Structural Features and Defect Equilibrium in Cubic PrBa1−xSrxFe2O6−δ
Abstract
1. Introduction
2. Experimental Section
3. Data Analysis Methods
3.1. Model of a Point Defect Equilibrium
3.2. Partial Molar Enthalpy and Entropy of Oxygen in Pr0.5Ba0.5−xSrxFeO3−δ
3.3. Statistical Thermodynamic Modeling
4. Results and Discussion
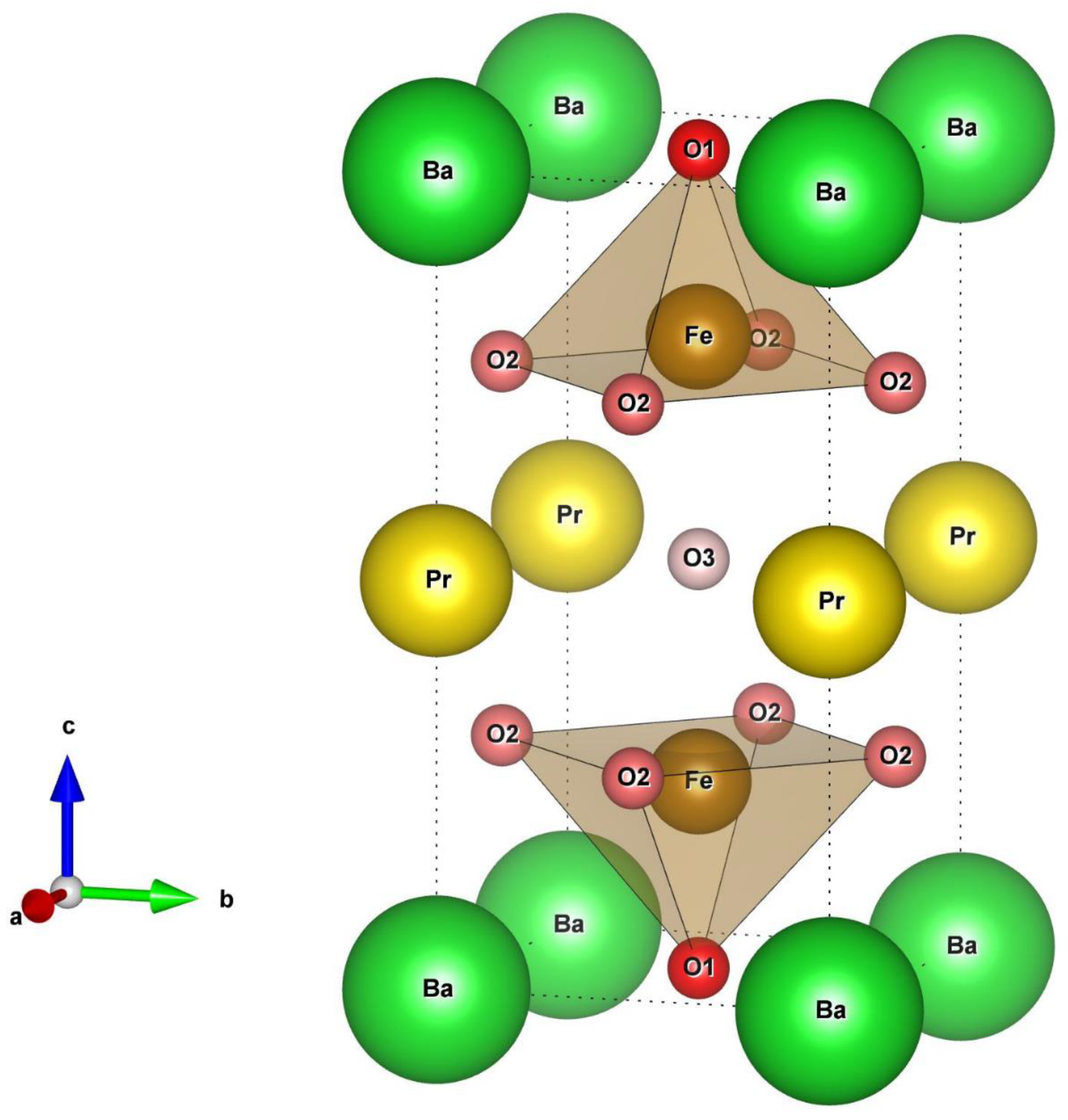
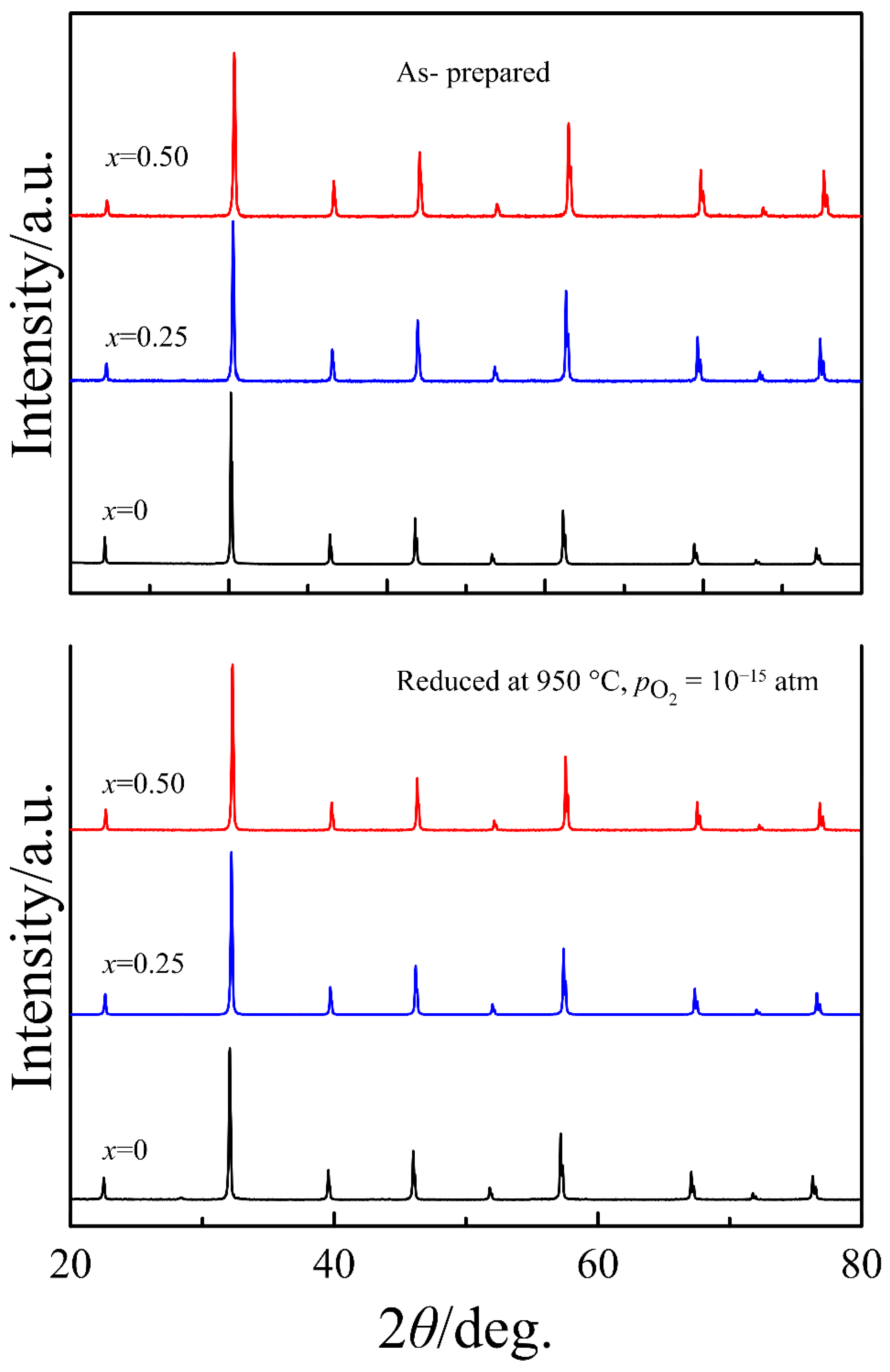
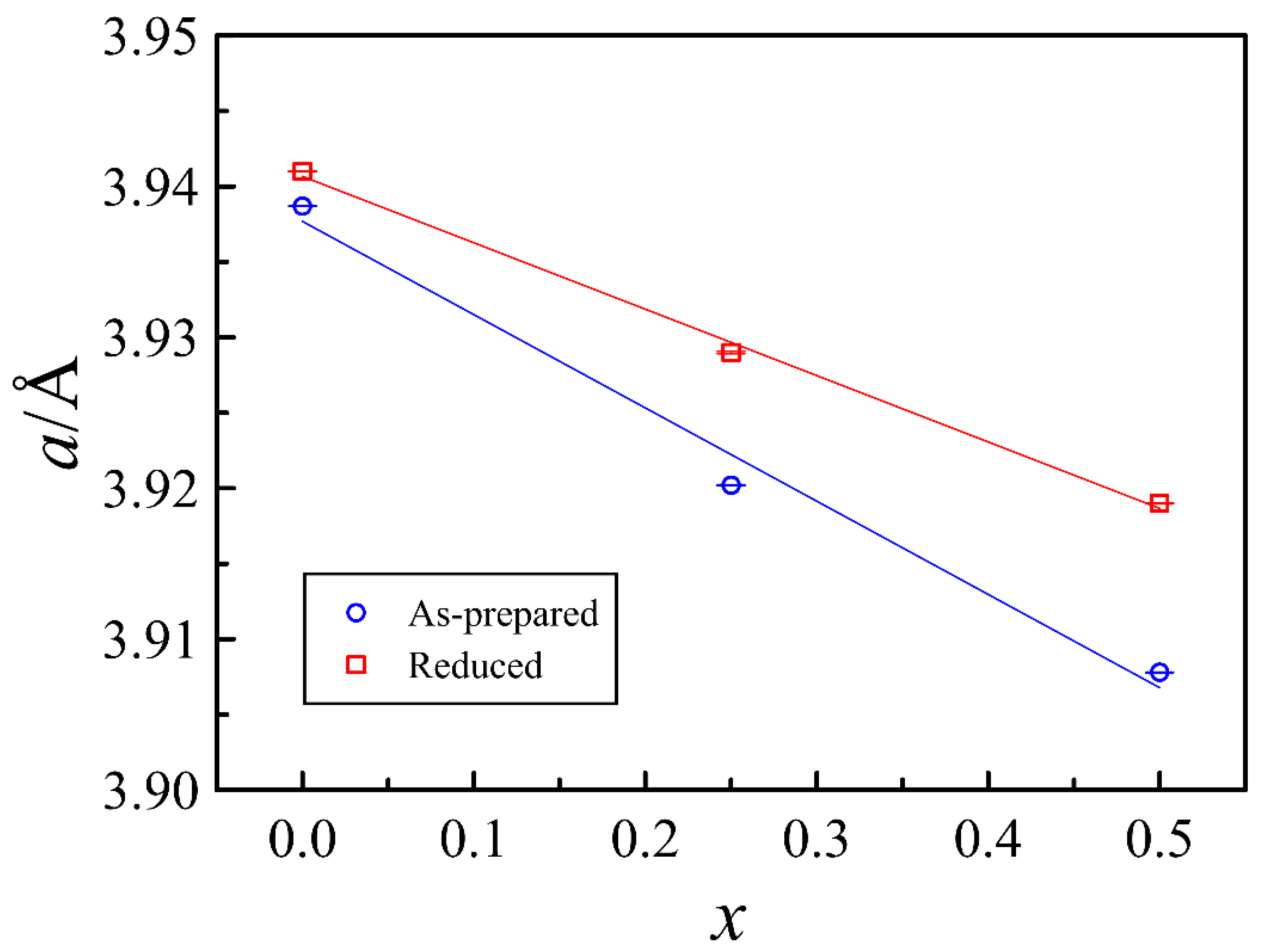
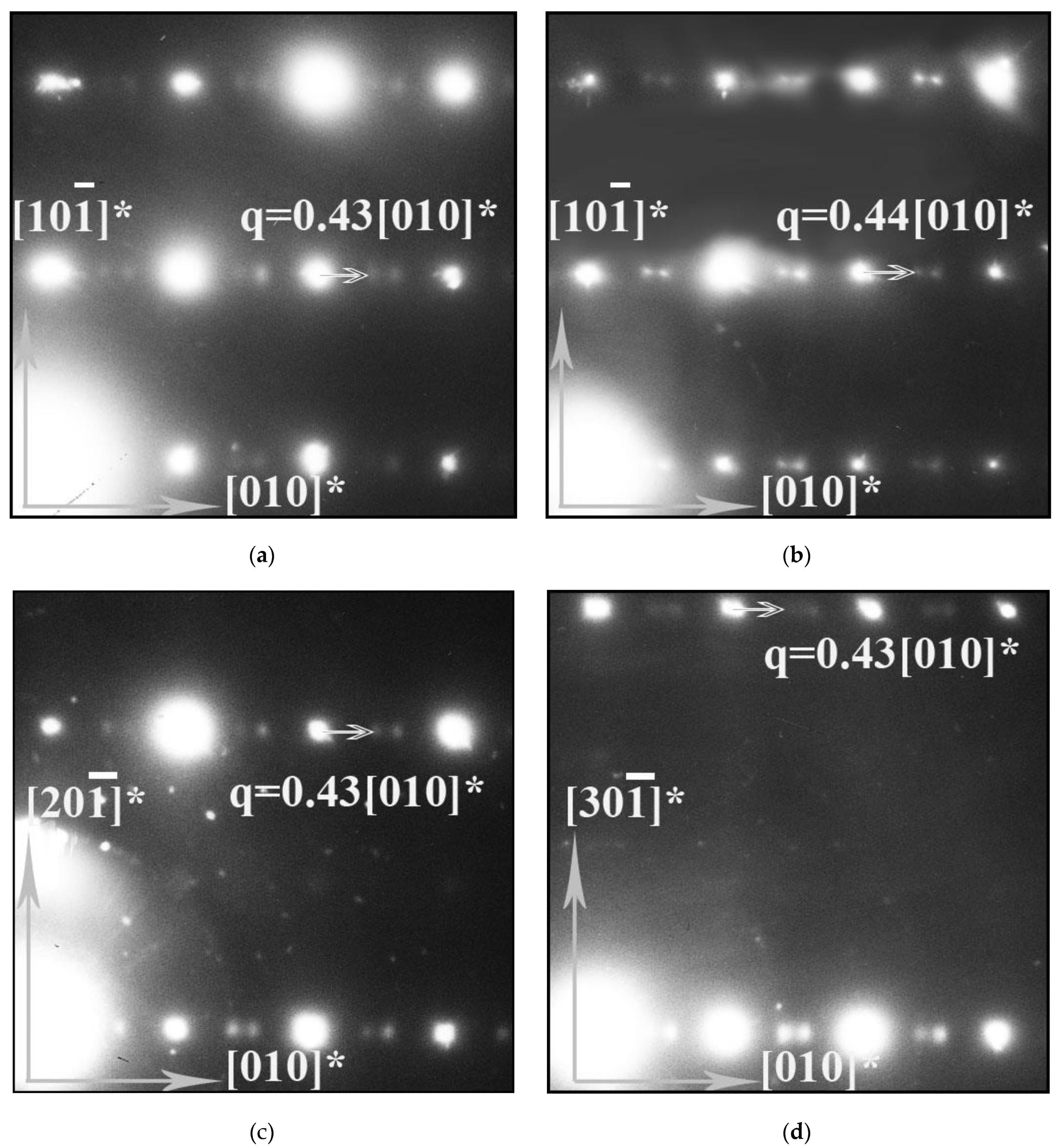
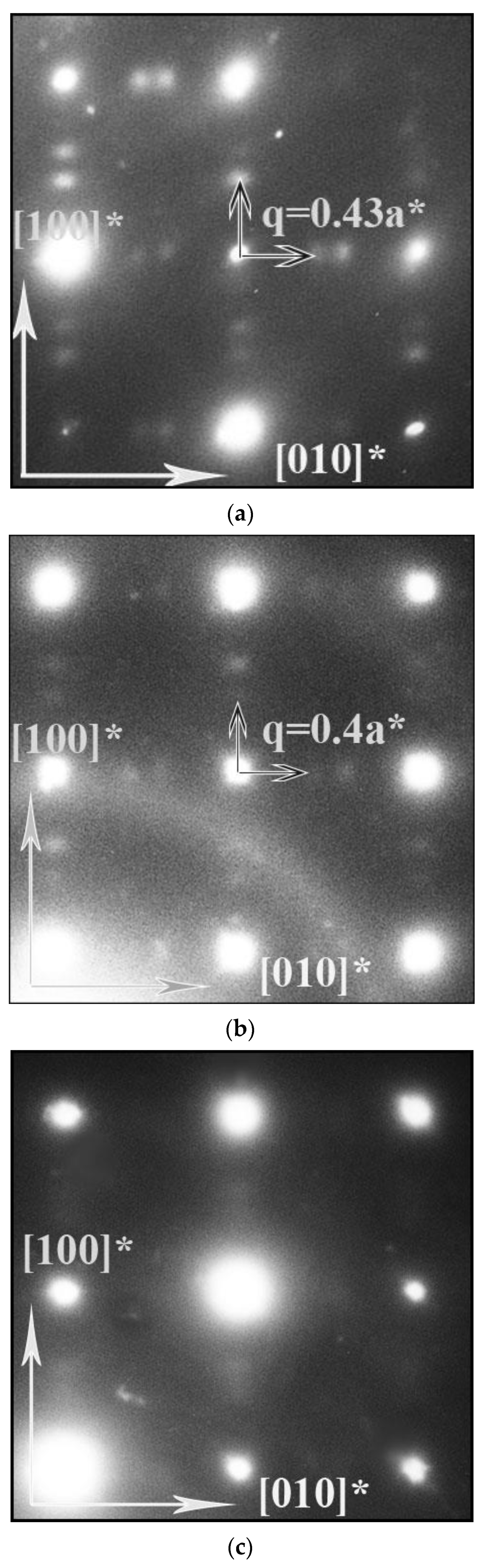
4.1. Structure and Oxygen Content
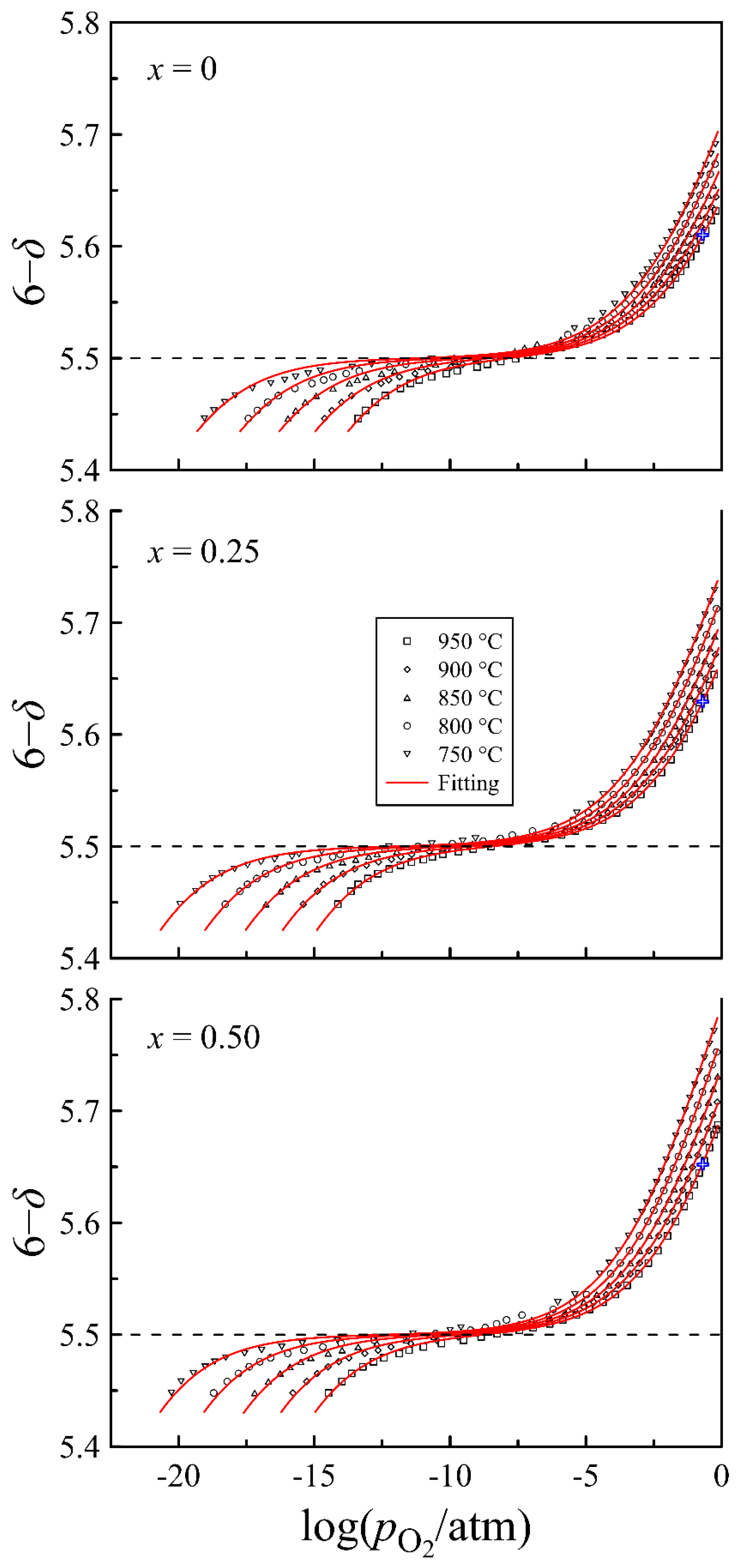
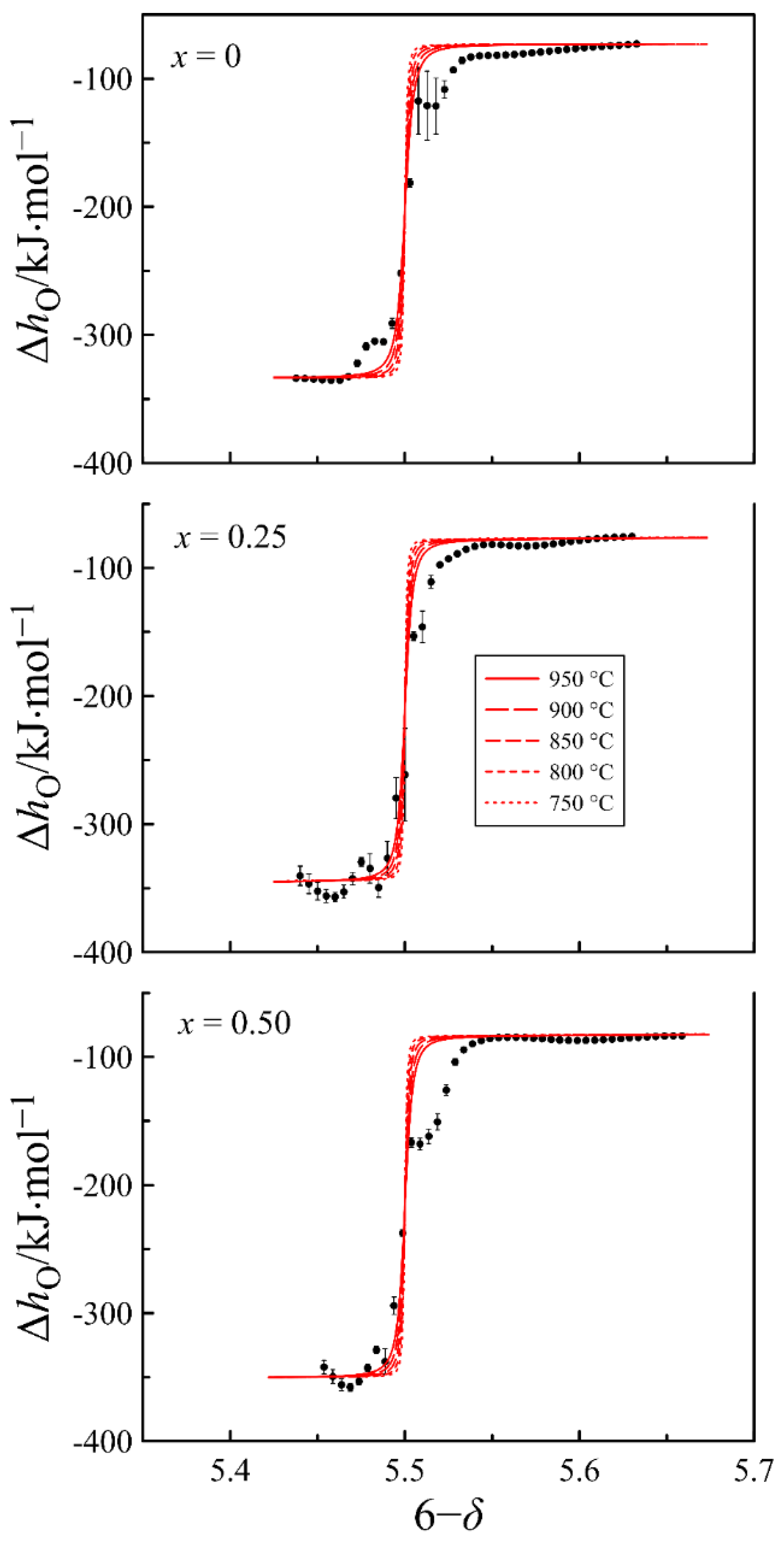
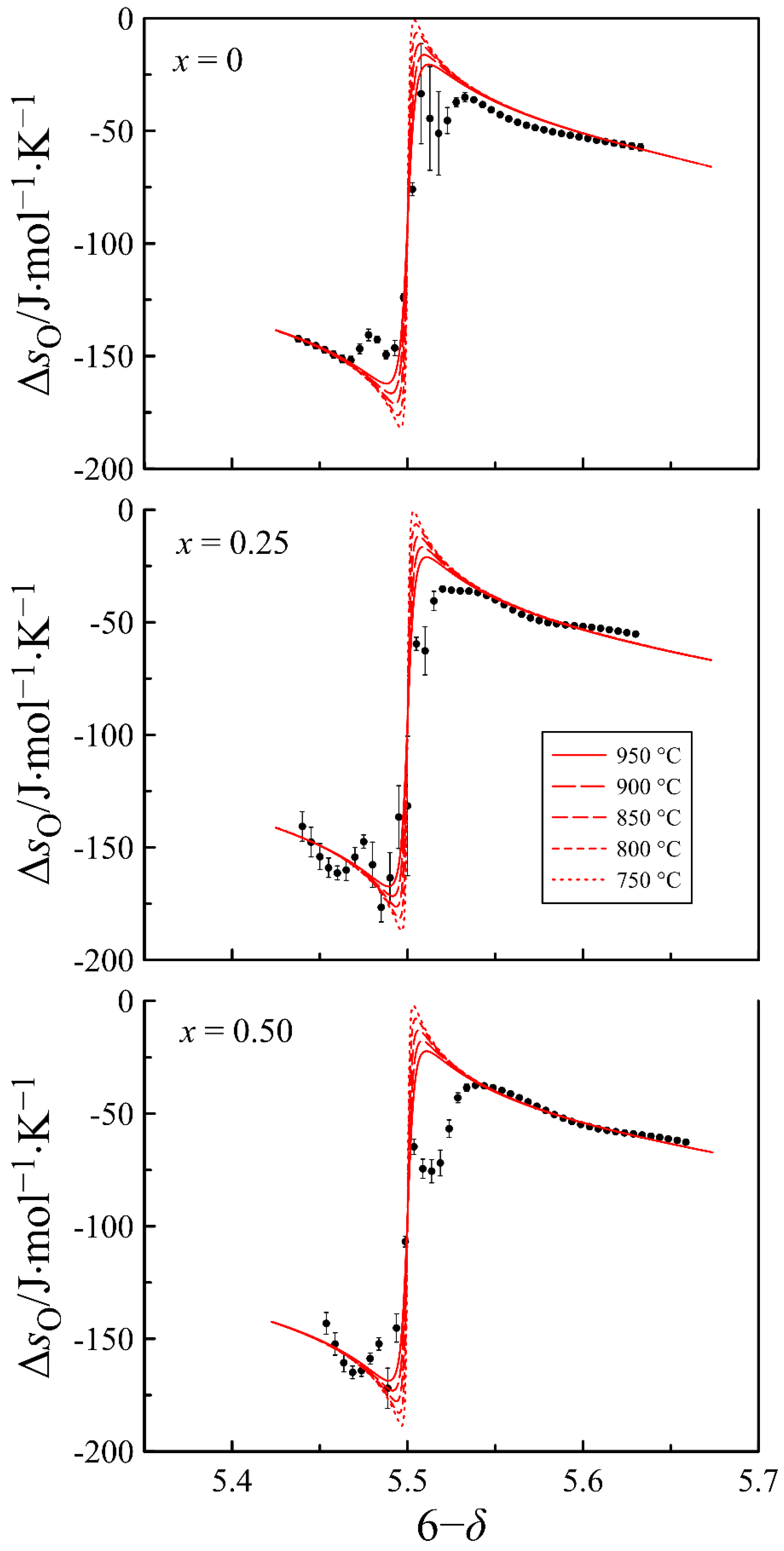
4.2. Defect Equilibrium Parameters and Thermodynamic Quantities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Zhang, Z.; Li, S.; Ni, J.; Wu, K.; Irvine, J.T.S. Iron-based electrode materials for solid oxide fuel cells and electrolysers. Energy Environ. Sci. 2021, 14, 6287–6319. [Google Scholar] [CrossRef]
- Lü, M.F.; Tsipis, E.V.; Waerenborgh, J.C.; Yaremchenko, A.A.; Kolotygin, V.A.; Bredikhin, S.; Kharton, V.V. Thermomechanical, transport and anodic properties of perovskite-type (La0.75Sr0.25)0.95Cr1−xFexO3−δ. J. Power Sources 2012, 206, 59–69. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Kozhevnikov, V.L.; Leonidov, I.A.; Patrakeev, M.V. Ceramic membranes with mixed conductivity and their application. Russ. Chem. Rev. 2013, 82, 772–782. [Google Scholar] [CrossRef]
