Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials
Abstract
:1. Introduction
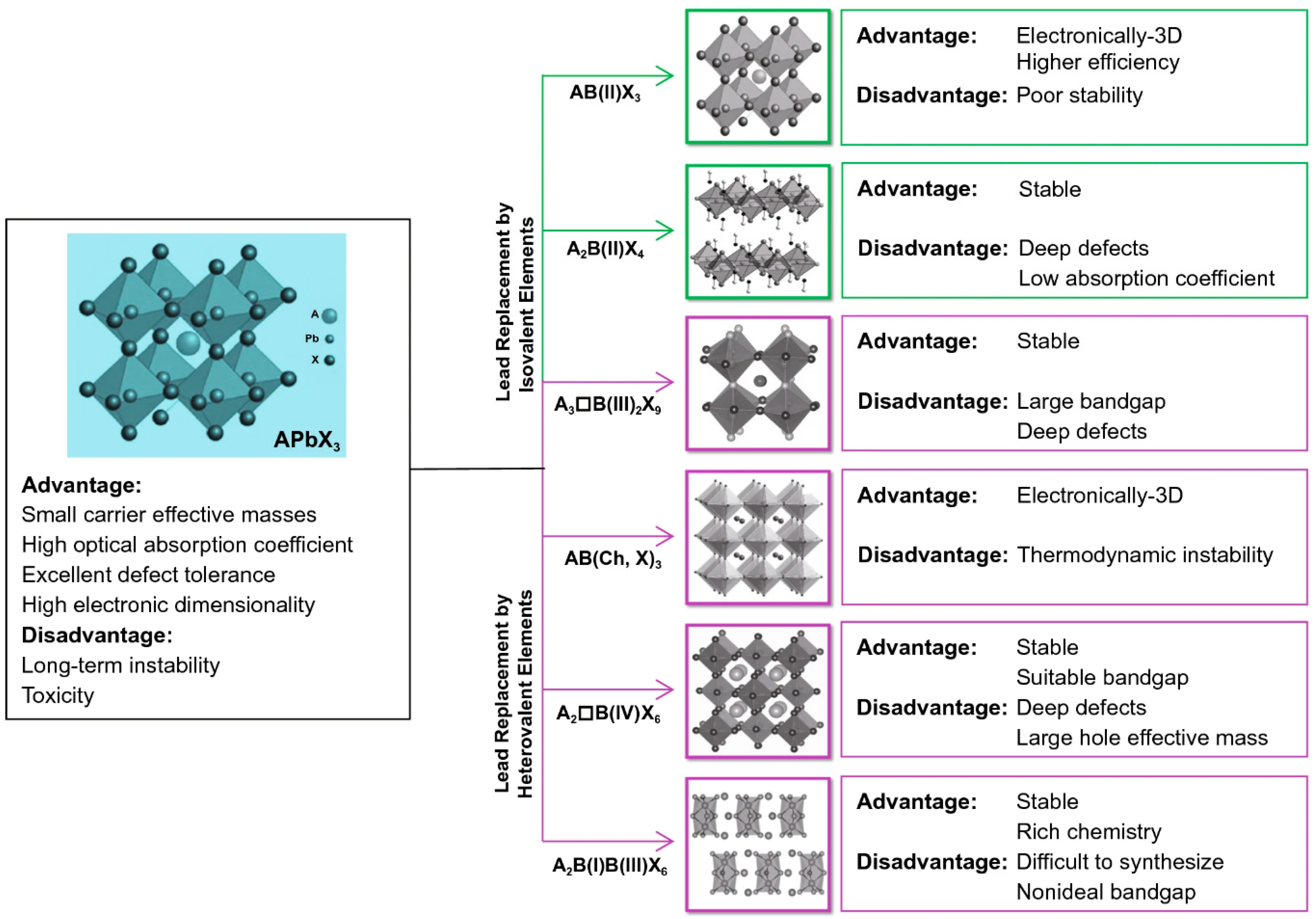
2. Lead-Free Halide Perovskites: Structure, Stability, and Characteristics
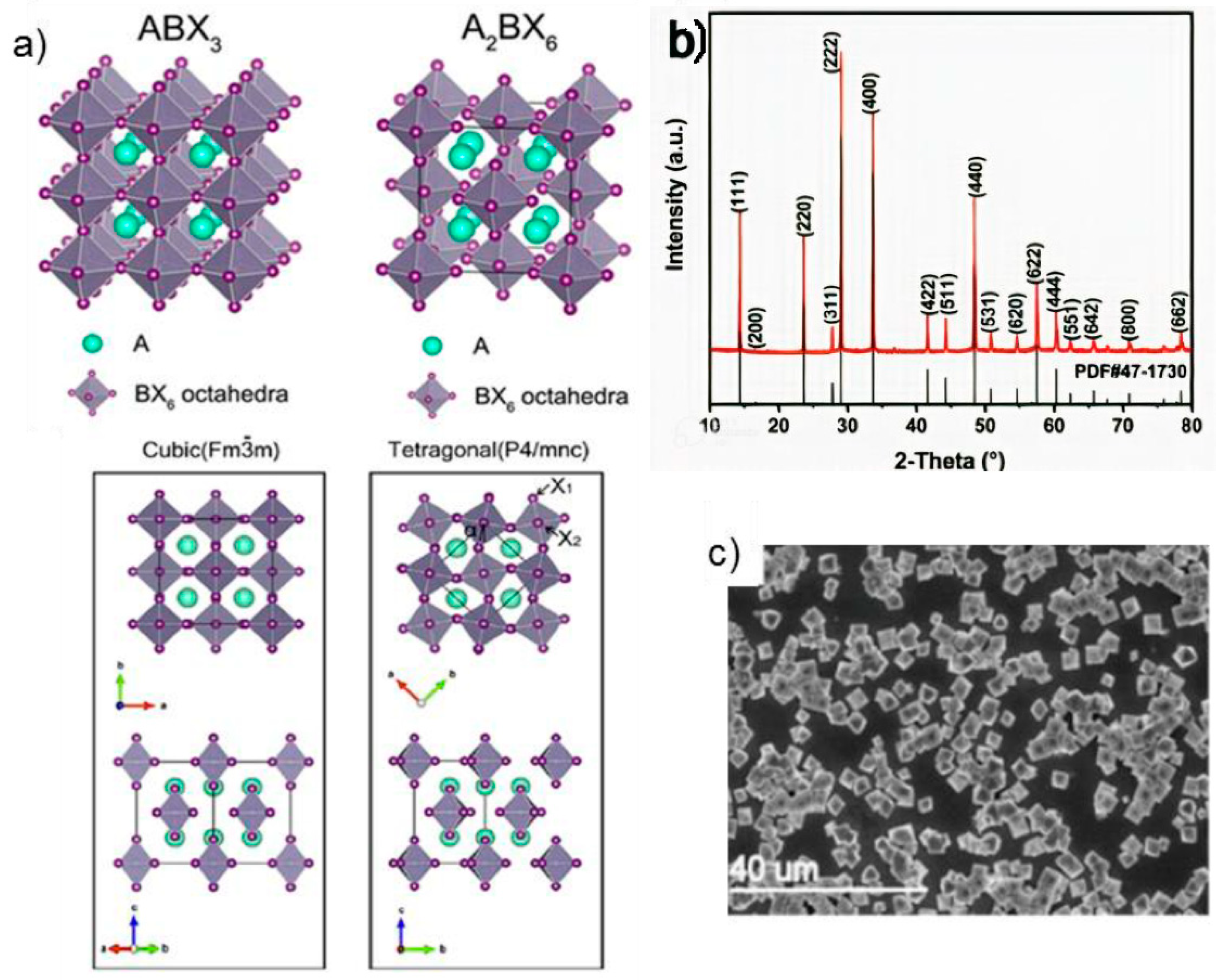
2.1. Vacancy Ordered and Double Perovskite Compounds
2.2. A2InX5∙H2O Perovskite Compounds
2.3. Factors That Make Lead-Free Inorganic Perovskite Materials Suitable for Humidity Sensing
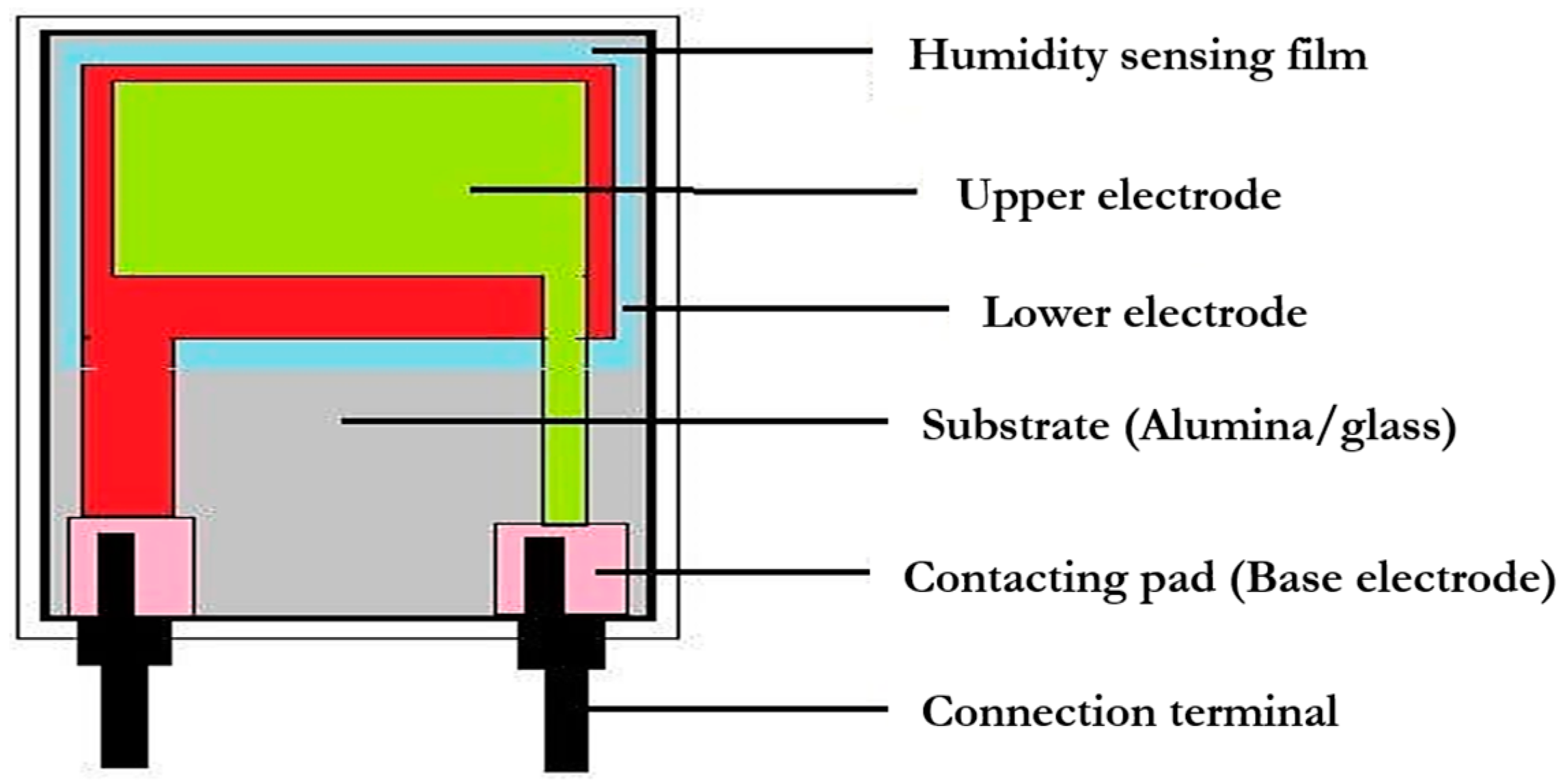
3. Applications
3.1. Humidity measurement
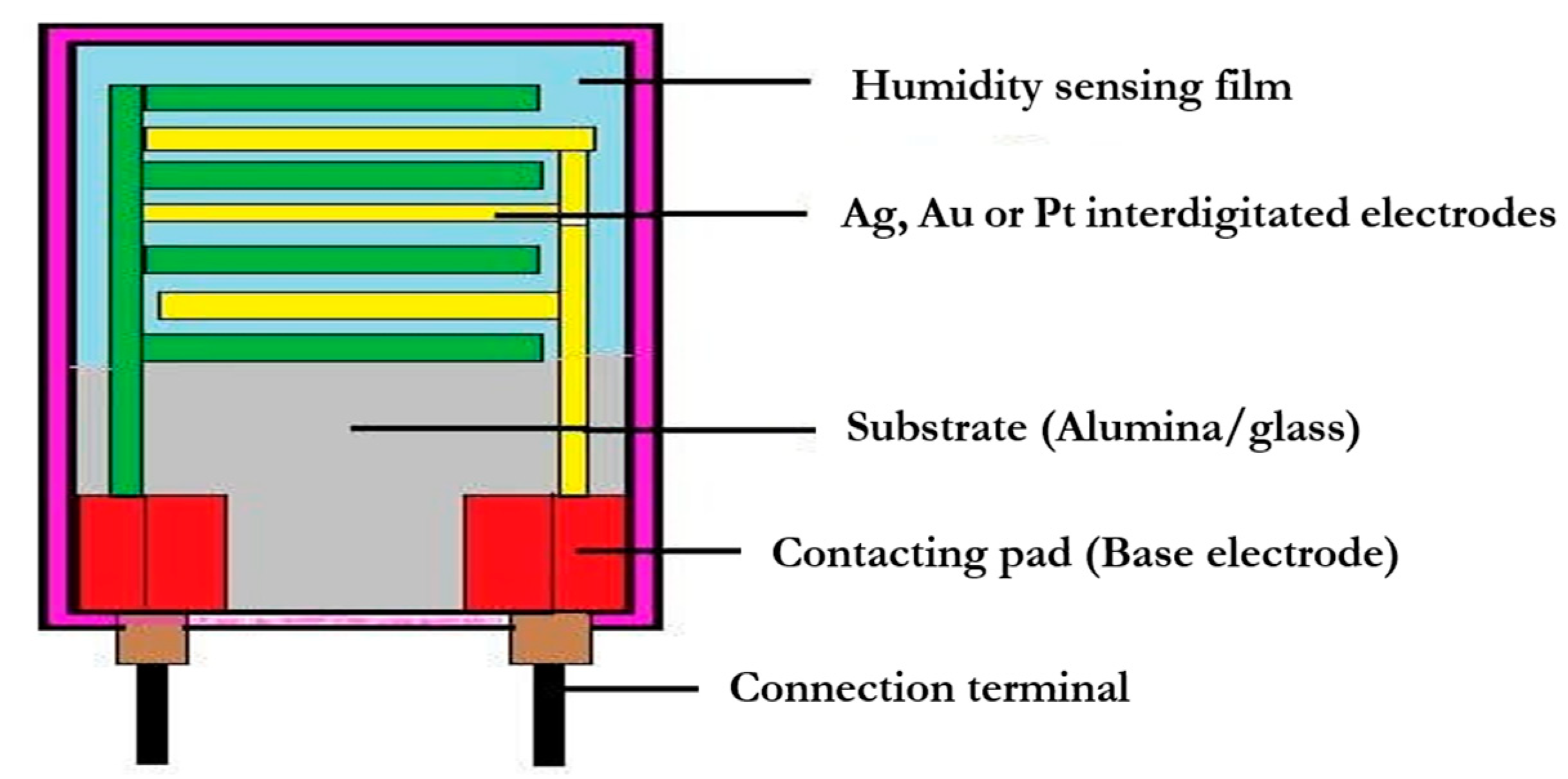
3.2. Classification of Humidity Sensors
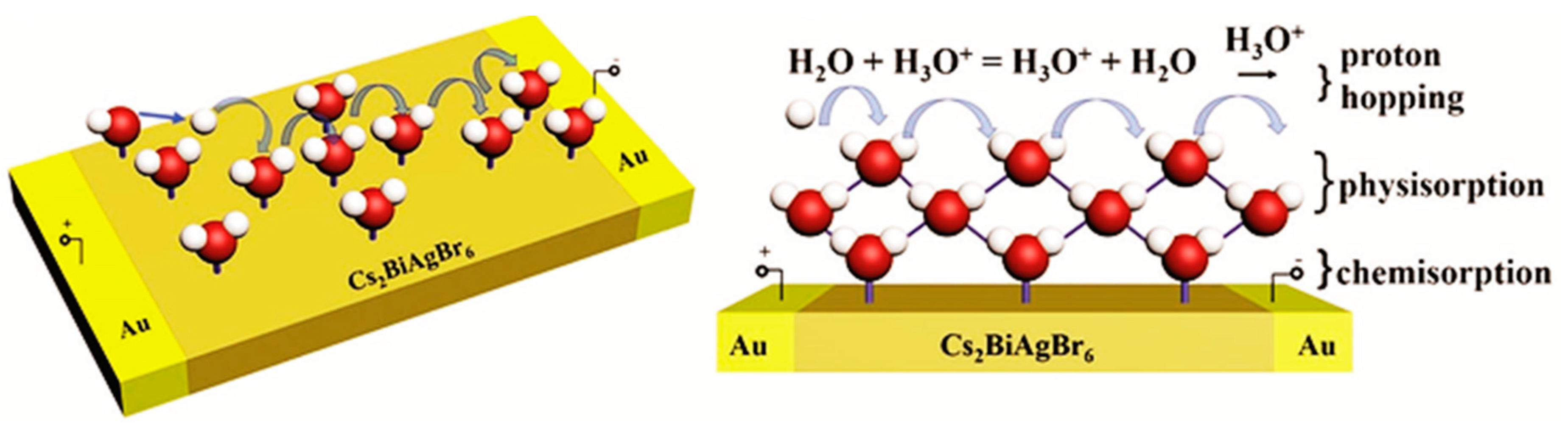
3.3. Humidity Sensing Mechanism of Perovskites
3.4. Sensing Applications of Lead-Free Halide Perovskites
| Compounds | Sensor Type | Coating Method | Morphology | Humidity Range (%) | Response and Recovery Time (tres/trec) (s) | Reference |
|---|---|---|---|---|---|---|
| ITO/alumina (4 cm2) | Capacitive | Screen-printing | Thin film | 11–95 | 21.4/4.8 | [6] |
| LiCl/ZnO | Capacitive | Screen-printing | Thin film | 11–95 | 3/6 | [92] |
| GO | Capacitive | Sputtering | Thin film | 11–97 | 15/2.5 | [93] |
| NFC/GO/PDMS 1 | Capacitive | Drop-coating Freeze drying | Thin Film | 11–97 | 57/2 | [8] |
| CaTiO3 | Capacitive | Solid-state step sintering | NPs | 33–95 | 14.5/34.27 | [94] |
| PMDS/PPDS 2 | Resistive | Drop casting | Thin film | 33–95 | 0.29/0.47 | [10] |
| PSDA-b-PEG 3 | Resistive | Electropolymerization | Thin film | 0–95 | 120/180 | [95] |
| CsPbBr3 | Resistive | Dip-coating | Thin film | 30–95 | 2 or 3/not measured | [96] |
| PbTiO3 | Resistive | Screen-printing | NPs | 80–95 | ------------ | [97] |
| ZnSnO3 | Resistive | Spin-coating | Thin film | 11–97 | 7/16 | [98] |
| NaTaO3 | Resistive | Doctor-blading | Thin film | 33–95 | 3/32 | [99] |
| Cs2PdBr6 | Resistive | Wet method | Single crystals | 11–95 | 0.7/1.7 | [37] |
| CH3NH3PbI0.2Cl2.8 | Resistive | Dip-coating | Thin film | 30–90 | 24/24 | [100] |
| Cs2BiAgBr6 | Resistive | Spin-coating | Thin film | 5–75 | 1.78/0.45 | [38] |
| Cs2InBr5·H2O | PL | crystallization method | Single crystals | 30–80 | 30/not measured | [39] |
3.4.1. Resistive Type Humidity Sensors
Polymer-Based Resistive Humidity Sensors
Ceramic Based Resistive Humidity Sensors
Perovskites and Perovskite-Type Ceramics-Based Resistive Humidity Sensors
3.4.2. Capacitive Type Humidity Sensors
Polymer-Based Capacitive Humidity Sensors
Ceramic-Based Capacitive Humidity Sensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ilin, A.S.; Forsh, P.A.; Martyshov, M.N.; Kazanskii, A.G.; Forsh, E.A.; Kashkarov, P.K. Humidity Sensing Properties of Organometallic Perovskite CH3NH3PbI3. ChemistrySelect 2020, 5, 6705–6708. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent advances in graphene-based humidity sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Fei, T.; Dai, J.; Jiang, K.; Zhao, H.; Zhang, T. Stable cross-linked amphiphilic polymers from a one-pot reaction for application in humidity sensors. Sens. Actuators B Chem. 2016, 227, 649–654. [Google Scholar] [CrossRef]
- Chou, K.-S.; Lee, C.-H.; Liu, B.-T. Effect of Microstructure of ZnO Nanorod Film on Humidity Sensing. J. Am. Ceram. Soc. 2016, 99, 531–535. [Google Scholar] [CrossRef]
- Seiyama, T.; Yamazoe, N.; Arai, H. Ceramic humidity sensors. Sens. Actuators 1983, 4, 85–96. [Google Scholar] [CrossRef]
- McGhee, J.R.; Sagu, J.S.; Southee, D.J.; Wijayantha, K.G.U. Humidity sensing properties of transparent sputter-coated indium-tin oxide and printed polymer structures. IEEE Sens. J. 2018, 18, 7358–7364. [Google Scholar] [CrossRef]
- Giradkar, P.; Rode, V. Formulation and evaluation of poly herbal anti aging face creams. J. Med. Pharm. Allied Sci. 2021, 10, 2920–2923. [Google Scholar] [CrossRef]
