Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Preparation of Ti3C2
2.3. Preparation of ZnO/Ti3C2 Composites
2.4. Characterization
2.5. Photocatalytic Activity
3. Results and Discussion
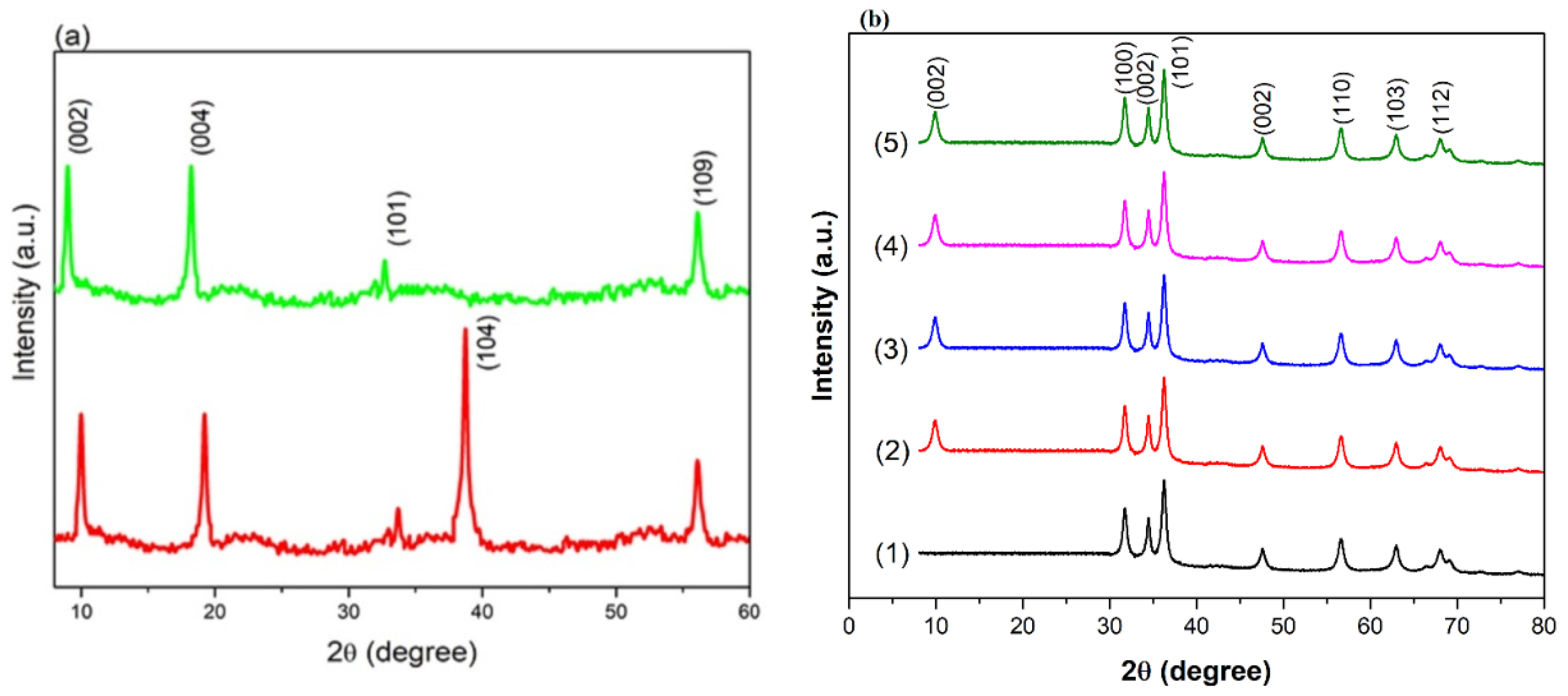
3.1. XRD Structural Analysis
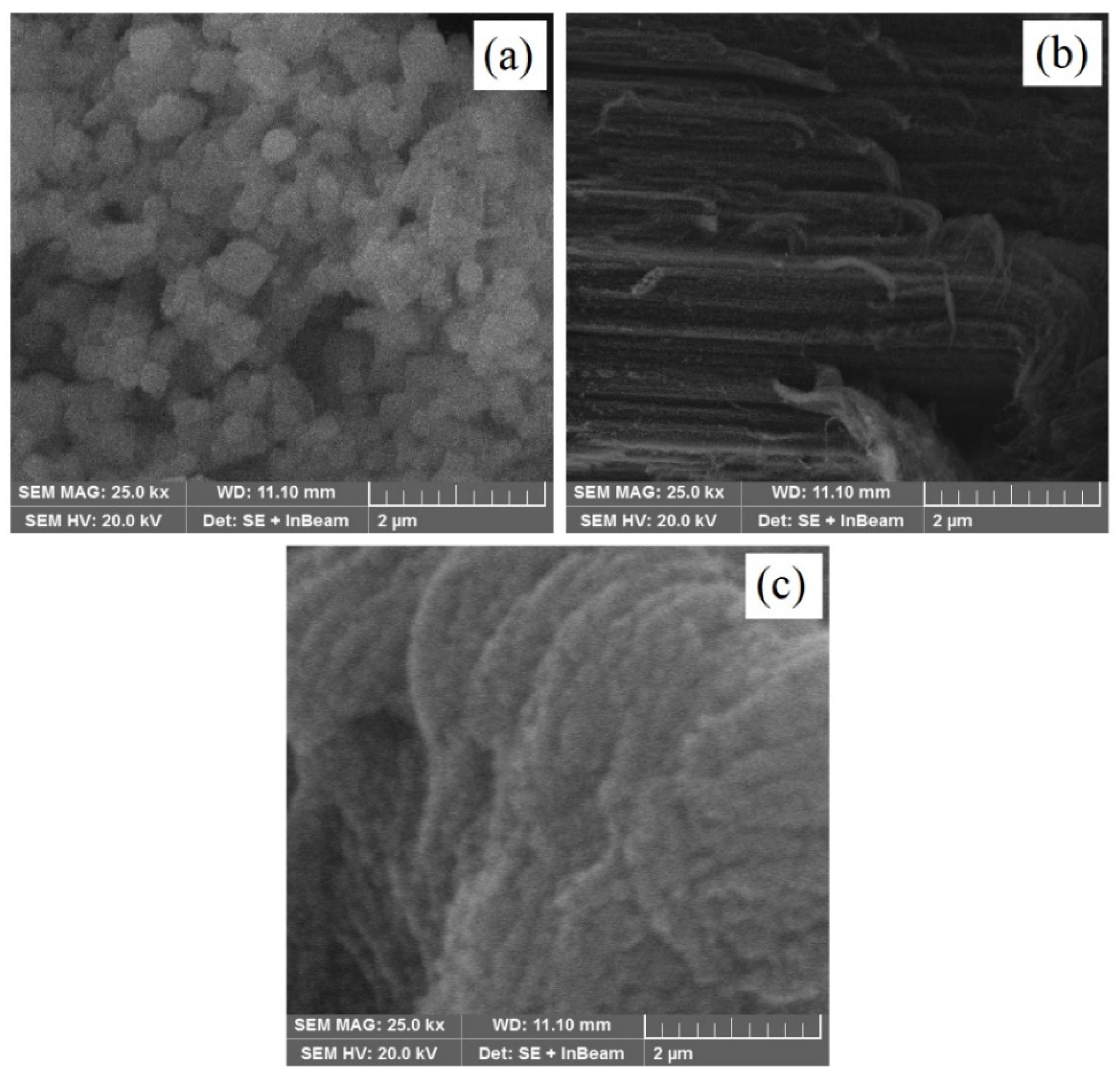
3.2. SEM Analysis
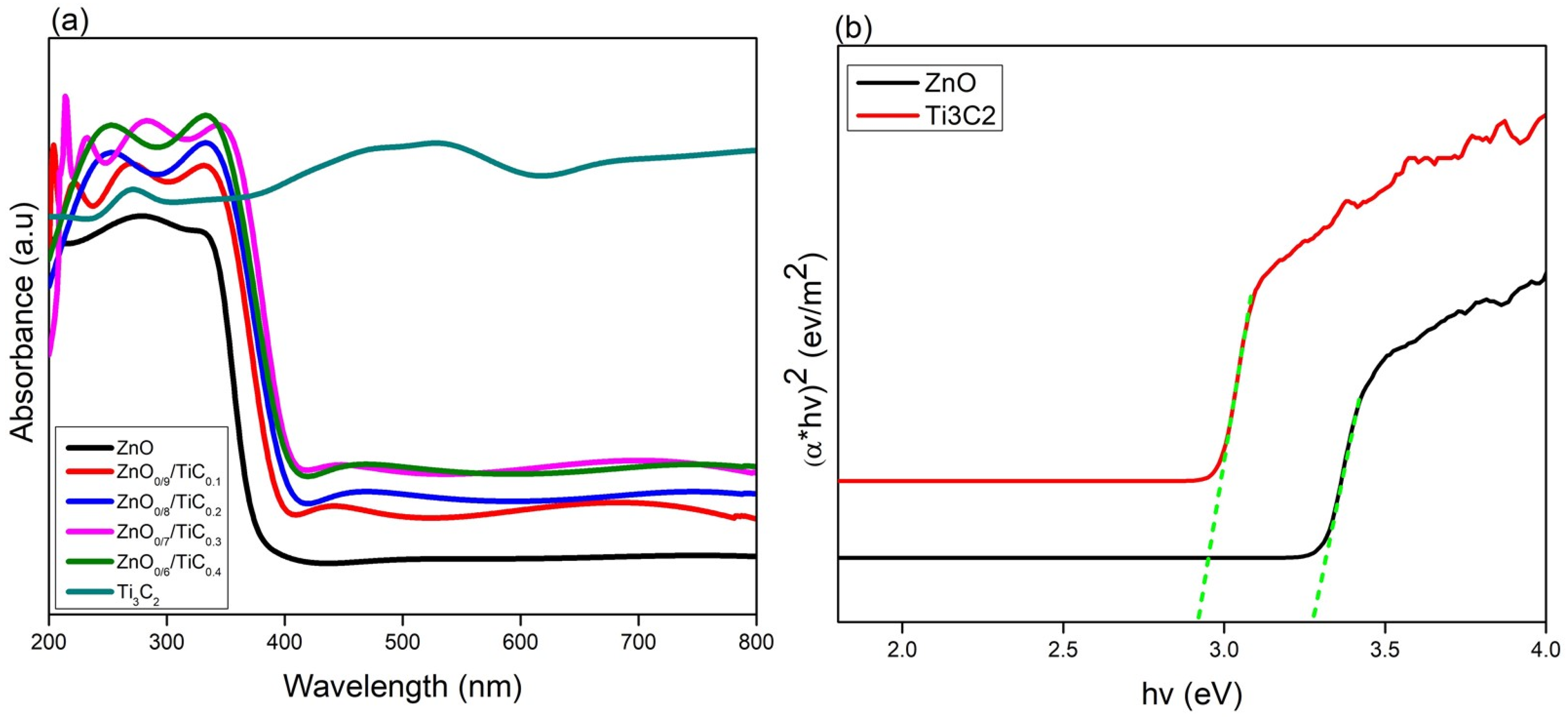
3.3. Optical Absorption
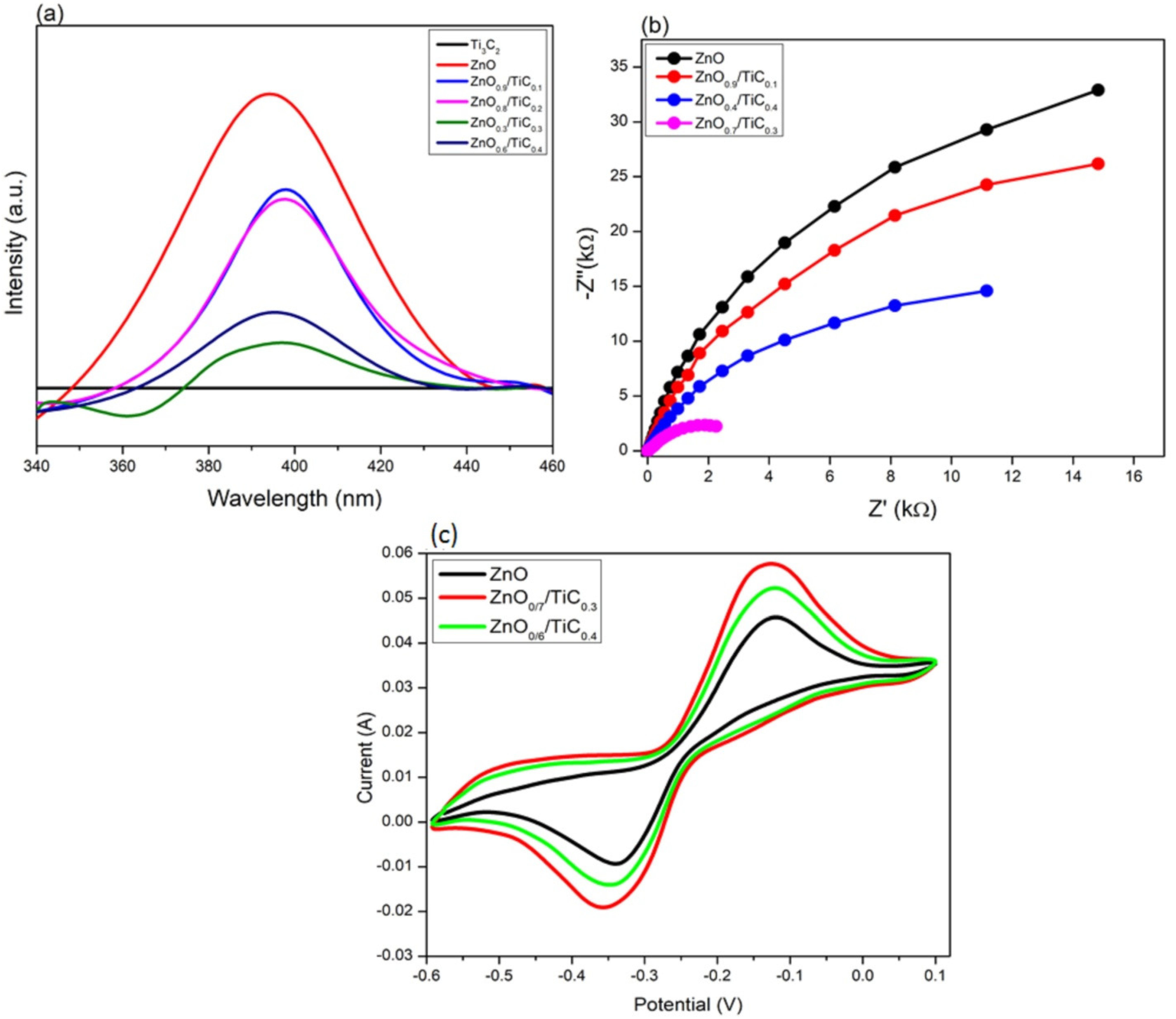
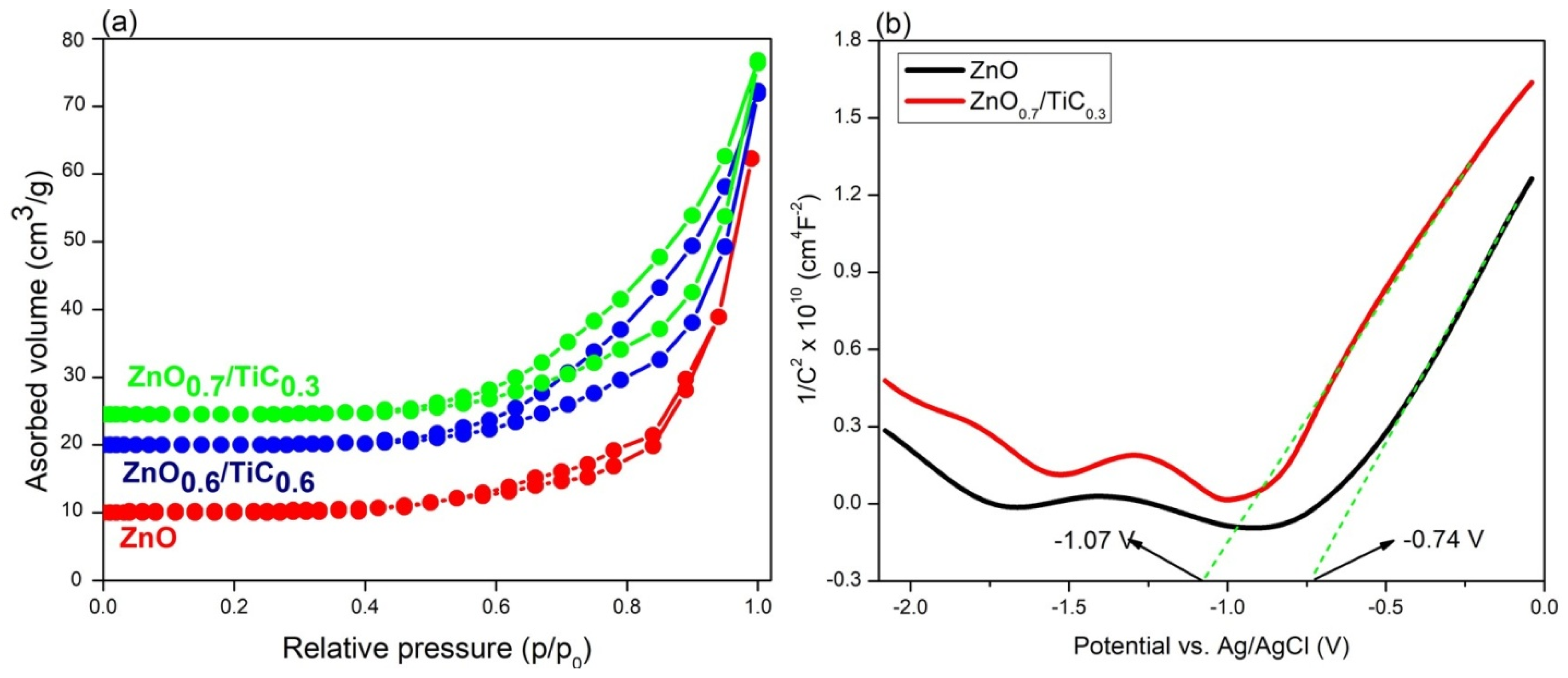
3.4. Spatial Charge Separation and Transfer Ability
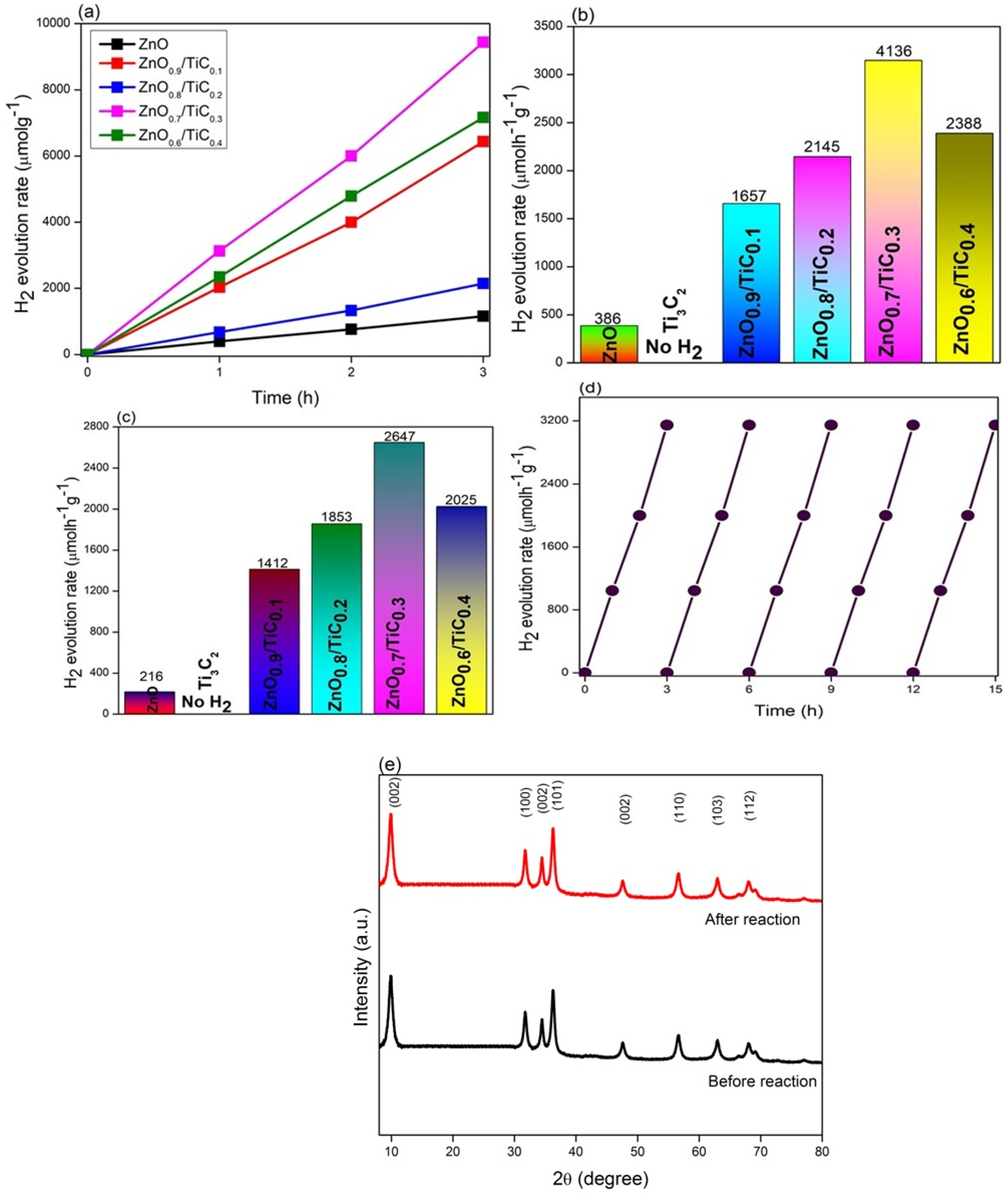
3.5. Photocatalytic H2 Evolution Activity and Stability Test
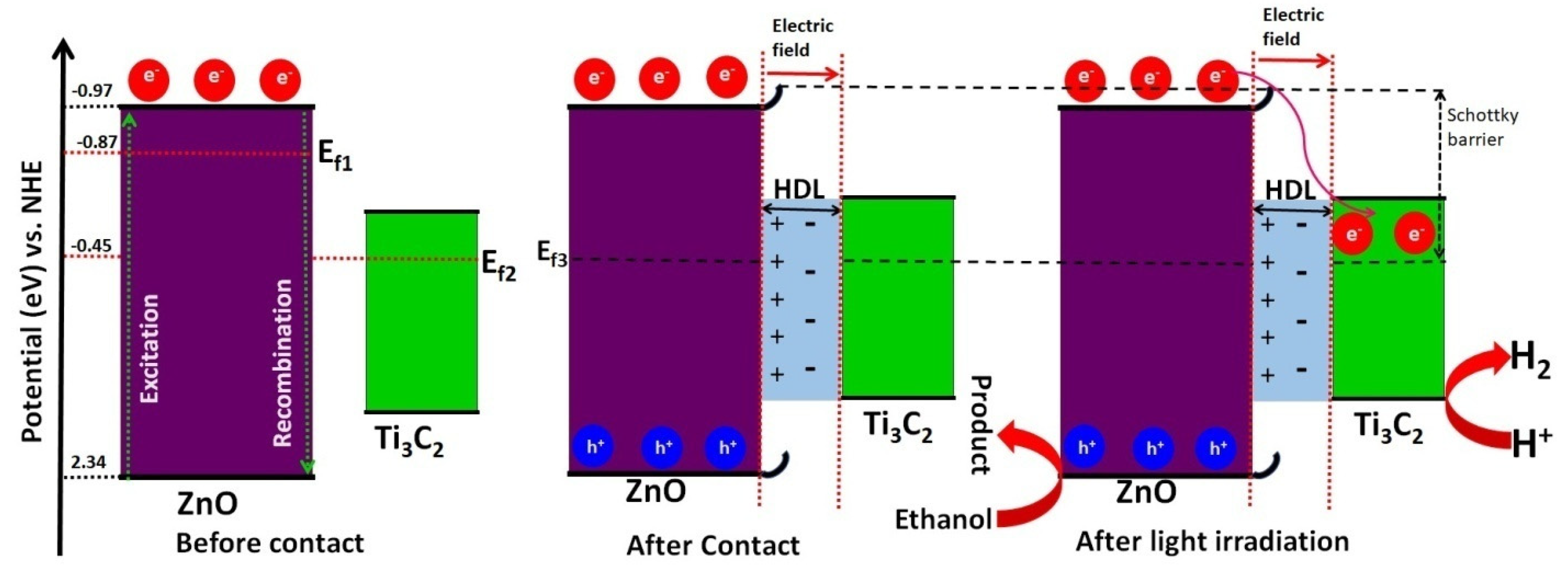
3.6. The Mechanism of Improved H2 Evolution Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production. Adv. Mater. 2018, 30, e1804282. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Qiao, P.; Li, Z.; Xie, Y.; Zhou, W. NiS/Pt nanoparticles co-decorated black mesoporous TiO2 hollow nanotube assemblies as efficient hydrogen evolution photocatalysts. Appl. Mater. Today 2021, 22, 100977. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, S.; Yang, F.; Zhou, W. Mesoporous black TiO2/MoS2/Cu2S hierarchical tandem heterojunctions toward optimized photothermal-photocatalytic fuel production. Chem. Eng. J. 2022, 427, 131830. [Google Scholar] [CrossRef]
- Hanh, N.T.; Tri, N.L.M.; Van Thuan, D.; Tung, M.H.T.; Pham, T.-D.; Minh, T.D.; Trang, H.T.; Binh, M.T.; Nguyen, M.V. Monocrotophos pesticide effectively removed by novel visible light driven Cu doped ZnO photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 382, 111923. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. The role of pH and nitrate concentration in the wet chemical growth of nano-rods shaped ZnO photocatalyst. Nano-Struct. Nano-Objects 2019, 18, 100250. [Google Scholar] [CrossRef]
- Vignesh, S.; Suganthi, S.; Sundar, J.K.; Raj, V.; Devi, P.R.I. Highly efficient visible light photocatalytic and antibacterial performance of PVP capped Cd:Ag: ZnO photocatalyst nanocomposites. Appl. Surf. Sci. 2019, 479, 914–929. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, L.; Yan, H.; Wang, X.; Liu, X.; Zhang, X.; Huang, X.; Hang, R.; Wang, Y.; Yao, X.; et al. Bovine serum albumin assisted synthesis of Ag/Ag2O/ZnO photocatalyst with enhanced photocatalytic activity under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 131–140. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced photocatalytic degradation of sulfamethoxazole by zinc oxide photocatalyst in the presence of fluoride ions: Optimization of parameters and toxicological evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef]
- Tawfik, W.Z.; Hassan, M.A.; Johar, M.A.; Ryu, S.-W.; Lee, J.K. Highly conversion efficiency of solar water splitting over p-Cu2O/ZnO photocatalyst grown on a metallic substrate. J. Catal. 2019, 374, 276–283. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, C.; Lu, B.; Li, P. Enhanced hydrogen production from water splitting by Sn-doped ZnO/BiOCl photocatalysts and Eosin Y sensitization. Int. J. Hydrog. Energy 2022, 47, 228–241. [Google Scholar] [CrossRef]
