Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Fungi and Preparation of AuNPs
2.2. Characterization of the AuNPs
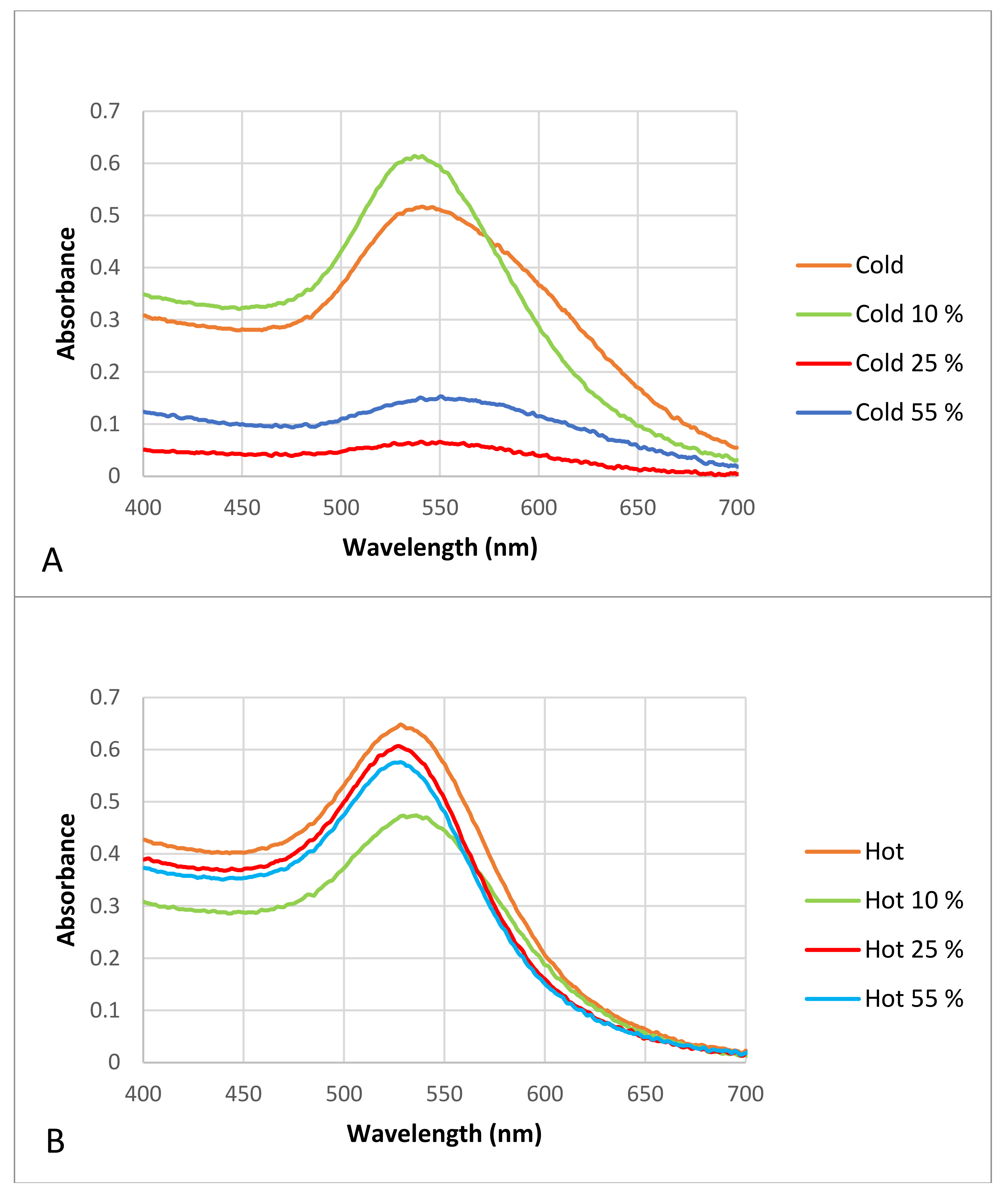
2.2.1. Characterization of the AuNPs Using Visible Spectrophotometry
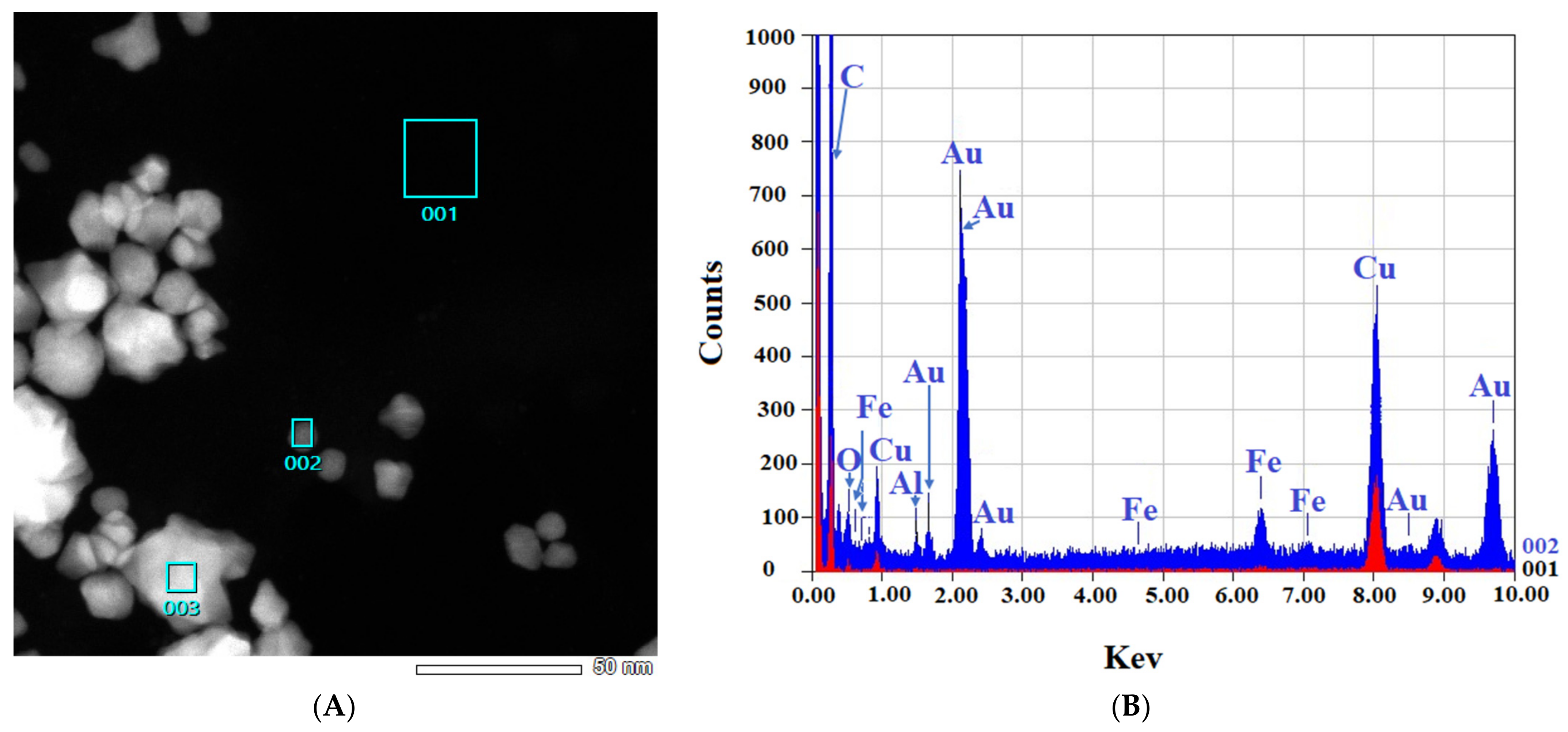
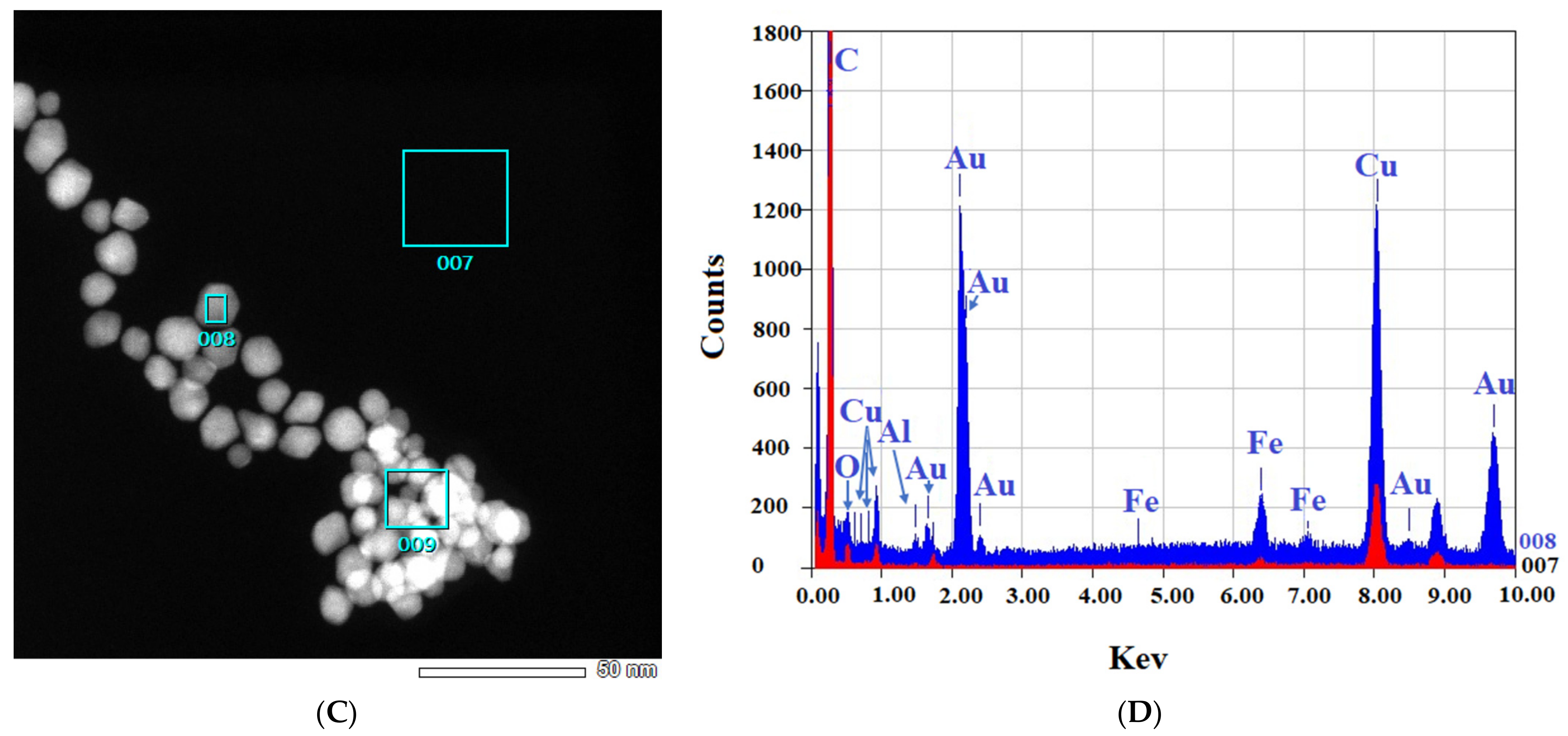
2.2.2. TEM and EDS
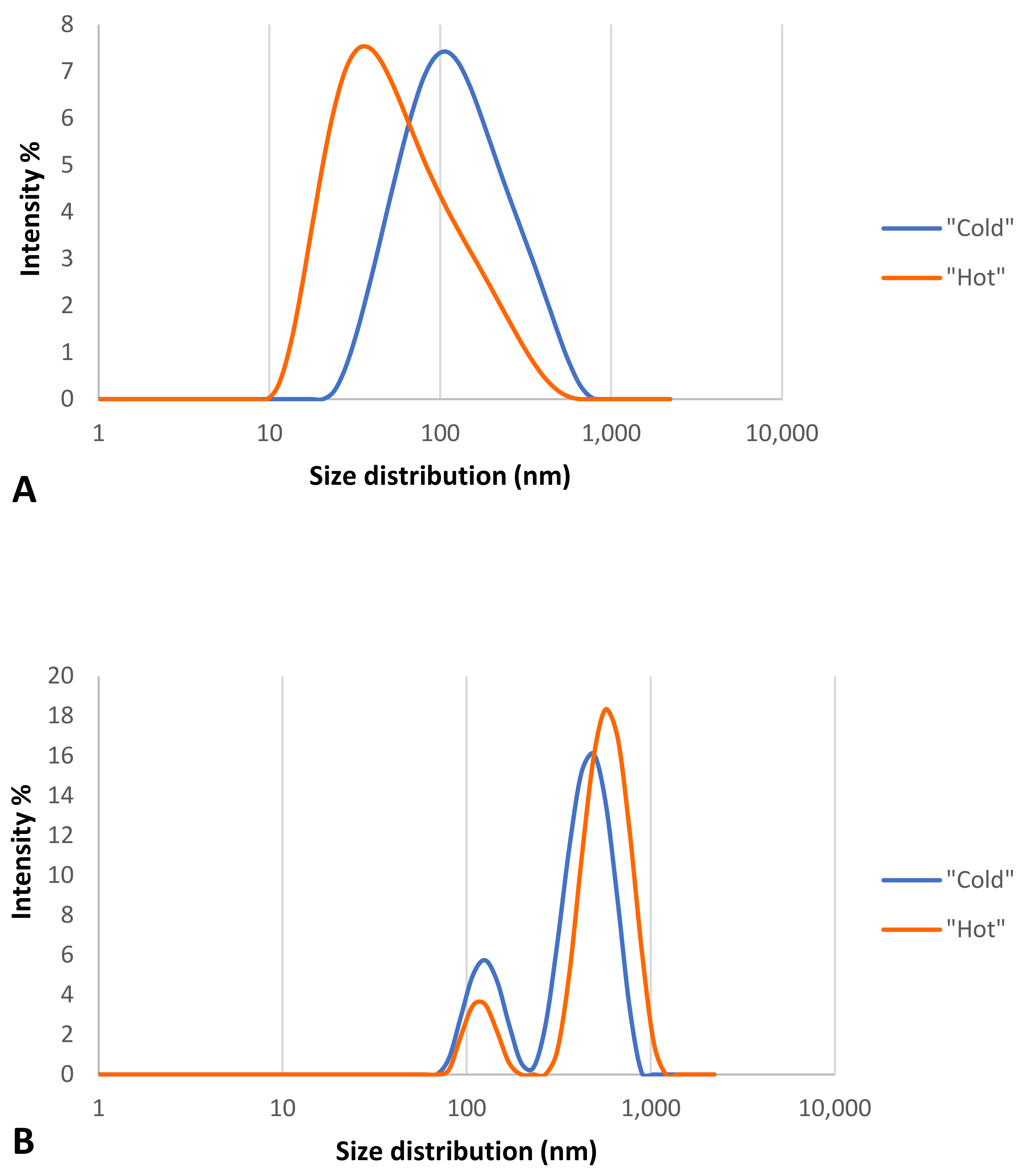
2.2.3. Characterization of the AuNPs: Z-Potential and DLS Measurements
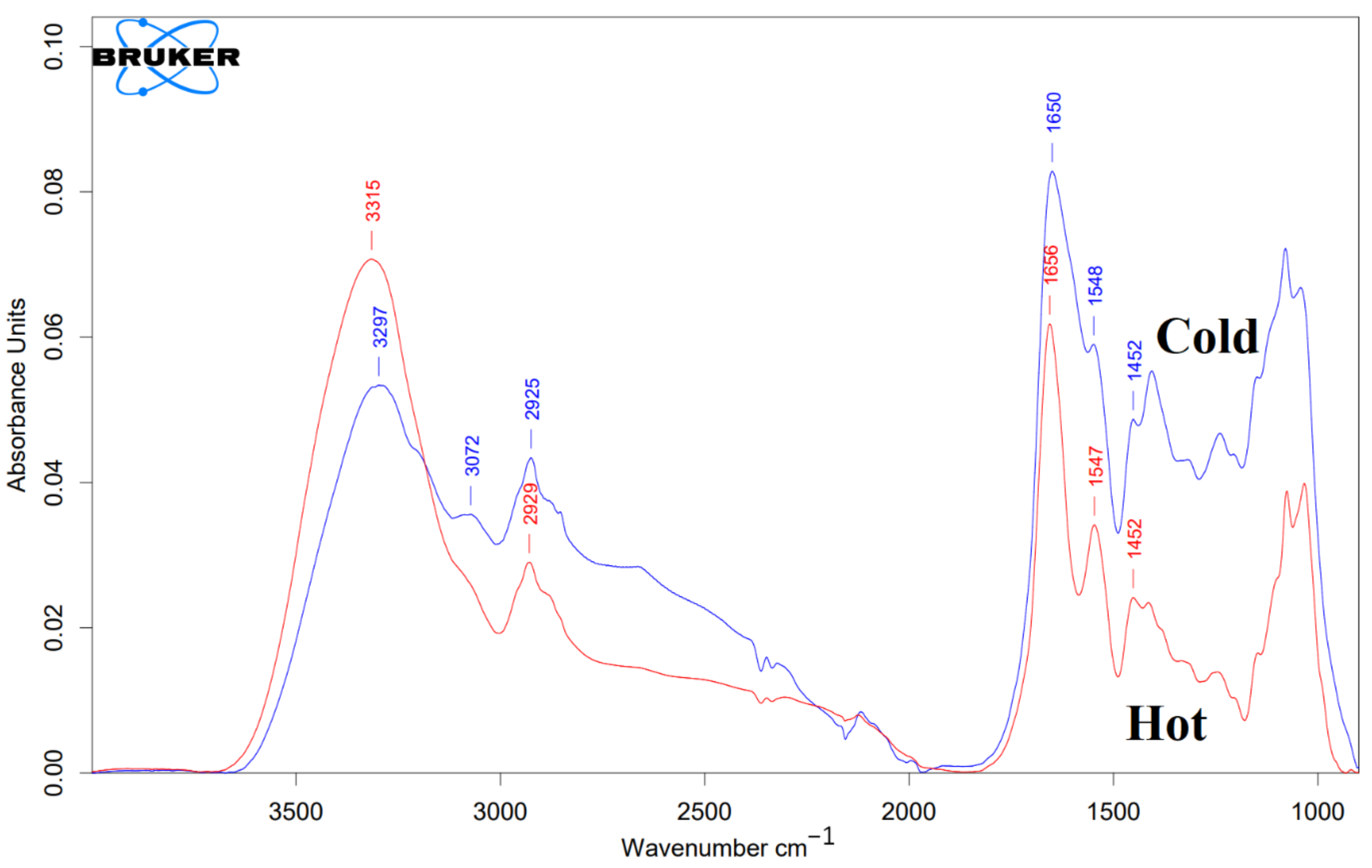
2.2.4. FTIR
2.3. Preparation of the Plasma
2.4. Preparation of Hard Protein Corona
2.5. Detection of the Hard Protein Corona
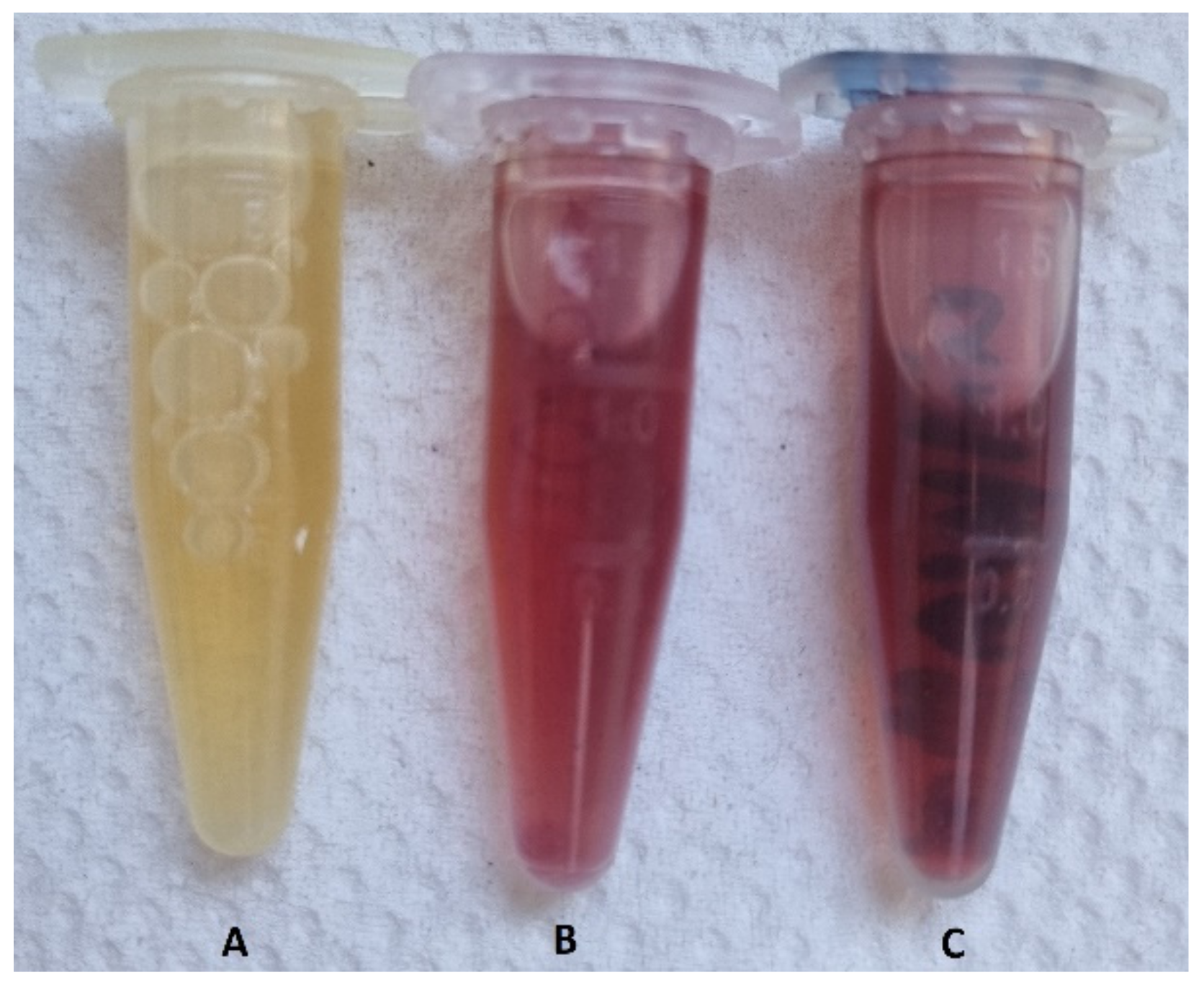
2.5.1. Detection of the Hard Protein Corona Using Visible Spectrophotometry
2.5.2. Detection of the Hard Protein Corona: Z-Potential and DLS Measurements
2.5.3. SDS-PAGE
2.5.4. LC-MS Analysis
3. Results and Discussion
3.1. Fungal Culturing and AuNP Production
3.2. Characterization of AuNPs
3.2.1. Characterization of AuNPs Using Visible Spectrophotometry
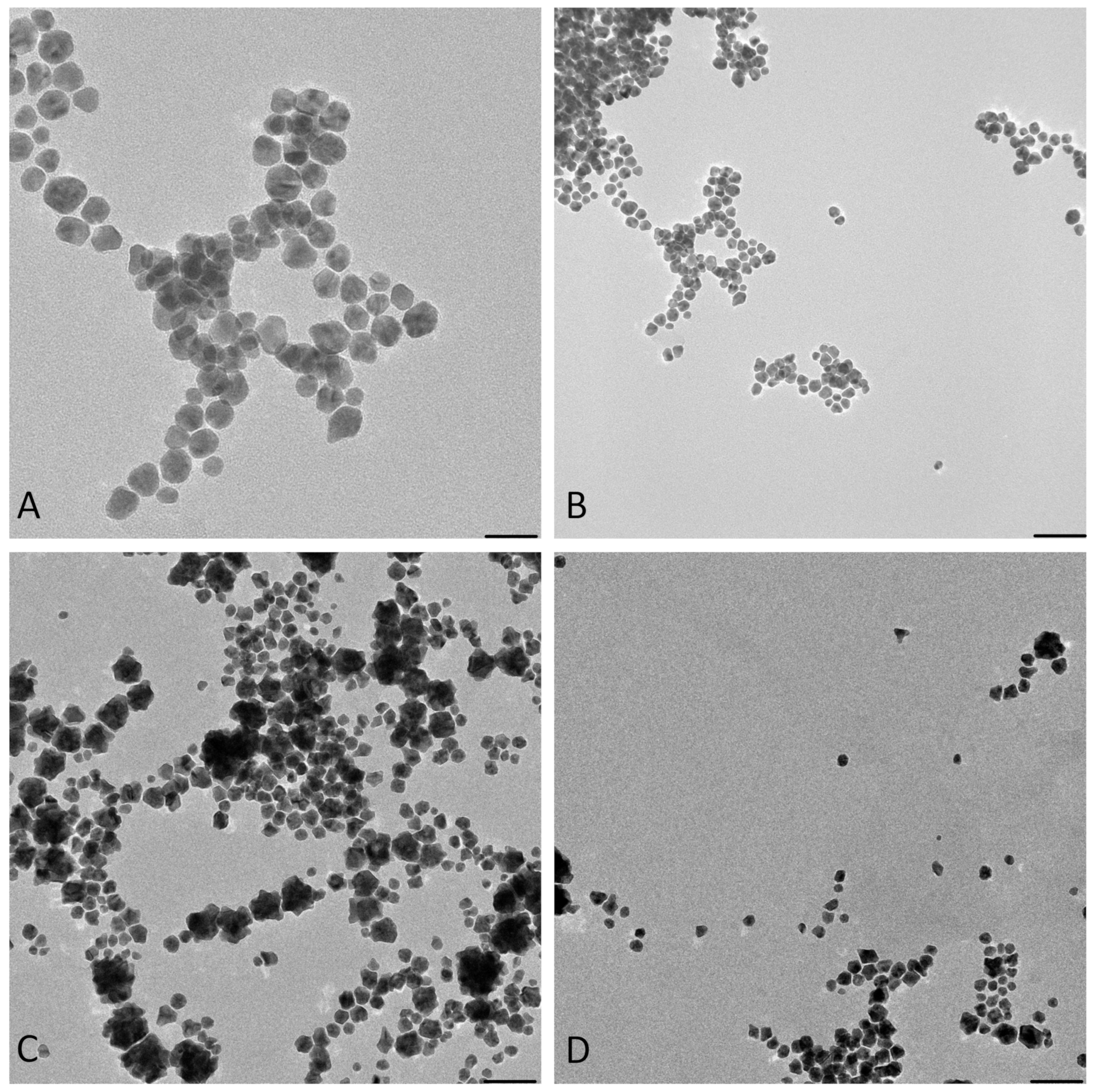
3.2.2. TEM Characterizations
3.2.3. Characterization of AuNPs: Z-Potential and DLS Measurements
3.2.4. FTIR Characterization
3.3. Detection of the Hard Protein Corona
3.3.1. Detection of the Hard Protein Corona Using Visible Spectrophotometry
3.3.2. Detection of the Hard Protein Corona: Z-Potential and DLS Measurements
3.4. SDS-PAGE and Detected Protein Coronas
3.5. LC-MS Analysis and Proteomics Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourali, P.; Baserisalehi, M.; Afsharnezhad, S.; Behravan, J.; Ganjali, R.; Bahador, N.; Arabzadeh, S. The effect of temperature on antibacterial activity of biosynthesized silver nanoparticles. Biometals 2013, 26, 189–196. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggard, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Salvati, A.; Dawson, K.A. What does the cell see? Nat. Nanotechnol. 2009, 4, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Pourali, P.; Benada, O.; Pátek, M.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Response of Biological Gold Nanoparticles to Different pH Values: Is It Possible to Prepare Both Negatively and Positively Charged Nanoparticles? Appl. Sci. 2021, 11, 1559. [Google Scholar] [CrossRef]
