Gallic Acid-Functionalized, TiO2-Based Nanomaterial—Preparation, Physicochemical and Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2-GA Nanomaterial
2.2. Physicochemical Characterization of the Material
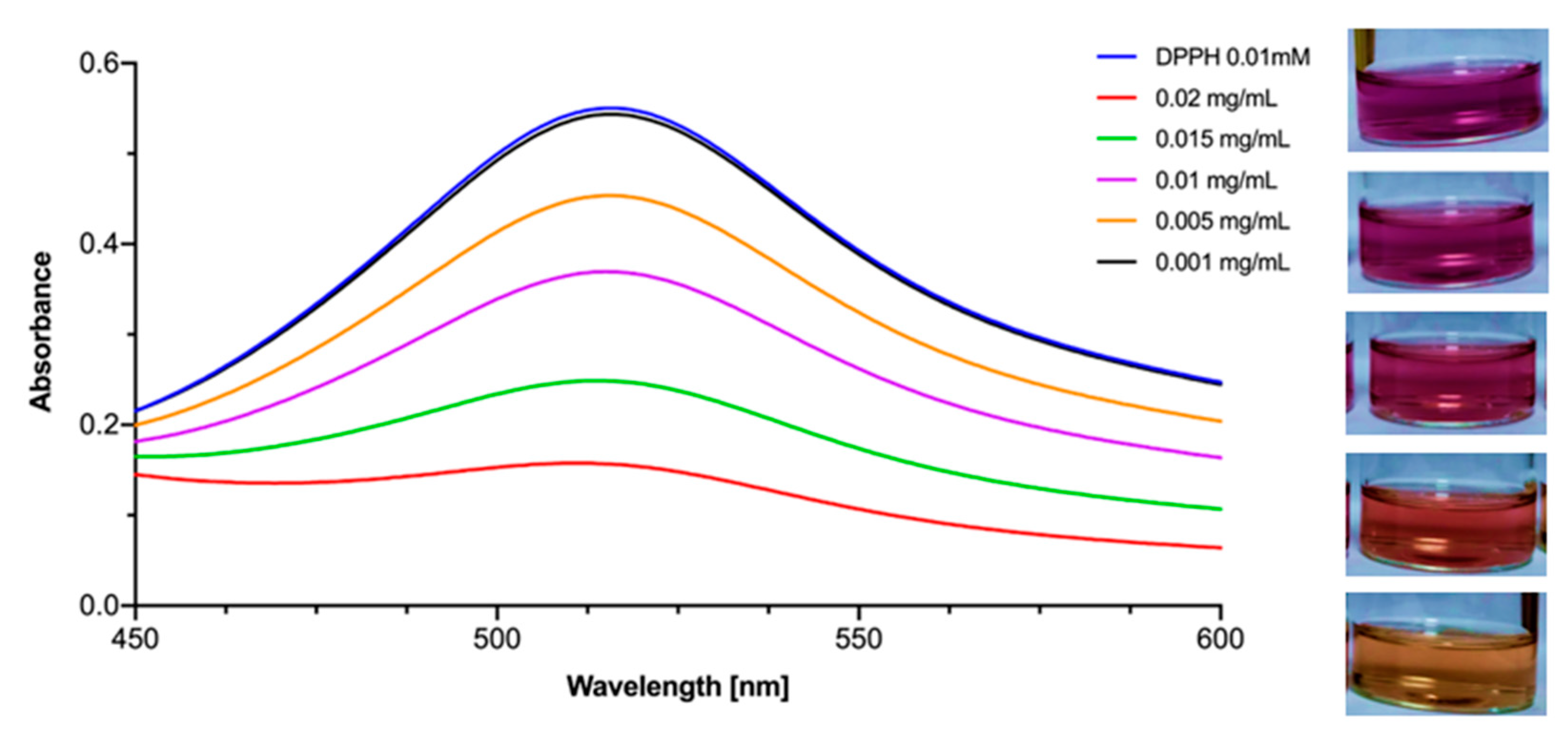
2.3. DPPH Antioxidant Assay
- I (%)—the percentage of inhibition.
- As—the absorbance of the compounds.
- Ac—the absorbance of the DPPH solution (control).
2.4. Microtox Assay
2.5. Microbial Strains and Cultivation
- N—number of CFU/mL.
- —sum of colonies on counted plates.
- V—inoculated volume [mL].
- n1—number of plates from the first calculated dilution.
- n2—number of plates from the second calculated dilution.
- d1—dilution factor corresponding to the first dilution taken into account.
