Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
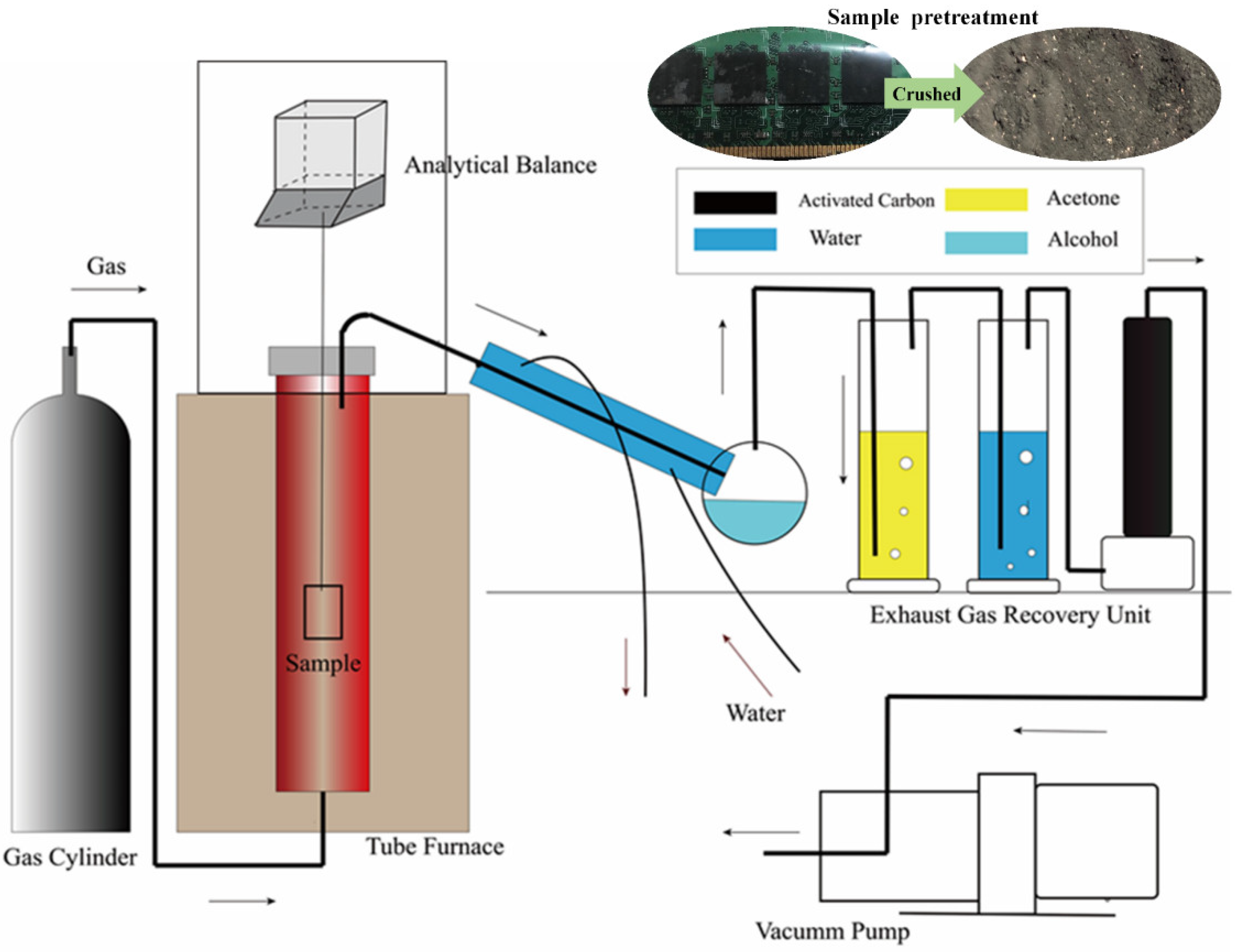
2.2. Experimental Methods
2.3. Thermogravimetric Analysis
2.4. Pyrolysis Kinetics
3. Results and Discussion
3.1. Pyrolysis Properties of ICs
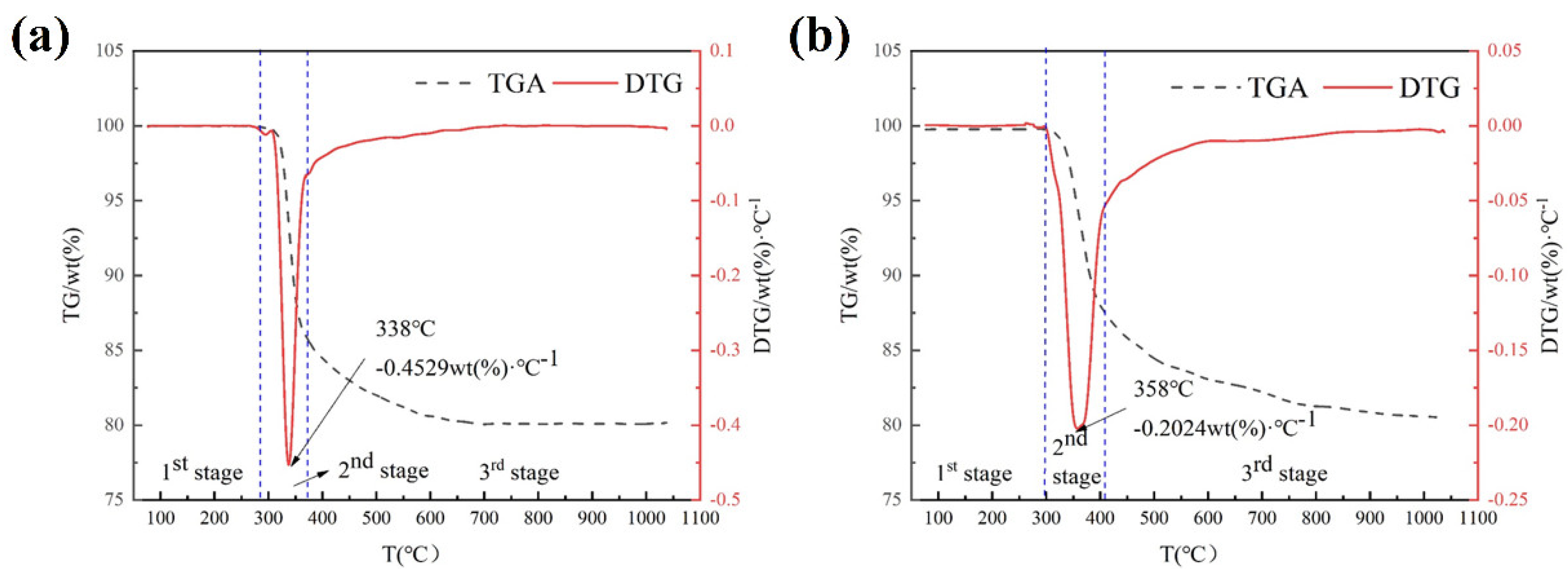
3.2. TG Analysis
3.3. Effect of Related Factors on Pyrolysis
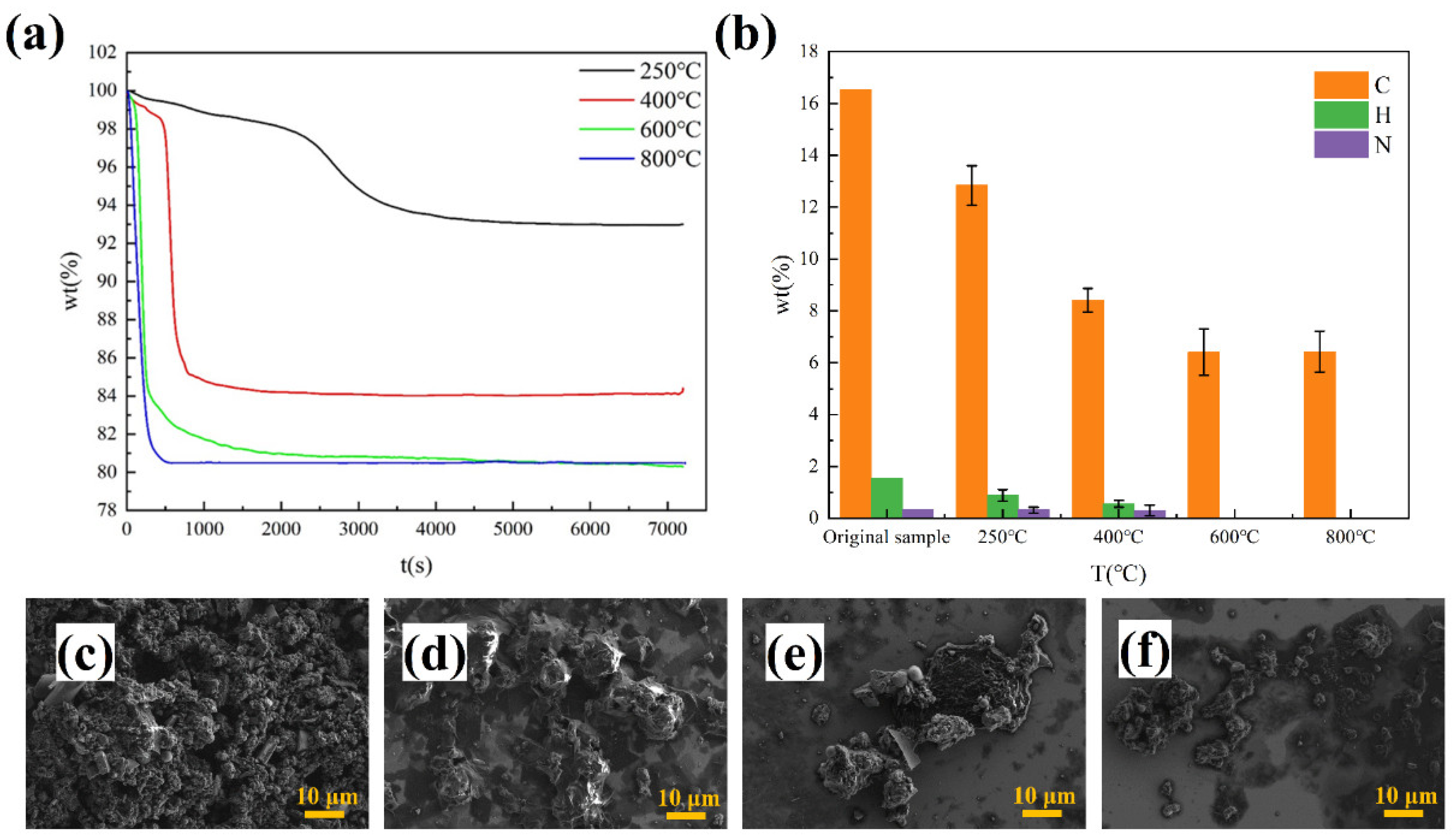
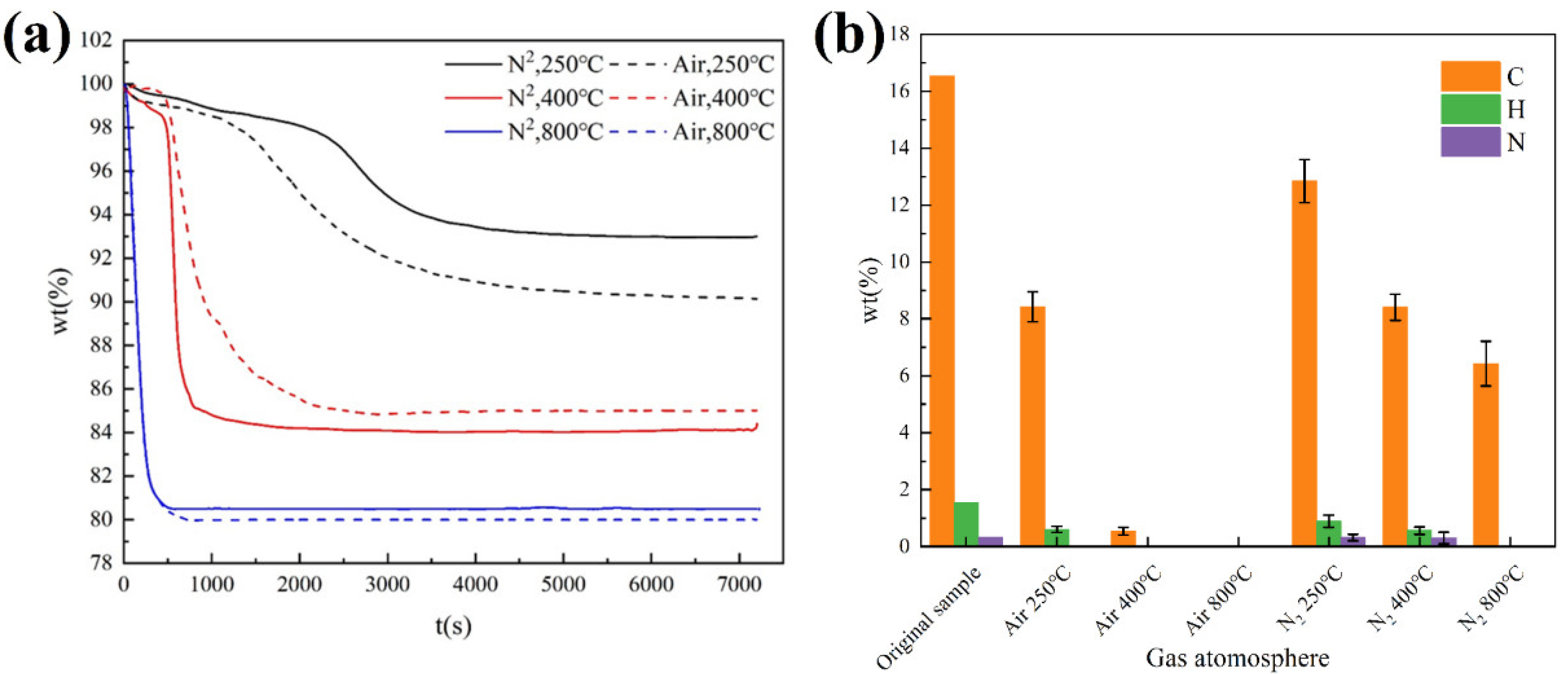
3.3.1. Effect of Temperature
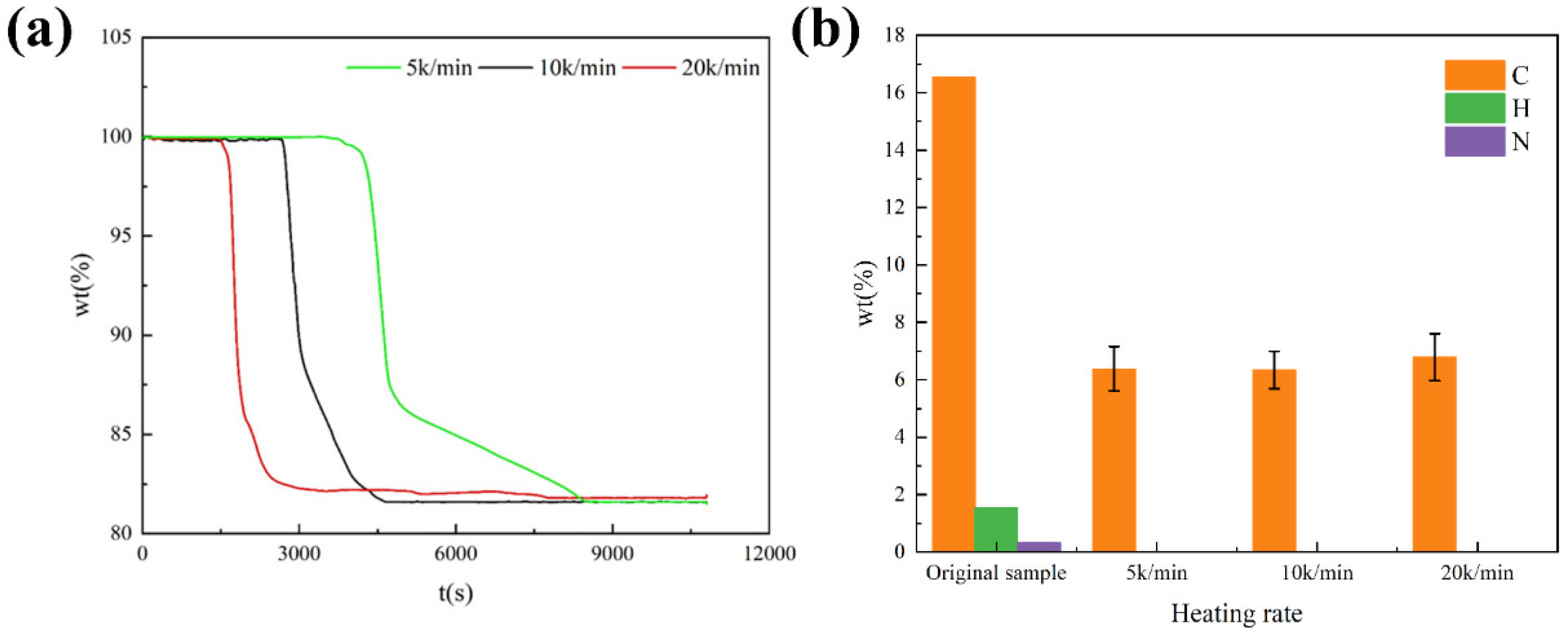
3.3.2. Effect of Heating Rate
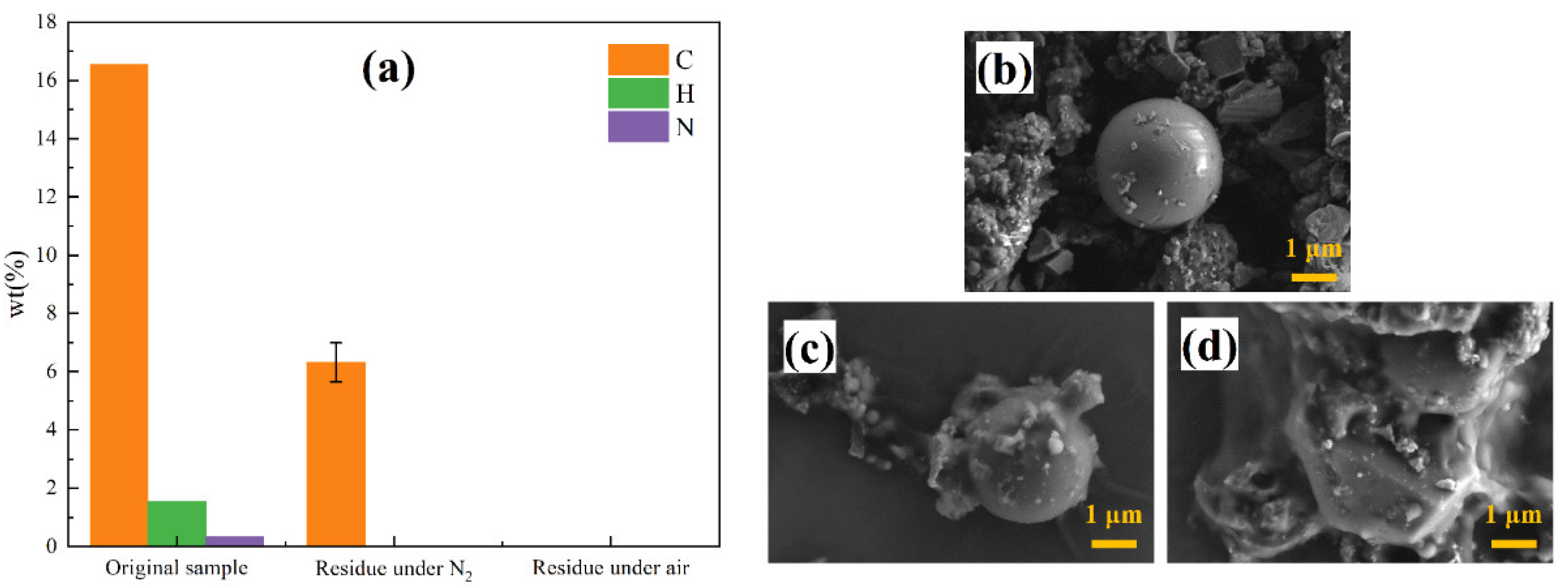
3.3.3. Effect of Gas Atmosphere
3.4. Non-Isothermal Kinetics of ICs
3.4.1. Reaction Activation Energy
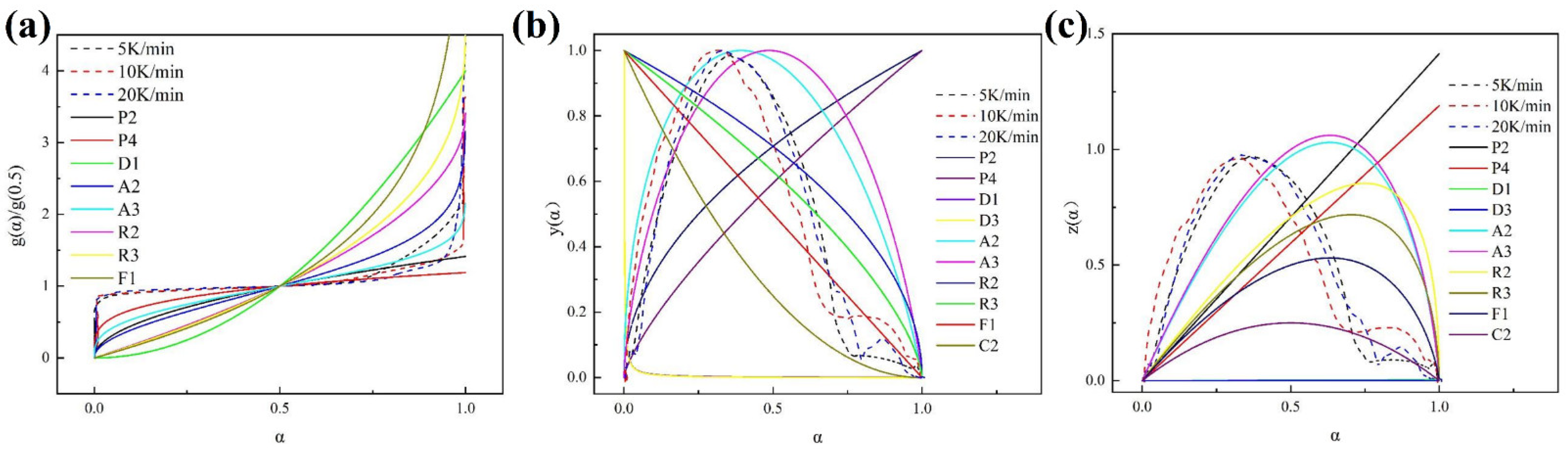
3.4.2. Kinetics Model for ICs Pyrolysis
3.4.3. Kinetic Parameters of ICs Pyrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.T.; Huda, N. Material flow analysis (MFA) as a strategic tool in E-waste management: Applications, trends and future directions. J. Environ. Manag. 2019, 244, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Metternicht, G. Assessing effectiveness of WEEE management policy in Australia. J. Environ. Manag. 2016, 181, 218–230. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xu, Z. Decomposition behavior and mechanism of epoxy resin from waste integrated circuits under supercritical water condition. J. Hazard. Mater. 2019, 374, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ogunseitan, O.A. The Basel Convention and e-waste: Translation of scientific uncertainty to protective policy. Lancet Glob. Health 2013, 1, e313–e314. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Chen, X.; Ge, X.; Chen, M. Chemical pyrolysis of E-waste plastics: Char characterization. J. Environ. Manag. 2018, 214, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Zhang, G.; Bai, X.; Zhao, X.; He, Y. Recycling organics from non-metallic fraction of waste printed circuit boards by a novel conical surface triboelectric separator. Resour. Conserv. Recycl. 2019, 146, 264–269. [Google Scholar] [CrossRef]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total. Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- Wong, M.H.; Wu, S.C.; Deng, W.J.; Yu, X.Z.; Luo, Q.; Leung, A.O.; Wong, C.S.; Luksemburg, W.J.; Wong, A.S. Export of toxic chemicals—A review of the case of uncontrolled electronic-waste recycling. Environ. Pollut. 2007, 149, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.O.; Duzgoren-Aydin, N.S.; Cheung, K.C.; Wong, M.H. Heavy metals concentrations of surface dust from e-waste recycling and its human health implications in southeast China. Environ. Sci. Technol. 2008, 42, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, C.; Wang, Y.; Liu, X.; Li, F.; Zhang, G.; Li, X. Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Guo, J.; Xu, Z. Impact of the operating conditions on the derived products and the reaction mechanism in vacuum pyrolysis treatment of the organic material in waste integrated circuits. J. Cleaner Prod. 2018, 197, 1488–1497. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Xu, Z. PBDEs emission from waste printed wiring boards during thermal process. Environ. Sci. Technol. 2015, 49, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.O.; Luksemburg, W.J.; Wong, A.S.; Wong, M.H. Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in southeast China. Environ. Sci. Technol. 2007, 41, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, P.; Shan, H.; Xie, Y. Environmental risk related to specific processes during scrap computer recycling and disposal. Environ. Technol. 2012, 33, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Flandinet, L.; Tedjar, F.; Ghetta, V.; Fouletier, J. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J. Hazard. Mater. 2012, 213–214, 485–490. [Google Scholar] [CrossRef]
- Jing-ying, L.; Xiu-li, X.; Wen-quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste. Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Pandey, B.D. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Zhou, Y. Application of corona discharge and electrostatic force to separate metals and nonmetals from crushed particles of waste printed circuit boards. J. Electrost. 2007, 65, 233–238. [Google Scholar] [CrossRef]
- Yoo, J.M.; Jeong, J.; Yoo, K.; Lee, J.C.; Kim, W. Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill. Waste. Manag. 2009, 29, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, Z. Application of supercritical water to decompose brominated epoxy resin and environmental friendly recovery of metals from waste memory module. Environ. Sci. Technol. 2015, 49, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Chen, Z.; Xu, Z. Recovery of Tantalum from Waste Tantalum Capacitors by Supercritical Water Treatment. ACS Sustainable Chem. Eng. 2017, 5, 4421–4428. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.S. Degradation of brominated flame retardant in computer housing plastic by supercritical fluids. J. Hazard. Mater. 2012, 205–206, 156–163. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Xu, Z. Vacuum pyrolysis characteristics and kinetic analysis of liquid crystal from scrap liquid crystal display panels. J. Hazard. Mater. 2017, 327, 55–63. [Google Scholar] [CrossRef]
- Diao, Z.; Zhao, Y.; Chen, B.; Duan, C.; Song, S. ReaxFF reactive force field for molecular dynamics simulations of epoxy resin thermal decomposition with model compound. J. Anal. Appl. Pyrol. 2013, 104, 618–624. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stabil. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Chen, W.-H.; Eng, C.F.; Wang, C.-W.; Liang, K.-C.; Kuo, J.-Y. Pyrolysis characteristics and non-isothermal torrefaction kinetics of industrial solid wastes. Fuel 2019, 251, 118–125. [Google Scholar] [CrossRef]
- Lu, K.-M.; Lee, W.-J.; Chen, W.-H.; Lin, T.-C. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl. Energy 2013, 105, 57–65. [Google Scholar] [CrossRef]
