Effect of Gamma Irradiation on the Structural, Optical, Electrical, and Ferroelectric Characterizations of Bismuth-Modified Barium Titanate Ceramics
Abstract
1. Introduction
2. Experimental Part
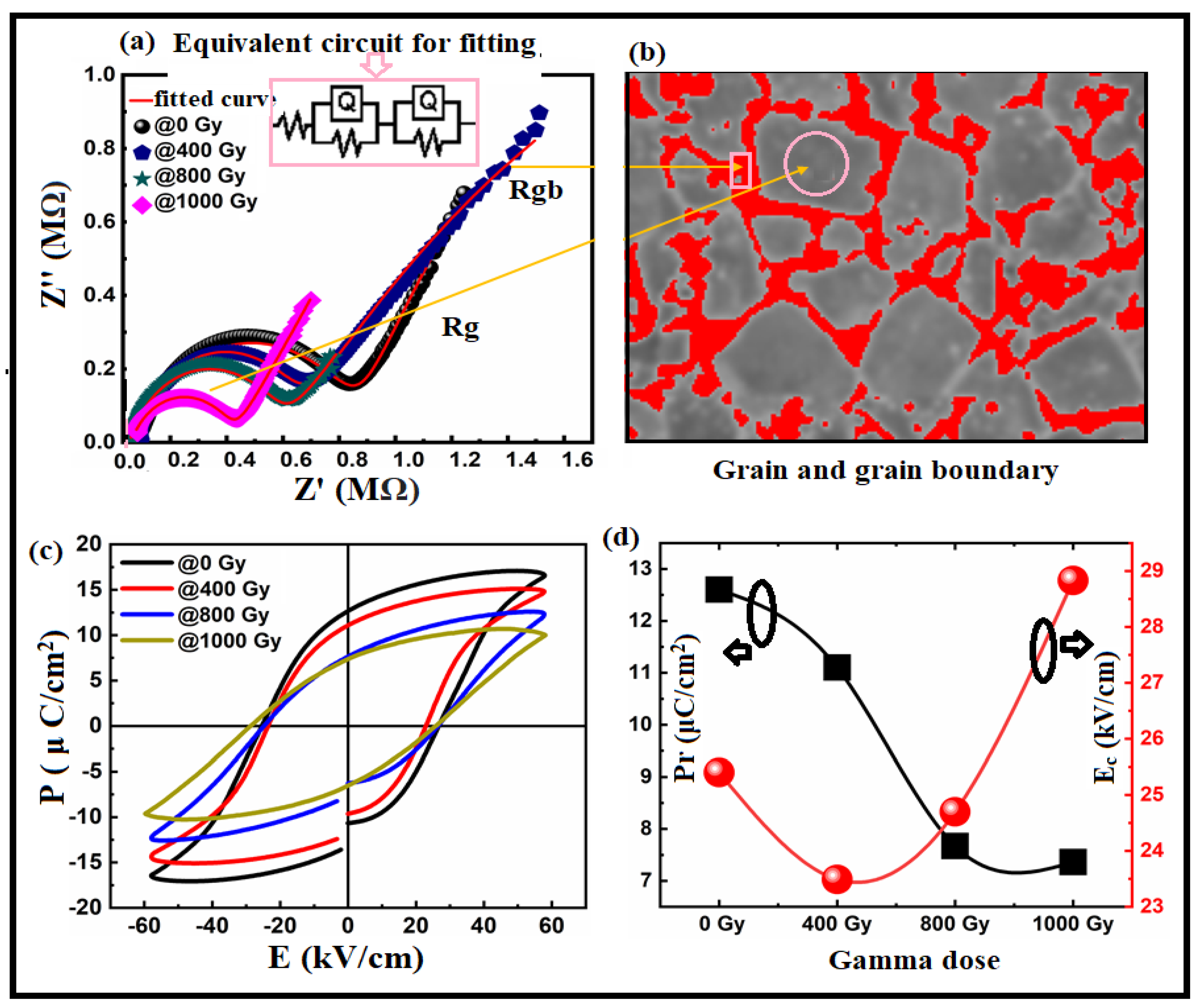
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Huang, Y.; Wu, J. Multifunctional barium titanate ceramics via chemical modification tuning phase structure. InfoMat 2020, 2, 1163–1190. [Google Scholar] [CrossRef]
- Jaffe, H. Piezoelectric Ceramics; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lines, M.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, UK, 1977. [Google Scholar]
- Haertling, G.H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Damjanovic, D. Ferroelectric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics. Prog. Phys. 1998, 61, 1267–1324. [Google Scholar] [CrossRef]
- Wang, S.F.; Dayton, G.O. Dielectric Properties of Fine-Grained Barium Titanate Based X7R Materials. J. Am. Ceram. Soc. 1999, 8, 2677–2682. [Google Scholar] [CrossRef]
- Hennings, D.; Klee, M.; Waser, R. Advanced dielectrics: Bulk ceramics and thin films. Adv. Mater. 1991, 3, 334–340. [Google Scholar] [CrossRef]
- Funakoshi, H.; Okamoto, A.; Sato, K. Long-term reading experiment on a photorefractive holographic memory with the hologram sustainment technique by optical feedback. J. Mod. Opt. 2005, 52, 1511–1527. [Google Scholar] [CrossRef]
- Tang, P.; Towner, D.; Meier, A.; Wessels, B. Low-Loss Electrooptic BaTiO3Thin Film Waveguide Modulator. IEEE Photon. Technol. Lett. 2004, 16, 1837–1839. [Google Scholar] [CrossRef]
- Petraru, A.; Schubert, J.; Schmid, M.; Buchal, C. Ferroelectric BaTiO3 thin-film optical waveguide modulators. Appl. Phys. Lett. 2002, 81, 1375–1377. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Zakaly, H.M.; Sekhar, K.C.; Issa, S.A.; Alharbi, T.; Badawi, A.; Shareefuddin, M. Investigations of mechanical and radiation shielding properties of BaTiO3-modified cadmium alkali borate glass. Appl. Phys. A 2022, 128, 260. [Google Scholar] [CrossRef]
- Kim, H.; Arbab, A.; Fenech-Salerno, B.; Yao, C.; Macpherson, R.; Kim, J.M.; Torrisi, F. Barium titanate-enhanced hexagonal boron nitride inks for printable high-performance dielectrics. Nanotechnology 2022, 33, 215704. [Google Scholar] [CrossRef]
- Moghtada, A.; Ashiri, R. Superiority of sonochemical processing method for the synthesis of barium titanate nanocrystals in contrast to the mechanochemical approach. Ultrason. Sonochem. 2018, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, L.; Ai, C.; Xie, P.; Lin, S.; Wang, C.-Z.; Lu, X. Tailoring Bandgap of Perovskite BaTiO3 by Transition Metals Co-Doping for Visible-Light Photoelectrical Applications: A First-Principles Study. Nanomaterials 2018, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumari, K.; Ahmed, F.; Alshoaibi, A.; Alvi, P.; Dalela, S.; Ahmad, M.M.; Aljawfi, R.N.; Dua, P.; Vij, A.; et al. Influence of Sm doping on structural, ferroelectric, electrical, optical and magnetic properties of BaTiO3. Vacuum 2020, 184, 109872. [Google Scholar] [CrossRef]