2.6. Photocytotoxicity Assessment against Bacteria and Fungi
2.7. Cell Culture
2.8. MTT Assay
3. Results and Discussions
3.1. Synthesis and Characterization of TiO2-GA Nanomaterial
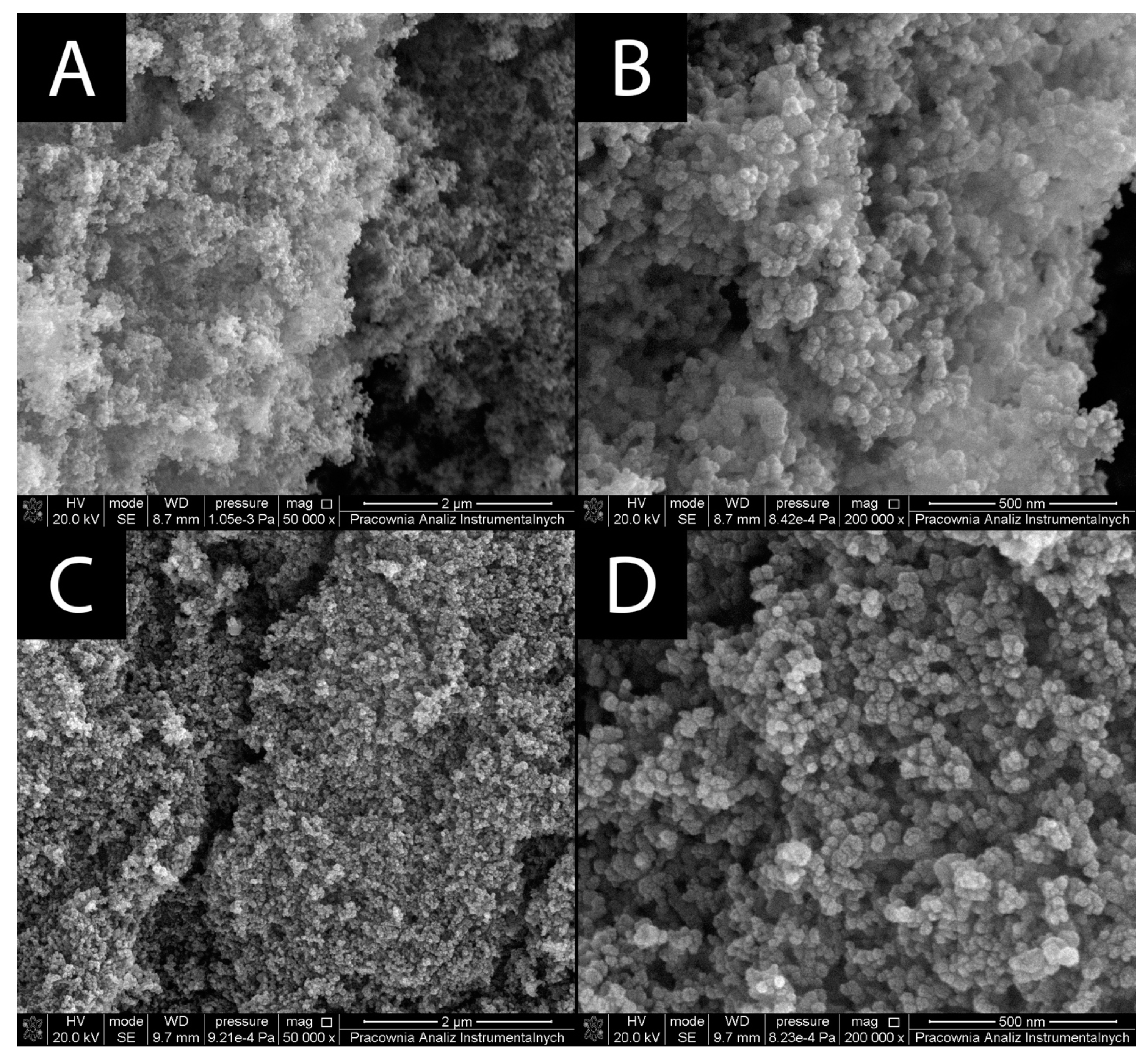
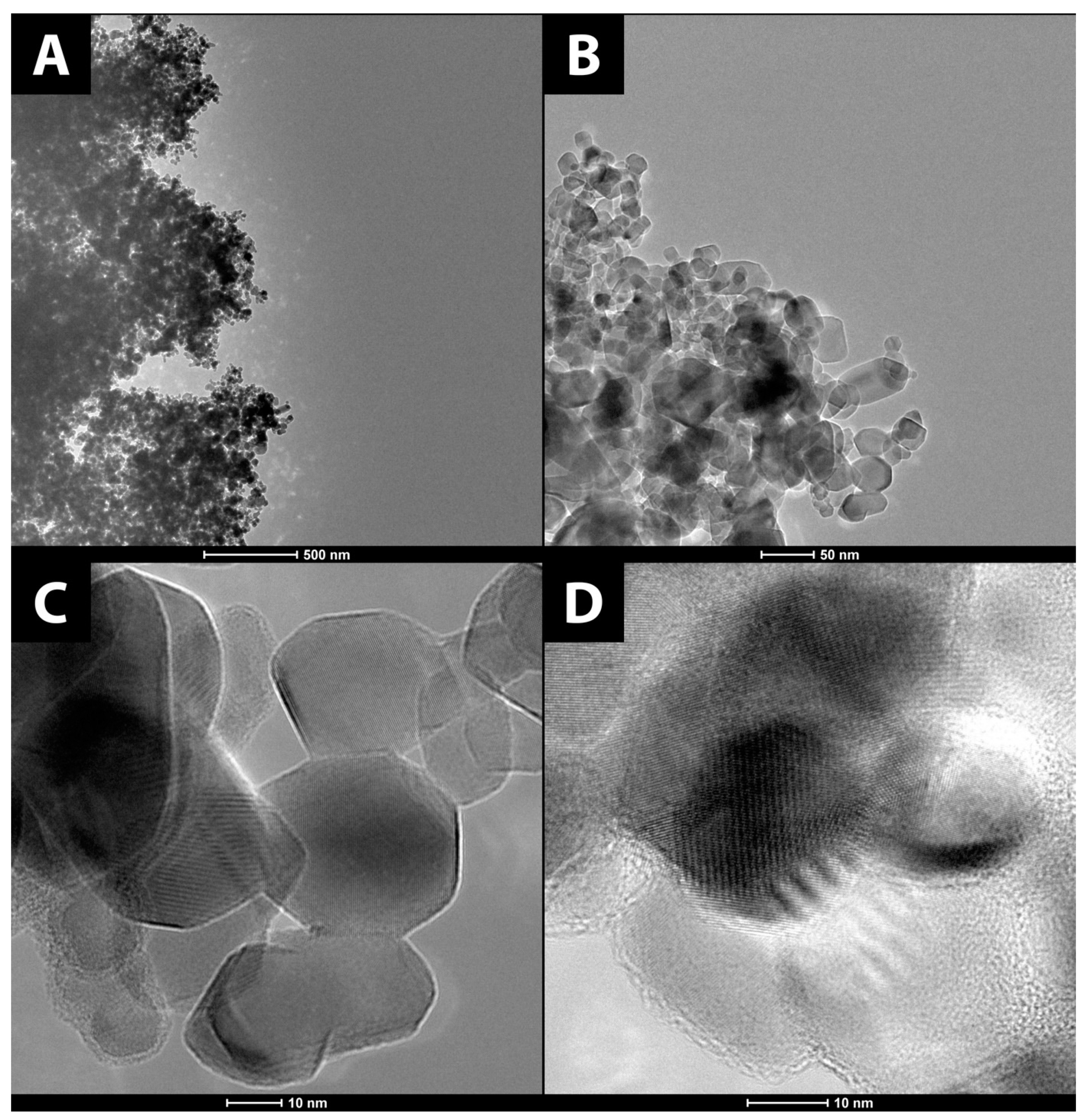
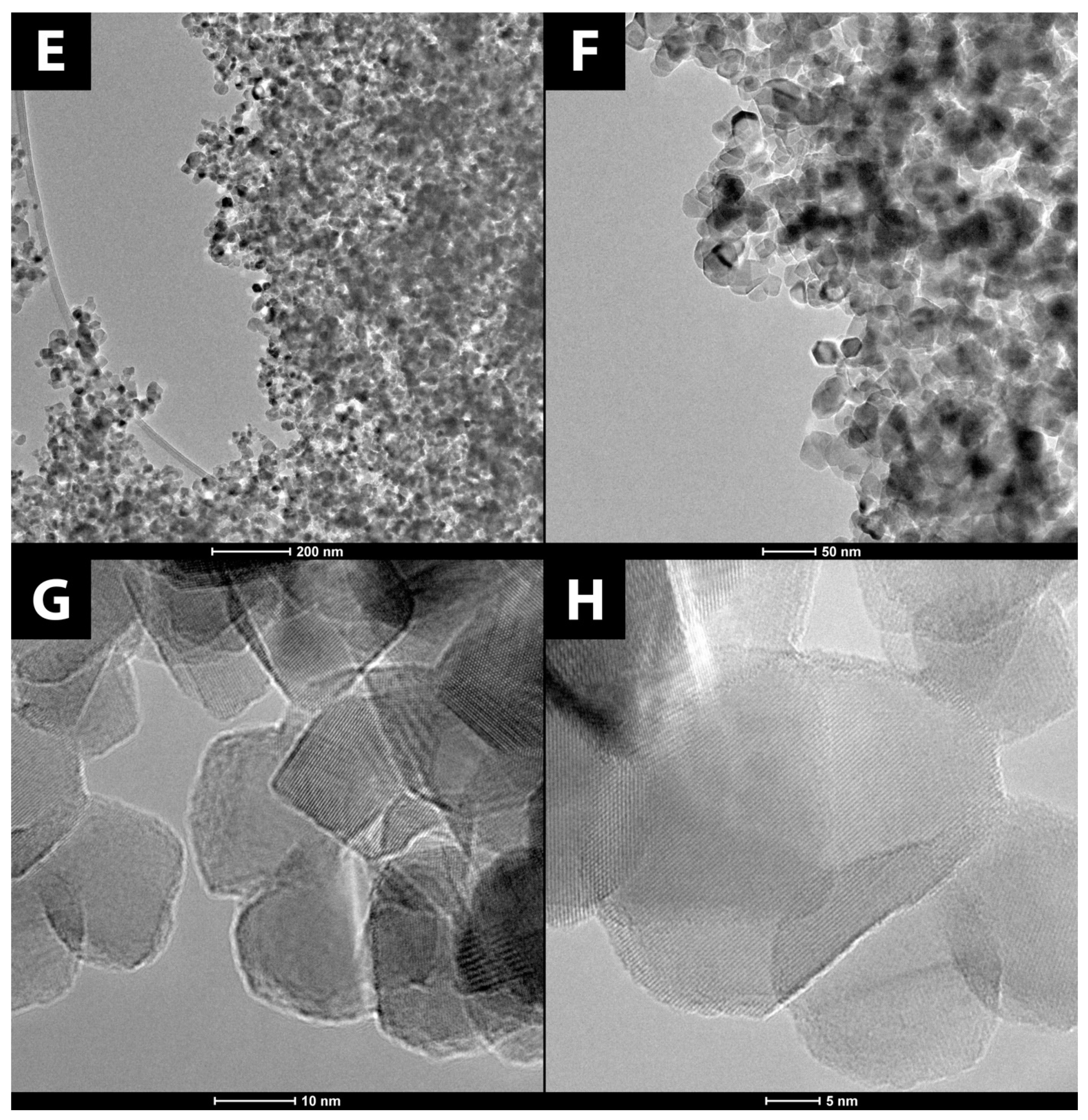
3.1.1. Surface Morphology
3.1.2. Nanoparticle Tracking Analysis
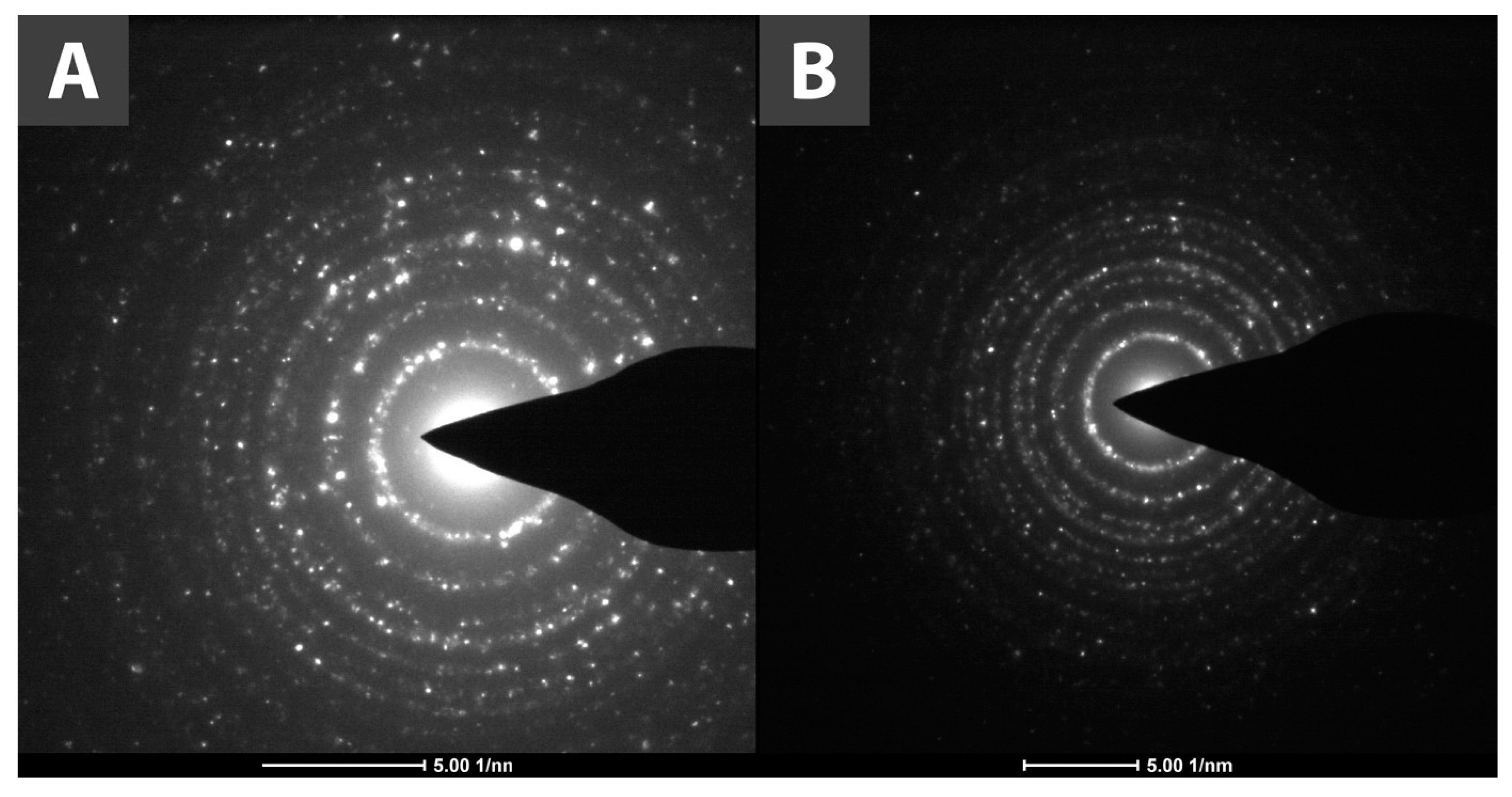
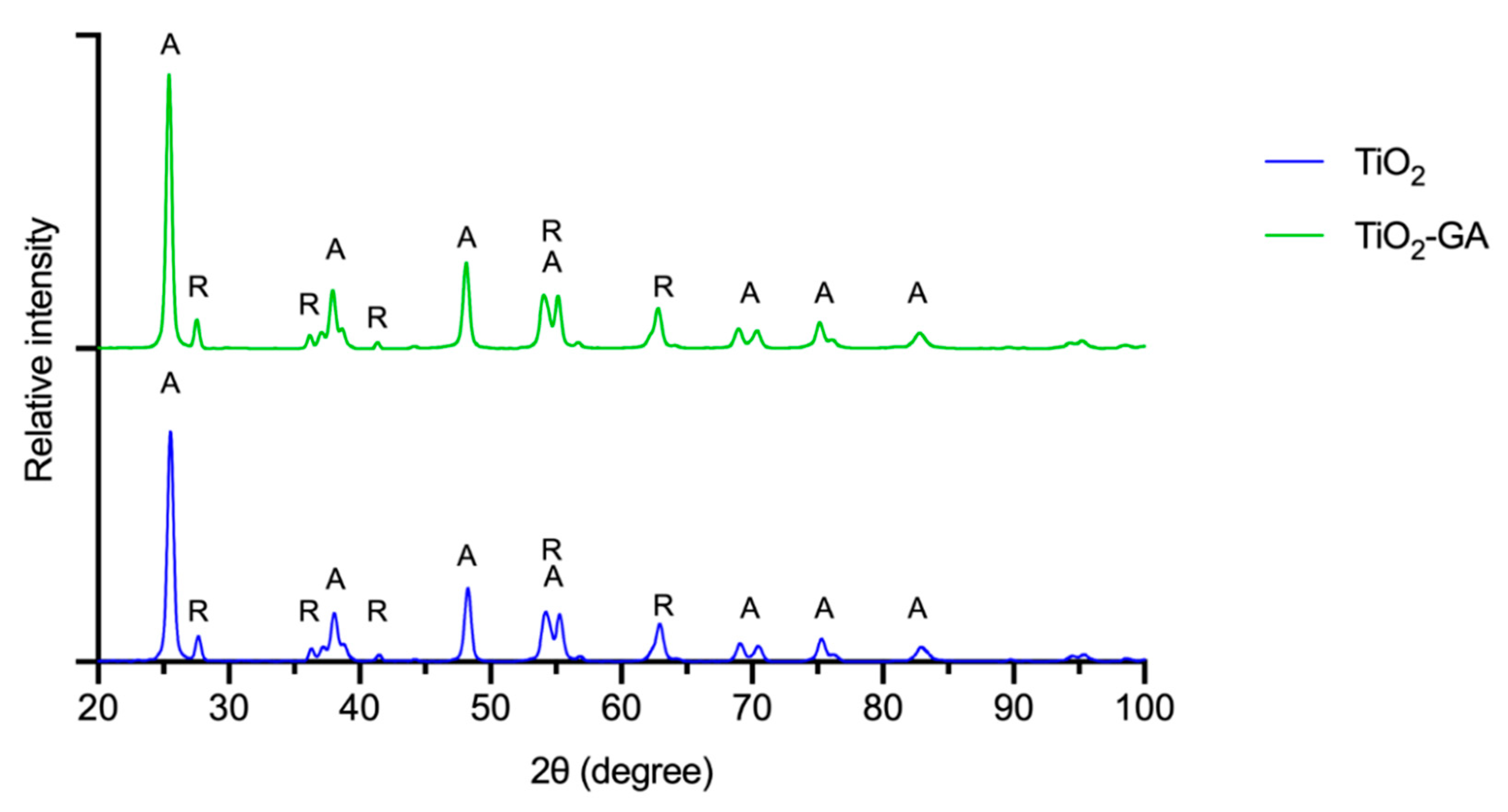
3.1.3. XRPD Analysis
3.1.4. Infrared Spectroscopy
3.1.5. Raman Spectroscopy
3.1.6. Thermogravimetric Analysis
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
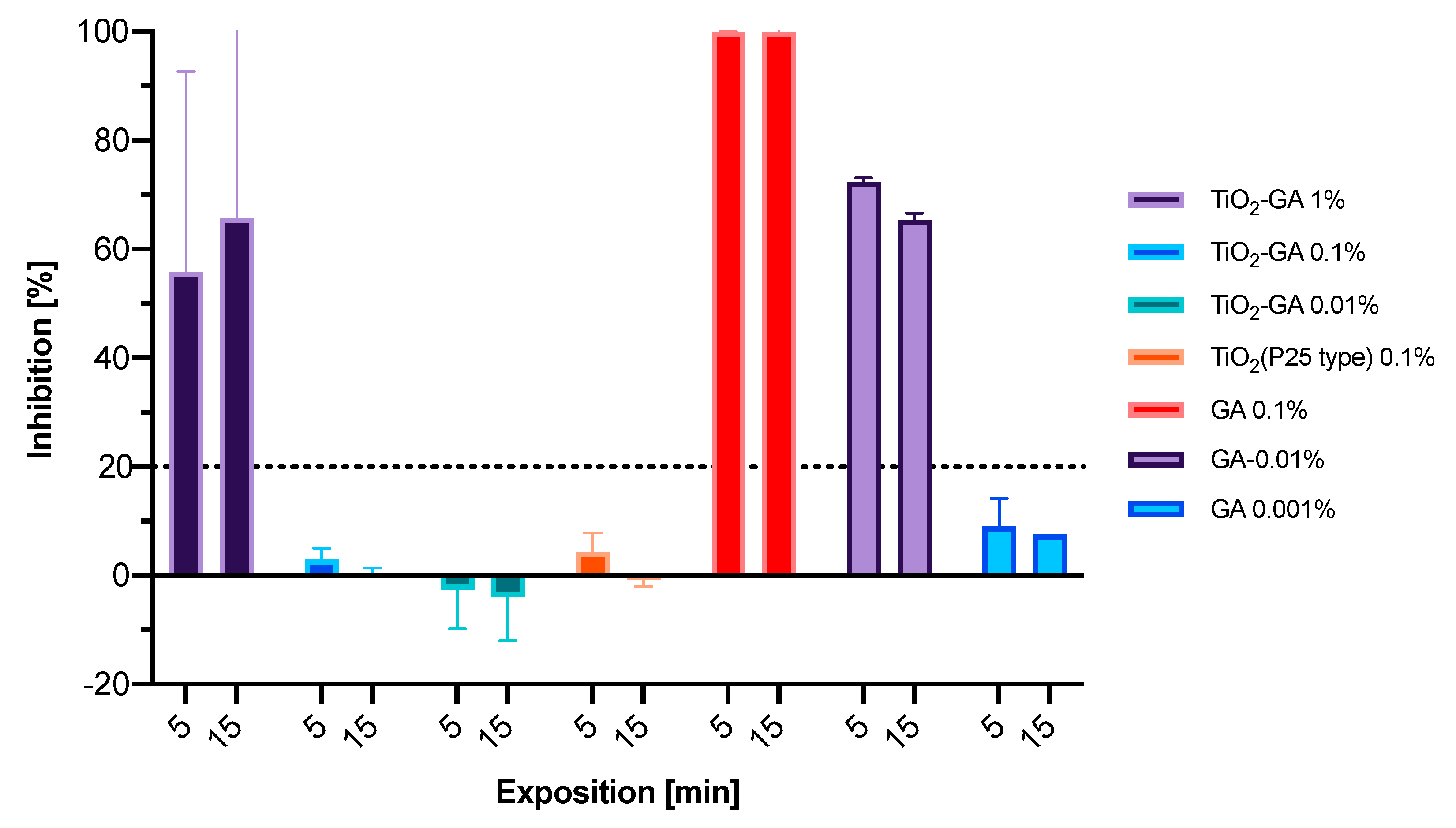
3.3.1. Microtox Acute Toxicity Evaluation
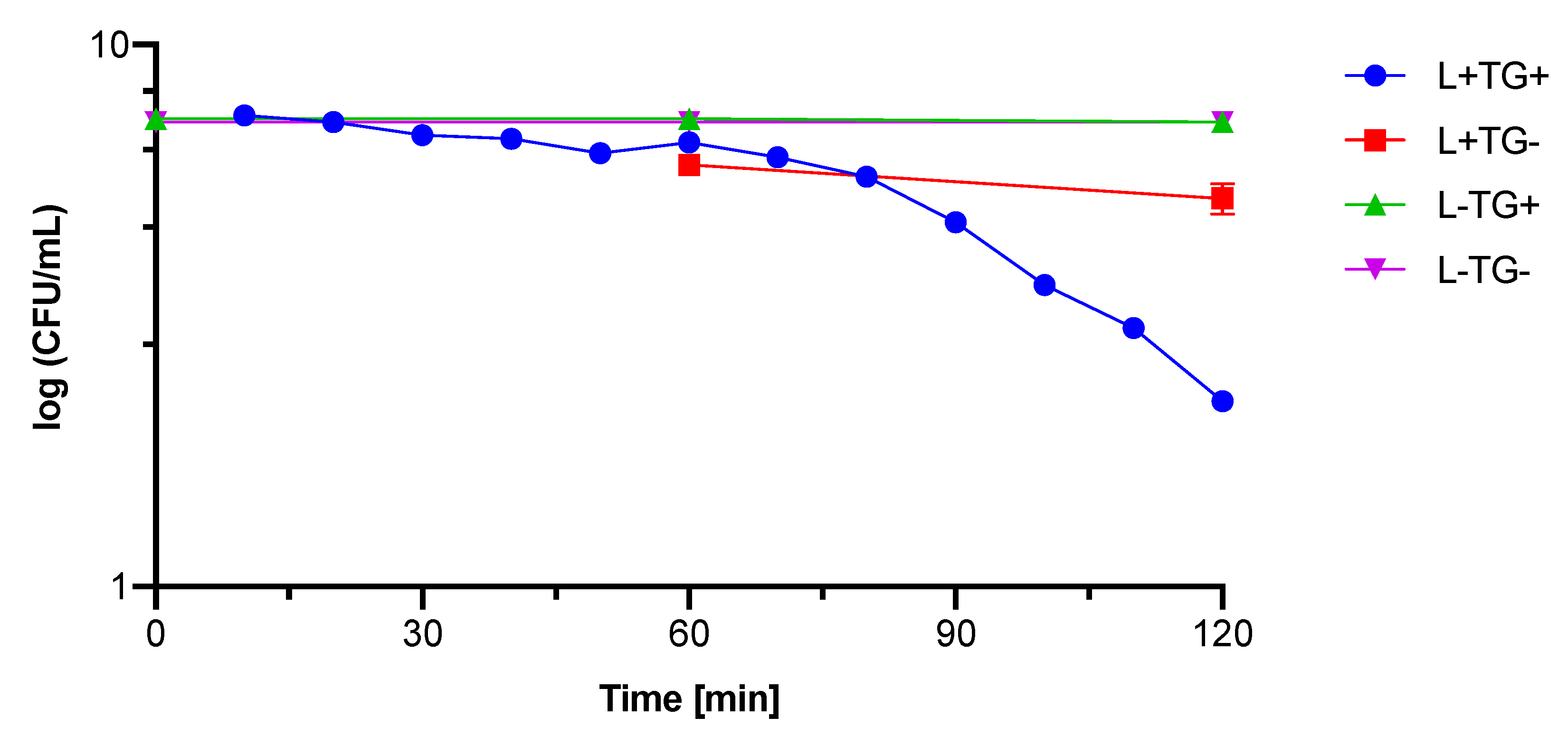
3.3.2. Photocytotoxicity against Bacteria and Fungi
3.4. Toxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broughton, G.; Janis, J.E.; Attinger, C.E. A Brief History of Wound Care. Plast. Reconstr. Surg. 2006, 117, 6S–11S. [Google Scholar] [CrossRef] [Green Version]
- Zarchi, K.; Martinussen, T.; Jemec, G.B.E. Wound Healing and All-Cause Mortality in 958 Wound Patients Treated in Home Care: Healing and Mortality in Wound Patients. Wound Rep. Reg. 2015, 23, 753–758. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1–32. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic Science of Wound Healing. Surg. Oxf. 2005, 23, 37–42. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active Silver Nanoparticles for Wound Healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.; Langemo, D.K.; Anderson, J.; Hanson, D.; Thompson, P. Hyperbaric Oxygen Therapy for Chronic Wounds. Adv. Ski. Wound Care 2010, 23, 116–119. [Google Scholar] [CrossRef]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as Wound Healing Materials. In Wound Healing Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 261–287. ISBN 978-1-78242-456-7. [Google Scholar]
