Experimental and Simulation Study for the Influence of Thermal Pre-Deformation on Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Influence of Thermal Pre-Deformation on Microhardness of Al-Zn-Mg-Cu Alloy during Subsequent Aging
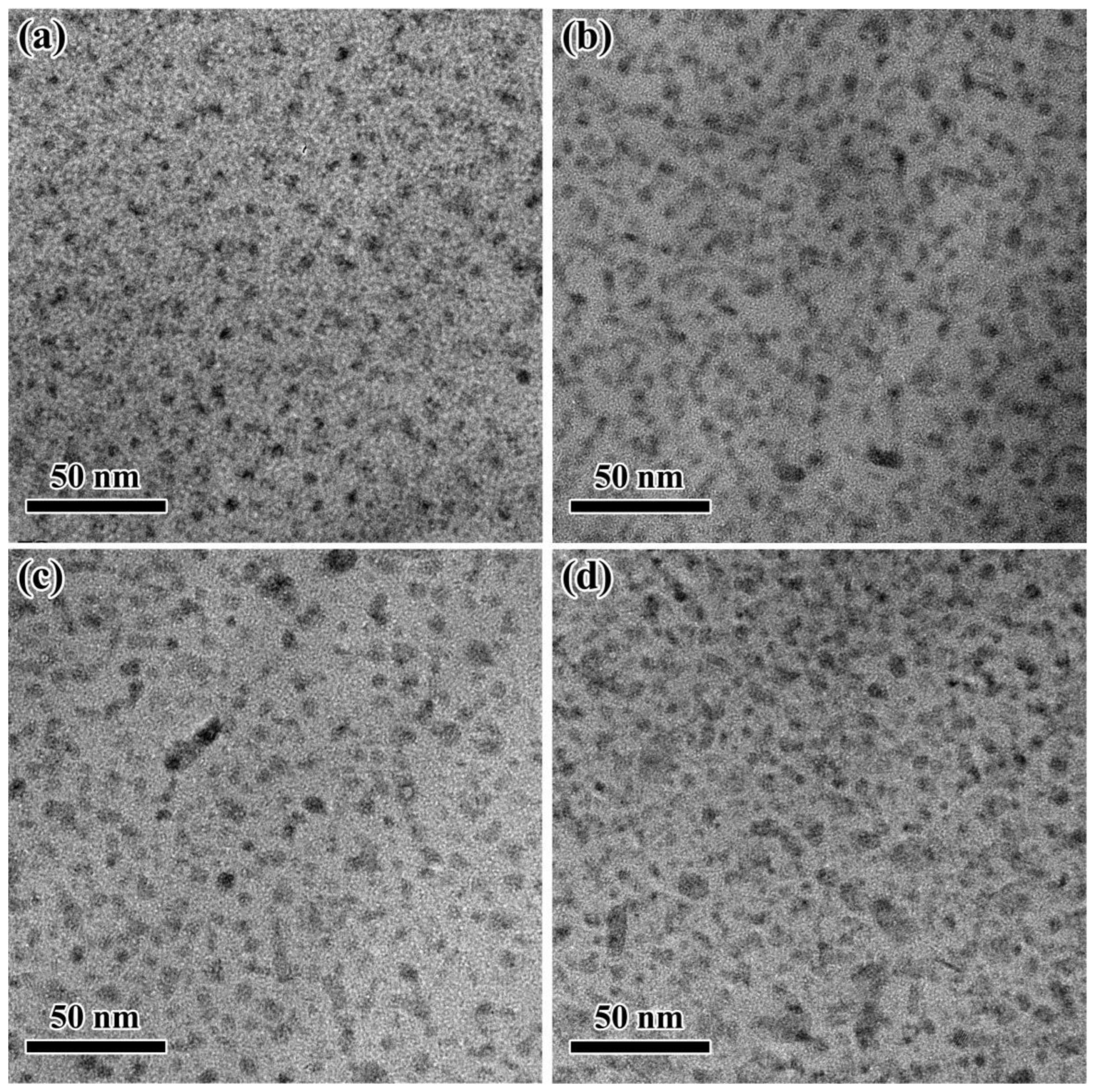
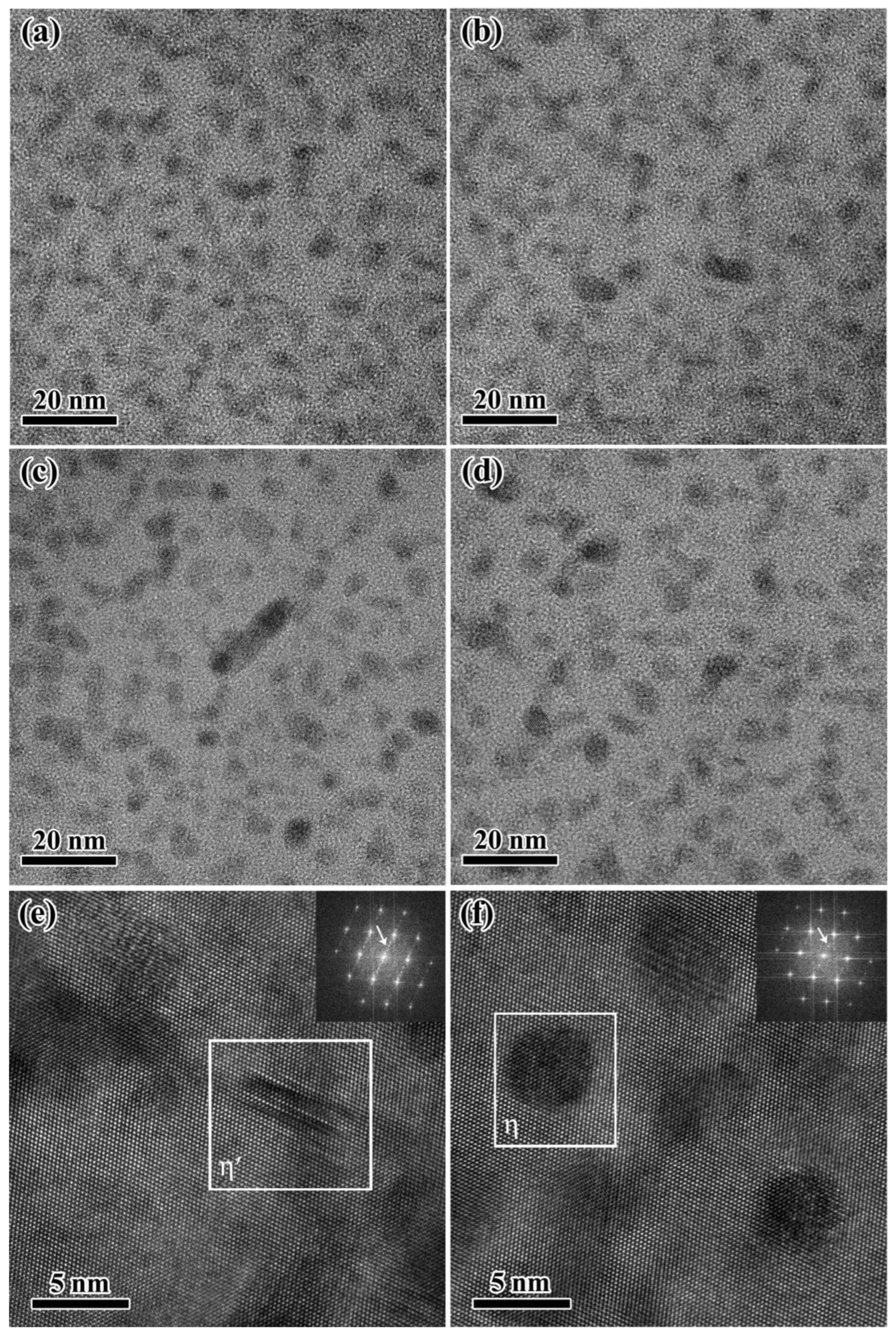
3.2. Effect of Thermal Pre-Deformation on Aging Precipitates Distribution of Al-Zn-Mg-Cu Alloy
4. Model
5. Calculation and Discussion
5.1. Influence of Thermal Pre-Deformation on the Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy
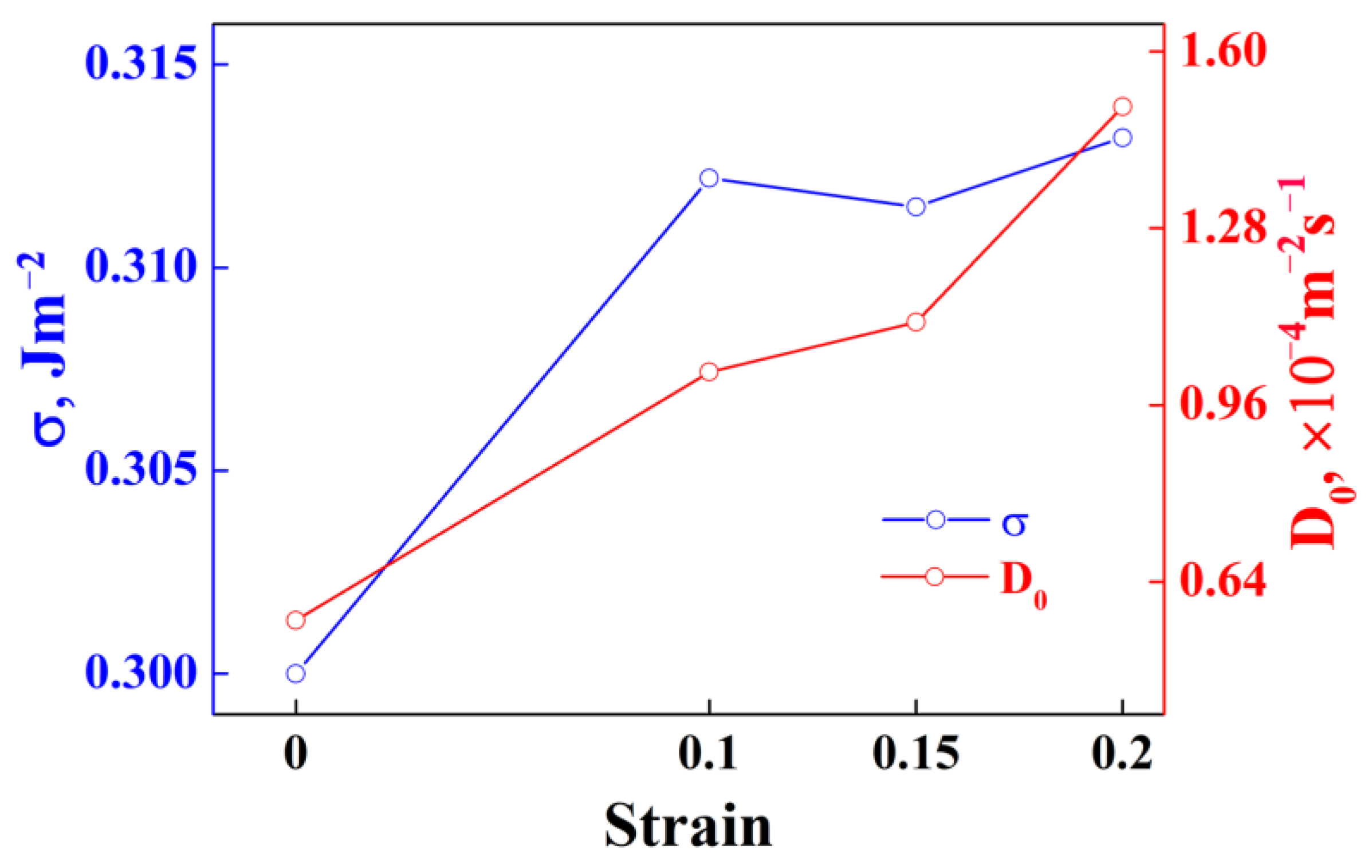
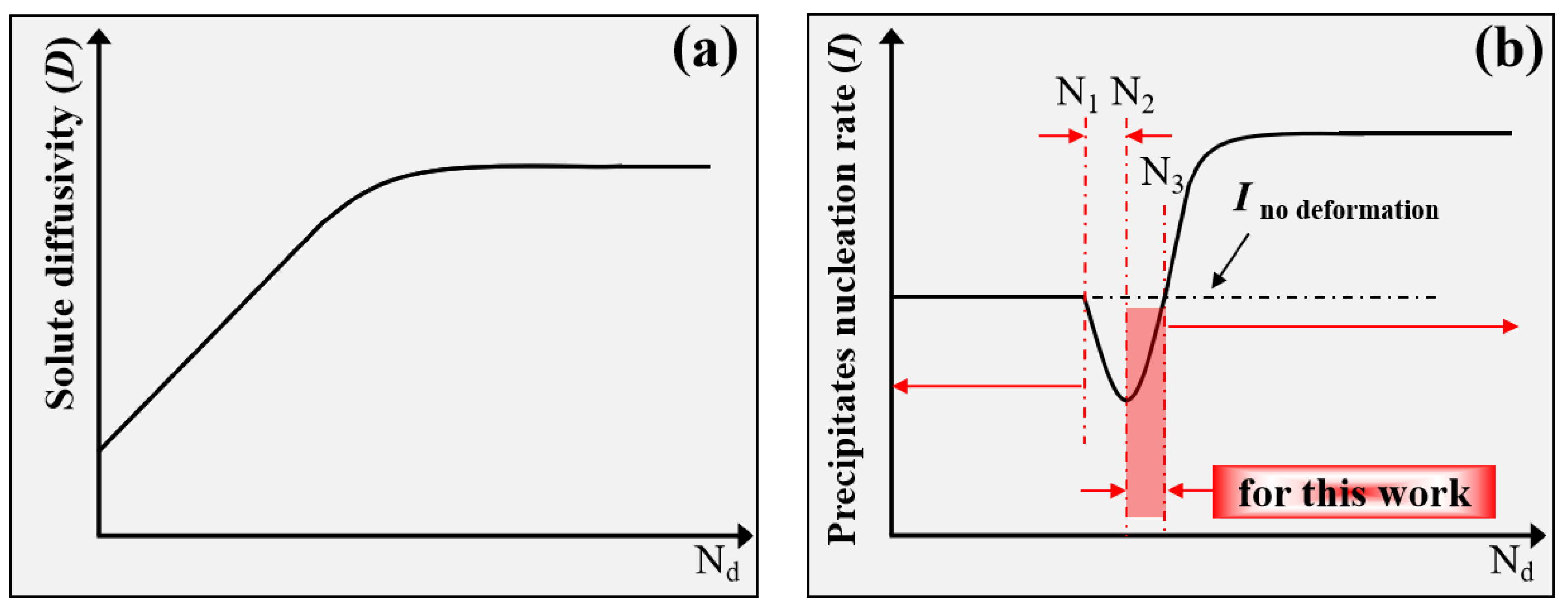
5.2. Role of Dislocations on the Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy
5.3. Influence of Pre-Deformation on the Microhardness of Al-Zn-Mg-Cu Alloy at Peak Aging
6. Conclusions
- (1)
- As the strain changes from 0 to 0.10, 0.15, 0.20 and 0.25, the peak aging microhardness of the alloy samples changes from 177.7 HV to 166.2 HV, 166.2 HV, 178.2 HV, 181.2 HV at aging times of 17 h, 11 h, 8 h, 7 h and 7 h, respectively, which indicate that thermal pre-deformation can shorten the peak aging time with the peak aging strength being guaranteed.
- (2)
- The calculation results demonstrate that, at peak aging, the precipitate average size almost remains unchanged, while the precipitate volume fraction shows a tendency to decrease and then increase with the strain changing from 0 to 0.10, 0.15 and 0.20, which is consistent with the change in microhardness of the Al-Zn-Mg-Cu alloy samples.
- (3)
- SAXS and TEM results, as well as the calculated results, demonstrate that thermal pre-deformation slightly inhibits nucleation and obviously promotes the growth of precipitates for the Al-Zn-Mg-Cu alloy during subsequent aging, thus further affecting the growth manner of precipitates.
- (4)