- Chen, G.; Feldhoff, A.; Weidenkaff, A.; Li, C.; Liu, S.; Zhu, X.; Sunarso, J.; Huang, K.; Wu, X.Y.; Ghoniem, A.F.; et al. Roadmap for Sustainable Mixed Ionic-Electronic Conducting Membranes. Adv. Funct. Mater. 2022, 32, 2105702. [Google Scholar] [CrossRef]
- Mizusaki, J.; Yoshihiro, M.; Yamauchi, S.; Fueki, K. Thermodynamic quantities and defect equilibrium in the perovskite-type oxide solid solution La1−xSrxFeO3−δ. J. Solid State Chem. 1987, 67, 1–8. [Google Scholar] [CrossRef]
- Song, J.; Ning, D.; Bouwmeester, H.J.M. Influence of alkaline-earth metal substitution on structure, electrical conductivity and oxygen transport properties of perovskite-type oxides La0.6A0.4FeO3−δ(A = Ca, Sr and Ba). Phys. Chem. Chem. Phys. 2020, 22, 11984–11995. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Patrakeev, M.V.; Tsipis, E.V.; Viskup, A.P.; Kolotygin, V.A.; Yaremchenko, A.A.; Shaula, A.L.; Kiselev, E.A.; Waerenborgh, J.C. Oxygen nonstoichiometry, mixed conductivity, and mössbauer spectra of Ln0.5A0.5FeO3−δ (Ln = La-Sm, A = Sr, Ba): Effects of cation size. Chem. Mater. 2008, 20, 6457–6467. [Google Scholar] [CrossRef][Green Version]