- Rajendran, S.; Hoang, T.K.; Trudeau, M.L.; Jalil, A.A.; Naushad, M.; Awual, M.R. Generation of novel npn (CeO2-PPy-ZnO) heterojunction for photocatalytic degradation of micro-organic pollutants. Environ. Pollut. 2022, 292, 118375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Y.; Li, J.; Li, P. Efficient hydrogen evolution with ZnO/SrTiO3 S-scheme heterojunction photocatalyst sensitized by Eosin Y. Int. J. Hydrog. Energy 2021, 46, 18922–18935. [Google Scholar] [CrossRef]
- Ramírez-Ortega, D.; Guerrero-Araque, D.; Acevedo-Peña, P.; Reguera, E.; Calderon, H.A.; Zanella, R. Enhancing the photocatalytic hydrogen production of the ZnO–TiO2 heterojunction by supporting nanoscale Au islands. Int. J. Hydrog. Energy 2021, 46, 34333–34343. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Heng, Q.; Chen, W.; Li, X.; Mao, L.; Shangguan, W. Promoted charge separation on 3D interconnected Ti3C2/MoS2/CdS composite for enhanced photocatalytic H2 production. Int. J. Hydrog. Energy 2022, 47, 8284–8293. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, H.; Zhang, Q.; Hu, H.; Liu, L.; Ye, J.; Wang, D. Surface-Alkalinized Ti3C2 MXene as an Efficient Cocatalyst for Enhanced Photocatalytic CO2 Reduction over ZnO. Catal. Sci. Technol. 2021, 11, 4953–4961. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, J.; Wu, Y.; Cai, Q. Hybridisation of ZnO with Ti3C2 as a co-catalyst for enhanced photocatalytic activity. Micro Nano Lett. 2020, 15, 764–768. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Rasheed, M.A.; Ahmad, M.; Ahmed, E.; Akhtar, M.S.; Khalid, N.; Hussain, A.; Khalid, S. Boosted hydrogen evolution activity from Sr doped ZnO/CNTs nanocomposite as visible light driven photocatalyst. Int. J. Hydrog. Energy 2021, 46, 26711–26724. [Google Scholar] [CrossRef]