- Yang, Y.; Su, G.; Li, Q.; Zhu, Z.; Liu, S.; Zhuo, B.; Li, X.; Ti, P.; Yuan, Q. Performance of the highly sensitive humidity sensor constructed with nanofibrillated cellulose/graphene oxide/polydimethylsiloxane aerogel via freeze drying. RSC Adv. 2021, 11, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.; Correia, D.C.; Lopes, A.C.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Esperança, J.M.S.S.; Laza, J.M.; Vilas, J.L.; et al. Development of poly(vinylidene fluoride)/ionic liquid electrospun fibers for tissue engineering applications. J. Mater. Sci. 2016, 51, 4442–4450. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Liu, Y.; Liu, X.; Fei, T.; Zhang, T. Ultrafast Response Polyelectrolyte Humidity Sensor for Respiration Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 6483–6490. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; de Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, N.; Gokhale, Y.; Trivedi, V.; Sudarshan, V. Perovskites—A complete overview. Int. J. Adv. Res. Electron. Commun. Eng. 2018, 7, 718–723. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- The National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiency Chart|Photovoltaic Research|NREL, National Renewable Energy Laboratory. 2021. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 22 August 2021).
- Park, N.G.; Zhu, K. Scalable fabrication and coating methods for perovskite solar cells and solar modules. Nat. Rev. Mater. 2020, 5, 333–350. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Z.; Huang, J. Light-induced self-poling effect on organometal trihalide perovskite solar cells for increased device efficiency and stability. Adv. Energy Mater. 2015, 5, 1500721. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, R.; Wang, Z.K.; Ma, X.J.; Wang, Q.; Wang, K.L.; Zhang, Y.; Liao, L.S.; Yang, Y. Composition Stoichiometry of Cs 2 AgBiBr 6 Films for Highly Efficient Lead-Free Perovskite Solar Cells. Nano Lett. 2019, 19, 2066–2073. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Gao, Z.; Lv, P.; Yang, Y.; Wu, J.; Hong, J.; Wang, X.; Zhou, Y. Local stress enhanced photocurrent of visible light photo-detection in Cs2AgBiBr6 single crystal. Appl. Phys. Lett. 2019, 115, 131103. [Google Scholar] [CrossRef]
- Xing, G.; Kumar, M.H.; Chong, W.K.; Liu, X.; Cai, Y.; Ding, H.; Asta, M.; Grätzel, M.; Mhaisalkar, S.; Mathews, N.; et al. Solution-Processed Tin-Based Perovskite for Near-Infrared Lasing. Adv. Mater. 2016, 28, 8191–8196. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, F.; Johnston, A.; Gao, C.; Choubisa, H.; Gao, Y.; Wang, Y.K.; Sagar, L.K.; Sun, B.; Li, P.; et al. High Color Purity Lead-Free Perovskite Light-Emitting Diodes via Sn Stabilization. Adv. Sci. 2020, 7, 1903213. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Z.; Zhang, G.; Yang, G.J. Lead-free perovskite [H3NC6H4NH3]CuBr4 with both a bandgap of 1.43 eV and excellent stability. J. Mater. Chem. A 2020, 8, 5484–5488. [Google Scholar] [CrossRef]
- Yu, X.; Tsao, H.N.; Zhang, Z.; Gao, P. Miscellaneous and Perspicacious: Hybrid Halide Perovskite Materials Based Photodetectors and Sensors. Adv. Opt. Mater. 2020, 8, 2001095. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S.; et al. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2AgBiBr6 Film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly Sensitive MoS2 Humidity Sensors Array for Noncontact Sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl phthalocyanine films and their hybrid structures with Pd nanoparticles: Structure and sensing properties. Sensors 2020, 20, 1893. [Google Scholar] [CrossRef]
- Szendrei-Temesi, K.; Sanchez-Sobrado, O.; Betzler, S.B.; Durner, K.M.; Holzmann, T.; Lotsch, B.V. Lithium Tin Sulfide—A High-Refractive-Index 2D Material for Humidity-Responsive Photonic Crystals. Adv. Funct. Mater. 2018, 28, 1705740. [Google Scholar] [CrossRef]