- Cai, C.; Yu, S.; Liu, Y.; Tao, S.; Liu, W. PBDE emission from E-wastes during the pyrolytic process: Emission factor, compositional profile, size distribution, and gas-particle partitioning. Environ. Pollut. 2018, 235, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Qiu, K. Recovery of materials from waste printed circuit boards by vacuum pyrolysis and vacuum centrifugal separation. Waste Manag. 2010, 30, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, P.-R.; Vuppaladadiyam, A.-K.; Prakash, A.; Pant, K.-K. Co-pyrolysis characteristics and kinetics of electronic waste and macroalgae: A synergy study based on thermogravimetric analysis. Algal Res. 2022, 61, 102601. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, R.; Chen, X.; Ge, X.; Chen, M. Co-pyrolysis of E-waste nonmetallic residues with biowastes. ACS Sustain. Chem. Eng. 2018, 6, 9086–9093. [Google Scholar] [CrossRef]
- Krishna, J.-V.-J.; Damir, S.-S.; Vinu, R. Pyrolysis of electronic waste and their mixtures: Kinetic and pyrolysate composition studies. J. Environ. Chem. Eng. 2021, 9, 105382. [Google Scholar] [CrossRef]
- Quan, C.; Li, A.; Gao, N. Combustion and pyrolysis of electronic waste: Thermogravimetric analysis and kinetic model. Proc. Environ. Sci. 2013, 18, 776–782. [Google Scholar] [CrossRef] [Green Version]


| Main Compositions | Content (wt.%) | Precious Metal | Content (g/t) |
|---|---|---|---|
| Cu | 17.82 | Au | 534.3 |
| C | 16.54 | Ag | 593 |
| SiO2 | 33.29 | Pt | 0.78 |
| Ca | 4.72 | Pd | 5.91 |
| Br | 9.17 | Rh | 0.2 |