- Patrakeev, M.V.; Bahteeva, J.A.; Mitberg, E.B.; Leonidov, I.A.; Kozhevnikov, V.L.; Poeppelmeier, K.R. Electron/hole and ion transport in La1−xSrxFeO3−δ. J. Solid State Chem. 2003, 172, 219–231. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.; He, B.; Chen, F.; Xia, C. Synthesis, characterization and evaluation of PrBaCo2−xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Karen, P. Chemistry and thermodynamics of the twin charge-ordering transitions in RBaFe2O5+w series. J. Solid State Chem. 2004, 177, 281–292. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Z.; Yang, Y.; Gu, Q.; Tian, D.; Lu, X.; Yu, W.; Lin, B. Novel quasi-symmetric solid oxide fuel cells with enhanced electrochemical performance. J. Power Sources 2016, 310, 109–117. [Google Scholar] [CrossRef]
- Dong, G.; Yang, C.; He, F.; Jiang, Y.; Ren, C.; Gan, Y.; Lee, M.; Xue, X. Tin doped PrBaFe2O5+δ anode material for solid oxide fuel cells. RSC Adv. 2017, 7, 22649–22661. [Google Scholar] [CrossRef]
- Lee, D.; Kim, D.; Son, S.J.; Kwon, Y.I.; Lee, Y.; Ahn, J.H.; Joo, J.H. Simultaneous A- and B- site substituted double perovskite (AA’B2O5+δ) as a new high-performance and redox-stable anode material for solid oxide fuel cells. J. Power Sources 2019, 434, 226743. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, Y.; Li, W.; Shao, J.; Bai, L.; Liu, X. Intermediate-temperature solid oxide fuel cells with high performance cobalt-doped Pr0.5Ba0.5FeO3−δ anodes. J. Alloys Compd. 2018, 741, 1091–1097. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Kolotygin, V.A.; Tsipis, E.V.; Bredikhin, S.I.; Kharton, V.V. Electrical Conductivity, Thermal Expansion and Electrochemical Properties of Perovskites PrBaFe2−xNixO5+δ. Russ. J. Electrochem. 2018, 54, 533–540. [Google Scholar] [CrossRef]
- Volkova, N.E.; Bazueva, M.V.; Aisarinova, D.T.; Alkhamova, A.D.; Gavrilova, L.Y.; Cherepanov, V.A.; Maignan, A. Influence of A- and B-site substitutions on crystal structure and oxygen content in air-prepared Ba1−xPrxFe1−yCoyO3−δ perovskites. J. Alloys Compd. 2021, 860, 158438. [Google Scholar] [CrossRef]
- Karen, P. Synthesis and equilibrium oxygen nonstoichiometry of PrBaFe2O5+w. J. Solid State Chem. 2021, 299, 122147. [Google Scholar] [CrossRef]
- Kim, D.; Son, S.J.; Kim, M.; Park, H.J.; Joo, J.H. PrBaFe2O5+δ promising electrode for redox-stable symmetrical proton-conducting solid oxide fuel cells. J. Eur. Ceram. Soc. 2021, 41, 5939–5946. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Zhu, Y.; Fu, X.; Huang, R.; Guo, Y.; Zhang, W.; Li, H.; Hou, L.; Meng, X. A-site deficient Fe-based double perovskite oxides PrxBaFe2O5+δ as cathodes for solid oxide fuel cells. J. Alloys Compd. 2022, 911, 165002. [Google Scholar] [CrossRef]
