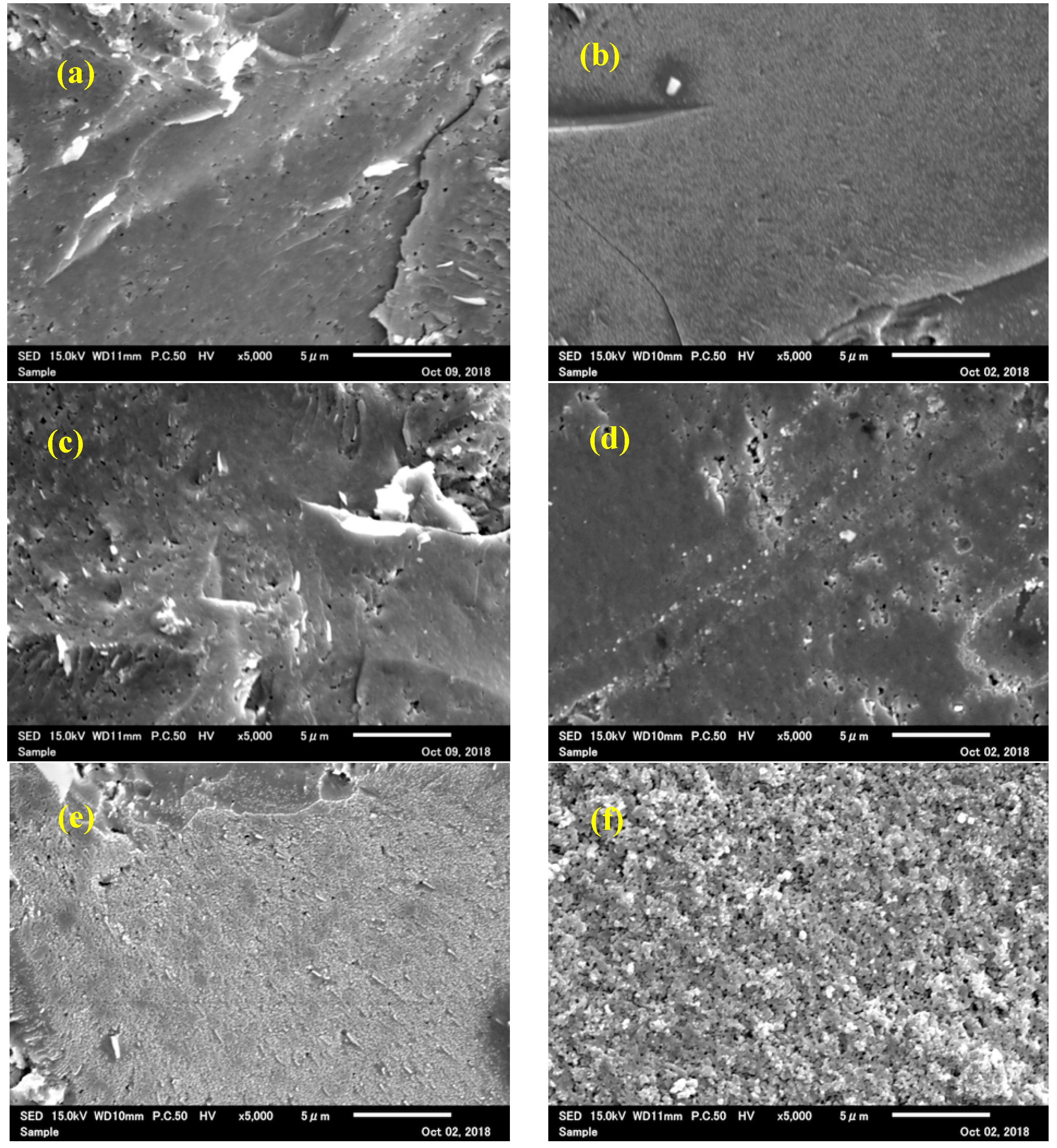
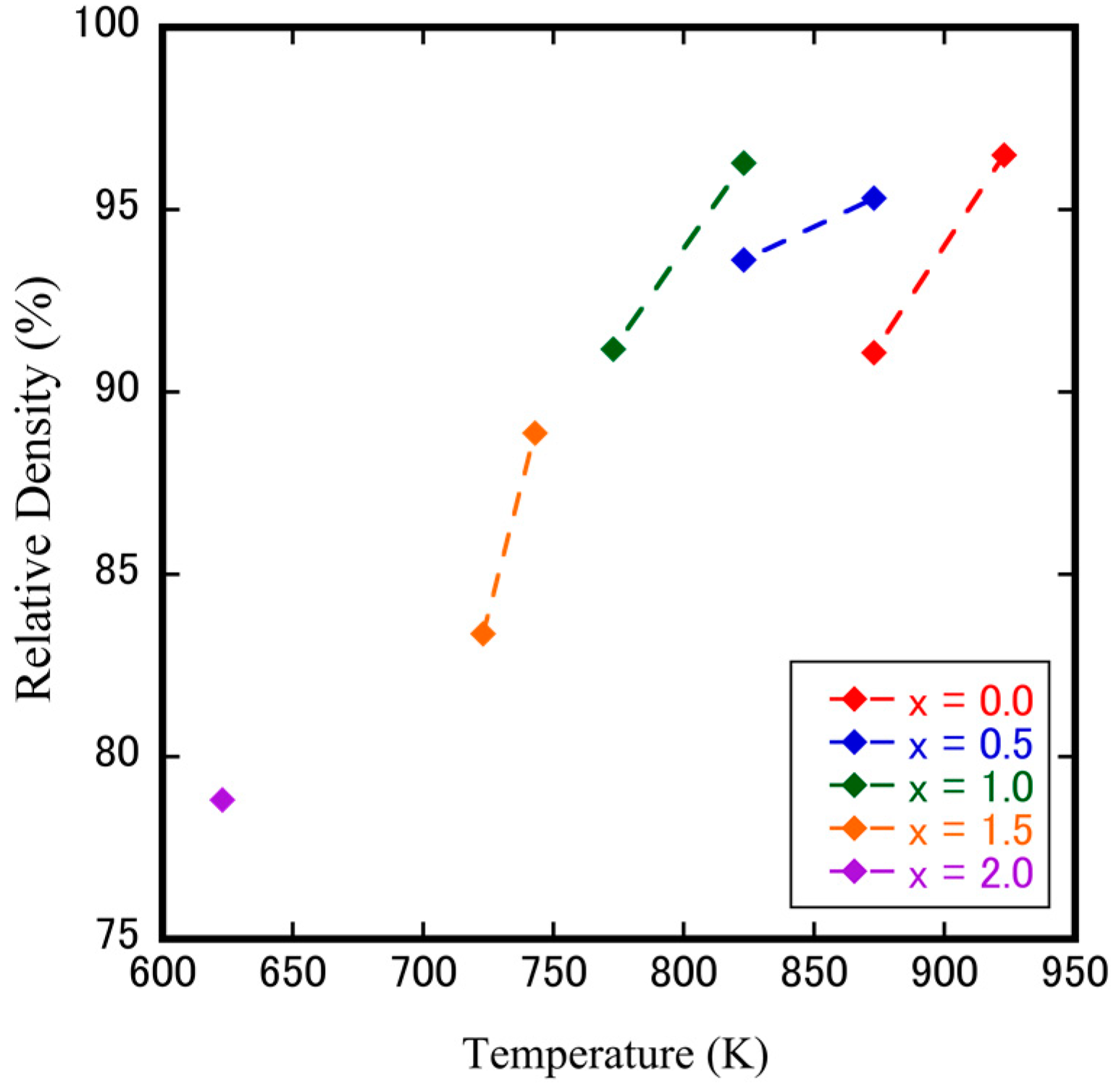
3.2. Fabrication of Dense Cubic-ZrW2–xMoxO8 Bulk Ceramics
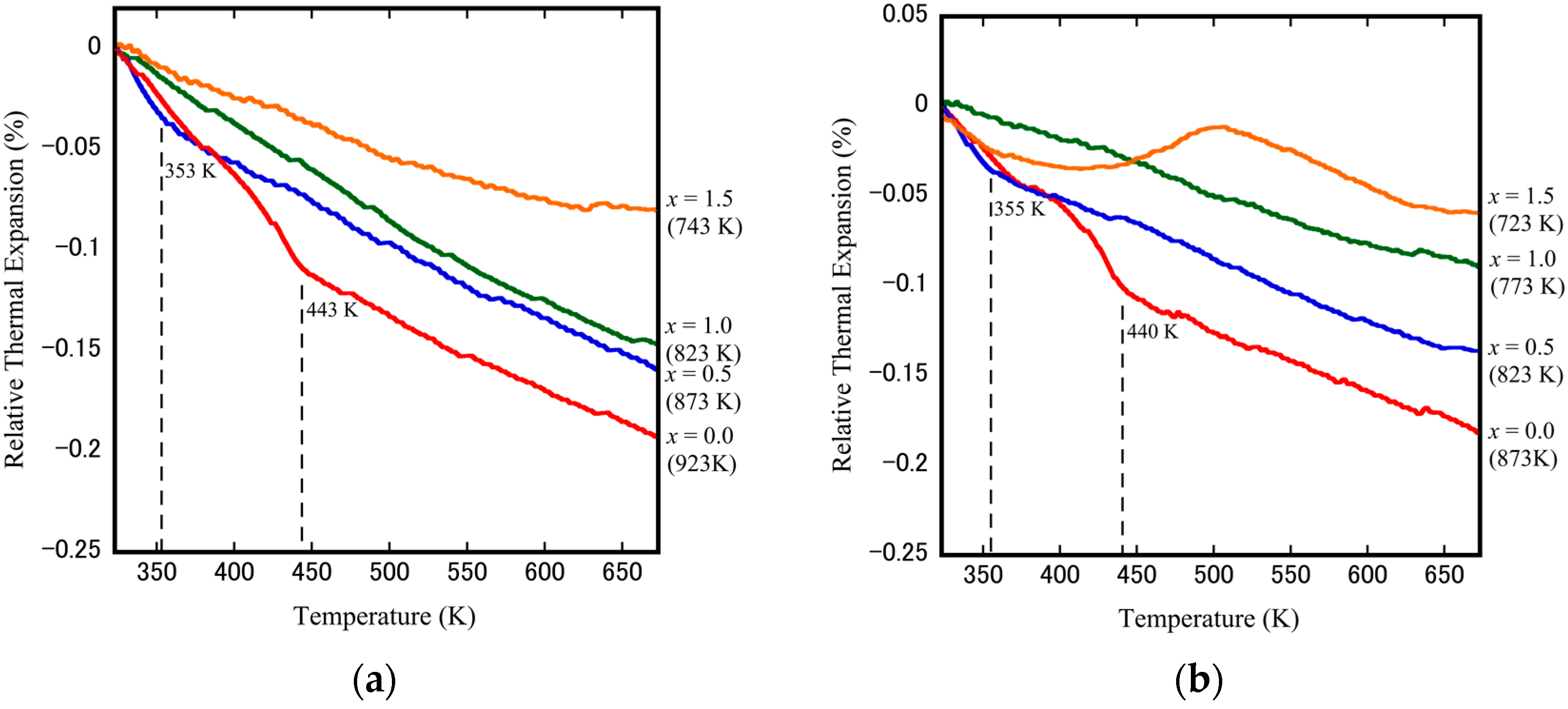
3.3. Evaluation of NTE Coefficients of Dense Cubic-ZrW2−xMoxO8 Bulk Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Closmann, C.; Sleight, A.W.; Haygarth, J.C. Low-Temperature Synthesis of ZrW2O8 and Mo-Substituted ZrW2O8. J. Solid State Chem. 1998, 139, 424–426. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hanson, P.A.; Ibberson, R.M.; Kameswari, U.; Duan, N.; Sleight, A.W. Low-Temperature Oxygen Migration and Negative Thermal Expansion in ZrW2−xMoxO8. J. Am. Chem. Soc. 2000, 122, 8694–8699. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Lindley, K.; Akinc, M. Hydrothermal Synthesis of ZrW2−δMoδO8 (δ = 0–0.91) and its α→β Transformation. J. Am. Ceram. Soc. 2011, 94, 2619–2624. [Google Scholar] [CrossRef]
- Shi, Y.F.; Chen, X.; Han, J.S.; Ma, H.; Li, X.X.; Yang, X.J.; Zhao, X.H. Phase transition behavior for ZrW2−xMoxO8 compositions at elevated temperatures. J. Solid State Chem. 2009, 182, 2030–2035. [Google Scholar] [CrossRef]
- Petrushina, M.Y.; Dedova, E.S.; Filatov, E.Y.; Plyusnin, P.E.; Korenev, S.V.; Kulkov, S.N.; Gubanov, A.I. Preparation of Zr(Mo,W)2O8 with a larger negative thermal expansion by controlling the thermal decomposition of Zr(Mo,W)2(OH,Cl)2·2H2O. Sci. Rep. 2018, 8, 5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.Q.; Yang, X.J.; Wang, H.L.; Han, J.S.; Ma, H.; Zhao, X. A novel route to synthesize cubic ZrW2−xMoxO8 (x = 0–1.3) solid solutions and their negative thermal expansion properties. J. Solid State Chem. 2007, 180, 3160–3165. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Q.G.; Ma, H.; Li, G.B.; Liao, F.H.; Qi, C.M.; Zhao, X.H. Phase Behaviors of the ZrMo2–xWxO8 (x = 0.2–2.0) System and the Preparation of an Mo-Rich Cubic Phase. Eur. J. Inorg. Chem. 2005, 2005, 4521–4526. [Google Scholar] [CrossRef]
- Jing, N.N.; Xu, S.; Wang, Z.Q.; Wang, G.X. Enhanced Electrochemical Performance and Safety of Silicon by a Negative Thermal Expansion Material of ZrW2O8. ACS Appl. Mater. Interfaces 2021, 13, 30468–30478. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.P.; Yang, D.L.; Li, S.; Qin, Y.Y.; Meng, S.H.; Du, S.Y. ZrW2O8 with Negative Thermal Expansion Fabricated at Ultralow Temperature: An Energy-Efficient Strategy for Metastable Material Fabrication. ACS Sustain. Chem. Eng. 2019, 7, 14747–14755. [Google Scholar] [CrossRef]
- Rottger, K.; Endriss, A.; Ihringer, J.; Doyle, S.; Kuhs, W.F. Lattice constants and thermal expansion of H2O and D2O Ice Ih between 10 and 265 K. Acta Crystallogr. B 1994, 50, 644–648. [Google Scholar] [CrossRef]
- Evans, J.S.O. Negative thermal expansion materials. Dalton Trans. 1999, 19, 3317–3326. [Google Scholar] [CrossRef]
- Rossetti, G.A.; Cline, J.P.; Navrotsky, A. Phase transition energetics and thermodynamic properties of ferroelectric PbTiO3. J. Mater. Res. 1998, 13, 3197–3206. [Google Scholar] [CrossRef]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative thermal expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.S.O.; Mary, T.A.; Vogt, T.; Subramanian, M.A.; Sleight, A.W. Negative Thermal Expansion in ZrW2O8 and HfW2O8. Chem. Mater. 1996, 8, 2809–2823. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hu, Z.; Jorgensenm, J.D.; Argyriou, D.N.; Short, S.; Sleight, A.W. Compressibility, Phase Transitions, and Oxygen Migration in Zirconium Tungstate, ZrW2O8. Science 1997, 275, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.W. Compounds That Contract on Heating. Inorg. Chem. 1998, 37, 2854–2860. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.X.; Zhang, Q.; Meng, Q.Y.; Zhang, L.Y.; Kobayashi, E.; Wu, G.H. Near-zero thermal expansion of ZrW2O8/Al–Si composites with three dimensional interpenetrating network structure. Compos. Part B 2021, 211, 108678. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.P.; Wang, X.F.; Yang, D.L.; Shi, H.F.; Meng, S.H.; Du, S.Y. ZrW2O8/ZrO2 Composites with Low/Near-Zero Coefficients of Thermal Expansion Fabricated at Ultralow Temperature: An Integration of Hydrothermal Assembly and a Cold Sintering Process. ACS Appl. Mater. Interfaces 2021, 13, 39738–39747. [Google Scholar] [CrossRef]
- Evans, J.S.O.; David, W.I.F.; Sleight, A.W. Structural investigation of the negative-thermal-expansion material ZrW2O8. Acta Crystallogr. B 1999, 5, 333–340. [Google Scholar] [CrossRef]
- Allen, S.; Evans, J.S.O. Negative thermal expansion and oxygen disorder in cubic ZrMo2O8. Phys. Rev. B 2003, 68, 134101–134103. [Google Scholar] [CrossRef]
- Kameswari, U.; Sleight, A.W.; Evans, J.S.O. Rapid synthesis of ZrW2O8 and related phases, and structure refinement of ZrWMoO8. Int. J. Inorg. Mater. 2000, 2, 333–337. [Google Scholar] [CrossRef]
- Readman, J.E.; Lister, S.E.; Peters, L.; Wright, J.; Evans, J.S.O. Direct Synthesis of Cubic ZrMo2O8 Followed by Ultrafast In Situ Powder Diffraction. J. Am. Chem. Soc. 2009, 131, 17560–17562. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Rawn, C.J.; Payzant, E.A. Preparation of the negative thermal expansion material cubic ZrMo2O8. J. Mater. Chem. 2001, 11, 3354–3359. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Hu, Z. Synthesis and properties of the negative thermal expansion material cubic ZrMo2O8. Chem. Mater. 1998, 10, 2335–2337. [Google Scholar] [CrossRef]
- Auray, M.; Quarton, M.; Tarte, P. New structure of high-temperature zirconium molybdate. Acta Cryst. 1986, 42, 257–259. [Google Scholar] [CrossRef]
- Chang, L.L.Y.; Scroger, M.G.; Phillips, B. Condensed Phase Relation in the Systems ZrO2–WO2–WO3 and HfO2–WO2–WO3. J. Am. Ceram. Soc. 1967, 50, 211–216. [Google Scholar] [CrossRef]
- Graham, J.; Wadsley, A.D.; Weymouth, J.H.; Williams, L.S. A New Ternary Oxide, ZrW2O8. J. Am. Ceram. Soc. 1959, 42, 570. [Google Scholar] [CrossRef]
- Martinek, C.; Hummel, F.A. Linear Thermal Expansion of Three Tungstates. J. Am. Ceram. Soc. 1968, 51, 227–228. [Google Scholar] [CrossRef]
- Buysser, K.D.; Driessche, I.V.; Schaubroeck, J.; Hoste, S. EDTA Assisted Sol–Gel Synthesis of ZrW2O8. J. Sol-Gel Sci. Technol. 2008, 46, 133–136. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P. Seeding and the Non-Hydrolytic Sol–Gel Synthesis of ZrW2O8 and ZrMo2O8. J. Sol-Gel Sci. Technol. 2002, 25, 51–56. [Google Scholar] [CrossRef]
- Wilkinson, A.P.; Lind, C.; Pattanaik, S. A New Polymorph of ZrW2O8 Prepared using Nonhydrolytic Sol-Gel Chemistry. Chem. Mater. 1999, 11, 101–108. [Google Scholar] [CrossRef]
- Kanamori, K.; Kineri, T.; Fukuda, R.; Nishio, K.; Hashimoto, M.; Mae, H. Spark Plasma Sintering of Sol-Gel Derived Amorphous ZrW2O8 Nanopowder. J. Am. Ceram. Soc. 2009, 92, 32–35. [Google Scholar] [CrossRef]