- Yahyaei, B.; Nouri, M.; Bakherad, S.; Hassani, M.; Pourali, P. Effects of biologically produced gold nanoparticles: Toxicity assessment in different rat organs after intraperitoneal injection. AMB Express 2019, 9, 38. [Google Scholar] [CrossRef]
- Yahyaei, B.; Pourali, P. One step conjugation of some chemotherapeutic drugs to the biologically produced gold nanoparticles and assessment of their anticancer effects. Sci. Rep. 2019, 9, 10242. [Google Scholar] [CrossRef] [Green Version]
- Hammami, I.; Alabdallah, N.M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B.; Ajoudanifar, H.; Taheri, R.; Alavi, H.; Hoseini, A. Impregnation of the bacterial cellulose membrane with biologically produced silver nanoparticles. Curr. Microbiol. 2014, 69, 785–793. [Google Scholar] [CrossRef]
- Pourali, P.; Razavian Zadeh, N.; Yahyaei, B. Silver nanoparticles production by two soil isolated bacteria, Bacillus thuringiensis and Enterobacter cloacae, and assessment of their cytotoxicity and wound healing effect in rats. Wound Repair Regen. 2016, 24, 860–869. [Google Scholar] [CrossRef]
- Abdolmaleki, H.; Sohrabi, M. Biosynthesis of silver nanoparticles by two lichens of “Usnea articulate” and “Ramalina sinensis” and investigation of their antibacterial activity against some pathogenic bacteria. Ebnesina 2016, 17, 33–42. [Google Scholar]
- Nikbakht, M.; Yahyaei, B.; Pourali, P. Green synthesis, characterization and antibacterial activity of silver nanoparticles using fruit aqueous and methanolic extracts of Berberis vulgaris and Ziziphus vulgaris. J. Pure Appl. Microbiol. 2015, 9, 349–355. [Google Scholar]
- Pourali, P.; Nouri, M.; Ameri, F.; Heidari, T.; Kheirkhahan, N.; Arabzadeh, S.; Yahyaei, B. Histopathological study of the maternal exposure to the biologically produced silver nanoparticles on different organs of the offspring. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 867–878. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.V.; Alam, M.; et al. Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005, 1, 47–53. [Google Scholar] [CrossRef]
