Effect of Uniaxial Compressive Stress on Phase Transformation Kinetics of Low-Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
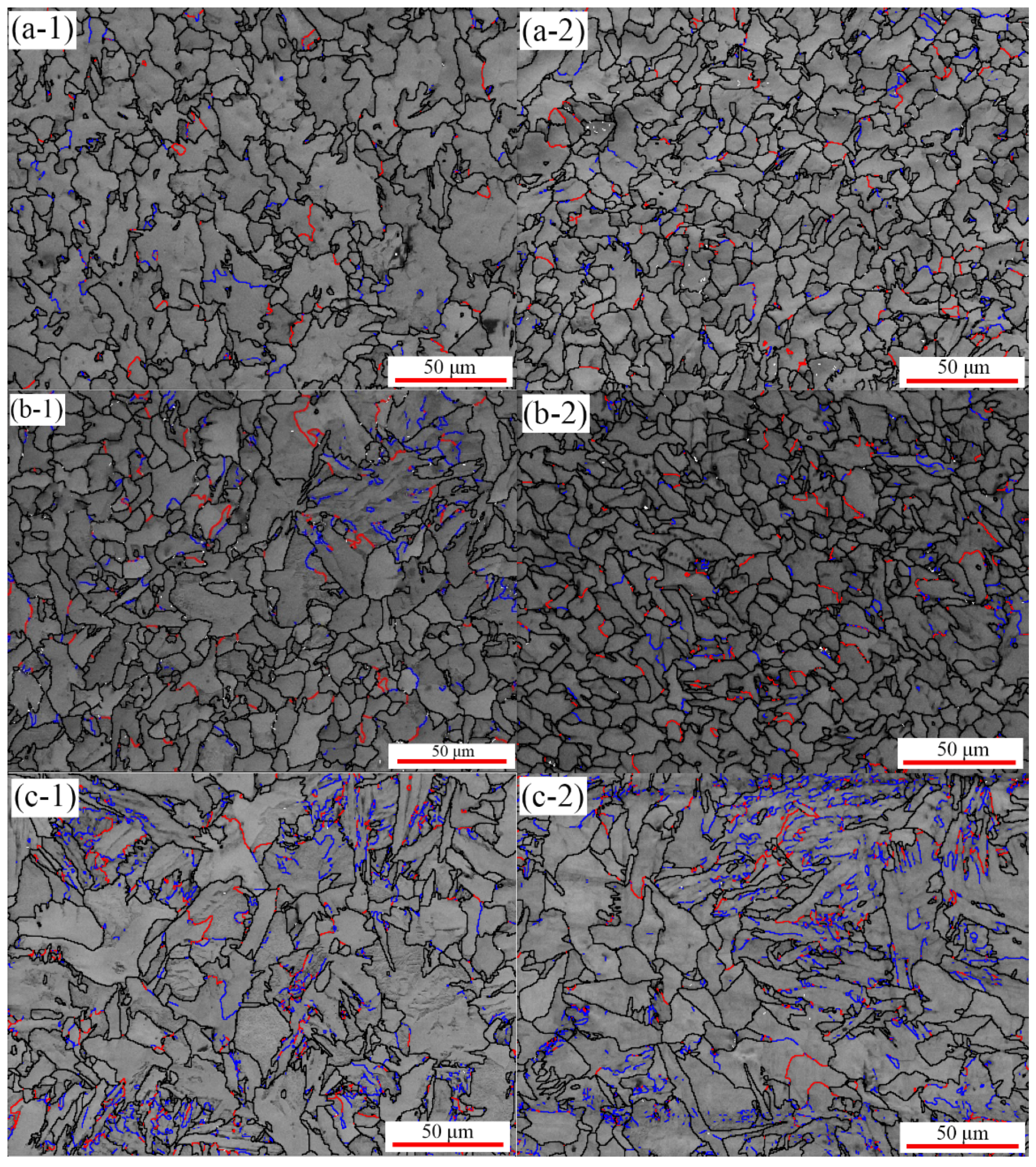
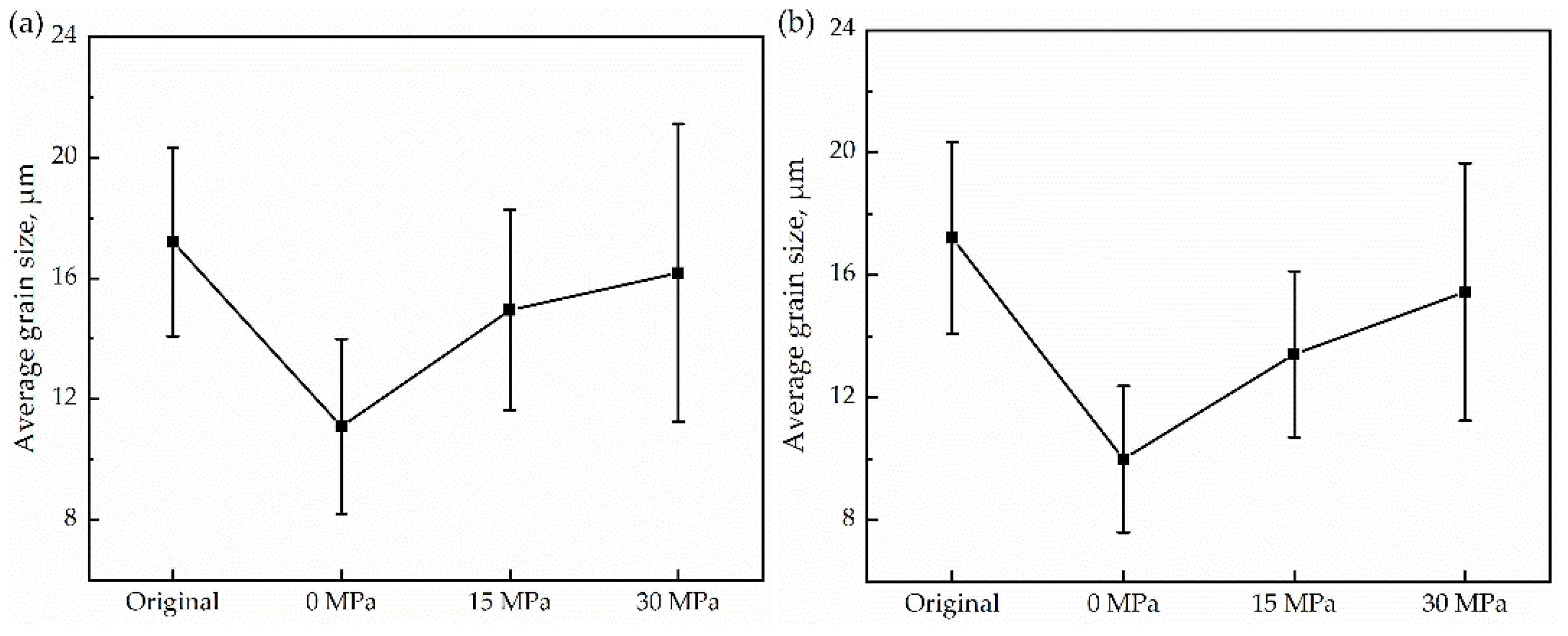
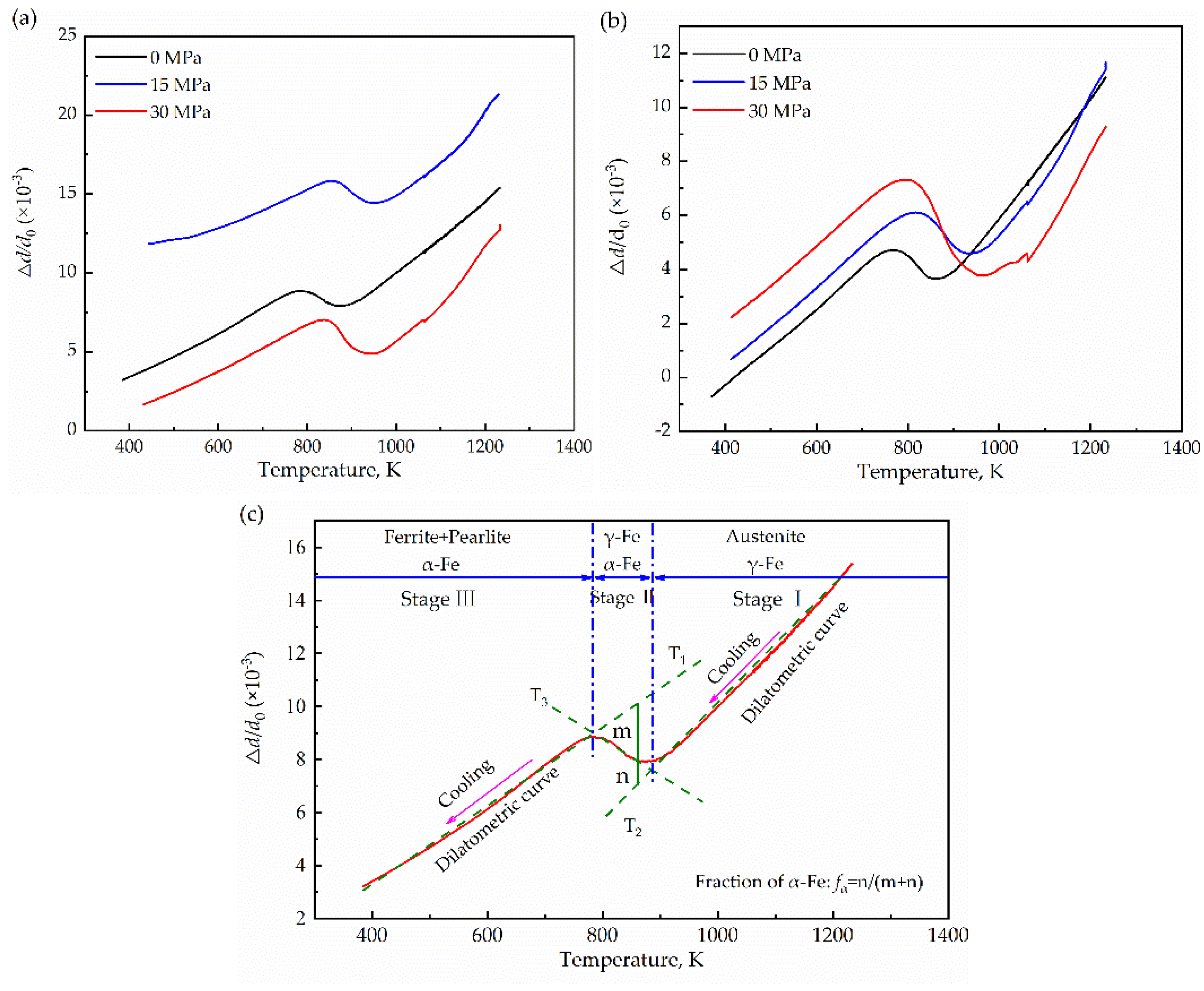
3.1. Microstructure Evolution
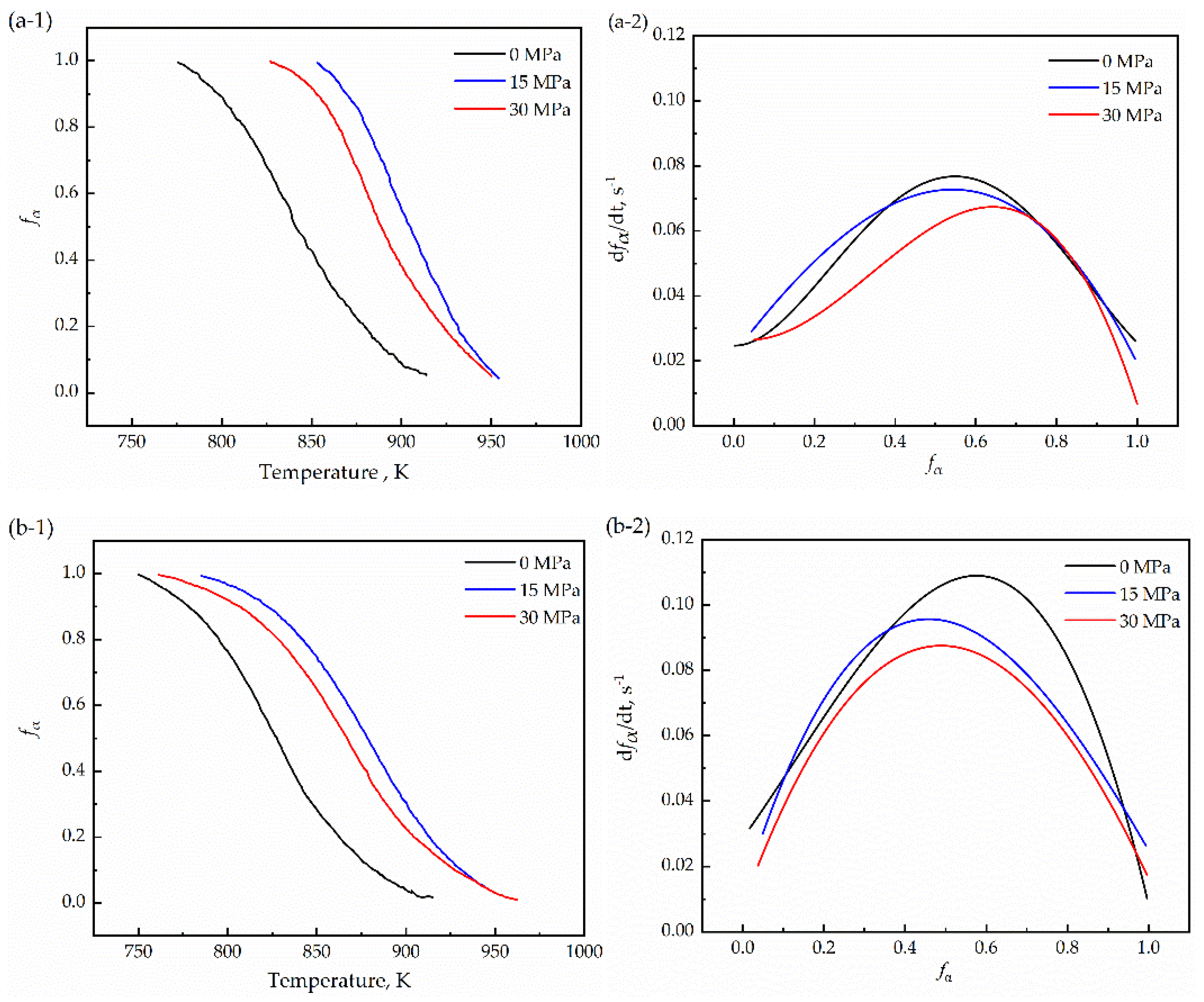
3.2. Phase-Transformation Kinetics Using the Modified JMA Model
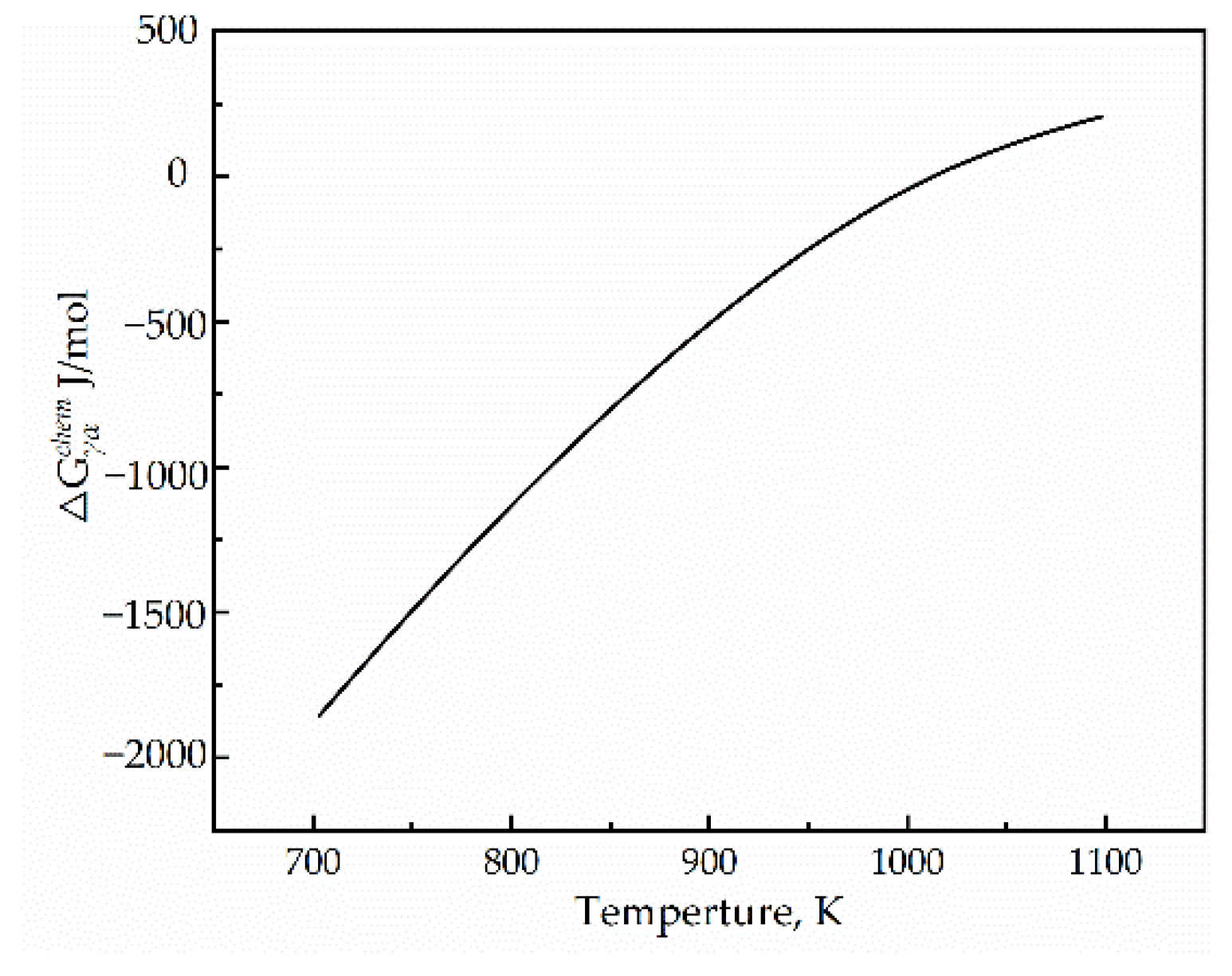
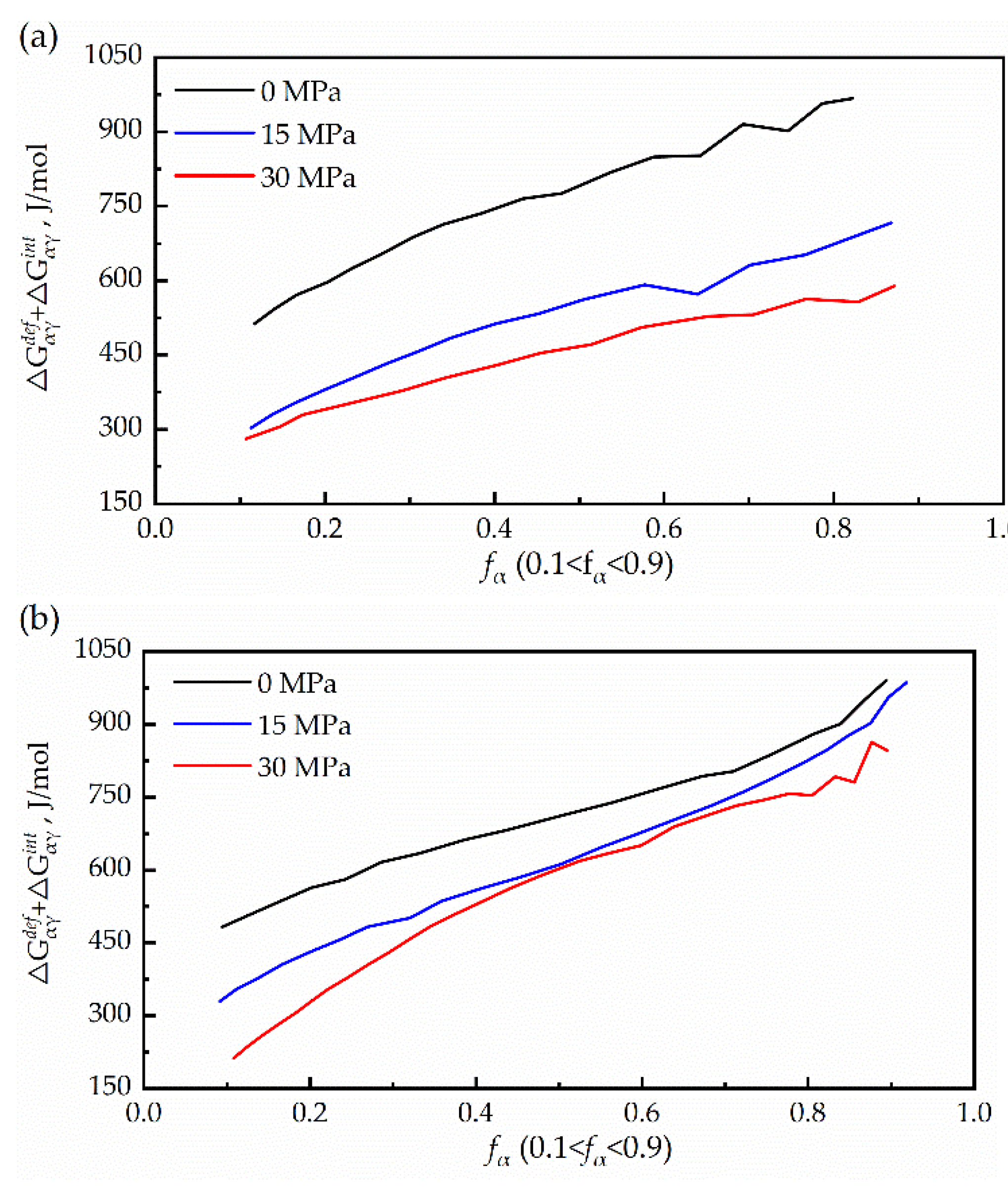
3.3. Driving Force for the Transformation of Austenite to Ferrite
4. Conclusions
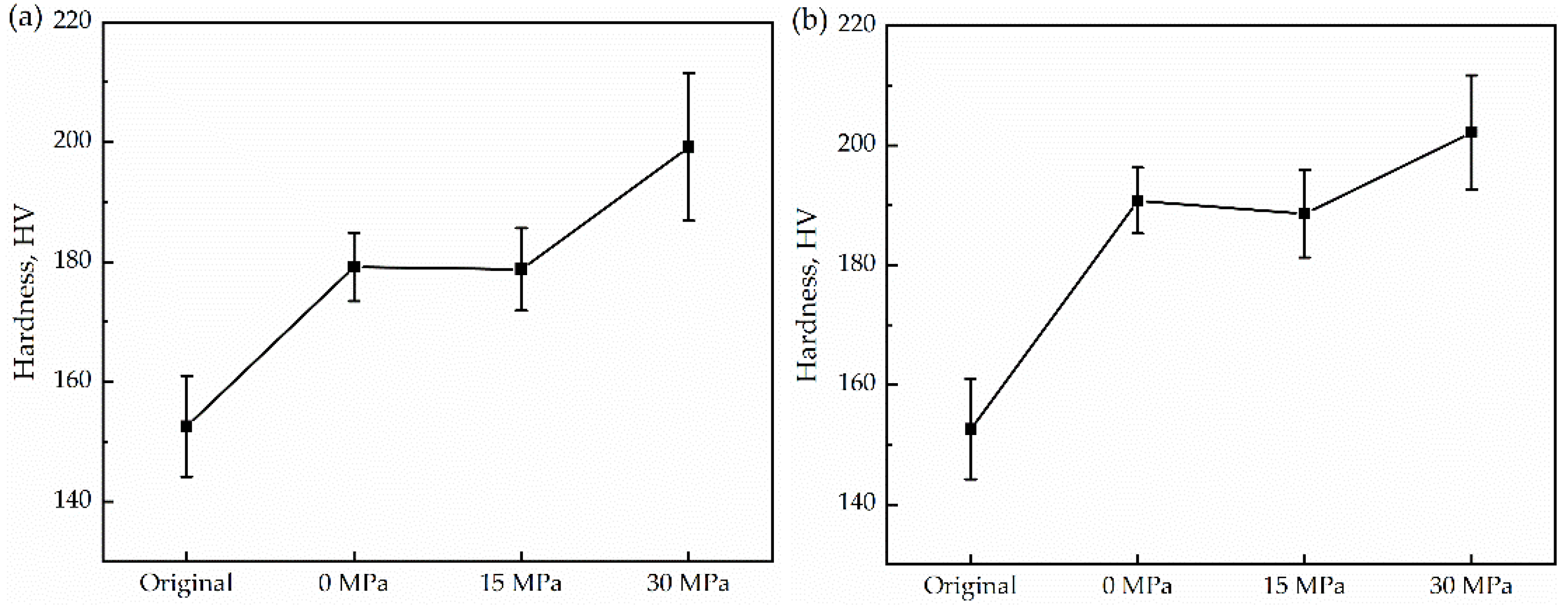
- Observing the microstructure, it was found that the grain size after thermal cycling was slightly finer than that of the original sample. Low-angle grain boundaries also increase with increasing stress. Meanwhile, additional energy was supplied to the grain growth, resulting in a decrease in the nucleation number and gradual coarsening. In addition, the microhardness increased with uniaxial compressive stress.
- The application of stress led to an increase in the grain size and a decrease in the density of the α/γ boundary, resulting in a decrease in the interfacial migration velocity with increasing stress. Therefore, the maximum transformation rate decreases with increasing compressive stress, and the single peak indicates that the transformation mode is a normal austenite-ferrite transformation under stress.
- Most of the chemical Gibbs free energy is dissipated by the sum of the misfit accommodation energy and interface energy during the γ→α transformation. The driving force of the γ→α transformation was very small compared with the sum energy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaper, L.; Tankova, T.; Simões da Silva, L.; Knobloch, M. A novel residual stress model for welded I-sections. J. Constr. Steel Res. 2022, 188, 107017. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L. The effect of joint configuration on residual stress and distortion of the 304 stainless steel multi-pass welded joints. Mater. Today Commun. 2022, 30, 103070. [Google Scholar] [CrossRef]
- Qiang, B.; Wang, X. Evaluating stress intensity factors for surface cracks in an orthotropic steel deck accounting for the welding residual stresses. Theor. Appl. Fract. Mech. 2020, 110, 102827. [Google Scholar] [CrossRef]
- Xu, K.; Qiao, G.-Y.; Shi, X.-B.; Xiao, F.-R. Effect of stress-relief annealing on the fatigue properties of X80 welded pipes. Mater. Sci. Eng. A 2021, 807, 140854. [Google Scholar] [CrossRef]
- Alves, E.M.M.; Silveira, C.C.; da Cunha, M.A. Influence of stress relief annealing temperature on the cutting edge microstructure and on the recovery of magnetic properties of grain oriented electrical steel. Mater. Charact. 2020, 166, 110408. [Google Scholar] [CrossRef]
- Jamei, F.; Mirzadeh, H.; Zamani, M. Synergistic effects of holding time at intercritical annealing temperature and initial microstructure on the mechanical properties of dual phase steel. Mater. Sci. Eng. A 2019, 750, 125–131. [Google Scholar] [CrossRef]
- Valdes-Tabernero, M.A.; Celada-Casero, C.; Sabirov, I.; Kumar, A.; Petrov, R.H. The effect of heating rate and soaking time on microstructure of an advanced high strength steel. Mater. Charact. 2019, 155, 109822. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, H.; Sun, H.; Chen, G. Effects of annealing temperature on the microstructure, textures and tensile properties of cold-rolled Fe–13Cr–4Al alloys with different Nb contents. Mater. Sci. Eng. A 2020, 798, 140236. [Google Scholar] [CrossRef]