- Wei, H.; Hasegawa, M.; Mizutani, S.; Aimi, A.; Fujimoto, K.; Nishio, K. Low-Temperature Spark Plasma Sintering of ZrW2−xMoxO8 Exhibiting Controllable Negative Thermal Expansion. Materials 2018, 11, 1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, N.; Kameswari, U.; Sleight, A.W. Further Contraction of ZrW2O8. J. Am. Chem. Soc. 1999, 121, 10432–10433. [Google Scholar] [CrossRef]
- Xing, Q.F.; Xing, X.R.; Yu, R.B.; Du, L.; Meng, J.; Luo, J.; Wang, D.; Liu, G.R. Single Crystal Growth of ZrW2O8 by Hydrothermal Route. J. Cryst. Growth 2005, 283, 208–214. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.P.; Shi, H.F.; Yang, D.L.; Meng, S.H.; Du, S.Y. Effects of the liquid phase content on the microstructure and properties of the ZrW2O8 ceramics with negative thermal expansion fabricated by the cold sintering process. J. Eur. Ceram. Soc. 2020, 40, 6079–6086. [Google Scholar] [CrossRef]
- Nishio, K.; Kawahara, T.; Fukuda, R.; Wada, T.; Yasumori, A.; Kineri, T. Preparation and Properties of Zirconium Tungstate with Isotropic Negative Thermal Expansion. J. Soc. Inorg. Mater. Jpn. 2007, 14, 69–74. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yang, J.; Cheng, X.N.; Sun, X.J.; Zang, C.L. Abnormal Positive Thermal Expansion In Mo Substituted ZrW2O8. Physica B 2011, 406, 3458–3464. [Google Scholar] [CrossRef]



| Measured NTE Coefficients of ZrW2−xMoxO8 Bulk Ceramics [×10−6 K−1] | Reported NTE Coefficients of ZrW2−xMoxO8 Powders [×10−6 K−1] | |||||
|---|---|---|---|---|---|---|
| α Phase | β Phase | α Phase | β Phase | α Phase | β Phase | |
| x = 0.0 | −8.68 (923 K) | −4.61 (923 K) | −8.44 (873 K) | −4.05 (873 K) | −8.80 [12] | −4.90 [12] |
| x = 0.1 | −7.90 (923 K) | −3.58 (923 K) | −7.85 (873 K) | −3.22 (873 K) | — | — |