- The XRD results and the calculated results for aging precipitate kinetics indicate that dislocations can promote the nucleation and growth of precipitates, while the actual effect of dislocations on the precipitate nucleation and growth might be closely related with the dislocation density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X.B.; He, Z.B.; Zhou, W.X.; Yuan, S.J. Formability and strengthening mechanism of solution treated Al-Mg-Si alloy sheet under hot stamping conditions. J. Mater. Proc. Technol. 2016, 228, 179–185. [Google Scholar] [CrossRef]
- Kaab, O.; Atekeh, A.; Samuel, K.; Tirdad, N.; Clifford, B.; Mary, W.; Shahrzad, E.; Michael, W. Process parameters for hot stamping of AA7075 and D-7xxx to achieve high performance aged products. J. Mater. Proc. Technol. 2018, 257, 170–179. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Zhou, J.; Dang, L.; Xiao, W.; Wang, Y. Influence of process parameters on deep drawing of 2060 Al–Li alloy under hot stamping process. Int. J. Lightweight Mater. Manuf. 2020, 3, 36–42. [Google Scholar] [CrossRef]
- Jin, S.; Ngai, T.; Li, L.; Jia, S.; Zhai, T.; Ke, D. Aging Response and Precipitation Behavior after 5% Pre-Deformation of an Al-Mg-Si-Cu Alloy. Materials 2018, 11, 1422. [Google Scholar] [CrossRef]
- Luo, L.; Liu, Z.; Bai, S.; Zhao, J.; Zeng, D.; Wang, J.; Cao, J.; Hu, Y. Hot Deformation Behavior Considering Strain Effects and Recrystallization Mechanism of an Al-Zn-Mg-Cu Alloy. Materials 2020, 13, 1743. [Google Scholar] [CrossRef]
- Gruber, B.; Grabner, F.; Fragner, W.; Schökel, A.; Spieckermann, F.; Uggowitzer, P.J.; Pogatscher, S. Ageing Behavior of Al–Mg–Si Alloys After Cryogenic and Room Temperature Deformation. Materials 2020, 13, 554. [Google Scholar] [CrossRef]
- Liang, W.J.; Pan, Q.L.; He, Y.B.; Zhu, Z.M.; Liu, Y.F. Effect of predeformation on microstructure and mechanical properties of Al-Cu-Li-Zr alloy containing Sc. Mater. Sci. Technol. 2007, 23, 395–399. [Google Scholar] [CrossRef]