- Yasaei, P.; Behranginia, A.; Foroozan, T.; Asadi, M.; Kim, K.; Khalili-Araghi, F.; Salehi-Khojin, A. Stable and Selective Humidity Sensing Using Stacked Black Phosphorus Flakes. ACS Nano 2015, 9, 9898–9905. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Zhong, L.W.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef]
- Katayama, K.; Hasegawa, H.; Noda, T.; Akiba, T.; Yanagida, H. Effect of alkaline oxide addition on the humidity sensitivitiy of Nb2O5-doped TiO2. Sens. Actuators B Chem. 1990, 2, 143–149. [Google Scholar] [CrossRef]
- Tilley, R.J.D. Perovskites: Structure-Property Relationship; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Agarwal, S.; Sharma, G.L. Humidity sensing properties of (Ba, Sr) TiO3 thin films grown by hydrothermal-electrochemical method. Sens. Actuators B Chem. 2002, 85, 205–211. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Radzi, Z.; Osman, N.A.A. Dielectric and AC conductivity studies of novel porous armalcolite nanocomposite-based humidity sensor. J. Am. Ceram. Soc. 2017, 100, 5131–5140. [Google Scholar] [CrossRef]
- Ye, W.; Cao, Q.; Cheng, X.F.; Yu, C.; He, J.H.; Lu, J.M. A lead-free Cs2PdBr6 perovskite-based humidity sensor for artificial fruit waxing detection. J. Mater. Chem. A 2020, 8, 17675–17682. [Google Scholar] [CrossRef]
- Weng, Z.; Qin, J.; Umar, A.A.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Li, X.; Zheng, L.; Zhan, Y. Lead-Free Cs2BiAgBr6 Double Perovskite-Based Humidity Sensor with Superfast Recovery Time. Adv. Funct. Mater. 2019, 29, 1902234. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Li, W.G.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.F.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Dahl, J.C.; Huang, J.; Osowiecki, W.T.; Swabeck, J.K.; Chan, E.M.; Yang, P.; Alivisatos, A.P. The Making and Breaking of Lead-Free Double Perovskite Nanocrystals of Cesium Silver-Bismuth Halide Compositions. Nano Lett. 2018, 18, 3502–3508. [Google Scholar] [CrossRef]
- Creutz, S.E.; Crites, E.N.; de Siena, M.C.; Gamelin, D.R. Colloidal Nanocrystals of Lead-Free Double-Perovskite (Elpasolite) Semiconductors: Synthesis and Anion Exchange to Access New Materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar] [CrossRef]
- Ravi, V.K.; Singhal, N.; Nag, A. Initiation and future prospects of colloidal metal halide double-perovskite nanocrystals: Cs2AgBiX6 (X = Cl, Br, I). J. Mater. Chem. A 2018, 6, 21666–21675. [Google Scholar] [CrossRef]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; et al. Colloidal Synthesis of Double Perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 Nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989–12995. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Yang, S.; Hong, F.; Sun, L.; Han, P.; Pullerits, T.; Deng, W.; Han, K. Lead-Free Silver-Bismuth Halide Double Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 5359–5363. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Scanlon, D.O.; Neilson, J.R. Perspectives and Design Principles of Vacancy-Ordered Double Perovskite Halide Semiconductors. Chem. Mater. 2019, 31, 1184–1195. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Bordelon, M.M.; Miller, E.M.; Scanlon, D.O.; Neilson, J.R. Defect Tolerance to Intolerance in the Vacancy-Ordered Double Perovskite Semiconductors Cs2SnI6 and Cs2TeI6. J. Am. Chem. Soc. 2016, 138, 8453–8464. [Google Scholar] [CrossRef]
- Rahim, W.; Cheng, A.; Lyu, C.; Shi, T.; Wang, Z.; Scanlon, D.O.; Palgrave, R.G. Geometric analysis and formability of the cubic A2BX6 vacancy-ordered double perovskite structure. Chem. Mater. 2020, 32, 9573–9583. [Google Scholar] [CrossRef]
