Effect of Boron Doping on the Interlayer Spacing of Graphite
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of Boron-Doped Graphite
2.2. Characterization of the Graphitized Samples
2.3. Models and Computational Details
3. Results and Discussion
3.1. Characterization of Boron-Doped Graphite
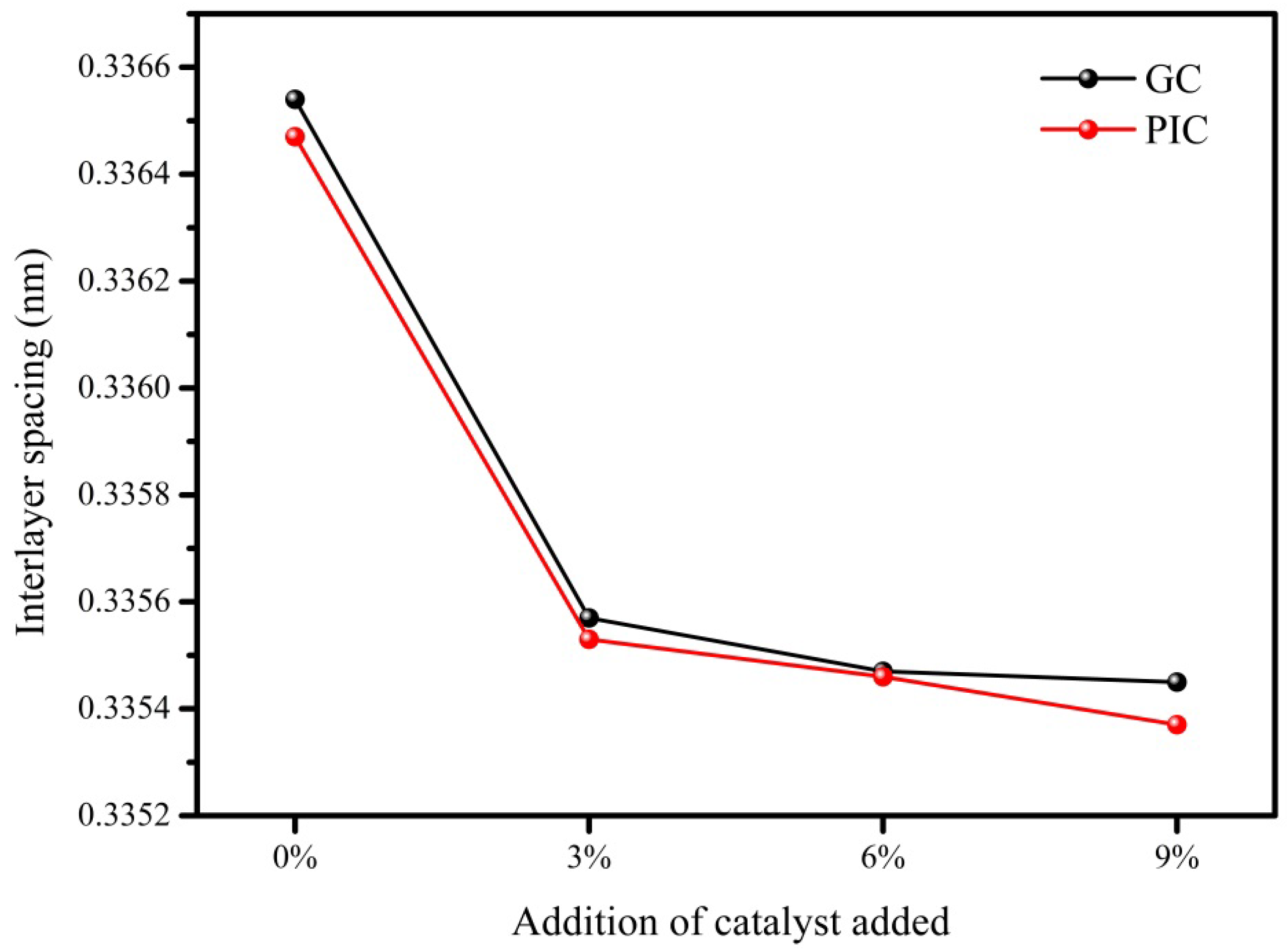
3.2. Effect of Substitutional Boron on the Interlayer Spacing
3.2.1. The Effect of Poisson Contraction on the Interlayer Spacing
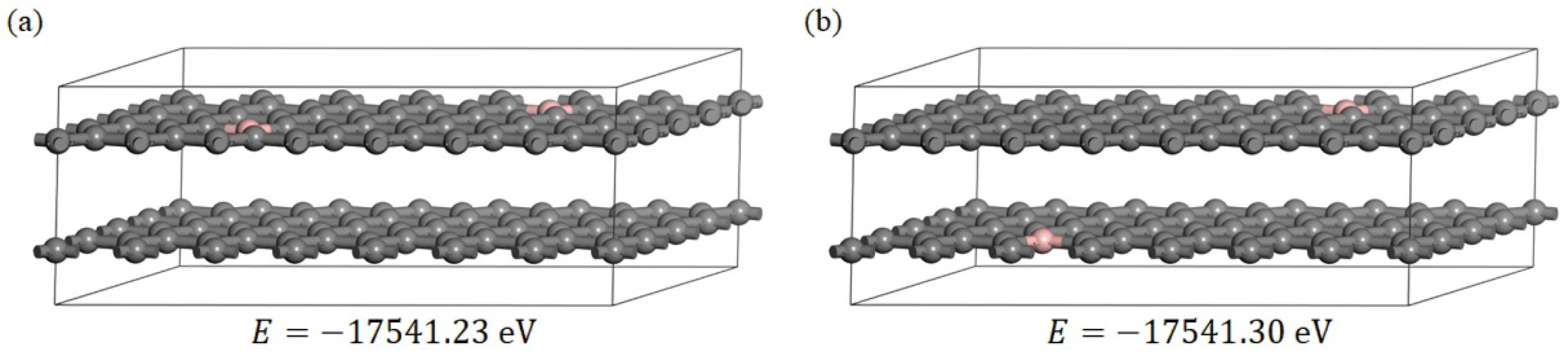
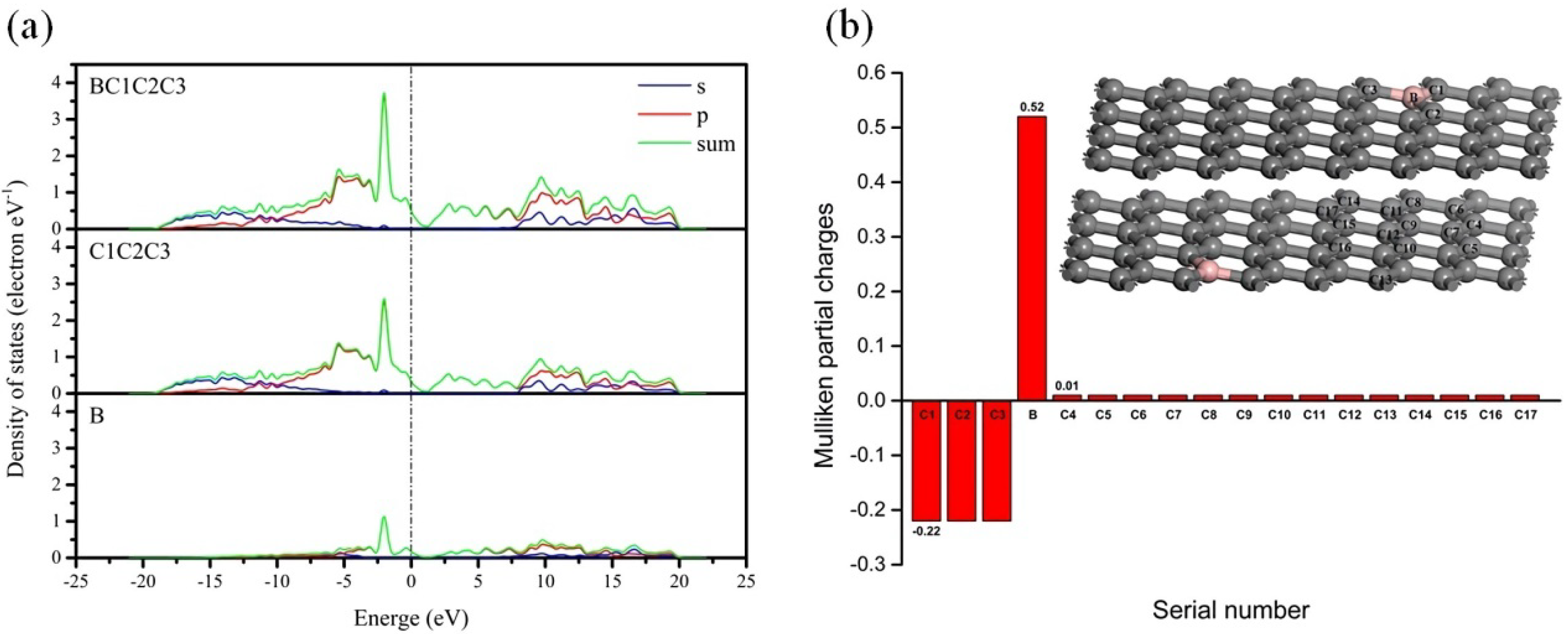
3.2.2. The Electronic Structure of the Boron-Doped Graphite
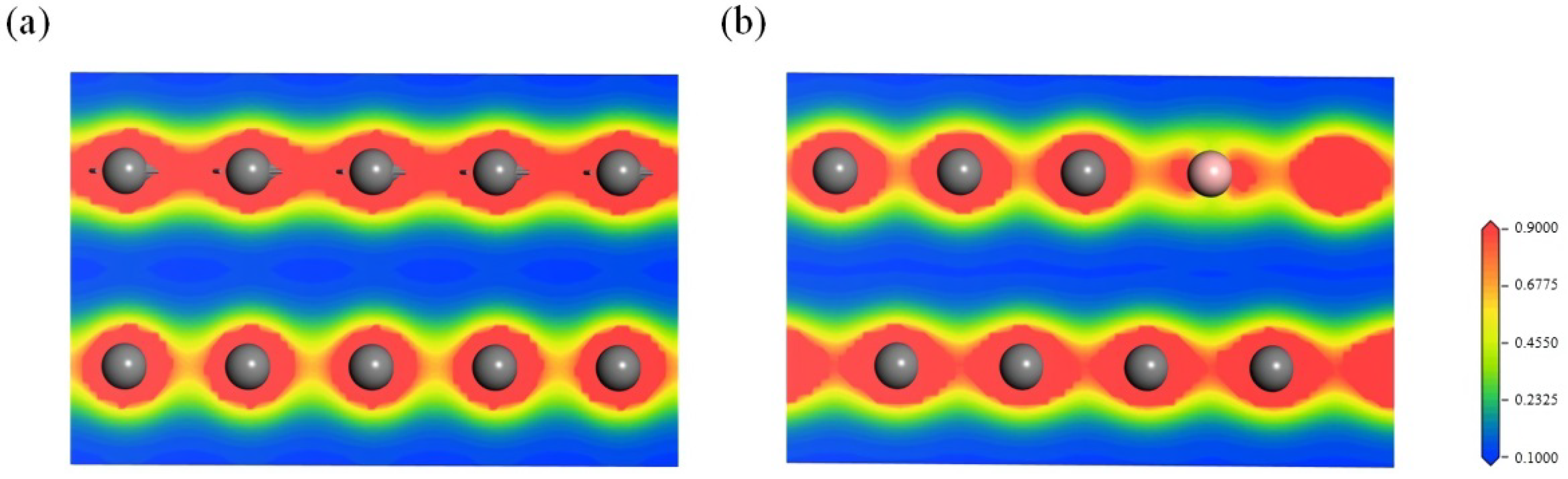
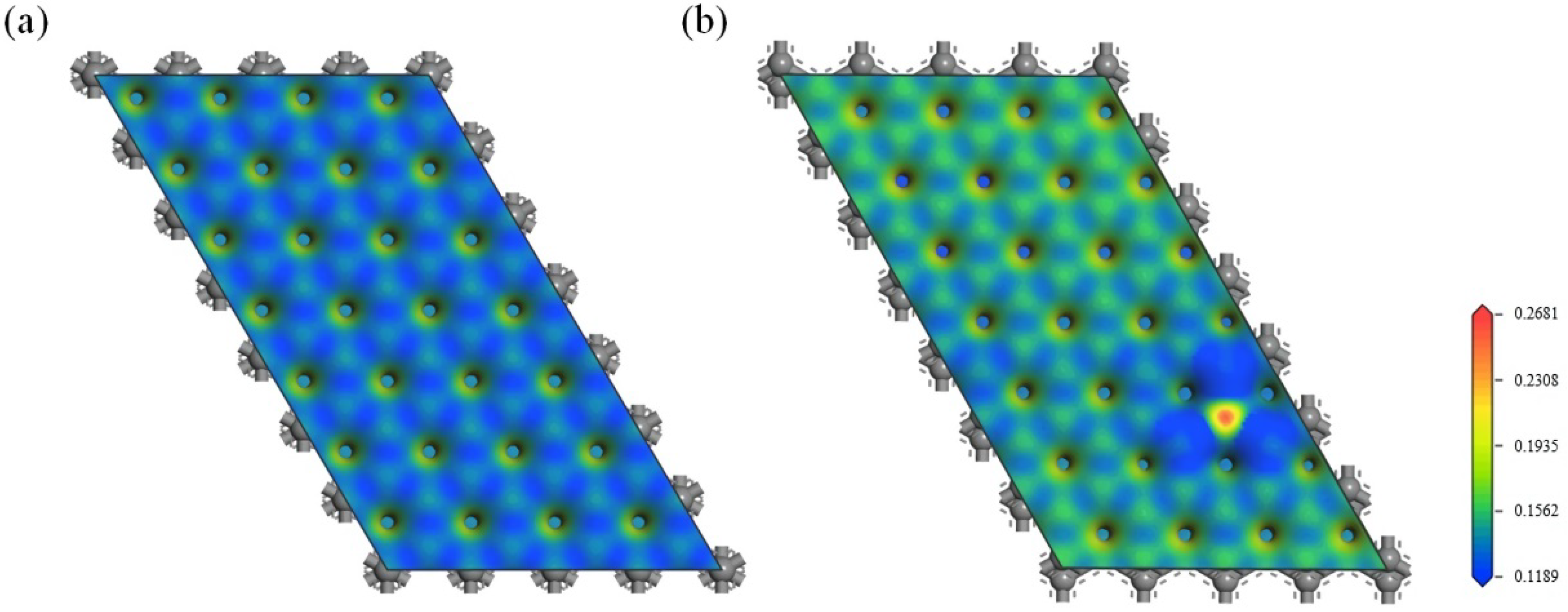
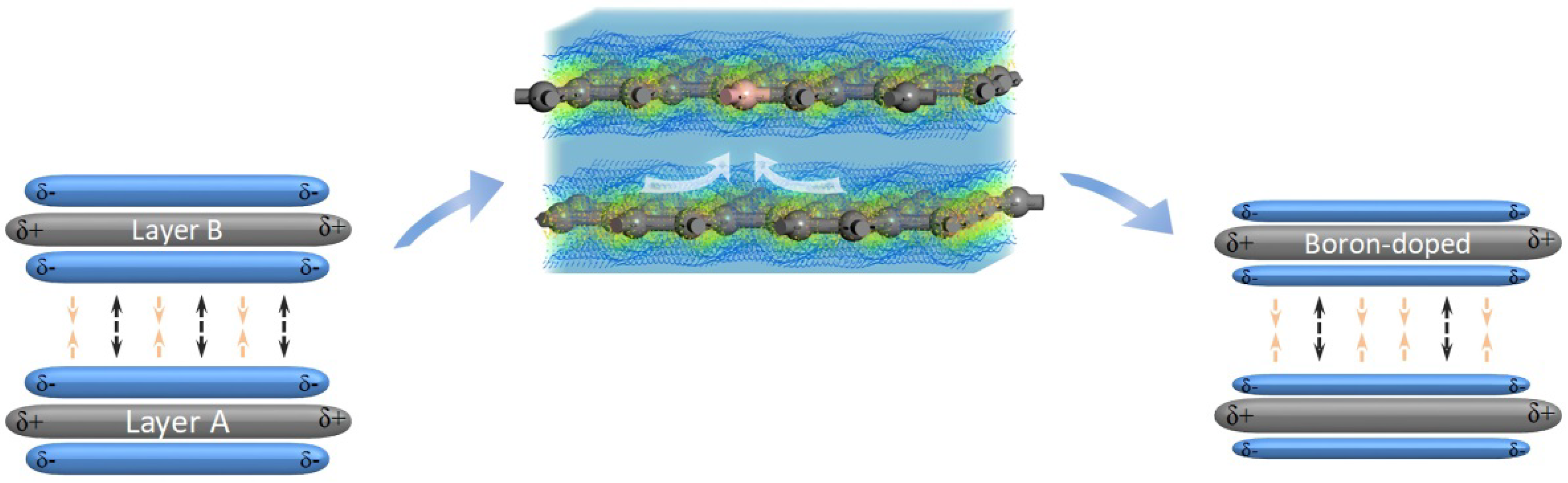