- Li, H.Z.; Liu, R.M.; Liang, X.P.; Deng, M.; Liao, H.J.; Huang, L. Effect of pre-deformation on microstructures and mechanical properties of high purity Al-Cu-Mg alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 1482–1490. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Z.Y. Effect of pre-strain on microstructure and stress corrosion cracking of over-aged 7050 aluminum alloy. J. Alloys Compd. 2009, 469, 445–450. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Wu, Q.; Yang, Z.; Zhou, K.; Fan, X.; Li, J.; Wang, J. Modulation of Multiple Precipitates for High Strength and Ductility in Al-Cu-Mn Alloy. Materials 2021, 14, 7383. [Google Scholar] [CrossRef]
- Lai, Y.X.; Fan, W.; Yin, M.J.; Wu, C.L.; Chen, J.H. Structures and formation mechanisms of dislocation-induced precipitates in relation to the age-hardening responses of Al-Mg-Si alloys. J. Mater. Sci. Technol. 2020, 41, 127–138. [Google Scholar] [CrossRef]
- Yu, X.W.; Chen, J.H.; Ming, W.Q.; Yang, X.B.; Zhao, T.T.; Shen, R.H.; He, Y.T.; Wu, C.L. Revisiting the Hierarchical Microstructures of an Al-Zn-Mg Alloy Fabricated by Pre-deformation and Aging. Acta Metall Sin. Engl. Lett. 2020, 33, 1518–1526. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. Effect of pre-straining on the aging behavior and mechanical properties of an Al-Cu-Mg-Ag alloy. Mater Sci. Eng. A 2015, 625, 119–130. [Google Scholar] [CrossRef]
- Ringer, S.P.; Muddle, B.C.; Polmear, I.J. Effects of cold work on precipitation in Al-Cu-Mg-(Ag) and Al-Cu-Li-(Mg-Ag) alloys. Metall Mater. Trans. 1995, 26, 1659–1671. [Google Scholar] [CrossRef]
- Waterloo, G.; Hansen, V.; Gjønnes, J.; Skjervold, S.R. Effect of predeformation and preaging at room temperature in Al-Zn-Mg-(Cu, Zr) alloys. Mater. Sci. Eng. 2001, 303, 226–233. [Google Scholar] [CrossRef]
- Ning, A.L.; Liu, Z.Y.; Zeng, S.M. Effect of large cold deformation after solution treatment on precipitation characteristic and deformation strengthening of 2024 and 7A04 aluminum alloys. Trans. Nonferrous Met. Soc. China 2006, 16, 1341–1347. [Google Scholar] [CrossRef]