- Lu, C.; Niu, B.; Yi, W.; Ji, Y.; Xu, B. Efficient symmetrical electrodes of PrBaFe2−xCoxO5+δ (x=0, 0.2, 0.4) for solid oxide fuel cells and solid oxide electrolysis cells. Electrochim. Acta 2020, 358, 136916. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Lankhorst, M.H.R.; Bouwmeester, H.J.M.; Verweij, H. Importance of electronic band structure to nonstoichiometric behaviour of La0.8Sr0.2CoO3−δ. Solid State Ionics 1997, 96, 21–27. [Google Scholar] [CrossRef]
- Patrakeev, M.V.; Leonidov, I.A.; Kozhevnikov, V.L. Applications of coulometric titration for studies of oxygen non-stoichiometry in oxides. J. Solid State Electrochem. 2011, 15, 931–954. [Google Scholar] [CrossRef]
- Merkulov, O.V.; Naumovich, E.N.; Patrakeev, M.V.; Markov, A.A.; Bouwmeester, H.J.M.; Leonidov, I.A.; Kozhevnikov, V.L. Oxygen nonstoichiometry and defect chemistry of perovskite-structured SrFe1−xMoxO3−δ solid solutions. Solid State Ion. 2016, 292, 116–121. [Google Scholar] [CrossRef]