- Hasan, M.; Hossain, A.A. Structural, electronic and optical properties of strontium and nickel co-doped BaTiO3: A DFT based study. Comput. Condens. Matter 2021, 28, e00578. [Google Scholar] [CrossRef]
- Apostolova, I.N.; Apostolov, A.T.; Wesselinowa, J.M. Phonon and optical properties of transition metal and rare earth ion doped BaTiO3. J. Appl. Phys. 2021, 130, 175103. [Google Scholar] [CrossRef]
- Naveed-Ul-Haq, M. Exploring Ba (Ti, Sn) O3: An experimental and theoretical study of structural, ferroelectric, electronic, and optical properties. Mater. Today Commun. 2021, 28, 102494. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Xie, S.; Tan, Z.; Nie, R.; Guan, Z.; Wang, Q.; Zhu, J. Ion Doping Effects on the Lattice Distortion and Interlayer Mismatch of Aurivillius-Type Bismuth Titanate Compounds. Materials 2018, 11, 821. [Google Scholar] [CrossRef]
- Medhi, N.; Nath, A.K. Gamma ray irradiation effects on the ferroelectric and piezoelectric properties of barium titanate ceramics. J. Mater. Eng. Perform. 2013, 22, 2716–2722. [Google Scholar] [CrossRef]
- Sharma, S.; Paliwal, A.; Tomar, M.; Singh, F.; Puri, N.; Gupta, V. Effect of ion beam irradiation on dielectric properties of BaTiO3 thin film using surface plasmon resonance. J. Mater. Sci. 2016, 51, 4055–4060. [Google Scholar] [CrossRef]
- Ahmed, B.S.; Nandaprakash, M.B.; Namratha, K.; Byrappa, K.; Somashekar, R. Structure and Electrical Conductivity of Irradiated BaTiO3 Nanoparticles. Phys. Status Solidi B 2018, 255, 1700581. [Google Scholar] [CrossRef]
- Kumar, P.; Saxena, N.; Gupta, V.; Singh, F.; Agarwal, A. Correlation between surface phonon mode and luminescence in nanocrystalline CdS thin films: An effect of ion beam irradiation. J. Appl. Phys. 2015, 116, 043517. [Google Scholar] [CrossRef]
- Gautam, S.K.; Singh, F.; Sulania, I.; Singh, R.G.; Kulriya, P.K.; Pippel, E. Micro-Raman study on the softening and stiffening of phonons in rutile titanium dioxide film: Competing effects of structural defects, crystallite size, and lattice strain. J. Appl. Phys. 2014, 115, 143504. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Q.; Lin, J.; Lin, C.; Zheng, X.; Lin, T.; Wu, X. Enhanced ferro-/piezoelectric properties of tape-casting-derived Er3+-doped Ba0.85Ca0.15Ti0.9Zr0.1O3 optoelectronic thick films. J. Adv. Ceram. 2020, 9, 693–702. [Google Scholar] [CrossRef]
- Singh, F.; Singh, R.G.; Kumar, V.; Khan, S.A.; Pivin, J.C. Softening of phonons by lattice defects and structural strain in heavy ion irradiated nanocrystalline zinc oxide films. J. Appl. Phys. 2011, 110, 083520. [Google Scholar] [CrossRef]