- Faizan, M.; Bhamu, K.C.; Murtaza, G.; He, X.; Kulhari, N.; AL-Anazy, M.M.; Khan, S.H. Electronic and optical properties of vacancy ordered double perovskites A2BX6 (A = Rb, Cs; B = Sn, Pd, Pt; and X = Cl, Br, I): A first principles study. Sci. Rep. 2021, 11, 6965. [Google Scholar] [CrossRef]
- Karim, M.M.S.; Ganose, A.M.; Pieters, L.; Leung, W.W.W.; Wade, J.; Zhang, L.; Scanlon, D.O.; Palgrave, R.G. Anion Distribution, Structural Distortion, and Symmetry-Driven Optical Band Gap Bowing in Mixed Halide Cs2SnX6 Vacancy Ordered Double Perovskites. Chem. Mater. 2019, 31, 9430–9444. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-Free Halide Double Perovskites via Heterovalent Substitution of Noble Metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P.H. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.G.; Carl, A.D.; Zong, Y.; Grimm, R.L.; Gu, J.; Zeng, X.C.; Zhou, Y.; Padture, N.P. Cesium Titanium(IV) Bromide Thin Films Based Stable Lead-free Perovskite Solar Cells. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef]
- Ghosh, J.; Sellin, P.J.; Giri, P.K. Recent Advances in Lead-free Double Perovskites for X-ray and Photodetection. Nanotechnology 2022, 33, 312001. [Google Scholar] [CrossRef]
- Khalfin, S.; Bekenstein, Y. Advances in lead-free double perovskite nanocrystals, engineering band-gaps and enhancing stability through composition tunability. Nanoscale 2019, 11, 8665–8679. [Google Scholar] [CrossRef]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Filip, M.R.; Hillman, S.; Haghighirad, A.A.; Snaith, H.J.; Giustino, F. Band Gaps of the Lead-Free Halide Double Perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from Theory and Experiment. J. Phys. Chem. Lett. 2016, 7, 2579–2585. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, W.; Wang, J.; Yan, Y. Thermodynamic Stability and Defect Chemistry of Bismuth-Based Lead-Free Double Perovskites. ChemSusChem 2016, 9, 2628–2633. [Google Scholar] [CrossRef]
- Hutter, E.M.; Gélvez-Rueda, M.C.; Bartesaghi, D.; Grozema, F.C.; Savenije, T.J. Band-Like Charge Transport in Cs2AgBiBr6 and Mixed Antimony-Bismuth Cs2AgBi1–xSbxBr6 Halide Double Perovskites. ACS Omega 2018, 3, 11655–11662. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Hu, Q.; Deng, Z.; Hu, M.; Zhao, A.; Zhang, Y.; Tan, Z.; Niu, G.; Wu, H.; Tang, J. X-ray scintillation in lead-free double perovskite crystals. Sci. China Chem. 2018, 61, 1581–1586. [Google Scholar] [CrossRef]
- Wu, C.; Du, B.; Luo, W.; Liu, Y.; Li, T.; Wang, D.; Guo, X.; Ting, H.; Fang, Z.; Wang, S.; et al. Highly Efficient and Stable Self-Powered Ultraviolet and Deep-Blue Photodetector Based on Cs2AgBiBr6/SnO2 Heterojunction. Adv. Opt. Mater. 2018, 6, 1800811. [Google Scholar] [CrossRef]
- Cai, Y.; Xie, W.; Ding, H.; Chen, Y.; Thirumal, K.; Wong, L.H.; Mathews, N.; Mhaisalkar, S.G.; Sherburne, M.; Asta, M. Computational Study of Halide Perovskite-Derived A2BX6 Inorganic Compounds: Chemical Trends in Electronic Structure and Structural Stability. Chem. Mater. 2017, 29, 7740–7749. [Google Scholar] [CrossRef]
- Sakai, N.; Haghighirad, A.A.; Filip, M.R.; Nayak, P.K.; Nayak, S.; Ramadan, A.; Wang, Z.; Giustino, F.; Snaith, H.J. Solution-Processed Cesium Hexabromopalladate(IV), Cs2PdBr6, for Optoelectronic Applications. J. Am. Chem. Soc. 2017, 139, 6030–6033. [Google Scholar] [CrossRef]
- Solans, X.; Moron, M.C.; Palacio, F. Structures of Rb 2 [InCl 5 (H 2 O)] and Cs 2 [InCl 5 (H 2 O)]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 965–967. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Filip, M.R.; Giustino, F. Computational Screening of Homovalent Lead Substitution in Organic-Inorganic Halide Perovskites. J. Phys. Chem. C 2016, 120, 166–173. [Google Scholar] [CrossRef]