- Hernandez-Duran, E.I.; Ros-Yanez, T.; Castro-Cerda, F.M.; Petrov, R.H. The influence of the heating rate on the microstructure and mechanical properties of a peak annealed quenched and partitioned steel. Mater. Sci. Eng. A 2020, 797, 140061. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Wang, Y.; Wang, Y.; Wang, T. Influence of annealing temperature on microstructure, tensile properties and tensile deformation mechanism of metastable austenitic stainless steel repetitively cold-rolled and annealed. Materialia 2019, 8, 100455. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Q.; Chen, L.; Guo, X.; Li, N.; Liu, X.; Jin, M. Influence of annealing temperature on the strain-hardening behavior of a lean duplex stainless steel. Mater. Sci. Eng. A 2018, 722, 216–224. [Google Scholar] [CrossRef]
- Cai, Z.H.; Li, H.Y.; Jing, S.Y.; Li, Z.C.; Ding, H.; Tang, Z.Y.; Misra, R.D.K. Influence of annealing temperature on microstructure and tensile property of cold-rolled Fe-0.2C-11Mn-6Al steel. Mater. Charact. 2018, 137, 256–262. [Google Scholar] [CrossRef]
- Olorundaisi, E.; Jamiru, T.; Adegbola, T.A. Response surface modelling and optimization of temperature and holding time on dual phase steel. Mater. Today Proc. 2021, 38, 1164–1169. [Google Scholar] [CrossRef]
- Balbi, M.; Alvarez-Armas, I.; Armas, A. Effect of holding time at an intercritical temperature on the microstructure and tensile properties of a ferrite-martensite dual phase steel. Mater. Sci. Eng. A 2018, 733, 1–8. [Google Scholar] [CrossRef]