- Poole, W.J.; Saeter, J.A.; Skjervold, S.; Waterloo, G. A model for predicting the effect of deformation after solution treatment on the subsequent artificial aging behavior of AA7030 and AA7108 alloys. Metall Mater. Trans. 2000, 31, 2327–2338. [Google Scholar] [CrossRef]
- Teichmann, K.; Marioara, C.D.; Andersen, S.J.; Marthinsen, K. The Effect of Preaging Deformation on the Precipitation Behavior of an Al-Mg-Si Alloy. Metall Mater. Trans. 2012, 43, 4006–4014. [Google Scholar] [CrossRef]
- Deschamps, A.; Livet, F.; Bréchet, Y. Influence of predeformation on ageing in an Al-Zn-Mg alloy-I. Microstructure evolution and mechanical properties. Acta Mater. 1998, 47, 281–292. [Google Scholar] [CrossRef]
- Hoyt, J.J. On the coarsening of precipitates located on grain boundaries and dislocations. Acta Metal. Mater. 1991, 39, 2091–2098. [Google Scholar] [CrossRef]
- Hornbogen, E. The role of strain energy during precipitation of copper and gold from alpha iron. Acta Metal. 1962, 10, 525–533. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, L.Y.; Sun, D.X.; Zhang, X.M. Effects of pre-deformation on microstructure and mechanical properties of 2519A-T9I6 aluminum alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 2434–2442. [Google Scholar] [CrossRef]
- Deschamps, A.; Bréchet, Y. Influence of predeformation on ageing in an Al-Zn-Mg alloy-II. Modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Dispersoid precipitation and process modelling in zirconium containing commercial aluminum alloys. Acta Mater. 2001, 49, 599–613. [Google Scholar] [CrossRef]
- Robson, J.D.; Jones, M.J.; Prangnell, P.B. Extension of the N-model to predict competing homogeneous and heterogeneous precipitation in Al-Sc alloys. Acta Mater. 2003, 51, 1453–1468. [Google Scholar] [CrossRef]
- Werenskiold, J.C.; Deschamps, A.; Bréchet, Y. Characterization and modeling of precipitation kinetics in an Al-Zn-Mg alloy. Mater. Sci. Eng. 2000, 293, 267–274. [Google Scholar] [CrossRef]