- Bhambure, R.; Bule, M.; Shaligram, N.; Kamat, M.; Singhal, R. Extracellular biosynthesis of gold nanoparticles using Aspergillus niger—Its characterization and stability. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2009, 32, 1036–1041. [Google Scholar] [CrossRef]
- Pourali, P.; Badiee, S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Ostrowski, M.; Trzcińska, J.; Rai, M.; Golińska, P. Biogenic silver nanoparticles: Assessment of their cytotoxicity, genotoxicity and study of capping proteins. Molecules 2020, 25, 3022. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J. Biomed. Nanotechnol. 2011, 7, 245–254. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B.; Afsharnezhad, S. Bio-Synthesis of Gold Nanoparticles by Fusarium oxysporum and Assessment of Their Conjugation Possibility with Two Types of β-Lactam Antibiotics without Any Additional Linkers. Microbiology 2018, 87, 229–237. [Google Scholar] [CrossRef]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. De Mycol. Med. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Ishida, T.; Harashima, H.; Kiwada, H. Interactions of liposomes with cells in vitro and in vivo: Opsonins and receptors. Curr. Drug Metab. 2001, 2, 397–409. [Google Scholar] [CrossRef]
- Camner, P.; Lundborg, M.; Låstbom, L.; Gerde, P.; Gross, N.; Jarstrand, C. Experimental and calculated parameters on particle phagocytosis by alveolar macrophages. J. Appl. Physiol. 2002, 92, 2608–2616. [Google Scholar] [CrossRef] [Green Version]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical−chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Pacheco, N.I.; Roubalova, R.; Dvorak, J.; Benada, O.; Pinkas, D.; Kofronova, O.; Semerad, J.; Pivokonsky, M.; Cajthaml, T.; Bilej, M.; et al. Understanding the toxicity mechanism of CuO nanoparticles: The intracellular view of exposed earthworm cells. Environ. Sci. Nano 2021, 8, 2464–2477. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Khiabani, M.S.; Hamishehkar, H.; Mokarram, R.R.; Amjadi, M. Antioxidant, antimicrobial and cytotoxic activities of biosynthesized gold nanoparticles (AuNPs) from Chinese lettuce (CL) leave extract (Brassica rapa var. pekinensis). Mater. Today Commun. 2021, 29, 102831. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar]