- Ahmadu, U.; Abubakar Soje, A.; Bidemi Usman, A.; Muhammad Musa, A.; Uthman Isah, K. Structural and microstructural study of gamma ray-irradiated co-doped barium titanate (Ba0. 88Ca0. 12Ti0. 975Sn0. 025O3). Process. Appl. Ceram. 2016, 10, 79–85. [Google Scholar] [CrossRef]
- Wu, S.; Wei, X.; Wang, X.; Yang, H.; Gao, S. Effect of Bi2O3 Additive on the Microstructure and Dielectric Properties of BaTiO3-Based Ceramics Sintered at Lower Temperature. J. Mater. Sci. Technol. 2010, 26, 472–476. [Google Scholar] [CrossRef]
- Kholodkova, A.; Smirnov, A.; Danchevskaya, M.; Ivakin, Y.; Muravieva, G.; Ponomarev, S.; Fionov, A.; Kolesov, V. Bi2O3 Modified Ceramics Based on BaTiO3 Powder Synthesized in Water Vapor. Inorganics 2020, 8, 8. [Google Scholar] [CrossRef]
- Yadav, A.K.; Fan, H.; Yan, B.; Wang, W.; Dong, W.; Wang, S. Structure evolutions with enhanced dielectric permittivity and ferroelectric properties of Ba (1−x)(La, Li) xTiO3 ceramics. J. Mater. Sci. Mater. Electro. 2021, 32, 23103–23115. [Google Scholar] [CrossRef]
- Abyshev, B.; Shlimas, D.I.; Zdorovets, M.V.; Arshamov, Y.K.; Kozlovskiy, A.L. Study of Radiation Resistance to Helium Swelling of Li2ZrO3/LiO and Li2ZrO3 Ceramics. Crystals 2022, 12, 384. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Grobe von Kolloidteilchen mittels Rontgenstrahlen. Gottinger Nachr. Ges. 1918, 2, 96–100. [Google Scholar]
- Mahapatra, A.; Parida, S.; Sarangi, S.; Badapanda, T. Dielectric and Ferroelectric Behavior of Bismuth-Doped Barium Titanate Ceramic Prepared by Microwave Sintering. JOM 2015, 67, 1896–1904. [Google Scholar] [CrossRef]
- Günay, S.D. Swelling Mechanisms of UO2 Lattices with Defect Ingrowths. PLoS ONE 2015, 10, e0134500. [Google Scholar]
- Zhang, H.; Su, R.; Szlufarska, I.; Shi, L.; Wen, H. Helium effects and bubbles formation in irradiated Ti3SiC2. J. Eur. Ceram. Soc. 2020, 41, 252–258. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Shen, T.; Chai, J.; Niu, L.; Li, S.; Jin, P.; Zheng, H.; Wang, Z. Irradiation response of Al2O3-ZrO2 ceramic composite under He ion irradiation. J. Eur. Ceram. Soc. 2021, 41, 2883–2891. [Google Scholar] [CrossRef]
- Schileo, G.; Luisman, L.; Feteira, A.; Deluca, M.; Reichmann, K. Structure–property relationships in BaTiO3–BiFeO3–BiYbO3 ceramics. J. Eur. Ceram. Soc. 2013, 33, 1457–1468. [Google Scholar] [CrossRef]
- Maxim, F.; Ferreira, P.; Vilarinho, P.M.; Reaney, I. Hydrothermal Synthesis and Crystal Growth Studies of BaTiO3 Using Ti Nanotube Precursors. Cryst. Growth Des. 2008, 8, 3309–3315. [Google Scholar] [CrossRef]
- Raddaoui, Z.; Smiri, B.; Maaoui, A.; Dhahri, J.; Ghaieth, R.M.; Abdelmoula, N.; Khiroun, K. Correlation of crystal structure and optical properties of Ba 0.97Nd0.0267Ti(1-x) WxO3 perovskite. RSC Adv. 2018, 8, 27870. [Google Scholar] [CrossRef]