- Chu, F.H.; Huang, C.W.; Hsin, C.L.; Wang, C.W.; Yu, S.Y.; Yeh, P.H.; Wu, W.W. Well-aligned ZnO nanowires with excellent field emission and photocatalytic properties. Nanoscale 2012, 4, 1471–1475. [Google Scholar] [CrossRef]
- Xiaoliang, W.; Shihua, D.; Yong, P.; Qin, X.; Yun, L. Study of the Photocatalytic Activity of Na and Al-doped ZnO Powders. Ferroelectrics 2013, 455, 90–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, J.-L.; Zheng, J.-H.; Lian, J.-S. Optical properties and photocatalytic activity of Nd-doped ZnO powders. Trans. Nonferrous Met. Soc. China 2014, 24, 1434–1439. [Google Scholar] [CrossRef]
- Perillo, P.M.; Atia, M.N. C-doped ZnOnanorods for photocatalytic degradation of p-aminobenzoic acid under sunlight. Nano-Struct. Nano-Objects 2017, 10, 125–130. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Su, C.; Che, J.; Sun, W.; Gao, H. Electrospun ZnO/Bi2O3 nanofibers with enhanced photocatalytic activity. J. Nanomater. 2014, 2014, 130539. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, G.; Chen, J.; Xu, H.; Shen, X.; Yuan, A. Co3O4/ZnO nanocomposites for gas-sensing applications. Appl. Surf. Sci. 2013, 265, 379–384. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun nanofibers of ZnO−SnO2 heterojunction with high photocatalytic activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 nanocomposites. J. Mol. Catal. A Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Flores, A.M.; Luévano-Hipólito, E.; Torres-Martínez, L.M.; Torres-Sánchez, A. Photocatalytic H2 production and CO2 reduction on Cu, Ni-doped ZnO: Effect of metal doping and oxygen vacancies. J. Mater. Sci. Mater. Electron. 2019, 30, 18506–18518. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, M.K.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and photocatalytic activity of Gd doped ZnO nanoparticles for enhanced degradation of methylene blue under visible light. Mater. Sci. Semicond. Process. 2019, 103, 104622. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M.; Keller, V.; Khan, W.Q.; Khalid, N.R. Rare earth co-doped ZnO photocatalysts: Solution combustion synthesis and environmental applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, E.; Ahmad, M. The excellent photocatalytic performances of silver doped ZnO nanoparticles for hydrogen evolution. SN Appl. Sci. 2019, 1, 327. [Google Scholar] [CrossRef] [Green Version]
- Beura, R.; Thangadurai, P. Effect of Sn doping in ZnO on the photocatalytic activity of ZnO-Graphene nanocomposite with improved activity. J. Environ. Chem. Eng. 2018, 6, 5087–5100. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Añó, E.; Simorte, J.; Valero-Gómez, A.; Bosch, F. Hybrid photocatalysts of ZnO obtained by waste valorization combined with reduced graphene oxide. Mater. Today Proc. 2020, 20, 356–364. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, E.; Ahmad, M.; Akhtar, M.S.; Basharat, M.A.; Khan, W.Q.; Ghauri, M.I.; Ali, A.; Manzoor, M.F. The investigation of hydrogen evolution using Ca doped ZnO catalysts under visible light illumination. Mater. Sci. Semicond. Process. 2020, 105, 104748. [Google Scholar] [CrossRef]