- Welz, B.; Sperling, M. Atomic Absorption Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jedlovszky-Hajdu, A.; Bombelli, F.B.; Monopoli, M.P.; Tombacz, E.; Dawson, K.A. Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Goldring, J. Protein quantification methods to determine protein concentration prior to electrophoresis. Protein Electrophor. 2012, 29–35. [Google Scholar]
- Brunelle, J.L.; Green, R. One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE). Methods Enzymol. 2014, 541, 151–159. [Google Scholar] [PubMed]
- Anwar, Z.M.; Azab, H.A. Ternary complexes in solution. Comparison of the coordination tendency of some biologically important zwitterionic buffers toward the binary complexes of some transition metal ions and some amino acids. J. Chem. Eng. Data 1999, 44, 1151–1157. [Google Scholar] [CrossRef]
- Azab, H.A.; Anwar, Z.M. Coordination tendency of some biologically important zwitterionic buffers toward metal ion nucleotide complexes at different temperatures. J. Chem. Eng. Data 2012, 57, 2890–2895. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Pinto, I.S.; Soares, E.V.; Soares, H.M. (Un) suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions—A review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef] [Green Version]
- Pavelek, Z.; Vyšata, O.; Tambor, V.; Pimková, K.; Vu, D.L.; Kuča, K.; Šťourač, P.; Vališ, M. Proteomic analysis of cerebrospinal fluid for relapsing-remitting multiple sclerosis and clinically isolated syndrome. Biomed. Rep. 2016, 5, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, J.R.; Granum, P.E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal. Biochem. 1980, 109, 156–159. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Nascimento, C.A.O.; Corrêa, B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci. Rep. 2014, 4, 6404. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Lepre, L.F.; Ando, R.A.; Oller do Nascimento, C.A.; Corrêa, B. Biosynthesis and uptake of copper nanoparticles by dead biomass of Hypocrea lixii isolated from the metal mine in the Brazilian Amazon region. PLoS ONE 2013, 8, e80519. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Ando, R.A.; Oller Nascimento, C.A.; Correa, B. Extra and intracellular synthesis of nickel oxide nanoparticles mediated by dead fungal biomass. PLoS ONE 2015, 10, e0129799. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.; Corrêa, B. Dead biomass of Amazon yeast: A new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J. Environ. Sci. Health Part A 2017, 52, 1112–1120. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014, 9, 365. [Google Scholar] [CrossRef] [Green Version]

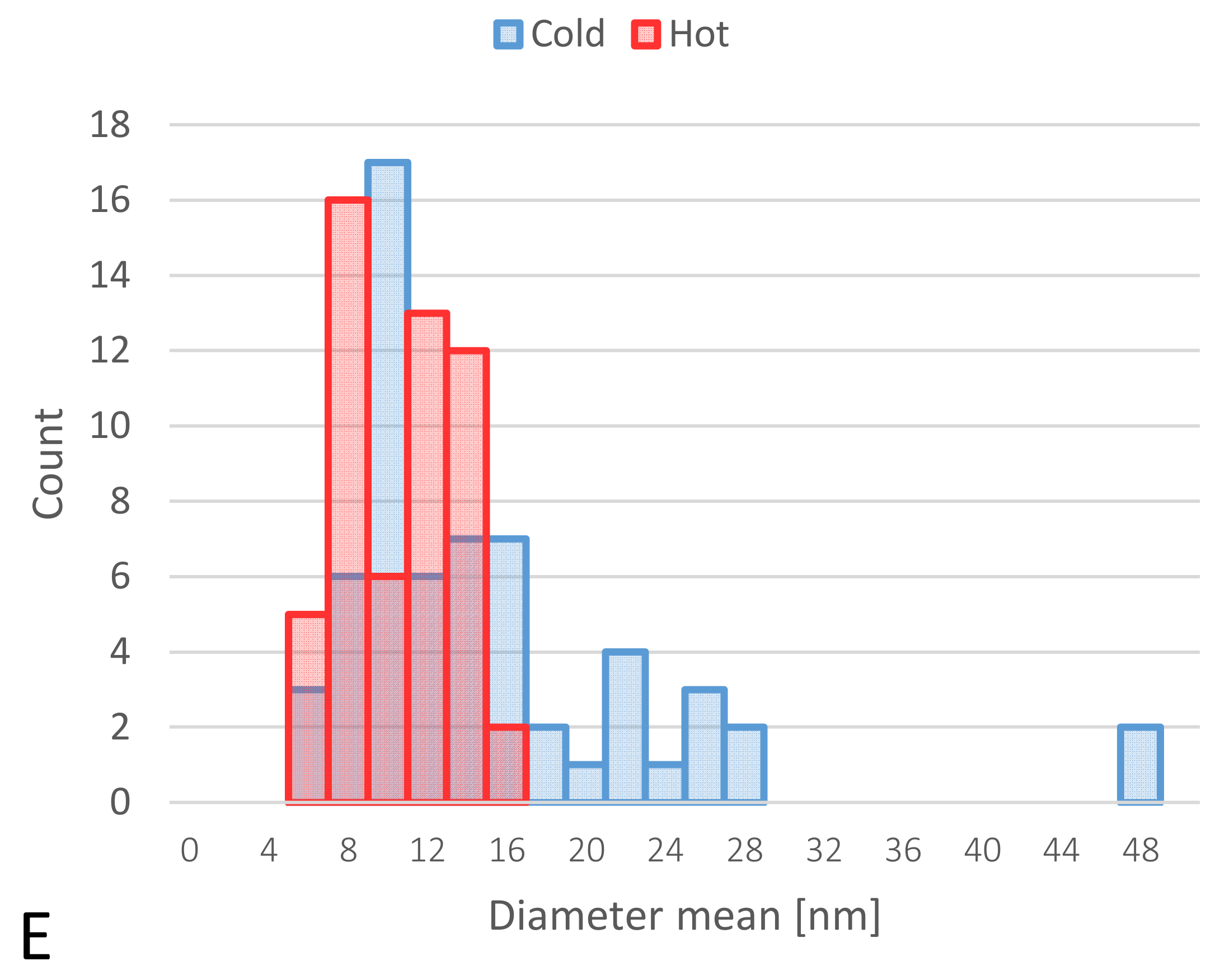
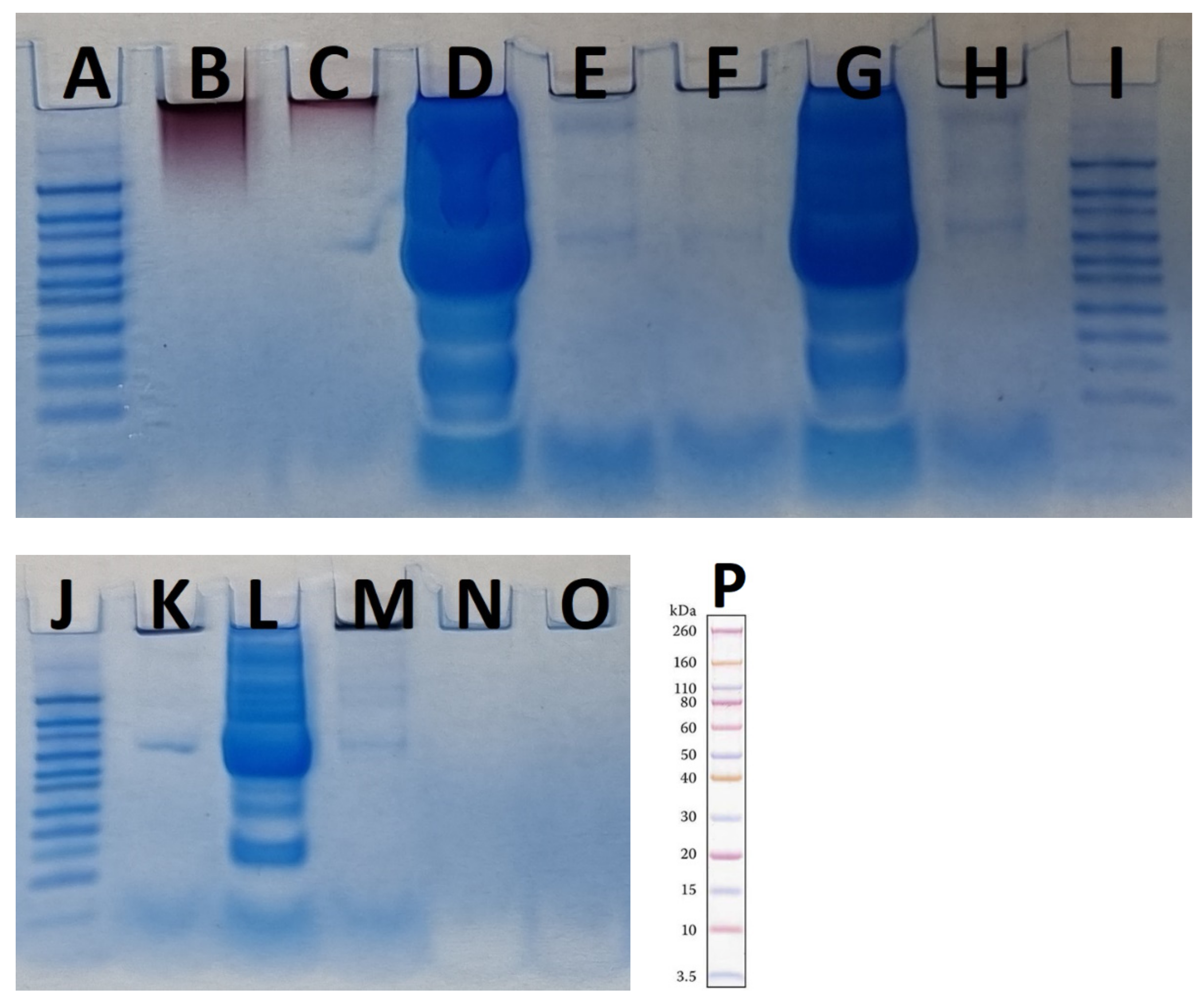
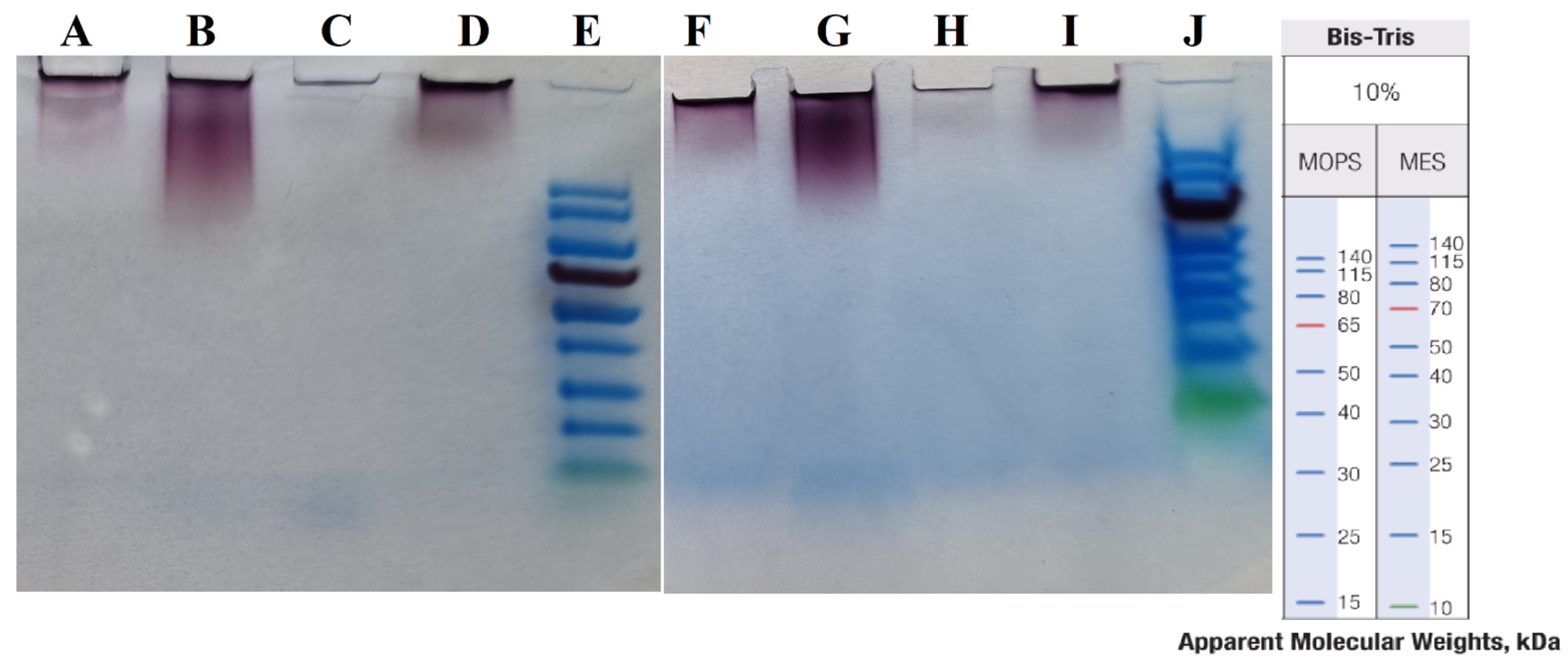



| AuNP Samples Before Protein Corona Formation | Mean | SD |
| Size of “cold” (nm) | 37.9 | 5.70 |
| Size of “hot” (nm) | 13.1 | 2.10 |
| Zeta potential (mV) of “cold” | −39.4 | 0.65 |
| Zeta potential (mV) of “hot” | −35.8 | 1.17 |
| AuNP Samples After Protein Corona Formation | Mean | SD |
| Size of “cold” peak I (nm) | 109.3 | 23.12 |
| peak II (nm) | 267.0 | 0 |
| Size of “hot” peak I (nm) | 86.7 | 25.04 |
| peak II (nm) | NA | NA |
| Zeta potential (mV) of “cold” | −37.7 | 5.13 |
| Zeta potential (mV) of “hot” | −30.5 | 4.15 |
| Protein ID | Description | Accession | Total Coverage (%) | Peptides | Unique | Avg. Mass |
|---|---|---|---|---|---|---|
| 28 | Fibrinogen beta chain OS = Rattus norvegicus OX = 10,116 GN = Fgb PE = 1 SV = 4 | P14480|FIBB_RAT | 42 | 24 | 15 | 54,235 |
| 556 | Hemoglobin subunit alpha OS = Otospermophilus beecheyi OX = 34,862 PE = 1 SV = 1 | B3EWC9|HBA_OTOBE | 52 | 8 | 4 | 15,023 |
| 92 | Fibrinogen beta chain OS = Cavia porcellus OX = 10,141 GN = FGB PE = 4 SV = 2 | tr|H0VD80|H0VD80_CAVPO | 28 | 16 | 6 | 54,105 |
| 258 | Fibrinogen beta chain OS = Oryctolagus cuniculus OX = 9986 GN = FGB PE = 4 SV = 1 | tr|A0A5F9D3P7|A0A5F9D3P7_RABIT | 24 | 13 | 4 | 53,716 |
| 278 | Fibrinogen beta chain OS = Oryctolagus cuniculus OX = 9986 GN = FGB PE = 4 SV = 3 | tr|G1T0W8|G1T0W8_RABIT | 23 | 13 | 4 | 56,507 |
| 818 | Hemoglobin subunit alpha OS = Blarina brevicauda OX = 9387 PE = 1 SV = 1 | B3EWE1|HBA_BLABR | 43 | 6 | 4 | 14,995 |
| 63 | Keratin 5 OS = Oryctolagus cuniculus OX = 9986 GN = KRT5 PE = 3 SV = 1 | tr|A0A5F9CNQ8|A0A5F9CNQ8_RABIT | 32 | 26 | 6 | 60,612 |