- González-Arévalo, N.E.; Velázquez, J.C.; Díaz-Cruz, M.; Cervantes-Tobón, A.; Terán, G.; Hernández-Sanchez, E.; Capula-Colindres, S. Influence of aging steel on pipeline burst pressure prediction and its impact on failure probability estimation. Eng. Fail. Anal. 2021, 120, 104950. [Google Scholar] [CrossRef]
- Bansal, G.K.; Rajinikanth, V.; Ghosh, C.; Srivastava, V.C.; Dutta, M.; Ghosh Chowdhury, S. Effect of cooling rate on the evolution of microstructure and mechanical properties of nonisothermally partitioned steels. Mater. Sci. Eng. A 2020, 788, 139614. [Google Scholar] [CrossRef]
- Dey, I.; Ghosh, S.K.; Saha, R. Effects of cooling rate and strain rate on phase transformation, microstructure and mechanical behaviour of thermomechanically processed pearlitic steel. J. Mater. Res. Technol. 2019, 8, 2685–2698. [Google Scholar] [CrossRef]
- Wu, M.; Hua, L.; Shao, Y.C.; Zhou, Q.J. Influence of the annealing cooling rate on the microstructure evolution and deformation behaviours in the cold ring rolling of medium steel. Mater. Des. 2011, 32, 2292–2300. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Meng, Q.; Hu, W.; Xu, D. Effect of heating rate on ferrite recrystallization and austenite formation of cold-roll dual phase steel. J. Alloys Compd. 2013, 578, 320–327. [Google Scholar] [CrossRef]
- Wei, Y.H.; Yu, X.F.; Su, Y.; Shen, X.Y.; Xia, Y.Z.; Yang, W.W. Effect of residual stress and microstructure evolution on size stability of M50 bearing steel. J. Mater. Res. Technol. 2021, 10, 651–661. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, J.; Chen, X.; Huang, G.; Jiang, B.; Tang, A.; Gavras, S.; Huang, Y.; Hort, N.; Pan, F. Effect of biaxial compressive stress state on the microstructure evolution and deformation compatibility of rolled sheet Mg alloy AZ31 at room temperature. Mater. Sci. Eng. A 2020, 789, 139599. [Google Scholar] [CrossRef]