- Suntsov, A.Y.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. Defect formation in double perovskites PrBaCo2−xCuxO5+δ at elevated temperatures. Solid State Ion. 2015, 274, 17–23. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Dezso, A.; Kaptay, G. On the configurational entropy of nanoscale solutions for more accurate surface and bulk nano-thermodynamic calculations. Entropy 2017, 19, 248. [Google Scholar] [CrossRef]
- Janssen, T.; Chapuis, G.; Boissieu, M. From Modulated Phases to Quasicrystals; University Press: Oxford, NY, USA, 2007; p. 46. [Google Scholar]
- Biendicho, J.J.; Shafeie, S.; Frenck, L.; Gavrilova, D.; Böhme, S.; Bettanini, A.M.; Svedlindh, P.; Hull, S.; Zhao, Z.; Istomin, S.Y.; et al. Synthesis and characterization of perovskite-type SrxY1−xFeO3−δ (0.63≤x<1.0) and Sr0.75Y0.25Fe1−yMyO3−δ (M = Cr, Mn, Ni). J. Solid State Chem. 2013, 200, 30–38. [Google Scholar] [CrossRef]
- Koryakov, A.D.; Markov, A.A.; Shalaeva, E.V.; Leonidov, I.A.; Patrakeev, M.V. The impact of structural features on ion and electron transport in Y0.25Sr0.75FeO3−δ. Mater. Lett. 2021, 301, 130261. [Google Scholar] [CrossRef]
- Bréard, Y.; Maignan, A.; Lechevallier, L.; Boulon, M.E.; Le Breton, J.M. The Sr0.8Y0.2Co1−xFexO3−δ oxygen deficient perovskites: Modulated structure, magnetic properties and magnetoresistance. Solid State Sci. 2006, 8, 619–624. [Google Scholar] [CrossRef]