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan Gum Incorporating Titanium Dioxide Nanoparticles Biofilm as Wound Dressing: Physicochemical, Mechanical, Antibacterial Properties and Wound Healing Studies. Mater. Sci. Eng. C 2019, 103, 109770. [Google Scholar] [CrossRef]
- Rouquerol, J.; Sing, K.S.W.; Llewellyn, P. Adsorption by Metal Oxides. In Adsorption by Powders and Porous Solids; Elsevier: Amsterdam, The Netherlands, 2014; pp. 393–465. ISBN 978-0-08-097035-6. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Mehrvar, M. Photocatalytic Treatment of an Actual Confectionery Wastewater Using Ag/TiO2/Fe2O3: Optimization of Photocatalytic Reactions Using Surface Response Methodology. Catalysts 2018, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Krakowiak, R.; Musial, J.; Frankowski, R.; Spychala, M.; Mielcarek, J.; Dobosz, B.; Krzyminiewski, R.; Sikorski, M.; Bendzinska-Berus, W.; Tykarska, E.; et al. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts 2020, 10, 1328. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Bakun, P.; Spychała, M.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Koczorowski, T.; Sobotta, L.; Stanisz, B.; Goslinski, T. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants Including Pharmaceutical Pollutants. Appl. Sci. 2021, 11, 8674. [Google Scholar] [CrossRef]
- Zhuang, H.-F.; Lin, C.-J.; Lai, Y.-K.; Sun, L.; Li, J. Some Critical Structure Factors of Titanium Oxide Nanotube Array in Its Photocatalytic Activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Wiesenthal, A.; Hunter, L.; Wang, S.; Wickliffe, J.; Wilkerson, M. Nanoparticles: Small and Mighty: Nanoparticles. Int. J. Dermatol. 