- Khan, H.; Jiang, Z.; Berk, D. Molybdenum doped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalytic applications. Sol. Energy 2018, 162, 420–430. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Wang, Y.; Wang, S.; Li, Z.; Sun, Q. Construction of three-dimensional g-C3N4/Gr-CNTs/TiO2 Z-scheme catalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 510, 145494. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Yang, G.; Chen, L.; Guo, P.; Zhang, L. A facile synthesis of ZnO/CNT hierarchical microsphere composites with enhanced photocatalytic degradation of methylene blue. RSC Adv. 2015, 5, 72476–72481. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Xu, Y.; Zhao, R.; Han, J.; Wang, L. Facile fabrication of CdSe/CuInS2 microflowers with efficient photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2022, 47, 8294–8302. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Markovskaya, D.V.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlova, E.A. New insights into the mechanism of photocatalytic hydrogen evolution from aqueous solutions of saccharides over CdS-based photocatalysts under visible light. Int. J. Hydrogen Energy 2020, 45, 30165–30177. [Google Scholar] [CrossRef]
- Khalid, N.; Bilal, M.; Tahir, M.; Shakil, M.; Iqbal, T.; Rafique, M.; Yousaf, N.; Gillani, S. Interfacial coupling effect of Ag2O nanorods over MoS2 microflowers for improved photocatalytic activity. Ceram. Int. 2020, 46, 6856–6859. [Google Scholar] [CrossRef]