| x = 0.2 | −8.26 (923 K) | −4.47 (923 K) | −7.79 (873 K) | −4.17 (873 K) | — | −3.5 [5] |
| x = 0.5 | −12.3 (873 K) | −4.12 (873 K) | −10.3 (823 K) | −3.80 (823 K) | −8.79 [38] | −4.77 [38] |
| x = 0.6 | −7.82 (873 K) | −4.60 (873 K) | −5.70 (823 K) | −4.01 (823 K) | — | −3.5 [5] |
| x = 0.7 | — | −3.52 (873 K) | — | −2.91 (823 K) | — | — |
| x = 1.0 | — | −4.28 (823 K) | — | −2.50 (773 K) | −9.00 [16] | −5.50 [16] |
| x = 1.5 | — | −2.79 (773 K) | — | — | — | −3.56 [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Mei, J.; Xu, Y.; Zhang, X.; Li, J.; Xu, X.; Zhang, Y.; Wang, X.; Li, M. Low-Temperature Rapid Sintering of Dense Cubic Phase ZrW2−xMoxO8 Ceramics by Spark Plasma Sintering and Evaluation of Its Thermal Properties. Materials 2022, 15, 4650. https://doi.org/10.3390/ma15134650
Wei H, Mei J, Xu Y, Zhang X, Li J, Xu X, Zhang Y, Wang X, Li M. Low-Temperature Rapid Sintering of Dense Cubic Phase ZrW2−xMoxO8 Ceramics by Spark Plasma Sintering and Evaluation of Its Thermal Properties. Materials. 2022; 15(13):4650. https://doi.org/10.3390/ma15134650
Chicago/Turabian StyleWei, Hui, Jian Mei, Yan Xu, Xu Zhang, Jing Li, Xiaoyong Xu, Yang Zhang, Xiaodong Wang, and Mingling Li. 2022. "Low-Temperature Rapid Sintering of Dense Cubic Phase ZrW2−xMoxO8 Ceramics by Spark Plasma Sintering and Evaluation of Its Thermal Properties" Materials 15, no. 13: 4650. https://doi.org/10.3390/ma15134650
APA StyleWei, H., Mei, J., Xu, Y., Zhang, X., Li, J., Xu, X., Zhang, Y., Wang, X., & Li, M. (2022). Low-Temperature Rapid Sintering of Dense Cubic Phase ZrW2−xMoxO8 Ceramics by Spark Plasma Sintering and Evaluation of Its Thermal Properties. Materials, 15(13), 4650. https://doi.org/10.3390/ma15134650