| 59 | Keratin 10 OS = Oryctolagus cuniculus OX = 9986 GN = KRT10 PE = 3 SV = 1 | tr|A0A5F9D8K0|A0A5F9D8K0_RABIT | 26 | 20 | 0 | 59,920 |
| 60 | Keratin 10 OS = Oryctolagus cuniculus OX = 9986 GN = KRT10 PE = 3 SV = 2 | tr|G1T1V0|G1T1V0_RABIT | 26 | 20 | 0 | 56,342 |
| 36 | Actin gamma 1 OS = Cavia porcellus OX = 10,141 GN = ACTG1 PE = 3 SV = 1 | tr|A0A286XYY5|A0A286XYY5_CAVPO | 55 | 18 | 7 | 41,793 |
| 95 | Keratin 10 OS = Myotis lucifugus OX = 59,463 GN = KRT10 PE = 3 SV = 1 | tr|G1P6A9|G1P6A9_MYOLU | 20 | 17 | 0 | 58,383 |
| 701 | Hemoglobin subunit alpha OS = Microtus pennsylvanicus OX = 10,058 PE = 1 SV = 1 | B3EWE3|HBA_MICPE | 45 | 8 | 3 | 15,073 |
| 120 | IF rod domain-containing protein OS = Cavia porcellus OX = 10,141 PE = 3 SV = 1 | tr|A0A286XNZ7|A0A286XNZ7_CAVPO | 23 | 17 | 2 | 58,626 |
| 363 | Hemoglobin subunit beta OS = Microtus pennsylvanicus OX = 10,058 PE = 1 SV = 1 | B3EWE4|HBB_MICPE | 62 | 9 | 6 | 15,677 |
| 585 | Hemoglobin subunit alpha OS = Peromyscus californicus OX = 42,520 PE = 1 SV = 1 | B3EWD5|HBA_PERCA | 68 | 8 | 2 | 14,869 |
| 1141 | Hemoglobin subunit alpha OS = Tamiasciurus hudsonicus OX = 10,009 PE = 1 SV = 1 | B3EWD7|HBA_TAMHU | 38 | 6 | 2 | 14,986 |
| 664 | Globin A1 OS = Myotis lucifugus OX = 59,463 GN = GLNA2 PE = 3 SV = 1 | tr|G1QEL0|G1QEL0_MYOLU | 47 | 8 | 4 | 15,885 |
| 437 | Hemoglobin subunit beta OS = Tamiasciurus hudsonicus OX = 10,009 PE = 1 SV = 1 | B3EWD8|HBB_TAMHU | 50 | 7 | 3 | 15,855 |
| 676 | Hemoglobin subunit beta OS = Otospermophilus beecheyi OX = 34,862 PE = 1 SV = 1 | B3EWD0|HBB_OTOBE | 31 | 6 | 3 | 15,825 |
| 767 | Hemoglobin subunit alpha OS = Sciurus carolinensis OX = 30,640 PE = 1 SV = 1 | B3EWD1|HBA_SCICA | 57 | 7 | 3 | 15,073 |
| 849 | Hemoglobin subunit alpha OS = Peromyscus crinitus OX = 144,753 PE = 1 SV = 1 | B3EWD3|HBA_PERCR | 48 | 6 | 2 | 14,986 |
| 426 | Hemoglobin subunit beta OS = Peromyscus crinitus OX = 144,753 PE = 1 SV = 1 | B3EWD4|HBB_PERCR | 47 | 6 | 1 | 15,807 |
| 727 | Biliverdin reductase B OS = Cavia porcellus OX = 10,141 GN = BLVRB PE = 4 SV = 1 | tr|H0UVS9|H0UVS9_CAVPO | 36 | 5 | 3 | 22,096 |