- Park, B.W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M.J. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef]
- Serrano-Lujan, L.; Espinosa, N.; Larsen-Olsen, T.T.; Abad, J.; Urbina, A.; Krebs, F.C. Tin- and lead-based perovskite solar cells under scrutiny: An environmental perspective. Adv. Energy Mater. 2015, 5, 1501119. [Google Scholar] [CrossRef]
- Liang, L.; Gao, P. Lead-Free Hybrid Perovskite Absorbers for Viable Application: Can We Eat the Cake and Have It too? Adv. Sci. 2018, 5, 1700331. [Google Scholar] [CrossRef]
- Abate, A. Perovskite Solar Cells Go Lead Free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
- Maughan, A.E.; Kurzman, J.A.; Neilson, J.R. Hybrid inorganic-organic materials with an optoelectronically active aromatic cation: (C7H7)2SnI6 and C7H7PbI3. Inorg. Chem. 2015, 54, 370–378. [Google Scholar] [CrossRef]
- Elsenety, M.M.; Kaltzoglou, A.; Antoniadou, M.; Koutselas, I.; Kontos, A.G.; Falaras, P. Synthesis, characterization and use of highly stable trimethyl sulfonium tin(IV) halide defect perovskites in dye sensitized solar cells. Polyhedron 2018, 150, 83–91. [Google Scholar] [CrossRef]
- Qiu, X.; Jiang, Y.; Zhang, H.; Qiu, Z.; Yuan, S.; Wang, P.; Cao, B. Lead-free mesoscopic Cs2SnI6 perovskite solar cells using different nanostructured ZnO nanorods as electron transport layers. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 587–591. [Google Scholar] [CrossRef]
- Lee, B.; Krenselewski, A.; Baik, S.I.; Seidman, D.N.; Chang, R.P.H. Solution processing of air-stable molecular semiconducting iodosalts, Cs2SnI6-: XBrx, for potential solar cell applications. Sustain. Energy Fuels 2017, 1, 710–724. [Google Scholar] [CrossRef]
- Kulwicki, B.M. Humidity Sensors. J. Am. Ceram. Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Yadav, B.C.; Singh, M.; Dwivedi, C.D. Optical characterization and humidity sensing properties of praseodymium oxide. Sens. Transducers 2011, 125, 68–75. [Google Scholar]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-fast response humidity sensor based on La0.7Sr0.3MnO3 nanocrystals prepared by PVP-assisted sol-gel method. Sens. Actuators B Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Sheng, M.; Gu, L.; Kontic, R.; Zhou, Y.; Zheng, K.; Chen, G.; Mo, X.; Patzke, G.R. Humidity sensing properties of bismuth phosphates. Sens. Actuators B Chem. 2012, 166, 642–649. [Google Scholar] [CrossRef]
- Anderson, J.H.; Parks, G.A.; Andersonl, J.H. The Electrical Conductivity of Silica Gel in the Presence of Adsorbed Water, (n.d.). Available online: https://pubs.acs.org/sharingguidelines (accessed on 30 October 2021).
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Kim, J.G. Electrical properties and fabrication of porous BaTiO3-based ceramics. J. Mater. Sci. Lett. 2002, 21, 477–479. [Google Scholar] [CrossRef]
- Perovskite, H.; Investigated, N. Photoluminescent Spectral Broadening of Lead. Molecules 2020, 25, 1151. [Google Scholar]
- Jong, U.G.; Yu, C.J.; Ri, J.S.; Kim, N.H.; Ri, G.C. Influence of halide composition on the structural, electronic, and optical properties of mixed CH3NH3Pb(I1-xBrx)3 perovskites calculated using the virtual crystal approximation method. Phys. Rev. B 2016, 94, 125139. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Ding, W.; Feng, L.; Gao, Y.; Guo, Z. Formability of ABX3 (X = F, Cl, Br, I) halide perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2008, 64, 702–707. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Liu, L.; Zhang, H.; Zheng, W.; Wang, Y.; Huang, H.; Wang, Z.; Wang, C. Humidity sensor based on LiCl-doped ZnO electrospun nanofibers. Sens. Actuators B Chem. 2009, 141, 404–409. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Bhuyan, S.; Shah, N.F.A.; Radzi, Z.; Osman, N.A.A. Design and development for capacitive humidity sensor applications of lead-free Ca, Mg, Fe, Ti-oxides-based electro-ceramics with improved sensing properties via physisorption. Sensors 2016, 16, 1135. [Google Scholar] [CrossRef]