3.2.3. Effect of the Electron Redistribution Caused by Boron Doping on the Interlayer Spacing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endo, M.; Kim, C.; Karaki, T.; Nishimura, Y.; Matthews, M.J.; Brown, S.; Dresselhaus, M.S. Anode performance of a Li ion battery based on graphitized and B-doped milled mesophase pitch-based carbon fibers. Carbon 1999, 37, 561–568. [Google Scholar] [CrossRef]
- Hagio, T.; Nakamizo, M.; Kobayashi, K. Studies on X-ray diffraction and Raman spectra of B-doped natural graphite. Carbon 1989, 27, 259–263. [Google Scholar] [CrossRef]
- Liu, T.; Luo, R.Y.; Yoon, S.H.; Mochida, I. Anode performance of boron-doped graphites prepared from shot and sponge cokes. J. Power Sources 2010, 195, 1714–1719. [Google Scholar] [CrossRef]
- Chen, M.H.; Wu, G.T.; Zhu, G.M.; You, J.K.; Lin, Z.G. Characterization and electrochemical investigation of boron-doped mesocarbon microbead anode materials for lithium ion batteries. J. Solid State Electrochem. 2002, 6, 420–427. [Google Scholar] [CrossRef]
- Radovic, L.R.; Karra, M.; Skokova, K.; Thrower, P.A. The role of substitutional boron in carbon oxidation. Carbon 1998, 36, 1841–1854. [Google Scholar] [CrossRef]
- Hamada, T.; Suzuki, K.; Kohno, T.; Sugiura, T. Structure of coke powder heat-treated with boron. Carbon 2002, 40, 1203–1210. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takahashi, T.; Tamaki, T.; Endo, M. Anode performance of B-doped mesophase pitch-based carbon fibers in lithium ion secondary batteries. Tanso 1996, 1996, 89–94. [Google Scholar] [CrossRef]