- Deschamps, A.; Militzer, M.; Poole, W.J. Precipitation Kinetics and Strengthening of a Fe-0.8wt%Cu Alloy. ISIJ Int. 2001, 41, 196–205. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Jin, S.B.; Trimby, P.W.; Liao, X.Z.; Murashkin, M.Y.; Valiev, R.Z.; Liu, J.Z.; Cairney, J.M.; Ringer, S.P.; Sha, G. Dynamic precipitation, segregation and strengthening of an Al-Zn-Mg-Cu alloy (AA7075) processed by high-pressure torsion. Acta Mater. 2018, 162, 19–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.D.; Tang, S.B.; Wang, Y.C.; Zhao, K.; Cao, L.F. The effect of pre-ageing/stretching on the ageing-hardening behavior of Al-Zn-Mg-Cu alloys correlated with Zn/Mg ratio. Mater. Sci. Eng. 2022, 830, 142331. [Google Scholar] [CrossRef]
- Conserva, M.; Buratti, M.; Dirusso, E.; Gatto, F. Age Hardening Behavior of TMT Processed AI-Zn-Mg-Cu Alloy. Mater. Sci. Eng. 1973, 11, 103–112. [Google Scholar] [CrossRef]
- Russo, E.D.; Conserva, M.; Gatto, F.; Markus, H. Thermomechanical treatments on high strength Al-Zn-Mg(Cu) alloys. Metal. Trans. 1973, 4, 1133–1144. [Google Scholar] [CrossRef]
- Russo, E.D.; Conserva, M.; Buratti, M.; Gatto, F. A new thermo-mechanical procedure for improving the ductility and toughness of Al-Zn-Mg-Cu alloys in the transverse directions. Mater. Sci. Eng. 1974, 14, 23–36. [Google Scholar] [CrossRef]
- Seon, H.-J.; Jongsup, L.; Megumi, K. Effects of Pre-Strain on the Aging Behavior of Al 7075 Alloy for Hot-Stamping Capability. Metals 2018, 8, 137. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Hua, L.; Sun, Q. Influence of thermal deformation parameters on the mechanical properties and microstructure evolution of AA7075 aluminum alloy during hot stamping-quenching process. JOM 2019, 71, 4778–4788. [Google Scholar] [CrossRef]
- Zhang, Y.; Pelliccia, D.; Milkereit, B.; Kirby, N.; Starink, M.J.; Rometsch, P.A. Analysis of Age Hardening Precipitates of Al-Zn-Mg-Cu Alloys in a Wide Range of Quenching Rates Using Small angle X-ray Scattering. Mater. Des. 2018, 142, 259–267. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, K.; Ren, C.; Naseem, S. Precipitation sequence and hardening effect in 7A85 aluminum alloy. J. Alloys Compd. 2021, 875, 159950. [Google Scholar] [CrossRef]