- Mondal, T.; Das, S.; Badapanda, T.; Sinha, T.P.; Sarun, P.M. Effect of Ca2+ substitution on impedance and electrical conduction mechanism of Ba1−xCaxZr0.1Ti0. 9O3 (0.00 ≤ x ≤ 0.20) ceramics. Phys. B 2017, 508, 124–135. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Reddy, V.R.; Lakshmi, N. Study of (1−x) BaTiO3–xNi0.5Zn0.5Fe2O4 (x = 5, 10 and 15%) magneto-electric ceramic composites. J. Asian Ceram. Social. 2013, 1, 346–350. [Google Scholar] [CrossRef]
- Alkathy, M.S.; Lente, M.H.; Eiras, J.A. Bandgap narrowing of Ba0. 92Na0. 04Bi0.04TiO3 ferroelectric ceramics by transition metals doping for photovoltaic applications. Mater. Chem. Phys. 2021, 257, 123791. [Google Scholar] [CrossRef]
- Alkathy, M.S.; James Raju, K.C. Study of diffuse Phase Transition behaviour in Bi and Li Co-substituted barium titanate ceramics. J. Electroceram. 2017, 38, 63–73. [Google Scholar] [CrossRef]
- Kreisel, J.; Glazer, A.M.; Jones, G.; Thomas, P.A.; Abello, L.; Lucazeau, G. An X-ray diffraction and Raman spectroscopy investigation of A-site substituted perovskite compounds: The (Na1-xKx)0.5Bi0. 5TiO3 solid solution. J. Phys. Condens. Matter 2000, 12, 3267. [Google Scholar] [CrossRef]
- Petzelt, J.; Kamba, S.; Fábry, J.; Noujni, D.; Porokhonskyy, V.; Pashkin, A.; Kugel, G.E. Infrared, Raman and high-frequency dielectric spectroscopy and the phase transitions in Na1/2Bi1/2TiO3. J. Condens. Matter Phys. 2004, 16, 2719–2731. [Google Scholar] [CrossRef]
- Kreisel, J.; Glazer, A.M.; Bouvier, P.; Lucazeau, G. High-pressure Raman study of a relaxor ferroelectric: The perovskite. Phys. Rev. B 2001, 63, 174106. [Google Scholar] [CrossRef]
- Ma, J.; Gu, J.; Su, D.; Wu, X.M.; Song, C.H.; Li, W.; Lu, X.M.; Zhu, J.S. Structural and ferroelectric properties of yttrium substituted bismuth titanium thin films. Thin Solid Films 2005, 492, 264–268. [Google Scholar] [CrossRef]
- Nath, A.; Medhi, N. Effect of gamma ray irradiation on the ferroelectric and piezoelectric properties of barium stannate titanate ceramics. Radiat. Phys. Chem. 2013, 91, 44–49. [Google Scholar] [CrossRef]
- Nath, A.K.; Medhi, A. Effects of gamma ray irradiation on the piezoelectric and ferroelectric properties of bismuth doped barium titanate ceramics. Indian J. Phys. 2014, 89, 131–136. [Google Scholar] [CrossRef]
- Lal, R.; Gokhale, N.M.; Krishnan, R.; Ramakrishnan, P. Effect of sintering parameters on the microstructure and properties of strontium modified PZT ceramics prepared using spray-dried powders. J. Mater. Sci. 1989, 24, 2911–2916. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Kasai, P.H. Electron Spin Resonance Studies of Donors and Acceptors in ZnO. Phys. Rev. B 1963, 130, 989–995. [Google Scholar] [CrossRef]
- Laguta, V.V.; Slipenyuk, A.M.; Bykov, I.P.; Glinchuk, M.D.; Maglione, M.; Bilous, A.G.; V’yunov, O.I.; Rosa, J.; Jastrabik, L. Electron spin resonance investigation of impurity and intrinsic defects in Nb-doped BaTiO3 single crystal and ceramics. J. Appl. Phys. 2005, 97, 073707. [Google Scholar] [CrossRef]