2011, 50, 247–254. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic Antibacterial Performance of TiO2 and Ag-Doped TiO2 against S. Aureus. P. Aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial Properties of TiO2 Modified with Reduced Graphene Oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Wang, Q.; de Oliveira, E.F.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On Mechanism behind UV-A Light Enhanced Antibacterial Activity of Gallic Acid and Propyl Gallate against Escherichia coli O157:H7. Sci. Rep. 2017, 7, 8325. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Moh, S.; Son, D.; You, S.; Kinyua, A.; Ko, C.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-Y.; Kim, M.; Shinde, S.; Sung, J.-S.; Ghodake, G. Cytotoxicity and Antibacterial Assessment of Gallic Acid Capped Gold Nanoparticles. Colloids Surf. B Biointerfaces 2017, 149, 162–167. [Google Scholar] [CrossRef]
- Shah, S.T.; A Yehya, W.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.; Al-Ani, L. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Długaszewska, J.; Antczak, M.; Kaczmarek, I.; Jankowiak, R.; Buszkiewicz, M.; Herkowiak, M.; Michalak, K.; Kukuła, H.; Ratajczak, M. In Vitro Biofilm Formation and Antibiotic Susceptibility of Pseudomonas Aeruginosa Isolated from Airways of Patients with Cystic Fibrosis. JMS 2016, 85, 245–253. [Google Scholar] [CrossRef]
- Birinci, Y.; Niazi, J.H.; Aktay-Çetin, O.; Basaga, H. Quercetin in the Form of a Nano-Antioxidant (QTiO2) Provides Stabilization of Quercetin and Maximizes Its Antioxidant Capacity in the Mouse Fibroblast Model. Enzym. Microb. Technol. 2020, 138, 109559. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Tu, K.-J.; Deng, J.-P.; Lo, Y.-S.; Wu, C.