- Ghasemi, A.; Gao, W. A method to predict energy barriers in stress modulated solid–solid phase transitions. J. Mech. Phys. Solids 2020, 137, 103857. [Google Scholar] [CrossRef]
- Aranas, C.; Rodrigues, S.; Siciliano, F.; Jonas, J. In-situ X-ray diffraction evidence of dynamic transformation of austenite to ferrite during hot compression test in the single austenite phase field. Scr. Mater. 2020, 177, 86–90. [Google Scholar] [CrossRef]
- Nakada, N.; Ishibashi, Y.; Tsuchiyama, T.; Takaki, S. Self-stabilization of untransformed austenite by hydrostatic pressure via martensitic transformation. Acta Mater. 2016, 110, 95–102. [Google Scholar] [CrossRef]
- Wan, J.-L.; Chen, Z.-B.; Qin, S.-J.; Shang, J.-X. Effect of hydrostatic pressure on thermally induced phase transformation in NiTi alloy: A molecular dynamics study. Comput. Mater. Sci. 2018, 153, 119–125. [Google Scholar] [CrossRef]
- Liu, Y.; China, P.R.; Sommer, F.; Mittemeijer, E.J. Nature and kinetics of the massive austenite-ferrite phase transformations in steels. In Phase Transformations in Steels; Woodhead Publishing: Sawston, UK, 2012; pp. 311–381. [Google Scholar]
- Hsu, T.Y. Phase Transformations Under Stress. Heat Treat. 2004, 19, 1–17. [Google Scholar]
- Hao, C.; Congyu, Z.; Jianing, Z.; Zenan, Y.; Ran, D.; Chi, Z.; Zhigang, Y. Austenite/Ferrite Interface Migration and Alloying Elements Partitioning: An Overview. Acta Metall. Sin. 2018, 54, 217–227. [Google Scholar] [CrossRef]
- Biglari, M.; Mittemeijer, E.J. Simulation of the massive austenite–ferrite transformation under uniaxial loading. Comput. Mater. Sci. 2013, 77, 214–223. [Google Scholar] [CrossRef]