- Scharfschwerdt, R.; Mazur, A.; Schirmer, O.F.; Hesse, H.; Mendricks, S. Oxygen vacancies in BaTiO3. Phys. Rev. B 1996, 54, 15284. [Google Scholar] [CrossRef] [PubMed]
- Lenjer, S.; Schirmer, O.F.; Hesse, H.; Kool, T.W. Conduction states in oxide perovskites: Three manifestations of Ti3+ Jahn-Teller polarons in barium titanate. Phys. Rev. B 2002, 66, 165106. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing and Sintering; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Dang, N.V.; Thanh, T.D.; Hong, L.V.; Lam, V.D.; Phan, T.L. Structural, optical and magnetic properties of polycrystalline BaTi1−xFexO3 ceramics. Appl. Phys. 2011, 110, 043914. [Google Scholar] [CrossRef]
- Evans, B.D. A review of the optical properties of anion lattice vacancies, and electrical conduction in α-Al2O3: Their relation to radiation-induced electrical degradation. J. Nucl. Mater. 1995, 219, 202–223. [Google Scholar] [CrossRef]
- Saidoh, M.; Townsend, P.D. Mechanisms of defect formation. Radiat. Eff. 1975, 27, 1–12. [Google Scholar] [CrossRef]
- Ewaida, M.; Sekkina, M.A.; Ebrahim, E.; Al-Adawy, A. Novel studies on the thermoelectro-mechanical properties of tantala-doped zirconia refractories. Polym. Degrad. Stab. 1988, 21, 227–235. [Google Scholar] [CrossRef]
- Muccillo, R.; Muccillo, E.N. Improved densification and ionic conductivity in flash-sintered gamma-ray irradiated yttria-stabilized zirconia. Scr. Mater. 1987, 23, 366–370. [Google Scholar] [CrossRef]
- Tawfik, A.; IAbd El-Ati, M.; MEl-Ashry, F.; Abou Sekkina, M.M. Further investigation on the effects of temperature and energetic ionizing radiation on monoclinic zirconia refractory. J. Phys. Soc. Jpn. 1985, 54, 3012–3017. [Google Scholar] [CrossRef]
- Thae-Khapp, K.; Il-Hiun, K.; Katano, Y.; Igawa, N.; Ohno, H. Effect of gamma-ray irradiation on in-situ electrical conductivity of ZrO2-10 mol% Gd2O3 single crystal at elevated temperatures. J. Nucl. Mater. 1994, 209, 321–325. [Google Scholar] [CrossRef]
- Claussen, N.; Ruhle, M.; Heuer, A.H. Science and Technology of Zirconia II; Claussen, N., Rühle, M., Heuer, A.H., Eds.; American Ceramic Society, Inc.: Columbus, OH, USA, 1983; p. 555. [Google Scholar]
- Tyunina, M. Oxygen vacancies in perovskite oxide piezoelectrics. Materials 2020, 13, 5596. [Google Scholar] [CrossRef] [PubMed]
- Jamil, T.S.; Abbas, H.A.; Youssief, A.M.; Mansor, E.S.; Hammad, F.F. The synthesis of nano-sized undoped, Bi doped and Bi, Cu co-doped SrTiO3 using two sol–gel methods to enhance the photocatalytic performance for the degradation of dibutyl phthalate under visible light. Comptes Rendus Chim. 2017, 20, 97–106. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Buscaglia, V.; Viviani, M.; Nanni, P. Atomistic Simulation of Dopant Incorporation in Barium Titanate. J. Am. Ceram. Soc. 2004, 84, 376–384. [Google Scholar] [CrossRef]