- Lowell, C. Solid solution of boron in graphite. J. Am. Ceram. Soc. 1967, 50, 142–144. [Google Scholar] [CrossRef]
- Hishiyama, Y.; Irumano, H.; Kaburagi, Y.; Soneda, Y. Structure, Raman scattering, and transport properties of boron-doped graphite. Phys. Rev. B 2001, 63, 245406. [Google Scholar] [CrossRef]
- Ōya, A.; Yamashita, R.; Ōtani, S. Catalytic graphitization of carbons by borons. Fuel 1979, 58, 495–500. [Google Scholar] [CrossRef]
- Murty, H.N.; Biederman, D.L.; Heintz, E.A. Apparent catalysis of graphitization. 3. Effect of boron. Fuel 1977, 56, 305–312. [Google Scholar] [CrossRef]
- Ōya, A.; Ōtani, S. Catalytic graphitization of carbons by various metals. Carbon 1979, 17, 131–137. [Google Scholar] [CrossRef]
- Kotlensky, W.V. Effect of boron level on graphitization of BPG. Carbon 1967, 5, 409-IN17. [Google Scholar] [CrossRef]
- Hishiyama, Y.; Inagaki, M. Lattice parameter changes in graphite with boron doping. Carbon 2001, 39, 150–152. [Google Scholar] [CrossRef]
- Iwashita, N.; Chong, R.P.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Cermignani, W.; Onneby, C.; Pantano, C.; Paulson, T. Synthesis and characterization of boron-doped carbons. Carbon 1995, 33, 367–374. [Google Scholar] [CrossRef]
- Shirasaki, T.; Derré, A.; Ménétrier, M.; Tressaud, A.; Flandrois, S. Synthesis and characterization of boron-substituted carbons. Carbon 2000, 38, 1461–1467. [Google Scholar] [CrossRef]