| Sn | 2.29 | ||
| Al | 2.65 | ||
| Fe | 1.63 | ||
| H | 1.43 | ||
| N | 0.33 | ||
| Pb | 0.87 | ||
| Mg | 0. 18 |
| Pyrolysis Related Factors | Independent Variables | Control Variables |
|---|---|---|
| Temperature | 250 °C | Heat rate: 10 k/min Reaction time: 2 h Gas atmosphere: N2 |
| 400 °C | ||
| 600 °C | ||
| 800 °C | ||
| Heating rate | 5 k/min | Temperature: 800 °C Reaction time: 3 h Gas atmosphere: N2 |
| 10 k/min | ||
| 20 k/min | ||
| Gas atmosphere | 250 °C, N2 | Heat rate: 10 k/min Reaction time: 2 h |
| 250 °C, Air | ||
| 400 °C, N2 | ||
| 400 °C, Air | ||
| 600 °C, N2 | ||
| 600 °C, Air |
| Nomenclature | Full Name | Nomenclature | Full Name |
|---|---|---|---|
| A | Pre-exponential factor (min−1) | T | Temperature (K) |
| B | Heating rate (K·min−1) | t | Heating time (min) |
| Ea | Activation energy (J·mol−1) | α | Conversion of sample |
| k | Rate constant (min−1) | m | Weight of sample (g) |
| n | Order of reaction | i | Initial state |
| R | Universal gas constant (J·mol−1·K−1) | f | Final state |
| Methods | Friedman | Kissinger | CR |
| Ea (kJ·mol−1) | 208.63 | 170.90 | 145.21 |
| Models | Heating Rate | wi | Parameters | f(α) | Root-Sum Square |
|---|---|---|---|---|---|
| Am-Cn | 5 K/min | w1 = 0.45 w2 = 0.55 | A1 = 2.58 × 1017, E1 = 205.69 kJ/mol A2 = 1.35 × 1013, E2 = 152.45 kJ/mol | Am:m1 = 10.0 Cn:m2 = 8.1 | 0.46157 |
| 10 K/min | w1 = 0.40 w2 = 0.60 | A1 = 3.63 × 1017, E1 = 285.62 kJ/mol A2 = 1.46 × 1014, E2 = 191.69 kJ/mol | Am:m1 = 0.2 Cn:m2 = 1.8 | 0.03042 | |