- Cankurtaran, H.; Yazici, O.; Dinc, S.; Karaman, F. Humidity sensitive properties of electronically conductive poly(diphenylamine sulfonic acid) and its block copolymer and blends. Int. J. Electrochem. Sci. 2013, 8, 3265–3278. Available online: https://www.researchgate.net/publication/277370235_Humidity_Sensitive_Properties_of_Electronically_Conductive_Polydiphenylamine_sulfonic_acid_and_Its_Block_Copolymer_and_Blends (accessed on 26 October 2021).
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.Z.; Qu, W.; Du, B.; et al. An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B Chem. 2021, 337, 129772. [Google Scholar] [CrossRef]
- Mahmoud, A.E.R.; Viola, G.; Afify, A.S.; Babeer, A.M.; Ferraris, M. Processing, structural and humidity sensing properties of PbTiO3 ceramic synthesized by solid state reaction. J. Porous Mater. 2020, 27, 947–958. [Google Scholar] [CrossRef]
- Bauskar, D.; Kale, B.B.; Patil, P. Synthesis and humidity sensing properties of ZnSnO3 cubic crystallites. Sens. Actuators B Chem. 2012, 161, 396–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cheng, X.; Feng, C.; Chen, L.; Zhou, J.; Ruan, S. A novel humidity sensor based on NaTaO3 nanocrystalline. Sens. Actuators B Chem. 2012, 174, 485–489. [Google Scholar] [CrossRef]
- Ren, K.; Huang, L.; Yue, S.; Lu, S.; Liu, K.; Azam, M.; Wang, Z.; Wei, Z.; Qu, S.; Wang, Z. Turning a disadvantage into an advantage: Synthesizing high-quality organometallic halide perovskite nanosheet arrays for humidity sensors. J. Mater. Chem. C 2017, 5, 2504–2508. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Pascal-Delannoy, F.; Sorli, B.; Boyer, A. Quartz Crystal Microbalance (QCM) used as humidity sensor. Sens. Actuators A Phys. 2000, 84, 285–291. [Google Scholar] [CrossRef]
- Lee, C.W.; Fung, Y.S.; Fung, K.W. A piezoelectric crystal detector for water in gases. Anal. Chim. Acta 1982, 135, 277–283. [Google Scholar] [CrossRef]
- Radeva, E.; Bobev, K.; Spassov, L. Study and application of glow discharge polymer layers as humidity sensors. Sens. Actuators B Chem. 1992, 8, 21–25. [Google Scholar] [CrossRef]
- Sun, H.T.; Cheng, Z.T.; Yao, X.; Wlodarski, W. Humidity sensor using sol—gel-derived silica coating on quartz crystal. Sens. Actuators B Chem. 1993, 13, 107–110. [Google Scholar] [CrossRef]
- Randin, J.P.; Züllig, F. Relative humidity measurement using a coated piezoelectric quartz crystal sensor. Sens. Actuators 1987, 11, 319–328. [Google Scholar] [CrossRef]
- Delapierre, G.; Grange, H.; Chambaz, B.; Destannes, L. Polymer-based capacitive humidity sensor: Characteristics and experimental results. Sens. Actuators 1983, 4, 97–104. [Google Scholar] [CrossRef]
- Koshigoe, M.; Shiota, I.; Shinohara, Y.; Imai, Y.; Nishida, I.A. Preparation and Thermoelectric Properties of Irsb3. In Functionally Graded Materials 1996; Elsevier: Amsterdam, The Netherlands, 1997; pp. 581–586. [Google Scholar] [CrossRef]
- Soni, A.K.; Joshi, R.; Ningthoujam, R.S. Hot Injection Method for Nanoparticle Synthesis: Basic Concepts, Examples and Applications. In Handbook on Synthesis Strategies for Advanced Materials; Springer: Singapore, 2021; pp. 383–434. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A review on molten salt synthesis of metal oxide nanomaterials: Status, opportunity, and challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Schaffer, J.; Herman, C. Precipitation Reactions—Chemistry LibreTexts, LibreTexts. 2021. Available online: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Main_Group_Reactions/Reactions_in_Aqueous_Solutions/Precipitation_Reactions (accessed on 3 June 2022).