- Hilliard, J.E.; Averbach, B.L.; Cohen, M. Self-and Interdiffusion in Aluminum-Zinc Alloys. Acta Metal. 1959, 7, 86–92. [Google Scholar] [CrossRef]
- Rothman, S.J.; Petrdon, N.L.; Nowicki, L.J.; Robinson, L.C. Tracer diffusion of magnesium in aluminum single crystals. Phys. Status Solidi 1974, 63, 29–33. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Wang, Q.L.; Li, H.L.; He, J. Modeling of the Precipitation Kinetics During Aging a Predeformed Fe-Cu Alloy. Metal. Mater. Trans. 2011, 42, 3200–3207. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al-Zn-Mg-Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef]
- Starink, M.J.; Li, X.M. A model for the electrical conductivity of peak-aged and overaged Al-Zn-Mg-Cu alloys. Metal. Mater. Trans. 2003, 34, 899–911. [Google Scholar] [CrossRef]
- Wan, C.Y.; Chen, J.H.; Yang, X.B.; Liu, J.Z.; Wu, C.L.; Zhao, X.Q. Study of early & mid-stage hardening precipitates in a 7xxx AlZnMgCu aluminum alloy. J. Chin. Electr. Microsc. Soc. 2010, 29, 454–460. [Google Scholar] [CrossRef]
- Luo, K.; Zang, B.; Fu, S.; Jiang, Y.; Yi, D.Q. Stress/strain aging mechanisms in Al alloys from first principles. Trans. Nonferrous Met. Soc. China 2014, 24, 2130–2137. [Google Scholar] [CrossRef]
- Hart, E.W. On the role of dislocations in bulk diffusion. Acta Metal. 1957, 5, 597. [Google Scholar] [CrossRef]
- Marqusee, J.A.; Ross, J. Theory of Ostwald ripening: Competitive growth and its dependence on volume fraction. J. Chem. Phys. 1984, 80, 536–543. [Google Scholar] [CrossRef]
- Moradpour, M.; Khodabakhshi, F.; Eskandari, H. Dynamic strain aging behavior of an ultra-fine grained Al-Mg alloy (AA5052) processed via classical constrained groove pressing. J. Mater. Res. Technol. 2019, 8, 630–643. [Google Scholar] [CrossRef]