- Noreen, Z.; Khalid, N.R.; Abbasi, R.; Javed, S.; Ahmad, I.; Bokhari, H. Visible light sensitive Ag/TiO2/graphene composite as a potential coating material for control of Campylobacter jejuni. Mater. Sci. Eng. C 2019, 98, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Je, M.; Ton, N.N.T.; Lei, W.; Taniike, T.; Yanagida, S.; Ogawa, D.; Suzuki, N.; Terashima, C.; Fujishima, A.; et al. C-doped ZnS-ZnO/Rh Nanosheets as Multijunctioned Photocatalysts for Effective H2 Generation from Pure Water under Solar Simulating Light. Appl. Catal. B Environ. 2021, 297, 120473. [Google Scholar] [CrossRef]
- Luan, Q.; Chen, Q.; Zheng, J.; Guan, R.; Fang, Y.; Hu, X. Construction of 2D-ZnS@ZnO Z-Scheme Heterostructured Nanosheets with a Highly Ordered ZnO Core and Disordered ZnS Shell for Enhancing Photocatalytic Hydrogen Evolution. ChemNanoMat 2020, 6, 470–479. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Zhang, Y.; Xu, H.; Cao, S.; Zhang, R. All-solid-state Z-scheme Pt/ZnS-ZnO heterostructure sheets for photocatalytic simultaneous evolution of H2 and O2. Chem. Eng. J. 2020, 385, 123782. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Aguilar-Martínez, O.; Acevedo-Peña, P.; Santolalla-Vargas, C.E.; Oros-Ruíz, S.; Galindo-Hernández, F.; Gómez, R.; Tzompantzi, F. Novel ZnS-ZnO composite synthesized by the solvothermal method through the partial sulfidation of ZnO for H2 production without sacrificial agent. Appl. Catal. B Environ. 2018, 230, 125–134. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Wang, K.; Zhang, G.; Li, Y.; Wu, X. Low boiling point solvent mediated strategy to synthesize functionalized monolayer carbon nitride for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 260, 118181. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Li, Y.; Jiang, L.; Zhang, G. Novel BiSbO4/BiOBr nanoarchitecture with enhanced visible-light driven photocatalytic performance: Oxygen-induced pathway of activation and mechanism unveiling. Appl. Surf. Sci. 2019, 498, 143850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Ahmad, I.; Shukrullah, S.; Hussain, H.; Atif, M.; Legutko, S.; Petru, J.; Hatala, M.; Naz, M.Y.; Rahman, S. Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution. Materials 2022, 15, 4557. https://doi.org/10.3390/ma15134557
Irfan M, Ahmad I, Shukrullah S, Hussain H, Atif M, Legutko S, Petru J, Hatala M, Naz MY, Rahman S. Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution. Materials. 2022; 15(13):4557. https://doi.org/10.3390/ma15134557
Chicago/Turabian StyleIrfan, Muhammad, Irshad Ahmad, Shazia Shukrullah, Humaira Hussain, Muhammad Atif, Stanislaw Legutko, Jana Petru, Michal Hatala, Muhammad Yasin Naz, and Saifur Rahman. 2022. "Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution" Materials 15, no. 13: 4557. https://doi.org/10.3390/ma15134557
APA StyleIrfan, M., Ahmad, I., Shukrullah, S., Hussain, H., Atif, M., Legutko, S., Petru, J., Hatala, M., Naz, M. Y., & Rahman, S. (2022). Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution. Materials, 15(13), 4557. https://doi.org/10.3390/ma15134557