| 778 | Biliverdin reductase B OS = Myotis lucifugus OX = 59,463 GN = BLVRB PE = 4 SV = 1 | tr|G1P4F0|G1P4F0_MYOLU | 25 | 4 | 2 | 21,982 |
| 660 | Hemoglobin subunit alpha OS = Tamias merriami OX = 123,787 PE = 1 SV = 1 | B3EWC7|HBA_TAMMR | 62 | 7 | 4 | 15,061 |
| 774 | Hemoglobin subunit beta OS = Blarina brevicauda OX = 9387 PE = 1 SV = 1 | B3EWE2|HBB_BLABR | 27 | 4 | 1 | 15,795 |
| 730 | Hemoglobin subunit beta OS = Tamias striatus OX = 45,474 PE = 1 SV = 1 | B3EWE0|HBB_TAMST | 27 | 4 | 1 | 15,869 |
| 795 | Hemoglobin subunit alpha OS = Vicugna pacos OX = 30,538 GN = HBA PE = 1 SV = 1 | P67816|HBA_VICPA | 38 | 4 | 2 | 15,126 |
| 896 | Hemoglobin subunit alpha OS = Lama vicugna OX = 9843 GN = HBA PE = 1 SV = 1 | P07425|HBA_LAMVI | 38 | 4 | 2 | 15,142 |
| 880 | Hemoglobin subunit alpha OS = Tamias striatus OX = 45,474 PE = 1 SV = 1 | B3EWD9|HBA_TAMST | 37 | 4 | 1 | 15,154 |
| 839 | Glutathione peroxidase OS = Oryctolagus cuniculus OX = 9986 PE = 3 SV = 1 | tr|A0A5F9CNR1|A0A5F9CNR1_RABIT | 21 | 4 | 2 | 23,298 |
| 1065 | Ubiquitin-like domain-containing protein OS = Oryctolagus cuniculus OX = 9986 PE = 4 SV = 1 | tr|A0A5F9CP41|A0A5F9CP41_RABIT | 44 | 3 | 3 | 8694 |
| 1067 | 60S ribosomal protein L40 OS = Takifugu rubripes OX = 31,033 GN = uba52 PE = 3 SV = 1 | tr|H2SBM2|H2SBM2_TAKRU | 27 | 3 | 3 | 14,745 |
| 1068 | 60S ribosomal protein L40 OS = Cavia porcellus OX = 10,141 GN = Uba52 PE = 3 SV = 1 | tr|A0A286XB24|A0A286XB24_CAVPO | 27 | 3 | 3 | 14,728 |
| Protein ID | Description | Accession | Coverage (%) | Peptides | Unique | Avg. Mass |
|---|---|---|---|---|---|---|
| HHot | ||||||
| 1383 | Ubiquitin OS = Encephalitozoon cuniculi (strain GB-M1) OX = 284,813 GN = ECU02_0740i PE = 1 SV = 1 | Q8SWD4|UBIQ_ENCCU | 12 | 1 | 1 | 8714 |
| Cold | ||||||
| 1389 | Ubiquitin-60S ribosomal protein L40 OS = Neurospora crassa (strain ATCC 24,698/74-OR23-1A/CBS 708.71/DSM 1257/FGSC 987) OX = 367,110 GN = crp-79 PE = 1 SV = 2 | P0C224|RL40_NEUCR | 20 | 2 | 2 | 14,637 |
| 1386 | Ubiquitin-60S ribosomal protein L40 OS = Schizosaccharomyces pombe (strain 972/ATCC 24843) OX = 284,812 GN = uep1 PE = 1 SV = 1 | P0CH07|RL402_SCHPO | 20 | 2 | 2 | 14,595 |