- Wen, Y.; Lu, Y.G.; Xiao, H.; Qin, X.Y. Further investigation on boric acid catalytic graphitization of polyacrylonitrile carbon fibers: Mechanism and mechanical properties. Mater. Des. (1980–2015) 2012, 36, 728–734. [Google Scholar] [CrossRef]
- Lee, Y.; Han, D.Y.; Lee, D.; Woo, A.J.; Lee, S.H.; Lee, D.; Kim, Y.K. 11 B NMR of boron-doped graphite as the negative electrode of a lithium secondary battery. Carbon 2002, 40, 403–408. [Google Scholar] [CrossRef]
- Wang, Y.C.; Scheerschmidt, K.; Gösele, U. Theoretical investigations of bond properties in graphite and graphitic silicon. Phys. Rev. B 2000, 61, 12864. [Google Scholar] [CrossRef]
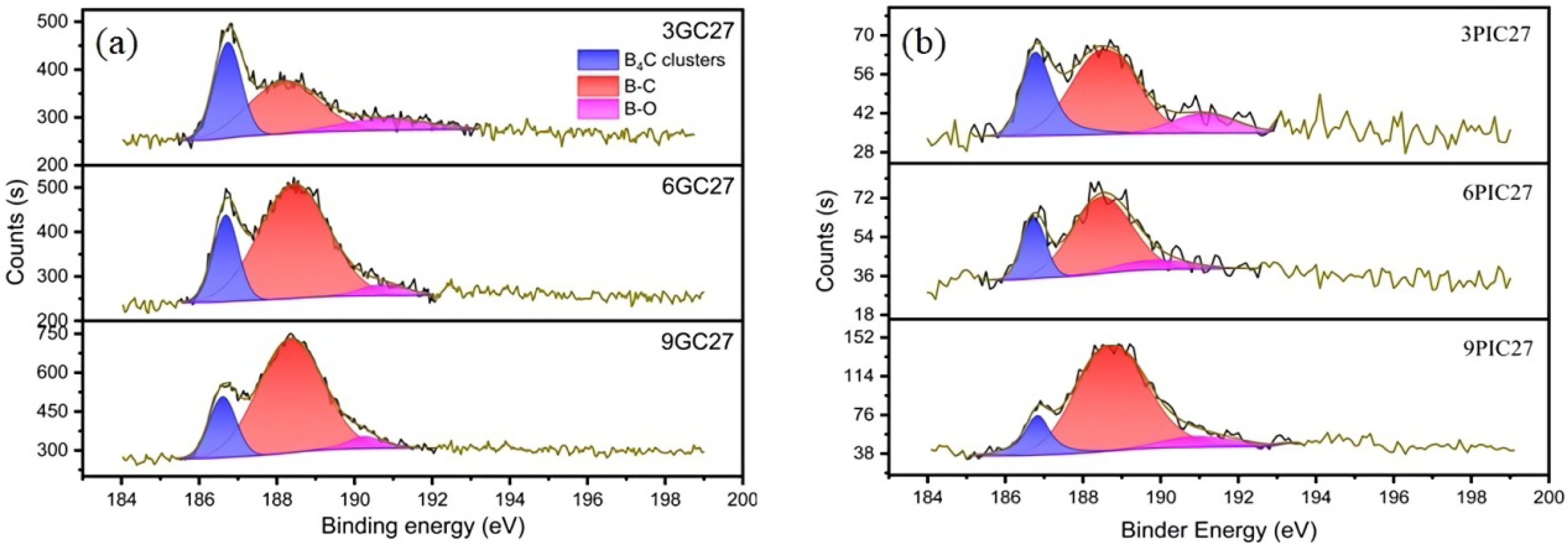
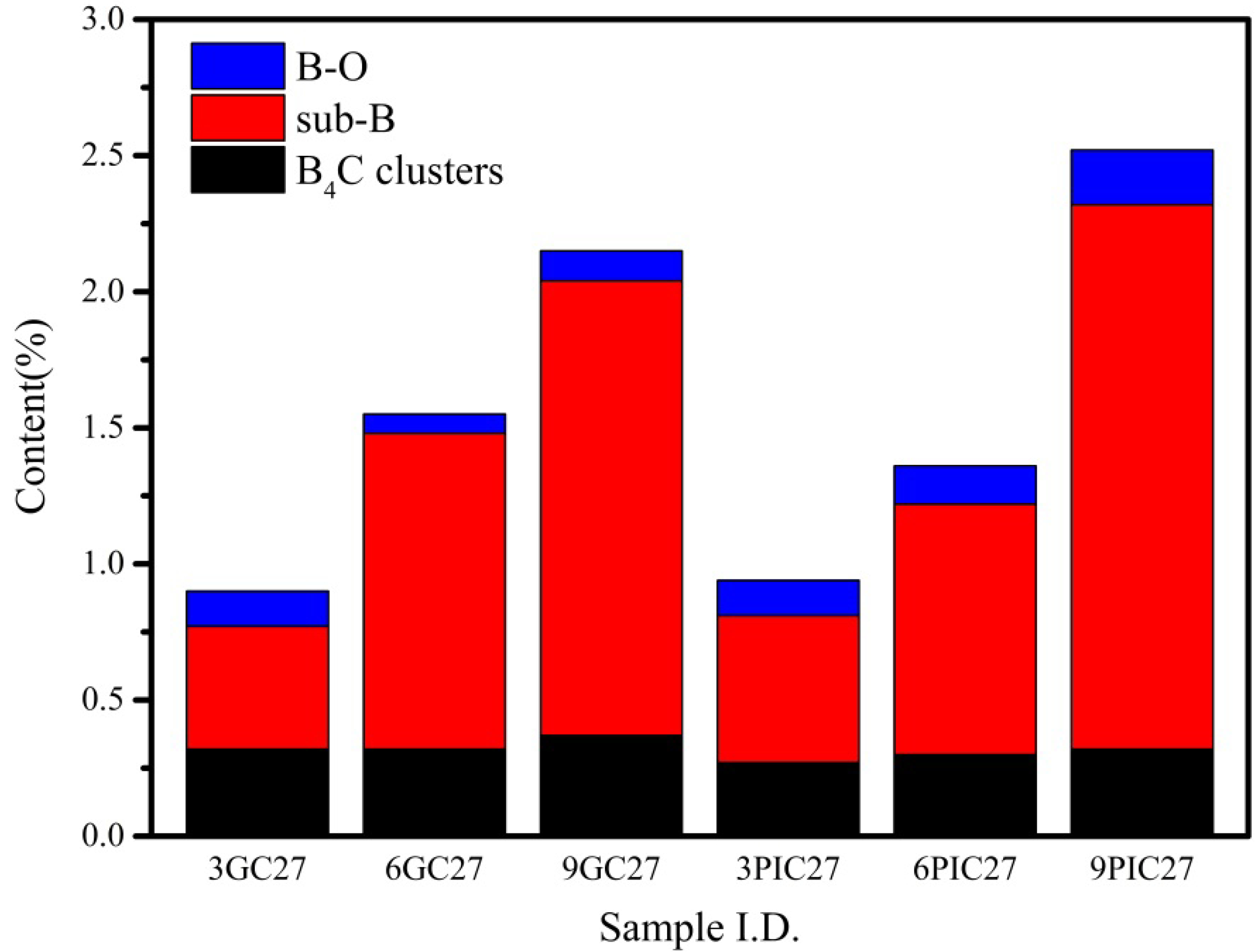
| Sample I.D. | B4C Clusters (at.%) | Sub-B (at.%) | B-O (at.%) | B (at.%) |
|---|---|---|---|---|
| GC27 | 0.32 | 0.45 | 0.13 | 0.90 |