- Luo, J.; Luo, H.Y.; Li, S.J.; Wang, R.; Ma, Y. Effect of pre-ageing treatment on second nucleating of GPII zones and precipitation kinetics in an ultrafine grained 7075 aluminum alloy. Mater. Des. 2019, 187, 108402. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, H.X.; Zhao, J.Z.; He, J. Microstructure evolution during the liquid-liquid phase transformation of Al-Bi alloys under the effect of TiC particles. Acta Mater. 2017, 129, 321–330. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, H.X.; Zhao, J.Z.; He, J. Effect of TiC particles on the liquid-liquid decomposition of Al-Pb alloys. Mater. Des. 2016, 91, 361–367. [Google Scholar] [CrossRef]
- Li, H.; Chen, P.; Wang, Z.X.; Zhu, F.; Song, R.G.; Zheng, Z.Q. Tensile properties, microstructures and fracture behaviors of an Al-Zn-Mg-Cu alloy during ageing after solution treating and cold-rolling. Mater Sci. Eng. 2019, 742, 798–812. [Google Scholar] [CrossRef]
- Mousavi Anijdan, S.H.; Sadeghi-Nezhad, D.; Lee, H.; Shin, W.; Park, N.; Nayyeri, M.J.; Jafarian, H.R.; Eivani, A.R. TEM study of S’ hardening precipitates in the cold rolled and aged AA2024 aluminum alloy influence on the microstructural evolution, tensile properties & electrical conductivity. J. Mater. Res. Technol. 2021, 13, 798–807. [Google Scholar] [CrossRef]
- Luo, J.; Luo, H.Y.; Zhao, T.S.; Wang, R.Z. Effect of magnetic field on dislocation morphology and precipitation behavior in ultrafine-grained 7075 aluminum alloy. J. Mater. Sci. Technol. 2021, 93, 128–146. [Google Scholar] [CrossRef]
- Anjabin, N.; Taheri, A.K.; Kim, H.S. Constitutive Modeling of Hot Deformation Behavior of the AA6063 Alloy with Different Precipitates. Metal. Mater.Trans. 2013, 44, 5853–5860. [Google Scholar] [CrossRef]
- Starink, M.J.; Deschamps, A.; Wang, S.C. The strength of friction stir welded and friction stir processed aluminum alloys. Scripta Mater. 2008, 58, 377–382. [Google Scholar] [CrossRef]







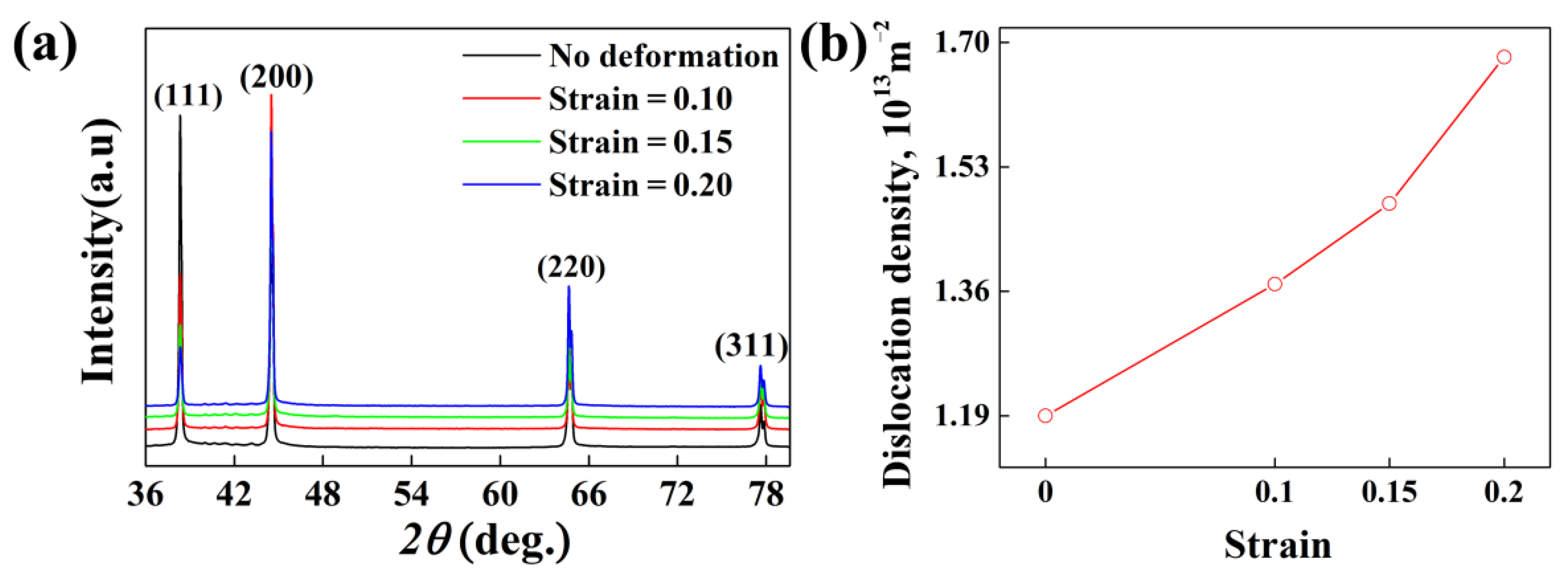

| Material | Zn | Mg | Cu | Fe | Si | Mn | Cr | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| AA7075 | 5.39 | 2.62 | 1.5 | 0.50 | 0.40 | 0.30 | 0.23 | 0.20 | Rem |