| 1387 | Ubiquitin-60S ribosomal protein L40 OS = Saccharomyces cerevisiae (strain ATCC 204508/S288c) OX = 559,292 GN = RPL40A PE = 1 SV = 1 | P0CH08|RL40A_YEAST | 20 | 2 | 2 | 14,554 |
| 1388 | Ubiquitin-60S ribosomal protein L40 OS = Saccharomyces cerevisiae (strain ATCC 204508/S288c) OX = 559,292 GN = RPL40B PE = 1 SV = 1 | P0CH09|RL40B_YEAST | 20 | 2 | 2 | 14,554 |
| 1390 | Ubiquitin-60S ribosomal protein L40 OS = Schizosaccharomyces pombe (strain 972/ATCC 24843) OX = 284,812 GN = ubi1 PE = 1 SV = 1 | P0CH06|RL401_SCHPO | 20 | 2 | 2 | 14,595 |
| 1391 | Ubiquitin-60S ribosomal protein L40 OS = Cryptococcus neoformans var. neoformans serotype D (strain JEC21/ATCC MYA-565) OX = 214,684 GN = UBI1 PE = 1 SV = 2 | P40909|RL40_CRYNJ | 19 | 2 | 2 | 14,653 |
| 1397 | Ubiquitin-40S ribosomal protein S27b OS = Schizosaccharomyces pombe (strain 972/ATCC 24843) OX = 284,812 GN = ubi5 PE = 1 SV = 2 | P0C8R3|RS27B_SCHPO | 17 | 2 | 2 | 17,215 |
| 1393 | Ubiquitin-40S ribosomal protein S27a OS = Schizosaccharomyces pombe (strain 972/ATCC 24843) OX = 284,812 GN = ubi3 PE = 1 SV = 2 | P0C016|RS27A_SCHPO | 17 | 2 | 2 | 17,258 |
| 1394 | Ubiquitin-40S ribosomal protein S31 OS = Saccharomyces cerevisiae (strain ATCC 204508/S288c) OX = 559,292 GN = RPS31 PE = 1 SV = 3 | P05759|RS31_YEAST | 16 | 2 | 2 | 17,216 |
| 1395 | Polyubiquitin OS = Candida albicans OX = 5476 GN = UBI1 PE = 1 SV = 1 | P0CG73|UBI1P_CANAX | 11 | 2 | 2 | 25,755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourali, P.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles. Materials 2022, 15, 4615. https://doi.org/10.3390/ma15134615
Pourali P, Neuhöferová E, Dzmitruk V, Benson V. Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles. Materials. 2022; 15(13):4615. https://doi.org/10.3390/ma15134615
Chicago/Turabian StylePourali, Parastoo, Eva Neuhöferová, Volha Dzmitruk, and Veronika Benson. 2022. "Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles" Materials 15, no. 13: 4615. https://doi.org/10.3390/ma15134615
APA StylePourali, P., Neuhöferová, E., Dzmitruk, V., & Benson, V. (2022). Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles. Materials, 15(13), 4615. https://doi.org/10.3390/ma15134615