-H. Markedly Enhanced Surface Hydroxyl Groups of TiO2 Nanoparticles with Superior Water-Dispersibility for Photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodríguez-González, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser-Assisted Production of Spherical TiO2 Nanoparticles in Water. Nanotechnology 2011, 22, 195606. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The Effect of Calcination Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of Structural Transformations in Nanophase Titanium Dioxide by Raman Spectroscopy. Appl. Phys. A Mater. Sci. Process. 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Huguenin, J.; Ould Saad Hamady, S.; Bourson, P. Monitoring Deprotonation of Gallic Acid by Raman Spectroscopy: Monitoring Deprotonation of Gallic Acid by Raman Spectroscopy. J. Raman Spectrosc. 2015, 46, 1062–1066. [Google Scholar] [CrossRef]
- Li, X.W.; Song, R.G.; Jiang, Y.; Wang, C.; Jiang, D. Surface Modification of TiO2 Nanoparticles and Its Effect on the Properties of Fluoropolymer/TiO2 Nanocomposite Coatings. Appl. Surf. Sci. 2013, 276, 761–768. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Bulich, A.A.; Isenberg, D.L. Microtox * Toxicity Test Systems—Where They Stand Today. In Microscale Testing in Aquatic Toxicology; Wells, P.G., Lee, K., Blaise, C., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 185–199. ISBN 978-0-203-74719-3. [Google Scholar]
- Cai, M.; Sugumaran, M.; Robinson, W.E. The Crosslinking and Antimicrobial Properties of Tunichrome. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 110–117. [Google Scholar] [CrossRef]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New Bioactive Fungal Molecules with High Antioxidant and Antimicrobial Capacity Isolated from Cerrena Unicolor Idiophasic Cultures. BioMed. Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okrągła, E.; Chraniuk, M.; Wolska, L. Microtox Test as a Tool to Assass Antimicrobial Properties of Herbal Infusions Used in Urinary Tract Infections. Acta Pol. Pharm. Drug Res. 2017, 74, 895–901. [Google Scholar]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. IJN 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guo, Q.; Wang, C.; Ma, X.; He, H.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. Titanium Dioxide Nanoparticles Increase Plasma Glucose via Reactive Oxygen Species-Induced Insulin Resistance in Mice: TiO2 Nanoparticle Increases Plasma Glucose. J. Appl. Toxicol. 2015, 35, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium Dioxide Nanoparticles Inhibit Proliferation and Induce Morphological Changes and Apoptosis in Glial Cells. Toxicology 2012, 302, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, M.; Anjaneyulu, U.; Manivasagam, G.; Vijayalakshmi, U. Preparation and Evaluation of the Cytotoxic Nature of TiO2 Nanoparticles by Direct Contact Method. Int. J. Nanomed. 2015, 10, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef]
- Muller, G.; Kramer, A. Biocompatibility Index of Antiseptic Agents by Parallel Assessment of Antimicrobial Activity and Cellular Cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of Blue Light Irradiation on Human Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef]
- Sha, B.; Gao, W.; Wang, S.; Xu, F.; Lu, T. Cytotoxicity of Titanium Dioxide Nanoparticles Differs in Four Liver Cells from Human and Rat. Compos. Part B Eng. 2011, 42, 2136–2144. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, B.; Ekdahl, K.N.; Vinogradov, V.V.; Kessler, V.G. Dispersion of TiO2 Nanoparticles Improves Burn Wound Healing and Tissue Regeneration through Specific Interaction with Blood Serum Proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, K.; Ruan, M.; Yang, J.; Gao, Z. Gallic Acid Inhibits Fibroblast Growth and Migration in Keloids through the AKT/ERK Signaling Pathway. Acta Biochim. Biophys. Sin. 2018, 50, 1114–1120. [Google Scholar] [CrossRef] [PubMed]



| L− T+ | L− TG+ | L− PS− | L+ T+ | L+ TG+ | L+ PS− | |
|---|---|---|---|---|---|---|
| 107 CFU/mL microorganisms | 150 µL | 150 µL | 150 µL | 150 µL | 150 µL | 150 µL |
| 0.9% NaCl | 150 µL | 150 µL | ||||
| 2 mg/mL TiO2-GA (TG) | 150 µL | 150 µL | ||||
| 2 mg/mL TiO2 (T) | 150 µL | 150 µL |
| L− TG+ | L− PS– | L+ TG+ | L+ PS− | |
|---|---|---|---|---|
| Time points, min | 0, 60, 120 | 0, 60, 120 | 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120 | 60, 120 |
| 107 CFU/mL microorganisms | 150 µL | 150 µL | 150 µL | 150 µL |
| 0.9% NaCl | 150 µL | 150 µL | ||
| 2 mg/mL TiO2-GA (TG) | 150 µL | 150 µL |
| Name | Mean [nm] | SD | PDI | SBET [m2/g] |
|---|---|---|---|---|
| TiO2 | 255.7 | 74.5 | 0.085 | 57 |
| TiO2-GA | 218.0 | 74.9 | 0.118 | 61 |
| First Stage | Second Stage | Third Stage | Fourth Stage | Residue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tp (°C) | Tk (°C) | Δm (%) | Tp (°C) | Tk (°C) | Δm (%) | Tp (°C) | Tk (°C) | Δm (%) | Tp (°C) | Tk (°C) | Δm (%) | (%) | |
| TiO2 | 29 | 222 | 1.04 | 389 | 800 | −0.89 | 99.85 | ||||||
| GA | 73 | 100 | 3.02 | 248 | 301 | 61.45 | 301 | 357 | 12.75 | 357 | 597 | 22.23 | 0.55 |
| TiO2-GA | 29 | 205 | 0.92 | 205 | 398 | 2.15 | 457 | 800 | −0.51 | 97.44 | |||
| Sample (Compound/Material) | IC50 ± S.E.M. (mg/L) |
|---|---|
| TiO2 | >1000 |
| TiO2-GA | 13.5 ± 0.3 |
| GA | 0.910 ± 0.002 |
| Vitamin C | 2.12 ± 0.03 |
| EGCG | 1.027 ± 0.003 |
| Curcumin | 4.43 ± 0.02 |