| 6GC27 | 0.32 | 1.16 | 0.07 | 1.55 |
| 9GC27 | 0.37 | 1.67 | 0.11 | 2.15 |
| 3PIC27 | 0.27 | 0.54 | 0.13 | 0.94 |
| 6PIC27 | 0.30 | 0.92 | 0.14 | 1.36 |
| 9PIC27 | 0.32 | 2.00 | 0.20 | 2.52 |
| Sample I.D. | d002 (nm) | Sample I.D. | d002 (nm) |
|---|---|---|---|
| 0GC27 | 0.33654 | 0PIC27 | 0.33647 |
| 3GC27 | 0.33557 | 3PIC27 | 0.33553 |
| 6GC27 | 0.33547 | 6PIC27 | 0.33546 |
| 9GC27 | 0.33545 | 9PIC27 | 0.33537 |
| Sample I.D. | d002 (nm) |
|---|---|
| Pristine graphite | 0.3369 |
| Model (b) | 0.3323 |
| Sample I.D. | ||||||
|---|---|---|---|---|---|---|
| Model b | 0.2459 nm | 0.6738 nm | 0.1485 nm | 0.1412 nm | 0.0009656 | −0.0001537 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, C.; Zeng, Q.; Li, F.; Shi, L.; Wu, W.; Yang, L.; Chen, Y.; Liu, H. Effect of Boron Doping on the Interlayer Spacing of Graphite. Materials 2022, 15, 4203. https://doi.org/10.3390/ma15124203
Bao C, Zeng Q, Li F, Shi L, Wu W, Yang L, Chen Y, Liu H. Effect of Boron Doping on the Interlayer Spacing of Graphite. Materials. 2022; 15(12):4203. https://doi.org/10.3390/ma15124203
Chicago/Turabian StyleBao, Chenguang, Qing Zeng, Fujin Li, Lei Shi, Wei Wu, Li Yang, Yuxi Chen, and Hongbo Liu. 2022. "Effect of Boron Doping on the Interlayer Spacing of Graphite" Materials 15, no. 12: 4203. https://doi.org/10.3390/ma15124203
APA StyleBao, C., Zeng, Q., Li, F., Shi, L., Wu, W., Yang, L., Chen, Y., & Liu, H. (2022). Effect of Boron Doping on the Interlayer Spacing of Graphite. Materials, 15(12), 4203. https://doi.org/10.3390/ma15124203