- Bretos, I.; Jiménez, R.; Ricote, J.; Calzada, M.L. Low-temperature crystallization of solution-derived metal oxide thin films assisted by chemical processes. Chem. Soc. Rev. 2018, 47, 291–308. [Google Scholar] [CrossRef]
- Traversa, E.; Sadaoka, Y.; Carotta, M.C.; Martinelli, G. Environmental monitoring field tests using screen-printed thick-film sensors based on semiconducting oxides. Sens. Actuators B Chem. 2000, 65, 181–185. [Google Scholar] [CrossRef]
- Kunte, G.V.; Shivashankar, S.A.; Umarji, A.M. Humidity sensing characteristics of hydrotungstite thin films. Bull. Mater. Sci. 2008, 31, 835–839. [Google Scholar] [CrossRef]
- Mamishev, A.V.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef]
- Moneyron, J.E.; de Roy, A.; Besse, J.P. Realisation of a Humidity Sensor Based on the Protonic Conductor Zn2Al(OH)6Cl.nH2O, Microelectron. Int. An Int. J. 1991, 8, 26–31. [Google Scholar] [CrossRef]
- Kim, E.; Kim, S.Y.; Jo, G.; Kim, S.; Park, M.J. Colorimetric and resistive polymer electrolyte thin films for real-time humidity sensors. ACS Appl. Mater. Interfaces 2012, 4, 5179–5187. [Google Scholar] [CrossRef]
- Anbia, M.; Fard, S.E.M.; Shafiei, K.; Hassanzadeh, M.A.; Mayahipour, A. Humidity sensing properties of the sensor based on V-doped nanoporous Ti0.9Sn0.1O2 thin film. Chin. J. Chem. 2012, 30, 842–846. [Google Scholar] [CrossRef]
- WTai, P.; Kim, J.G.; Oh, J.H.; Lee, C.; Park, D.W.; Ahn, W.S. Humidity sensing properties of nanostructured- bilayered potassium tantalate: Titania films. J. Mater. Sci. Mater. Electron. 2005, 16, 517–521. [Google Scholar] [CrossRef]
- Racheva, T.M.; Stambolova, I.D.; Donchev, T. Humidity-sensitive characteristics of SnO2-Fe2O3 thin films prepared by spray pyrolysis. J. Mater. Sci. 1994, 29, 281–284. [Google Scholar] [CrossRef]
- Niranjan, R.S.; Sathaye, S.D.; Mulla, I.S. Bilayered tin oxide: Zirconia thin film as a humidity sensor. Sens. Actuators B Chem. 2001, 81, 64–67. [Google Scholar] [CrossRef]
- Harris, K.D.; Huizinga, A.; Brett, M.J. High-speed porous thin film humidity sensors. Electrochem. Solid-State Lett. 2002, 5, H27–H29. [Google Scholar] [CrossRef]
- Bagum, N.; Gafur, M.A.; Bhuiyan, A.H.; Saha, D.K. MgCl2 doped CuxZn1-xFe2O4 ferrite humidity sensors. Phys. Status Solidi Appl. Mater. Sci. 2010, 207, 986–992. [Google Scholar] [CrossRef]
- Koo, J.S.; Gong, M.S. Preparation and humidity-sensitive properties of novel photocurable sulfonated polyimides. Macromol. Res. 2012, 20, 1226–1233. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M. Humidity sensors based on polymer thin films. Sens. Actuators B Chem. 1996, 35, 85–90. [Google Scholar] [CrossRef]
- Sakai, Y. Humidity sensors using chemically modified polymeric materials. Sens. Actuators B Chem. 1993, 13, 82–85. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]
- Prudenziati, M. Thick-film technology. Sens. Actuators A Phys. 1990, 25, 227–234. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Galvagno, S.; Pace, C.; Patanè, S.; Arena, A. Humidity sensing properties of Li–iron oxide based thin films. Sens. Actuators B Chem. 2001, 73, 89–94. [Google Scholar] [CrossRef]
- Sin, N.D.M.; Mamat, M.H.; Malek, M.F.; Rusop, M. Fabrication of nanocubic ZnO/SnO2 film-based humidity sensor with high sensitivity by ultrasonic-assisted solution growth method at different Zn:Sn precursor ratios. Appl. Nanosci. 2013, 4, 829–838. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, M.C.; Zhen, C. Humidity sensors with reactively evaporated Al2O3 films as porous dielectrics. Sens. Actuators B Chem. 1990, 2, 167–171. [Google Scholar] [CrossRef]
- Nitta, T.; Terada, J.; Fukushima, F. Multifunctional Ceramic Sensors: Humidity-Gas Sensor And Temperature-Humidity Sensor. IEEE Trans. Electron Devices 1982, 29, 95–101. [Google Scholar] [CrossRef]
- Yokomizo, Y.; Uno, S.; Harata, M.; Hiraki, H.; Yuki, K. Microstructure and humidity-sensitive properties of ZnCr2O4-LiZnVO4 ceramic sensors. Sens. Actuators 1983, 4, 599–606. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Tseng, T.; Chang, D.A. Electrical Properties of TiO2-K2Ti6O13 Porous Ceramic Humidity Sensor. J. Am. Ceram. Soc. 1990, 73, 1992–1998. [Google Scholar] [CrossRef]
- Wu, L.; Wu, C.C.; Her, J.C. Ni(Al, Fe)2O4-TiO2 ceramic humidity sensors. J. Mater. Sci. 1991, 26, 3874–3878. [Google Scholar] [CrossRef]
- Nenov, T.; Yordanov, S. Ceramic sensor device materials. Sens. Actuators B. Chem. 1992, 8, 117–122. [Google Scholar] [CrossRef]
- Nitta, T.; Terada, Z.; Hayakawa, S. Humidity-Sensitive Electrical Conduction of MgCr2O4-TiO2 Porous Ceramics. J. Am. Ceram. Soc. 1980, 63, 295–300. [Google Scholar] [CrossRef]
- Hwang, T.J.; Choi, G.M. Humidity Response Characteristics of Barium Titanate. J. Am. Ceram. Soc. 1993, 76, 766–768. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Liu, H.; Xiong, Y.; Zhang, Z. A rapid-response humidity sensor based on BaNbO3 nanocrystals. Sens. Actuators B Chem. 2009, 136, 128–132. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Zhang, Y.; Zheng, X. Humidity sensing properties of Bi0.5(Na0.85K0.15)0.5Ti0.97Zr0.03O3 microspheres: Effect of A and B sites co-substitution. Sens. Actuators B Chem. 2014, 190, 305–310. [Google Scholar] [CrossRef]