| 20 K/min | w1 = 0.29 w2 = 0.71 | A1 = 1.55 × 1015, E1 = 288.09 kJ/mol; A2 = 6.559 × 1013, E2 = 216.03 kJ/mol | Am:m1 = 0.2 Cn:m2 = 1.4 | 0.14106 | |
| Am-Cn-Cn | 5 K/min | w1 = 0.11 w2 = 0.24 w3 = 0.65 | A1 = 7.2 × 1016, E1 = 219.54 kJ/mol A2 = 2.03 × 1014, E2 = 184.10 kJ/mol A3 = 1.97 × 1015, E3 = 174.24 kJ/mol | Am:m1 = 0.3 Cn:m2 = 6.9 Cn:m3 = 1.5 | 0.0021 |
| 10 K/min | w1 = 0.12 w2 = 0.23 w3 = 0.65 | A1 = 3.63 × 1017, E1 = 235.62 kJ/mol A2 = 3.46 × 1014, E2 = 191.69 kJ/mol A3 = 1.14 × 1015, E3 = 183.80 kJ/mol | Am:m1 = 0.4 Cn:m2 = 6.1 Cn:m3 = 1.8 | 0.00831 | |
| 20 K/min | w1 = 0.15 w2 = 0.22 w3 = 0.65 | A1 = 4.97 × 1017, E1 = 248.09 kJ/mol; A2 = 2.44 × 1014, E2 = 210.15 kJ/mol A3 = 2.05 × 1015, E3 = 203.80 kJ/mol | Am:m1 = 0.5 Cn:m2 = 6.1 Cn:m3 = 1.5 | 0.01682 | |
| Am-Cn-D1 | 5 K/min | w1 = 0.26 w2 = 0.73 w3 = 0.01 | A1 = 3.38 × 1020, E1 = 302.65 kJ/mol A2 = 5.81 × 1014, E2 = 169.20 kJ/mol A3 = 7.15 × 1010, E3 = 146.12 kJ/mol | Am:m1 = 0.2 Cn:m2 = 1.7 | 0.10047 |
| 10 K/min | w1 = 0.38 w2 = 0.61 w3 = 0.07 | A1 = 2.41 × 1018, E1 = 244.34 kJ/mol A2 = 5.42 × 1013, E2 = 194.99 kJ/mol A3 = 1.24 × 1010, E3 = 112.52 kJ/mol | Am:m1 = 0.7 Cn:m2 = 8.2 | 0.04883 | |
| 20 K/min | w1 = 0.17 w2 = 0.82 w3 = 0.01 | A1 = 4.95 × 1020, E1 = 385.22 kJ/mol; A2 = 2.62 × 1013, E2 = 210.24 kJ/mol A3 = 2.17 × 1013, E3 = 198.02 kJ/mol | Am:m1 = 0.5 Cn:m2 = 1.9 | 0.20789 | |