- Morrison, F.D.; Coats, A.M.; Sinclair, D.C.; West, A.R. Charge compensation mechanisms in La-doped BaTiO3. J. Electroceram. 2001, 6, 219–232. [Google Scholar] [CrossRef]
- Alonso, J.A.; Martinez-Lope, M.J.; Casais, M.T.; Fernandez-Diaz, M.T. Evolution of the Jahn−Teller Distortion of MnO6 Octahedra in RMnO3 Perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A Neutron Diffraction Study. Inorg. Chem. 2000, 39, 917–923. [Google Scholar] [CrossRef]
- Lufaso, M.; Woodward, P.M. Jahn–Teller distortions, cation ordering and octahedral tilting in perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 10–20. [Google Scholar] [CrossRef]
- Alkathy, M.S.; Eiras, J.A.; Zabotto, F.L.; Raju, K.C.J. Structural, optical, dielectric, and multiferroic properties of sodium and nickel co-substituted barium titanate ceramics. J. Mater. Sci. Mater. Electron. 2020, 32, 12828–12840. [Google Scholar] [CrossRef]
- Alkathy, M.S.; Zabotto, F.L.; Manuel, H.; Lente, J.; Eiras, A. Octahedral distortion and oxygen vacancies induced band-gap narrowing and enhanced visible light absorption of Co/Fe co-doped Bi3.25Nd0.75Ti3O12 ferroelectrics for photovoltaic applications. J. Phys. D Appl. Phys. 2020, 53, 465106. [Google Scholar] [CrossRef]
- Alkathy, M.; Zabotto, F.; Milton, F.P.; Eiras, J. Bandgap tuning in samarium-modified bismuth titanate by site engineering using iron and cobalt co-doping for photovoltaic application. J. Alloy. Compd. 2022, 908, 164222. [Google Scholar] [CrossRef]
- Kolte, J.; Salame, P.H.; Daryapurkar, A.S.; Gopalan, P. Impedance and AC conductivity study of nano crystalline, fine grained multiferroic bismuth ferrite (BiFeO3), synthesized by microwave sintering. Advances 2015, 5, 097164. [Google Scholar] [CrossRef]
- Van Dijk, M.P.; Ter Maat, J.H.; Roelofs, G.; Bosch, H.; Van de Velde, G.M.; Gellings, P.J.; Burggraaf, A.J. Electrical and catalytic properties of some oxides with the fluorite or pyrochlore structure: Part 1: Synthesis, characterization and conductivity. Mater. Res. Bull. 1984, 19, 1149–1156. [Google Scholar] [CrossRef][Green Version]
- Mao, Y.P.; Mao, S.Y.; Ye, Z.G.; Xie, Z.X.; Zheng, L.S. Size-dependences of the dielectric and ferroelectric properties of/polyvinylidene fluoride nanocomposites. Appl. Phys. 2010, 108, 014102. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Effect of grain size on the domain structures and electromechanical responses of ferroelectric polycrystal. Smart Mater. Struct. 2016, 26, 015013. [Google Scholar] [CrossRef]
- Amorín, H.; Venet, M.; Chinarro, E.; Ramos, P.; Algueró, M.; Castro, A. Lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ferroelectric ceramics with refined microstructure and high strain under electric field by mechanosynthesis. J. Eur. Ceram. Soc. 2022, 42, 4907–4916. [Google Scholar] [CrossRef]