- Markov, A.A.; Nikitin, S.S.; Politov, B.V.; Shalaeva, E.V.; Tyutyunnik, A.P.; Leonidov, I.A.; Patrakeev, M.V. The impact of cerium content on oxygen stoichiometry, defect equilibrium, and thermodynamic quantities of Sr1−xCexFeO3−δ. J. Alloys Compd. 2021, 875, 160051. [Google Scholar] [CrossRef]
- Karen, P.; Woodward, P.M. Synthesis and structural investigations of the double perovskites REBaFe2O5+w (RE = ND, Sm). J. Mater. Chem. 1999, 9, 789–797. [Google Scholar] [CrossRef]
- Withers, R.L. ‘Disorder’, structured diffuse scattering and local crystal chemistry. In Advances in Imaging and Electron Physics; Hawkes, P.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 303–337. [Google Scholar]
- Withers, R.L. A modulation wave approach to the order hidden in disorder. IUCrJ 2015, 2, 74–84. [Google Scholar] [CrossRef]
- Leonidov, I.A.; Markov, A.A.; Patrakeev, M.V. Thermodynamic quantities and defect formation in solid solution La0.49Sr0.5−xBaxFeO3−δ. Mater. Lett. 2019, 235, 107–110. [Google Scholar] [CrossRef]

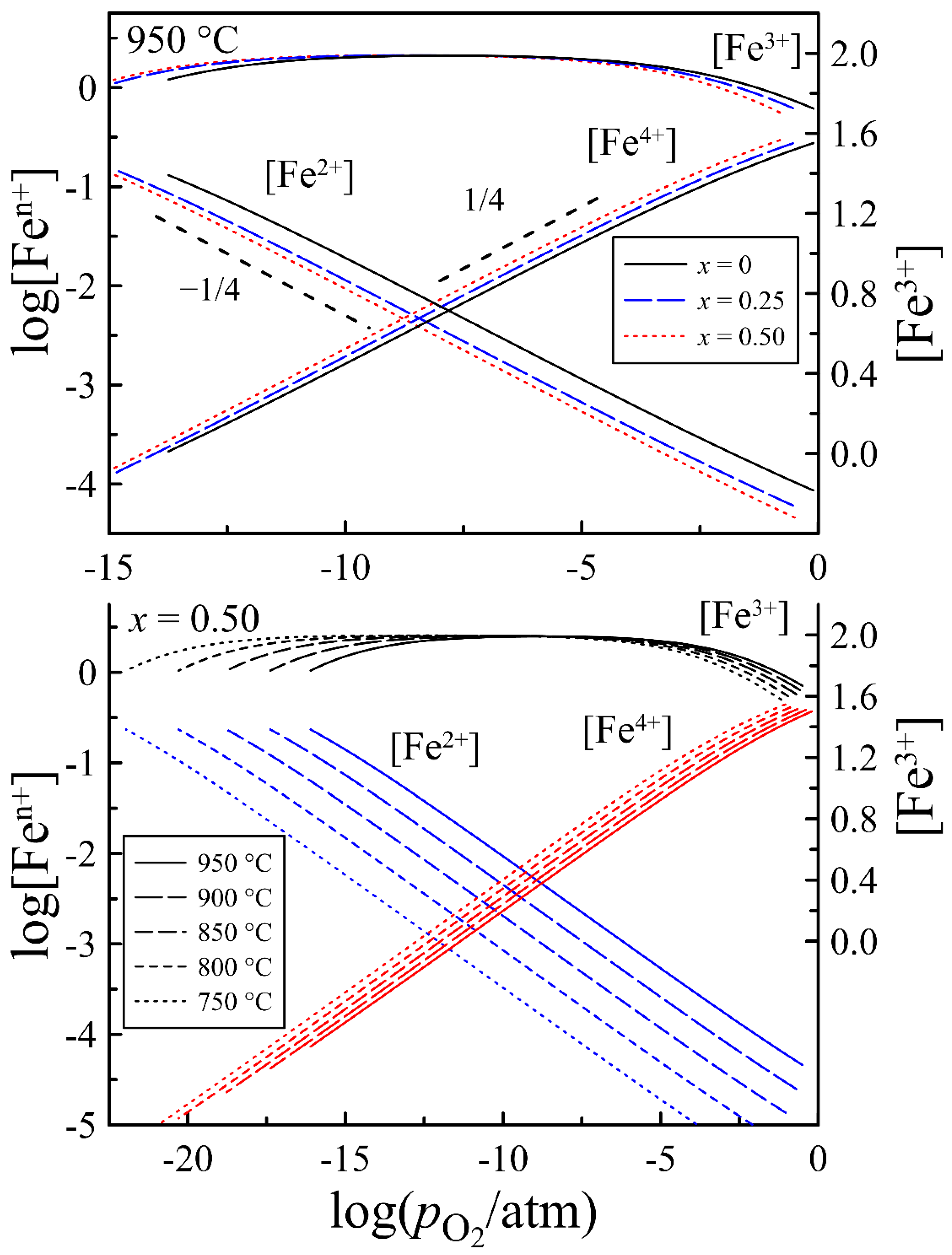
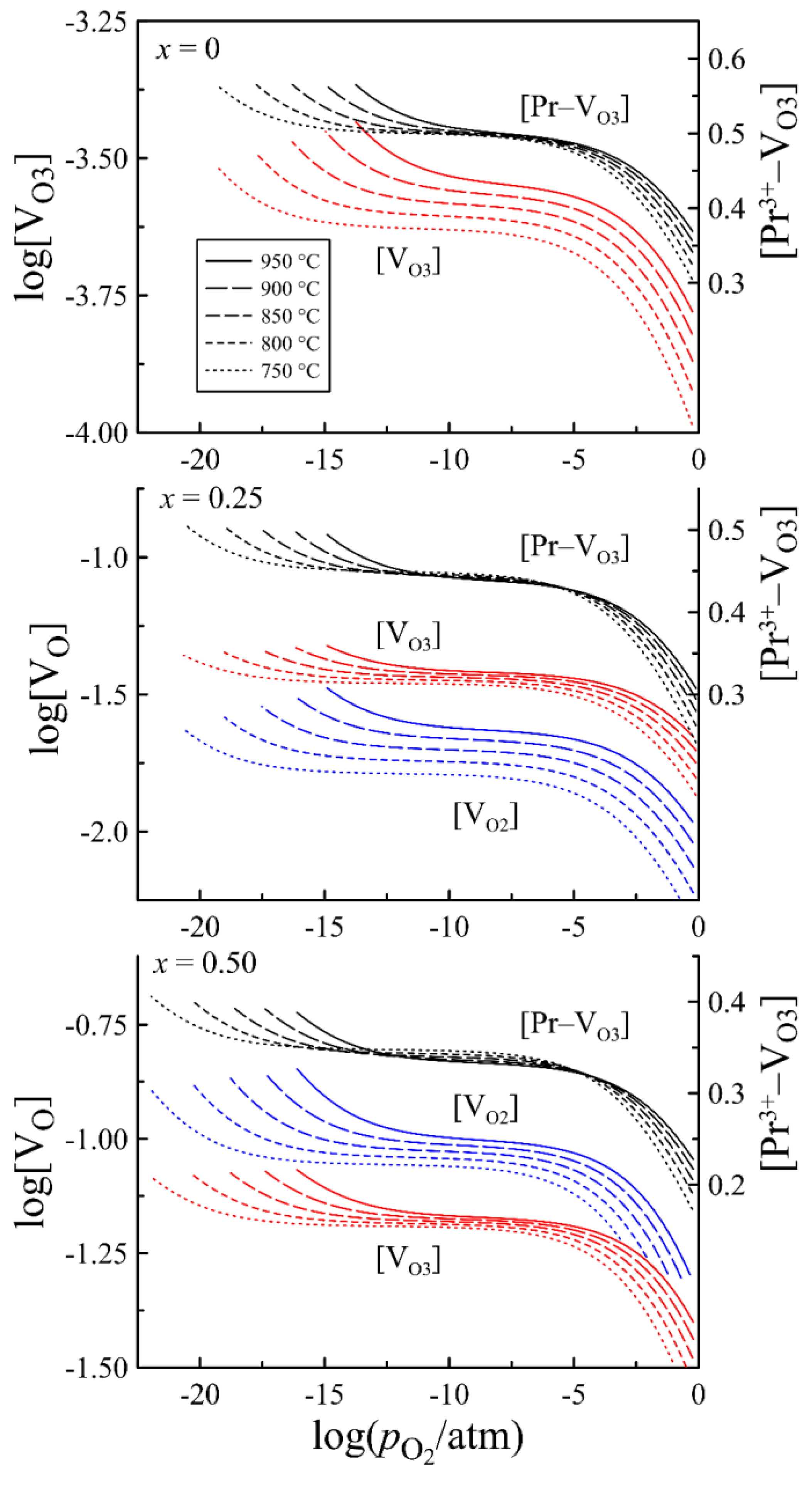
| x = 0 | x = 0.25 | x = 0.50 | |
|---|---|---|---|
| ΔHox/kJ·mol−1 | −80 ± 2 | −82 ± 1 | −85 ± 1 |
| ΔSox/J·mol−1K−1 | −59 ± 2 | −65 ± 1 | −68 ± 1 |
| ΔHd/kJ·mol−1 | 132 ± 4 | 133 ± 3 | 133 ± 3 |
| ΔSd/J·mol−1K−1 | 10 ± 1 | 9 ± 1 | 8 ± 1 |
| ΔHod/kJ·mol−1 | – | 15 ± 1 | 5.3 ± 0.6 |