| Am-Cn-Cn-Cn | 5 K/min | w1 = 0.48 w2 = 0.23 w3 = 0.23 w4 = 0.16 | A1 = 2.72 × 1018, E1 = 198.26 kJ/mol A2 = 9.62 × 1014, E2 = 176.53 kJ/mol A3 = 9.6 × 1014, E3 = 176.53 kJ/mol A4 = 9.84 × 1014, E4 = 179.06 kJ/mo | Am:m1 = 0.8 Cn:m2 = 5.1 Cn:m3 = 5.1 Cn:m4 = 4.2 | 0.03323 |
| 10 K/min | w1 = 0.19 w2 = 0.28 w3 = 0.28 w4 = 0.25 | A1 = 9.12 × 1040, E1 = 500.00 kJ/mol A2 = 1.68 × 1014, E2 = 194.19 kJ/mol A3 = 1.66 ×1014, E3 = 194.16 kJ/mol A4 = 3.85 × 1012, E4 = 211.72 kJ/mol | Am:m1 = 0.4 Cn:m2 = 2.7 Cn:m3 = 2.8 Cn:m4 = 3.7 | 0.00875 | |
| 20 K/min | w1 = 0.46 w2 = 0.24 w3 = 0.24 w4 = 0.16 | A1 = 1.99 × 1015, E1 = 237.60 kJ/mol; A2 = 3.48 × 1015, E2 = 252.56 kJ/mol A3 = 3.48 × 1015, E3 = 252.56 kJ/mol A4 = 5.54 × 1016, E4 = 285.47 kJ/mol | Am:m1 = 1.1 Cn:m2 = 5.0 Cn:m3 = 5.0 Cn:m4 = 1.4 | 0.00829 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Liu, L.; Wang, H.; Liu, L.; Wang, X. Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits. Materials 2022, 15, 4460. https://doi.org/10.3390/ma15134460
Chen Z, Liu L, Wang H, Liu L, Wang X. Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits. Materials. 2022; 15(13):4460. https://doi.org/10.3390/ma15134460
Chicago/Turabian StyleChen, Ziwei, Linhao Liu, Hao Wang, Lili Liu, and Xidong Wang. 2022. "Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits" Materials 15, no. 13: 4460. https://doi.org/10.3390/ma15134460
APA StyleChen, Z., Liu, L., Wang, H., Liu, L., & Wang, X. (2022). Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits. Materials, 15(13), 4460. https://doi.org/10.3390/ma15134460