- Tumarkin, A.; Tyurnina, N.; Mukhin, N.; Tyurnina, Z.; Sinelshchikova, O.; Gagarin, A.; Sapego, E.; Kretser, Y. Glass-ceramic ferroelectric composite material BaTiO3/KFeSi for microwave applications. Compos. Struct. 2021, 281, 114992. [Google Scholar] [CrossRef]
- Slimani, Y.; Selmi, A.; Hannachi, E.; Almessiere, M.; AlFalah, G.; AlOusi, L.F.; Yasin, G.; Iqbal, M. Study on the addition of SiO2 nanowires to BaTiO3: Structure, morphology, electrical and dielectric properties. J. Phys. Chem. Solids. 2021, 156, 110183. [Google Scholar] [CrossRef]
- Li, W.-B.; Zhou, D.; Liu, W.-F.; Su, J.-Z.; Hussain, F.; Wang, D.-W.; Wang, G.; Lu, Z.-L.; Wang, Q.-P. High-temperature BaTiO3-based ternary dielectric multilayers for energy storage applications with high efficiency. Chem. Eng. J. 2021, 414, 128760. [Google Scholar] [CrossRef]
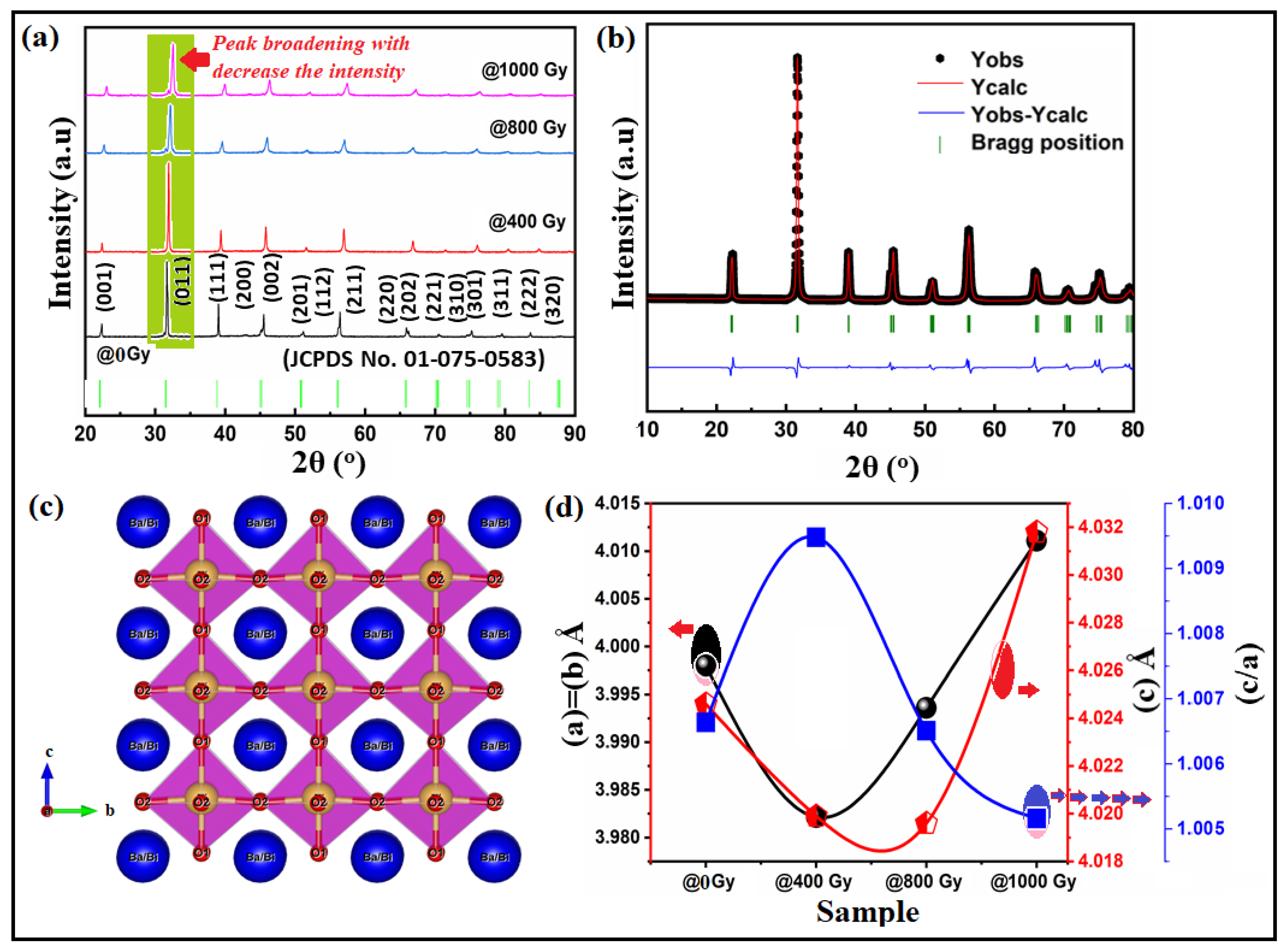
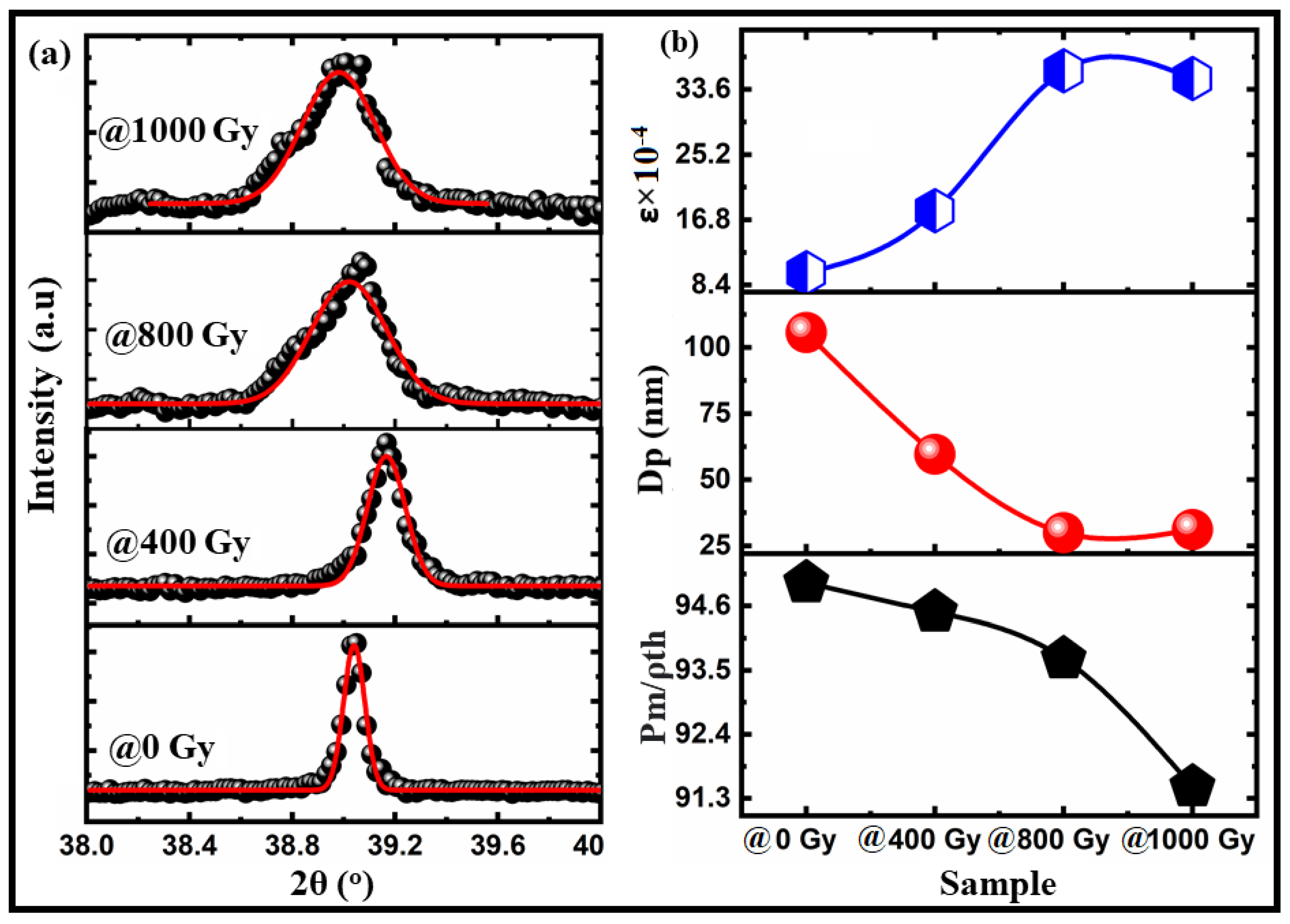
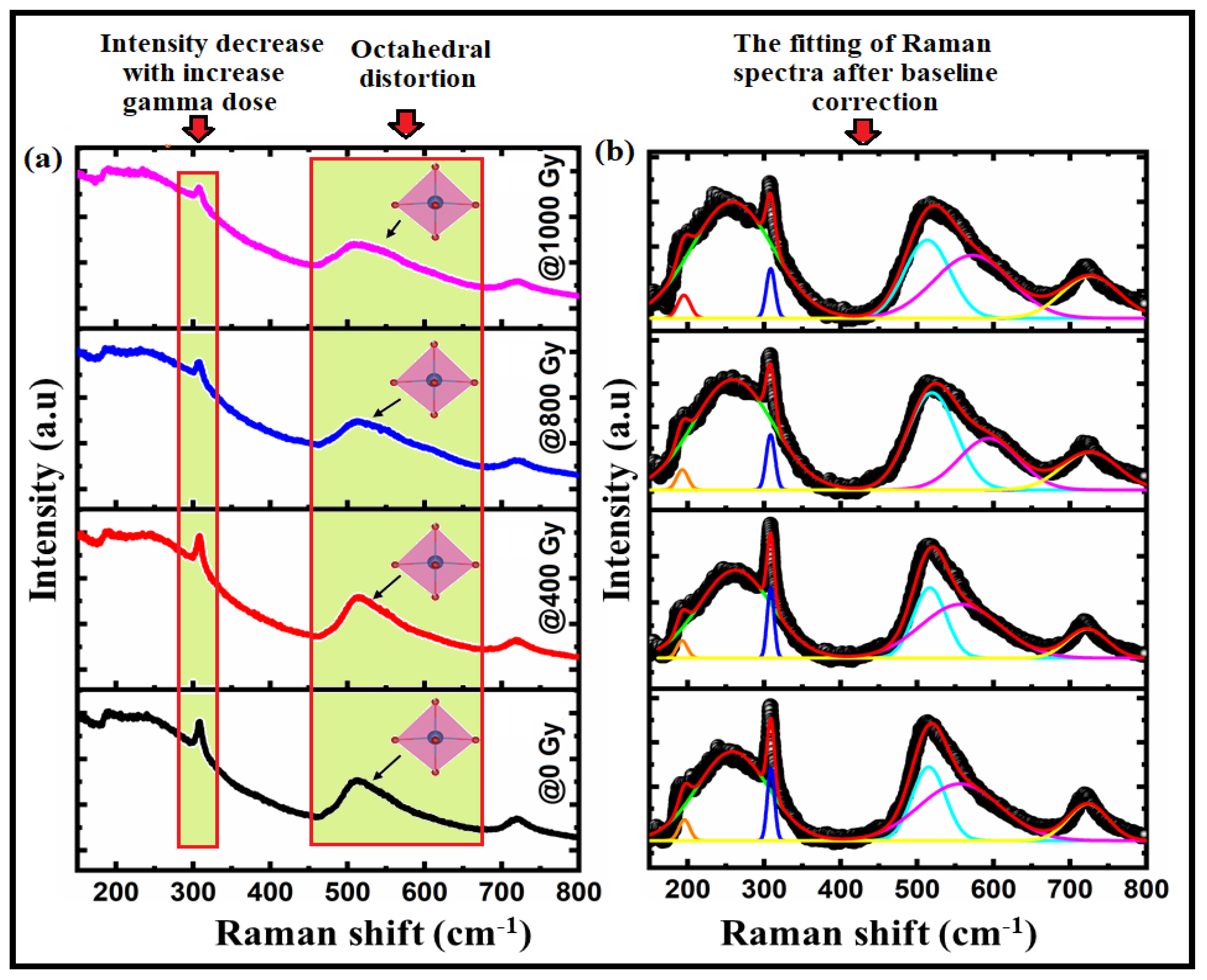

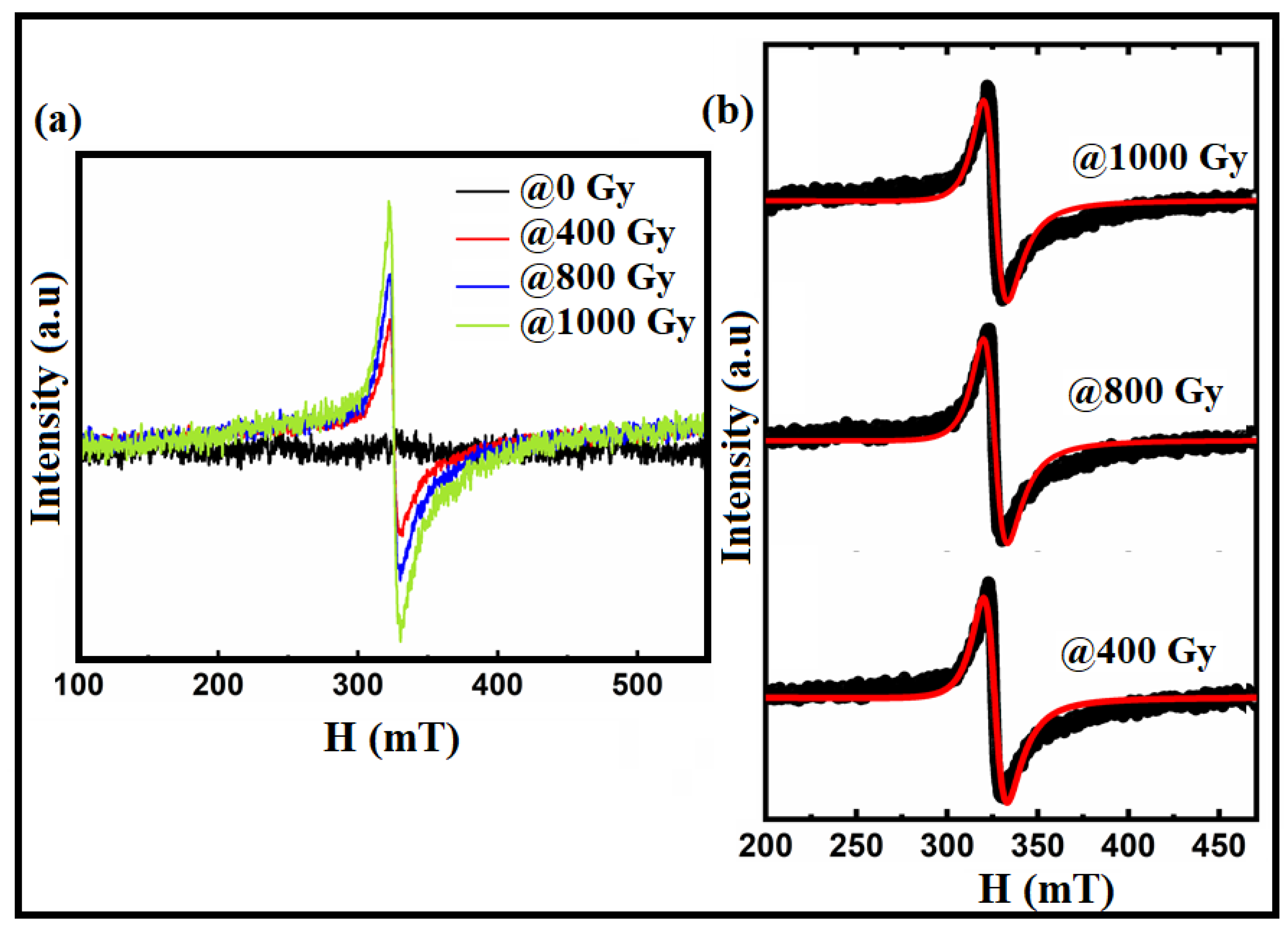

| Sample/Parameters | @0 Gy | @400 Gy | @800 Gy | @1000 Gy | |
|---|---|---|---|---|---|
| Crystal structure | Tetragonal | Tetragonal | Tetragonal | Tetragonal | |
| Lattice constant a = b (Å) | 3.99802 | 3.98215 | 3.9936 | 4.0111 | |
| c (Å) | 4.02460 | 4.0199 | 4.0196 | 4.0318 | |
| c/a | 1.00664 | 1.00948 | 1.00651 | 1.005161 | |
| V (Å)3 | 64.329864 | 64.34994 | 64.52533 | 65.20208 | |
| Space group | P4mm | P4mm | P4mm | P4mm | |
| Space group number | 99 | 99 | 99 | 99 | |
| Theoretical density g/cm3 | 6.19 | 6.073 | 6.103 | 6.106 | |
| Measured density g/cm3 | 5.881 | 5.741 | 5.718 | 5.588 | |
| Relative density (%) | 95 | 94.5 | 93.7 | 91.5 | |
| Porosity (%) | 5 | 5.5 | 5.3 | 8.5 | |
| Distortion crystal lattice (a) | - | −0.00397 | 0.00288 | 0.00438 | |
| Distortion crystal lattice (c) | - | −0.00117 | −7.46 × 10−5 | 0.00304 | |
| Crystallite size (nm) | 105.59 | 59.62 | 29.95 | 31.110 | |
| Lattice strain | 0.0010 | 0.0018 | 0.0036 | 0.0035 | |
| Ba/Bi | x | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| y | 0.00000 | 0.00000 | 0.00000 | 0.00000 | |
| z | 0.00000 | 0.00000 | 0.00000 | 0.00000 | |
| Occ | 1.000 | 1.000 | 1.000 | 1.000 | |
| Site | 1a | 1a | 1a | 1a | |
| Sym | 4 mm | 4 mm | 4 mm | 4 mm | |
| Ti/Co | x | 0.50000 | 0.50000 | 0.50000 | 0.50000 |
| y | 0.50000 | 0.50000 | 0.50000 | 0.50000 | |
| z | 0.52430 | 0.53082 | 0.52376 | 0.52835 | |
| Occ | 1.134 | 1.295 | 0.97340 | 0.96697 | |
| Site | 1b | 1b | 1b | 1b | |
| Sym | 4 mm | 4 mm | 4 mm | 4 mm | |
| O1 | x | 0.50000 | 0.50000 | 0.50000 | 0.50000 |
| y | 0.50000 | 0.50000 | 0.50000 | 0.50000 | |
| z | 0.03260 | 0.05652 | 0.03152 | 0.05517 | |
| Occ | 0.872 | 1.371 | 1.23106 | 1.34734 | |
| Site | 1b | 1b | 1b | 1b | |
| Sym | 4 mm | 4 mm | 4 mm | 4 mm | |
| O2 | x | 0.50000 | 0.50000 | 0.50000 | 0.50000 |
| y | 0.00000 | 0.00000 | 0.00000 | 0.00000 | |