| Light | 365 nm | 425 nm | ||
|---|---|---|---|---|
| Nanomaterial | TiO2 | TiO2-GA | TiO2 | TiO2-GA |
| C. albicans | no effect | |||
| E. coli | 1.3 log | no effect | 2.1 log | 4.1 log |
| S. aureus | 3.1 log | no effect | 1.4 log | 4.2 log |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakun, P.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Musielak, M.; Mylkie, K.; Dlugaszewska, J.; Koczorowski, T.; Suchorska, W.M.; Ziegler-Borowska, M.; Goslinski, T.; et al. Gallic Acid-Functionalized, TiO2-Based Nanomaterial—Preparation, Physicochemical and Biological Properties. Materials 2022, 15, 4177. https://doi.org/10.3390/ma15124177
Bakun P, Czarczynska-Goslinska B, Mlynarczyk DT, Musielak M, Mylkie K, Dlugaszewska J, Koczorowski T, Suchorska WM, Ziegler-Borowska M, Goslinski T, et al. Gallic Acid-Functionalized, TiO2-Based Nanomaterial—Preparation, Physicochemical and Biological Properties. Materials. 2022; 15(12):4177. https://doi.org/10.3390/ma15124177
Chicago/Turabian StyleBakun, Pawel, Beata Czarczynska-Goslinska, Dariusz T. Mlynarczyk, Marika Musielak, Kinga Mylkie, Jolanta Dlugaszewska, Tomasz Koczorowski, Wiktoria M. Suchorska, Marta Ziegler-Borowska, Tomasz Goslinski, and et al. 2022. "Gallic Acid-Functionalized, TiO2-Based Nanomaterial—Preparation, Physicochemical and Biological Properties" Materials 15, no. 12: 4177. https://doi.org/10.3390/ma15124177
APA StyleBakun, P., Czarczynska-Goslinska, B., Mlynarczyk, D. T., Musielak, M., Mylkie, K., Dlugaszewska, J., Koczorowski, T., Suchorska, W. M., Ziegler-Borowska, M., Goslinski, T., & Krakowiak, R. (2022). Gallic Acid-Functionalized, TiO2-Based Nanomaterial—Preparation, Physicochemical and Biological Properties. Materials, 15(12), 4177. https://doi.org/10.3390/ma15124177