- Mohapatra, G.; Sommer, F.; Mittemeijer, E.J. The austenite to ferrite transformation of Fe–Ni under the influence of a uniaxially applied tensile stress. Acta Mater. 2007, 55, 4359–4368. [Google Scholar] [CrossRef]
- Rafiei, M.; Mirzadeh, H.; Malekan, M. Precipitation kinetics of γ″ phase and its mechanism in a Nb-bearing nickel-based superalloy during aging. Vacuum 2020, 178, 109456. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y.; Agarwal, S.; Henry, J.; Zinkle, S.J. Toward accurate evaluation of bulk hardness from nanoindentation testing at low indent depths. Mater. Des. 2022, 213, 110317. [Google Scholar] [CrossRef]
- Wang, J.; Hong, H.; Huang, A.; Yang, X.; Qian, R.; Shang, C. New insight into the relationship between grain boundaries and hardness in bainitic/martensitic steels from the crystallographic perspective. Mater. Lett. 2022, 308, 131105. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Q. The role of low angle grain boundary in deformation of titanium and its size effect. Scr. Mater. 2019, 163, 148–151. [Google Scholar] [CrossRef]
- Sedláček, R.; Blum, W.; Kratochvíl, J.; Forest, S. Subgrain formation during deformation: Physical origin and consequences. Metall. Mater. Trans. A 2002, 33, 319–327. [Google Scholar] [CrossRef]
- Li, H.; Gai, K.; He, L.; Zhang, C.; Cui, H.; Li, M. Non-isothermal phase-transformation kinetics model for evaluating the austenization of 55CrMo steel based on Johnson–Mehl–Avrami equation. Mater. Des. 2016, 92, 731–741. [Google Scholar] [CrossRef]
- Liu, Y.C.; Sommer, F.; Mittemeijer, E.J. Austenite–ferrite transformation kinetics under uniaxial compressive stress in Fe–2.96at.% Ni alloy. Acta Mater. 2009, 57, 2858–2868. [Google Scholar] [CrossRef]