| Strain Rate (s−1) | Strain | Aging Time (h) |
|---|---|---|
| 0.1 | 0, 0.10, 0.15, 0.20, 0.25 | 2, 6, 8, 9, 11, 14, 16, 17, 18, 21, 24, 26, 28 |
| Parameter | Significance | Value | Origin |
|---|---|---|---|
| interfacial energy between the precipitate and the Al-rich matrix | 0.30 Jm−2 | [23,26] | |
| constant for the dissolution enthalpy of the precipitate | 3.85 | [40,41] | |
| pre-exponential factor for the diffusion coefficient of solute Zn | 6.2 × 10−4 m2·s−1 | [37] + this work | |
| diffusion activation energy of Zn | 129.3 kJ/mol | [37] | |
| ratio between diameter and thickness of the disc-shaped precipitated phase | 3.2 | [26,40,42] + this work | |
| gas constant | 8.314 Jmol−1·K−1 | / | |
| Boltzmann’s constant | 1.38 × 10−23 J/K | / | |
| average lattice parameter of the nucleus and the matrix | 0.451 nm | / | |
| constant | 1.05 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Yu, S.; Wang, H.; Ma, H.; Li, H.; Hu, Z. Experimental and Simulation Study for the Influence of Thermal Pre-Deformation on Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy. Materials 2022, 15, 4634. https://doi.org/10.3390/ma15134634
Sun Q, Yu S, Wang H, Ma H, Li H, Hu Z. Experimental and Simulation Study for the Influence of Thermal Pre-Deformation on Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy. Materials. 2022; 15(13):4634. https://doi.org/10.3390/ma15134634
Chicago/Turabian StyleSun, Qian, Sha Yu, Hong Wang, Huijuan Ma, Huanhuan Li, and Zhili Hu. 2022. "Experimental and Simulation Study for the Influence of Thermal Pre-Deformation on Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy" Materials 15, no. 13: 4634. https://doi.org/10.3390/ma15134634
APA StyleSun, Q., Yu, S., Wang, H., Ma, H., Li, H., & Hu, Z. (2022). Experimental and Simulation Study for the Influence of Thermal Pre-Deformation on Subsequent Aging Precipitation Kinetics of Al-Zn-Mg-Cu Alloy. Materials, 15(13), 4634. https://doi.org/10.3390/ma15134634