| ΔSod/J·mol−1K−1 | – | −9.4 ± 0.9 | −8.5 ± 0.7 |
| ΔHc/kJ·mol−1 | −10 ± 1 | −7 ± 1 | −5.7 ± 0.8 |
| ΔSc/J·mol−1K−1 | 26 ± 2 | 19 ± 2 | 12 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonidov, I.A.; Markov, A.A.; Zavyalov, M.A.; Merkulov, O.V.; Shalaeva, E.V.; Nikitin, S.S.; Tsipis, E.V.; Patrakeev, M.V. Structural Features and Defect Equilibrium in Cubic PrBa1−xSrxFe2O6−δ. Materials 2022, 15, 4390. https://doi.org/10.3390/ma15134390
Leonidov IA, Markov AA, Zavyalov MA, Merkulov OV, Shalaeva EV, Nikitin SS, Tsipis EV, Patrakeev MV. Structural Features and Defect Equilibrium in Cubic PrBa1−xSrxFe2O6−δ. Materials. 2022; 15(13):4390. https://doi.org/10.3390/ma15134390
Chicago/Turabian StyleLeonidov, Ilia A., Alexey A. Markov, Mikhail A. Zavyalov, Oleg V. Merkulov, Elisaveta V. Shalaeva, Sergey S. Nikitin, Ekaterina V. Tsipis, and Mikhail V. Patrakeev. 2022. "Structural Features and Defect Equilibrium in Cubic PrBa1−xSrxFe2O6−δ" Materials 15, no. 13: 4390. https://doi.org/10.3390/ma15134390
APA StyleLeonidov, I. A., Markov, A. A., Zavyalov, M. A., Merkulov, O. V., Shalaeva, E. V., Nikitin, S. S., Tsipis, E. V., & Patrakeev, M. V. (2022). Structural Features and Defect Equilibrium in Cubic PrBa1−xSrxFe2O6−δ. Materials, 15(13), 4390. https://doi.org/10.3390/ma15134390