- McNamara, K.; Ji, Y.; Lia, F.; Promoppatum, P.; Yao, S.-C.; Zhou, H.; Wang, Y.; Chen, L.-Q.; Martukanitz, R.P. Predicting phase transformation kinetics during metal additive manufacturing using non-isothermal Johnson-Mehl-Avrami models: Application to Inconel 718 and Ti-6Al-4V. Addit. Manuf. 2022, 49, 102478. [Google Scholar] [CrossRef]
- He, J.; Han, G.; Feng, Y. Phase transformation and plastic behavior of QP steel sheets: Transformation kinetics-informed modeling and forming limit prediction. Thin-Walled Struct. 2022, 173, 108977. [Google Scholar] [CrossRef]
- Poyraz, O.; Ögel, B. Recrystallization, grain growth and austenite formation in cold rolled steels during intercritical annealing. J. Mater. Res. Technol. 2020, 9, 11263–11277. [Google Scholar] [CrossRef]
- Kempen, A.T.W.; Sommer, F.; Mittemeijer, E.J. The kinetics of the austenite–ferrite phase transformation of Fe-Mn: Differential thermal analysis during cooling. Acta Mater. 2002, 50, 3545–3555. [Google Scholar] [CrossRef]
- Liu, Y.C.; Sommer, F.; Mittemeijer, E.J. Kinetics of the abnormal austenite–ferrite transformation behaviour in substitutional Fe-based alloys. Acta Mater. 2004, 52, 2549–2560. [Google Scholar] [CrossRef]
- Sharma, H.; Huizenga, R.M.; Bytchkov, A.; Sietsma, J.; Offerman, S.E. Observation of changing crystal orientations during grain coarsening. Acta Mater. 2012, 60, 229–237. [Google Scholar] [CrossRef]
- Curnan, M.T.; Shin, D.; Saidi, W.A.; Yang, J.C.; Han, J.W. Universally characterizing atomistic strain via simulation, statistics, and machine learning: Low-angle grain boundaries. Acta Mater. 2022, 226, 117635. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, L.; Xiong, Z.; Yang, J.; Tan, Y.; Liu, Y.; Zhang, Y.; Tan, Q.; Hao, C.; Cao, L.; et al. The response of dislocations, low angle grain boundaries and high angle grain boundaries at high strain rates. Mater. Sci. Eng. A 2021, 822, 141704. [Google Scholar] [CrossRef]