- Feng, C.; Ruan, S.; Li, J.; Zou, B.; Luo, J.; Chen, W.; Dong, W.; Wu, F. Ethanol sensing properties of LaCoxFe1−xO3 nanoparticles: Effects of calcination temperature, Co-doping, and carbon nanotube-treatment. Sens. Actuators B Chem. 2011, 155, 232–238. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Hayashi, T.; Ito, A.; Matsuguchi, M.; Sadaoka, Y.; Sakai, Y. A thin film polyimide based capacitive type relative humidity sensor. Sens. Actuators B. Chem. 1993, 13, 89–91. [Google Scholar] [CrossRef]
- Xu, W.; Li, F.; Cai, Z.; Wang, Y.; Luo, F.; Chen, X. An ultrasensitive and reversible fluorescence sensor of humidity using perovskite CH3NH3PbBr3. J. Mater. Chem. C 2016, 4, 9651–9655. [Google Scholar] [CrossRef]
- Park, B.W.; Seok, S.I. Intrinsic Instability of Inorganic–Organic Hybrid Halide Perovskite Materials. Adv. Mater. 2019, 31, 1805337. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-Induced Degradation via Grain Boundaries of HC(NH2)2PbI3 Planar Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Sun, S. Synthesis, Characterization and Properties of Hybrid Organic-Inorganic Perovskites for Photovoltaic Applications; University of Cambridge: Cambridge, UK, 2017. [Google Scholar]
- Steele, J.A.; Pan, W.; Martin, C.; Keshavarz, M.; Debroye, E.; Yuan, H.; Banerjee, S.; Fron, E.; Jonckheere, D.; Kim, C.W.; et al. Photophysical Pathways in Highly Sensitive Cs2AgBiBr6 Double-Perovskite Single-Crystal X-Ray Detectors. Adv. Mater. 2018, 30, 1804450. [Google Scholar] [CrossRef] [PubMed]
- Fenner, R.; Zdankiewicz, E. Micromachined Water Vapor Sensors: A Review of Sensing Technologies. IEEE Sens. J. 2001, 1, 309–317. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zafar, Q.; Sulaiman, K.; Akram, R.; Karimov, K.S. A humidity sensing organic-inorganic composite for environmental monitoring. Sensors 2013, 13, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Misevich, K.W. Capacitive Humidity Transducer. Digit. Comput. Cycloconverter Oper. 1969, IECI-16, 6–12. [Google Scholar] [CrossRef]
- Laconte, J.; Wilmart, V.; Raskin, J.P.; Flandre, D. Capacitive humidity sensor using a polyimide sensing film. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS 2003, Cannes, France, 7 May 2003; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2003; pp. 223–228. [Google Scholar] [CrossRef]
- Denton, D.D.; Ho, C.N.; He, S.G. A Solid-State Relative Humidity Measurement System. IEEE Trans. Instrum. Meas. 1990, 39, 508–511. [Google Scholar] [CrossRef]
- Laville, C.; Pellet, C.; N’Kaoua, G. Interdigitated humidity sensors for a portable clinical microsystem. IEEE Trans. Biomed. Eng. 2000, 49, 572–577. [Google Scholar] [CrossRef]
- Dokmeci, M.; Najafi, K. High-sensitivity polyimide humidity sensor for monitoring hermetic micropackages; In Proceedings of the Technical Digest. IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 99CH36291), Orlando, FL, USA, 21–21 January 1999; pp. 279–284. [Google Scholar] [CrossRef]
- Yang, M.J.; Casalbore-Miceli, G.; Camaioni, N.; Mari, C.M.; Sun, H.; Li, Y.; Ling, M. Characterization of capacitive humidity sensors based on doped poly(propargyl-alcohol). J. Appl. Electrochem. 2000, 30, 753–756. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Shinmoto, M.; Sadaoka, Y.; Kuroiwa, T.; Sakai, Y. Effect of cross-linking degree of PVCA film on the characteristics of capacitive-type humidity sensor. In Proceedings of the International Solid-State Sensors and Actuators Conference-TRANSDUCERS ’95, Stockholm, Sweden, 25–29 June 1995; pp. 825–828. [Google Scholar] [CrossRef]
- Yeow, J.T.W.; She, J.P.M. Carbon nanotube-enhanced capillary condensation for a capacitive humidity sensor. Nanotechnology 2006, 17, 5441–5448. [Google Scholar] [CrossRef]
- Shamala, K.S.; Murthy, L.C.S.; Radhakrishna, M.C.; Rao, K.N. Characterization of Al2O3 thin films prepared by spray pyrolysis method for humidity sensor. Sens. Actuators A 2006, 135, 552–557. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, K.; Zhou, Y.; Li, J.; Mo, X.; Patzke, G.R.; Chen, G. Humidity sensors based on ZnO/TiO2 core/shell nanorod arrays with enhanced sensitivity. Sens. Actuators B Chem. 2011, 159, 1–7. [Google Scholar] [CrossRef]

| Advantages | Disadvantages | Applications | |
|---|---|---|---|
| Capacitive |
|
|
|
| Resistive |
|
|
|
| Thermal conductivity |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambwe, K.; Ross, N.; Baker, P.; Bui, T.-T.; Goubard, F. Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials. Materials 2022, 15, 4146. https://doi.org/10.3390/ma15124146
Tambwe K, Ross N, Baker P, Bui T-T, Goubard F. Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials. Materials. 2022; 15(12):4146. https://doi.org/10.3390/ma15124146
Chicago/Turabian StyleTambwe, Kevin, Natasha Ross, Priscilla Baker, Thanh-Tuân Bui, and Fabrice Goubard. 2022. "Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials" Materials 15, no. 12: 4146. https://doi.org/10.3390/ma15124146
APA StyleTambwe, K., Ross, N., Baker, P., Bui, T.-T., & Goubard, F. (2022). Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials. Materials, 15(12), 4146. https://doi.org/10.3390/ma15124146