| z | 0.48920 | 0.51915 | 0.48851 | 0.61300 | |
| Occ | 1.191 | 1.467 | 1.32096 | 1.41480 | |
| Site | 2c | 2c | 2c | 2c | |
| Sym | 2 mm | 2 mm | 2 mm | 2 mm | |
| Rp% | 10.7 | 4.61 | 4.87 | 5.16 | |
| Rwp% | 7.41 | 5.77 | 6.14 | 7.21 | |
| Rex% | 6.26 | 5.19 | 5.33 | 6.17 | |
| χ2 | 1.4 | 1.23 | 1.32 | 1.36 | |
| Modes | @0 Gy | @400 Gy | @800 Gy | @1000 Gy |
|---|---|---|---|---|
| E(TO2) | 197 | 195 | 194.5 | 194.12 |
| A1(TO2) | 245 | 244 | 243.8 | 243.6 |
| E(TO3) | 309 | 308 | 307 | 306.6 |
| A1(TO3) | 512 | 509 | 508.5 | 506 |
| E(LO4) | 561 | 555 | 554 | 553 |
| A1(LO3) | 724 | 721 | 720 | 719 |
| Sample/Parameters | @0 Gy | @400 Gy | @800 Gy | @1000 Gy |
|---|---|---|---|---|
| (Ba/Bi-O1)×4 Å | 2.83007 (5) | 2.80775 (0) | 2.8197 (3) | 2.8379 (0) |
| (Ba/Bi-O2)×4 Å | 2.86744 (4) | 2.85473 (0) | 2.8663 (3) | 2.5370 (17) |
| (Ba/Bi-O2) ×4 Å | 2.83007 (5) | 2.74735 (0) | 2.8004 (3) | 2.5370 (17) |
| (Ti-O1) Å | 1.9789 (0) | 1.97964 (0) | 1.9535 (3) | 1.9074 (17) |
| (Ti-O1) Å | 2.0457 (0) | 2.05334 (0) | 2.0461 (3) | 2.1244 (17) |
| (Ti-O2) ×4 Å | 1.9789 (5) | 1.9795 (0) | 1.9692 (3) | 2.02944 (4) |
| (O2-Ti-O2) deg | 177.3226 (0) | 173.9156 (3) | 170.8418 (13) | 160.637 (4) |
| (O1-Ti-O1) deg | 180 | 180 | 180 | 180 |
| Octahedral distortion × 10−4 | 1.23712 | 1.505405 | 2.066853 | 9.66842 |
| Bandgap energy (eV) | 3.14 | 3.019 | 2.89 | 2.80 |
| @0 Gy | @400 Gy | @800 Gy | @1000 Gy | |
|---|---|---|---|---|
| Rs (Ω) | 81.73 | 81.54 | 53.38 | 23.44 |
| Q1 (Fsn) | 5.127 × 10−5 | 6.877 × 10-5 | 2.479 × 10-5 | 5.968 × 10-5 |
| Rg (k Ω) | 620.7 | 578.4 | 367.3 | 389.8 |
| Q2 (Fsn) | 3.654 × 10-7 | 8.694 × 10-8 | 5.649 × 10-7 | 5.117 × 10-6 |
| Rgb (k Ω) | 1759.6 | 1508.7 | 1522.9 | 1505.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamdi, H.; Almuqrin, A.H.; Kassim, H. Effect of Gamma Irradiation on the Structural, Optical, Electrical, and Ferroelectric Characterizations of Bismuth-Modified Barium Titanate Ceramics. Materials 2022, 15, 4337. https://doi.org/10.3390/ma15124337
Al-Ghamdi H, Almuqrin AH, Kassim H. Effect of Gamma Irradiation on the Structural, Optical, Electrical, and Ferroelectric Characterizations of Bismuth-Modified Barium Titanate Ceramics. Materials. 2022; 15(12):4337. https://doi.org/10.3390/ma15124337
Chicago/Turabian StyleAl-Ghamdi, Hanan, Aljawhara H. Almuqrin, and Hamoud Kassim. 2022. "Effect of Gamma Irradiation on the Structural, Optical, Electrical, and Ferroelectric Characterizations of Bismuth-Modified Barium Titanate Ceramics" Materials 15, no. 12: 4337. https://doi.org/10.3390/ma15124337
APA StyleAl-Ghamdi, H., Almuqrin, A. H., & Kassim, H. (2022). Effect of Gamma Irradiation on the Structural, Optical, Electrical, and Ferroelectric Characterizations of Bismuth-Modified Barium Titanate Ceramics. Materials, 15(12), 4337. https://doi.org/10.3390/ma15124337