- Lim, A.T.; Srolovitz, D.J.; Haataja, M. Low-angle grain boundary migration in the presence of extrinsic dislocations. Acta Mater. 2009, 57, 5013–5022. [Google Scholar] [CrossRef]
- Hillert, M. Diffusion and Interface Reactions in Alloys Control of Reactions in Alloys. Metall. Trans. A 1975, 6, 5–19. [Google Scholar] [CrossRef]
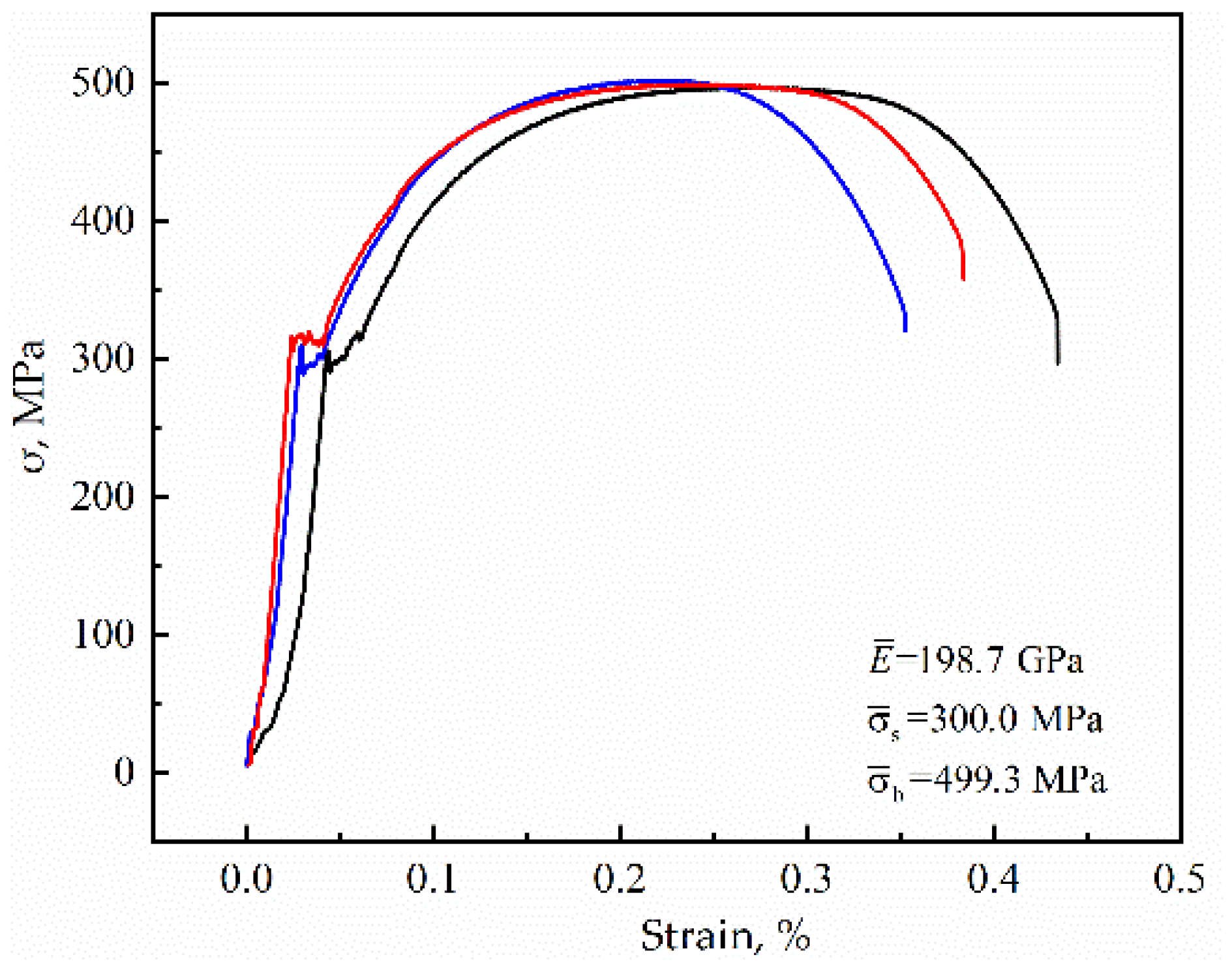
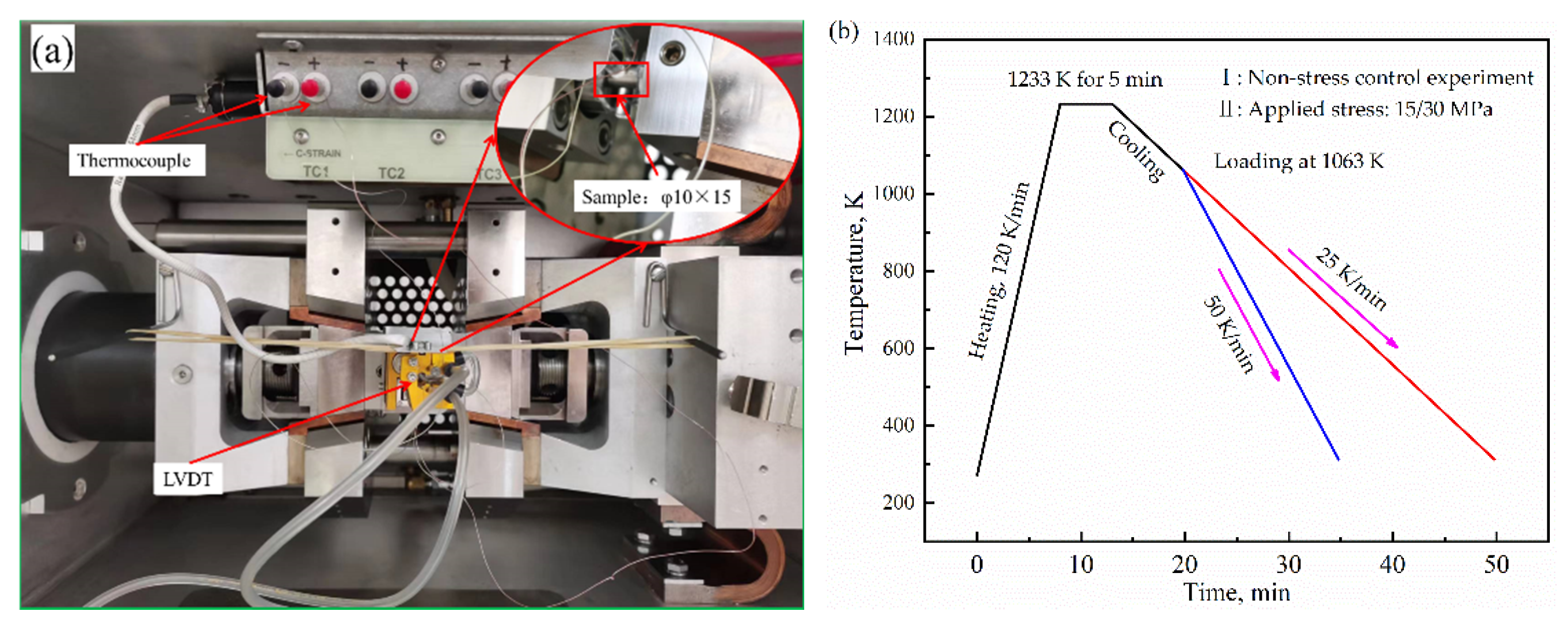

| Composition | C | Si | Mn | P | S | Cr | Fe |
|---|---|---|---|---|---|---|---|
| Content | 0.15 | 0.14 | 0.71 | 0.03 | 0.03 | 0.24 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, S.; Cheng, P.; Wang, D.; Du, B.; Guan, K.; Zhang, J. Effect of Uniaxial Compressive Stress on Phase Transformation Kinetics of Low-Carbon Steel. Materials 2022, 15, 4477. https://doi.org/10.3390/ma15134477
Zuo S, Cheng P, Wang D, Du B, Guan K, Zhang J. Effect of Uniaxial Compressive Stress on Phase Transformation Kinetics of Low-Carbon Steel. Materials. 2022; 15(13):4477. https://doi.org/10.3390/ma15134477
Chicago/Turabian StyleZuo, Shanchao, Peng Cheng, Decheng Wang, Bing Du, Keming Guan, and Jing Zhang. 2022. "Effect of Uniaxial Compressive Stress on Phase Transformation Kinetics of Low-Carbon Steel" Materials 15, no. 13: 4477. https://doi.org/10.3390/ma15134477
APA StyleZuo, S., Cheng, P., Wang, D., Du, B., Guan, K., & Zhang, J. (2022). Effect of Uniaxial Compressive Stress on Phase Transformation Kinetics of Low-Carbon Steel. Materials, 15(13), 4477. https://doi.org/10.3390/ma15134477





